Abstract
The present study explored the immobilization of laccase onto iron magnetic nanoparticles (MNPs) to enhance its enzymatic properties and applications. The immobilization process was optimized using Box–Behnken design (BBD). BBD showed significance towards the quadratic model with experimental data. Maximum laccase activity recovery (99%) of the predicted model was observed at 0.75 mg/mL of laccase concentration, 200 mg/mL of MNPs, 0.3% cross linking with carbodiimide, and 3 h of cross-linking time. The magnetization activity of MNPs (8 emu/g) and the immobilized laccase with MNPs (4 emu/g) was analyzed using vibrating sample magnetometer (VSM). Maximum activity of immobilized laccase was observed at pH 7.0 and 55 °C. The immobilized laccase has greater stability (100 h) and significant chlorpyrifos (pesticide) degradation activity. High-performance liquid chromatography (HPLC) results confirmed the degraded metabolic products of chlorpyrifos. In all, the immobilized laccase was superior to free laccase, showing promising structural and application characteristics.
Keywords: Immobilization, Laccase, Magnetic nanoparticles, Optimization, Pesticides
Introduction
Synthetic pesticides have become an inevitable part of modern agriculture, which contributes to their extensive distribution throughout the ecosystem (Kumar et al. 2018). Although the use of pesticides has contributed to better crop yields, the dispersion of their residues in the environment is disastrous to aquatic as well as to terrestrial eco systems (Li 2018; Villarreal-Chiu et al. 2017). Exposure to pesticides causes serious health problems, such as neurotoxic disorders, and can lead to death (Pereira et al. 2015; Simonelli et al. 2007). Researchers have developed a wide range of physico-chemical methods to overcome the adverse effects of pesticides on the ecosystem (Mir-Tutusaus et al. 2018; Maqbool et al. 2016). However, these physico-chemical methods are inefficient, expensive, and time consuming (Zeng et al. 2017). Thus, there is a need to develop simple, highly efficient, and environmentally sustainable processes for the removal of pesticides from the environment.
Enzymatic catalysis is considered as a simple, eco-friendly, green bioremediation method for the degradation and/or detoxification of pesticides from soil and water (Ahmed et al. 2017; Bilal et al. 2017a, b). Several studies reported that enzyme-based degradation methods have maximum degradation efficiency and stability against pH, temperature, and salinity (Kupski et al. 2019). Thus, enzyme-mediated green remediation method shave found attention as an attractive alternative to the physico-chemical methods. Among the microbial enzymes, lignolytic laccases have gained much popularity due to their wide range of substrate and environmental applications (Bilal et al. 2019; Bilal et al. 2017a, b).
Microbial laccases (E.C. 1.10.3.2) are lignolytic multi-copper containing monomeric extra-cellular glycoproteins that originate from various bacteria, fungi, and plants, and are abundant in nature. Because of their wide distribution and abundance, laccases have been widely applied in food, paper and pulp, and textile industries (Gursharan et al. 2019; Nandhini et al. 2019; Jordan et al. 2018; Bilal et al. 2017a). Several studies reported the laccase-mediated bioremediation of a wide range of pollutants including aromatics, xenobiotics, and phenolics (Vera et al. 2019; Antecka et al. 2018; Zdarta et al. 2018; Zeng et al. 2017). Recent studies have addressed the improvement of laccase efficiency, through its immobilization, for the bioremediation of different groups of environmental contaminants (Bilal et al. 2019). Additionally, it has been reported that immobilization enhances the enzyme’s properties (Chen et al. 2016) and increases its stability (Lai et al. 2019).
The immobilization of enzymes onto nanopolymers and nanoparticles has received great interest because of the small size of the enzyme carrier materials, which is known to improve the efficiency of the immobilized enzymes (Govarthanan et al. 2020). Magnetic nanoparticles represent materials with required magnetic properties mostly used for environmental applications. Magnetic iron oxide nanoparticles (MNPs) are inexpensive to produce and exhibit sufficient physical and chemical stability, in addition to being biocompatible and environmentally safe. MNPs also confer specific applications like magnetic separation and pesticide and dye degradation (Laurent et al. 2008; Gupta and Gupta 2005). Of the several types of nanoparticles, MNPs are potential candidates for enzyme immobilization due to their low toxicity and ease of recovery. Amin et al. (2016) and Dyla et al. (2003) reported that enzyme immobilized on MNPs showed enhanced activity and stability. Also, immobilized laccase enzyme has been reported to degrade a wide range of dyes such as Direct Blue 78, Acid Blue 225, Reactive Red 195, Acid Blue 74, and Phenol Red (Jořenek and Zajoncova 2015) and pesticides (Das et al. 2020). Thus, the present study was aimed to (i) synthesize MNPs for laccase immobilization, (ii) optimize immobilization efficiency, (iii) compare the laccase properties before and after immobilization, (iv) characterize the enzyme immobilized onto the MNPs, and (v) evaluate pesticide removal by the immobilized laccase.
Materials and methods
Materials
Laccase from Bacillus sp., (Accession number: MF957300) (Srinivasan et al. 2019), carbodiimide, and 2, 2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) were purchased from Hi-Media (Mumbai, India). Chlorpyrifos (20% EC) was obtained from Agro-Service Center, Mallasamudram, Tamil Nadu, and India.
Preparation of MNPs
MNPs (Fe3O4) were prepared by co-precipitating Fe2+ and Fe3+ ions. Briefly, ferric and ferrous chlorides were dissolved in a 2:1 molar ratio in water, and chemical precipitation was achieved at 25–30 °C under vigorous mixing by adding 28% NH4OH solution. The resultant precipitate was heated at 100 °C for 15 min and washed three times with water and once with anhydrous ethanol. The particles were dried for 24 h (Govarthanan et al. 2020; Selvam et al. 2016) for further use.
Optimization of laccase immobilization
The purified MNPs (5 mg/mL) were suspended in 20 mL of acetate buffer solution (pH 4.0) with a designed amount of laccase solution (0.75 mg/mL) and the immobilization process was performed in a shaking incubator at 37 °C for 30–50 min. After incubation, the supernatant was collected for further studies. In addition, to explore the optimal immobilization conditions, the response surface methodology (RSM) based on Box–Behnken design (BBD) was adopted using the Design Expert software (9.0.0.7 trial version). Four factors [enzyme concentration, magnetic nanoparticle concentration, cross-linking (carbodiimide %), and cross-linking time] were optimized for immobilization. The results were assessed by applying the coefficient of determination (R2), analysis of variance, and response plots. RSM was employed with the most widely used second‐order polynomial equation developed to fit the experimental results and to identify the relevant model terms:
| 1 |
where Y is the predicted response, β0, βi, and βij are the constant regression coefficients of the model, and Xi and Xj represent independent variables.
Characterization of MNPs
The morphology of the MNPs and their elemental composition before and after laccase cross-linking were evaluated using a scanning electron microscope coupled with an energy-dispersive spectroscope (SEM–EDS; Jeol JSM 6390). The functional groups of the MNPs and immobilized laccase were analyzed using Fourier-Transform infrared spectroscopy (FT-IR, Nicolet iS5, Thermo). The magnetic of the synthesized nanoparticles were studied using a vibrating sample magnetometer (VSM, Lake Shore, Westerville, OH, USA) at room temperature.
Determination of free enzyme and immobilized laccase activity
Free enzyme and immobilized laccase activity was determined according to Wolfenden and Wilson (1982) using ABTS (2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) as substrate. One unit of laccase activity was defined as the amount of required enzyme to oxidize one µmol of ABTS per min under the assay conditions.
Effect of pH, temperature, and storage time on the stability of immobilized laccase
The effect of pH on free and immobilized laccase activity was analyzed by measuring the reaction at different pH levels (5–11). The effects of incubation temperature on free and immobilized laccase activities were determined by performing the reaction temperature ranging at 40–80 °C. The stabilities of free and immobilized laccase were determined after storage in phosphate buffer (0.02 mol/L, pH 7.0) at 4 °C for 100 h. Retained activities were measured as described above, and the activity of each preparation was expressed as a percentage of its retained activity compared to its initial activity.
Degradation of pesticide by immobilized MNPs’ laccase
The pesticide degradation ability of immobilized laccase was tested according to Das et al. (2017) with minor modifications. Briefly, 20 mg of immobilized laccase was mixed with 100 mL of chlorpyrifos (Tricel) at a final concentration of 1000 mg/L. The reaction mixer was incubated in a shaking incubator at 300 rpm for 18 h. After the incubation, 10 mL of sample was collected and extracted with hexane in a 1:1 ratio and stored in amber colored bottles for high-performance liquid chromatography (HPLC, LC-20AD, Shimadzu, Tokyo, Japan) analysis according to Srinivasan et al. (2019).
Statistical analysis
All the experiments presented in this study were mean values of three replicates, and standard deviations were used to analyze the data. Effect of pH, temperature, and storage time on the stability of immobilized laccase results were subjected to two-way analysis of variance (ANOVA) using SPSS software v 12 (Chicago, USA).
Results and discussion
Optimization of the conditions for laccase immobilization
The laccase immobilization process is influenced by several biotic (laccase concentration) and abiotic factors (pH, temperature, incubation time) (Liu et al. 2012). Thus, BBD was designed to determine the optimal conditions for the immobilization of laccase. The BBD model is shown in Table 1. The BBD based on the analysis of variance of the quadratic regression model demonstrated high significance (Table 2). The significance of the model was further validated by the Fisher’s F test with a very low probability value (F value = 397.82). Values of ‘Probability > F’ (0.0500) indicate that the model was significant. The predicted R2 (0.9856) and adjusted R2 (0.9950) values for optimal conditions were in reasonable agreement with the value of R2 (0.9856), which is closer to 1.0, indicating better fit of the model in the experimental data. There is only a 0.01% probability that a model F value could occur because of noise. The model was further validated by sequential model sum of squares, lack of fit tests, and model summary statistics. The 3D plots generated showed significant relationship among enzyme concentration, MNPs’ concentration, cross-linking (carbodiimide %), and cross-linking time (Fig. 1). The model predicted a maximum laccase activity recovery of 99% for an enzyme concentration of 0.75 mg/mL, MNPs concentration of 200 mg/mL, cross linking with carbodiimide at 0.3%, and a cross-linking time of 3 h. The predicted model values were in good agreement with the values measured in these experiments, mitigating the validity of the response model and the necessity for optimal conditions. The graphs highlighted the parts played by the variables for the production of laccase. The coefficients of the regression equation were calculated, and the following regression equation was obtained:
| 2 |
where Y is laccase activity, A is enzyme concentration, B is the cross-linked MNPs concentration (carbodiimide %), and D is the cross-linking time. High degrees of similarity of experimental values were observed, reflecting the precision and applicability of RSM to optimize enzyme immobilization (Soozanipour et al. 2015; Halim et al. 2009; Krishna et al. 2001). Our results are in agreement with earlier studies that reported laccase concentration and immobilization time to remarkably influence the immobilization of laccase (Wen et al. 2019; Huang et al. 2017; Zheng et al. 2016; Lin et al. 2007).
Table 1.
Box–Behnken design for the variables and the experimental observed responses
| Run | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Response 1 |
|---|---|---|---|---|---|
| A: Enzyme conc | B: MNPs conc | C: Cross linking (carbodiamide) | D: Time course of cross linking | Enzyme activity | |
| mg/mL | mg/mL | % | h | % | |
| 1 | 0.75 | 200 | 0.3 | 3 | 99 |
| 2 | 0.75 | 300 | 0.1 | 3 | 70 |
| 3 | 0.75 | 100 | 0.3 | 1 | 68 |
| 4 | 0.75 | 200 | 0.3 | 3 | 99 |
| 5 | 0.75 | 200 | 0.3 | 3 | 99 |
| 6 | 0.5 | 200 | 0.1 | 3 | 54 |
| 7 | 0.75 | 100 | 0.3 | 5 | 74 |
| 8 | 0.75 | 100 | 0.1 | 3 | 62 |
| 9 | 0.75 | 200 | 0.1 | 5 | 69 |
| 10 | 0.5 | 100 | 0.3 | 3 | 57 |
| 11 | 0.75 | 200 | 0.3 | 3 | 99 |
| 12 | 0.5 | 200 | 0.3 | 1 | 63 |
| 13 | 0.5 | 200 | 0.5 | 3 | 71 |
| 14 | 1 | 100 | 0.3 | 3 | 70 |
| 15 | 0.75 | 200 | 0.3 | 3 | 99 |
| 16 | 0.75 | 300 | 0.3 | 5 | 89 |
| 17 | 1 | 200 | 0.5 | 3 | 82 |
| 18 | 1 | 200 | 0.3 | 5 | 82 |
| 19 | 1 | 200 | 0.3 | 1 | 78 |
| 20 | 0.75 | 200 | 0.5 | 1 | 81 |
| 21 | 0.75 | 200 | 0.5 | 5 | 92 |
| 22 | 0.75 | 200 | 0.1 | 1 | 73 |
| 23 | 0.5 | 300 | 0.3 | 3 | 69 |
| 24 | 0.75 | 100 | 0.5 | 3 | 73 |
| 25 | 1 | 200 | 0.1 | 3 | 70 |
| 26 | 1 | 300 | 0.3 | 3 | 83 |
| 27 | 0.5 | 200 | 0.3 | 5 | 72 |
| 28 | 0.75 | 300 | 0.3 | 1 | 84 |
| 29 | 0.75 | 300 | 0.5 | 3 | 92 |
Table 2.
Analysis of variance (ANOVA) for the response surface quadratic model
| Source | Sum of squares | df | Mean square | F value | p value | |
|---|---|---|---|---|---|---|
| Model | 4906.49 | 14 | 350.46 | 397.82 | < 0.0000 | significant |
| A-Enzyme conc | 520.08 | 1 | 520.08 | 590.36 | < 0.0001 | |
| B-MNPs conc | 574.08 | 1 | 574.08 | 651.66 | < 0.0001 | |
| C-Cross linking (carbodiamide) | 720.75 | 1 | 720.75 | 818.15 | < 0.0001 | |
| D-Time course of cross linking | 80.08 | 1 | 80.08 | 90.91 | < 0.0001 | |
| AB | 0.2500 | 1 | 0.2500 | 0.2838 | 0.6026 | |
| AC | 6.25 | 1 | 6.25 | 7.09 | 0.0185 | |
| AD | 6.25 | 1 | 6.25 | 7.09 | 0.0185 | |
| BC | 30.25 | 1 | 30.25 | 34.34 | < 0.0001 | |
| BD | 0.2500 | 1 | 0.2500 | 0.2838 | 0.6026 | |
| CD | 56.25 | 1 | 56.25 | 63.85 | < 0.0001 | |
| A2 | 1920.82 | 1 | 1920.82 | 2180.39 | < 0.0001 | |
| B2 | 966.77 | 1 | 966.77 | 1097.41 | < 0.0001 | |
| C2 | 1006.77 | 1 | 1006.77 | 1142.82 | < 0.0001 | |
| D2 | 410.82 | 1 | 410.82 | 466.34 | < 0.0000 | significant |
| Residual | 12.33 | 14 | 0.8810 | – | – | |
| Lack of fit | 12.33 | 10 | 1.23 | – | – | |
| Pure error | 0.0000 | 4 | 0.0000 | – | – | |
| Cor total | 4918.83 | 28 |
Fig. 1.
Response 3-D plots of laccase immobilization magnetic nanoparticles under BBD optimized conditions
Characterization studies
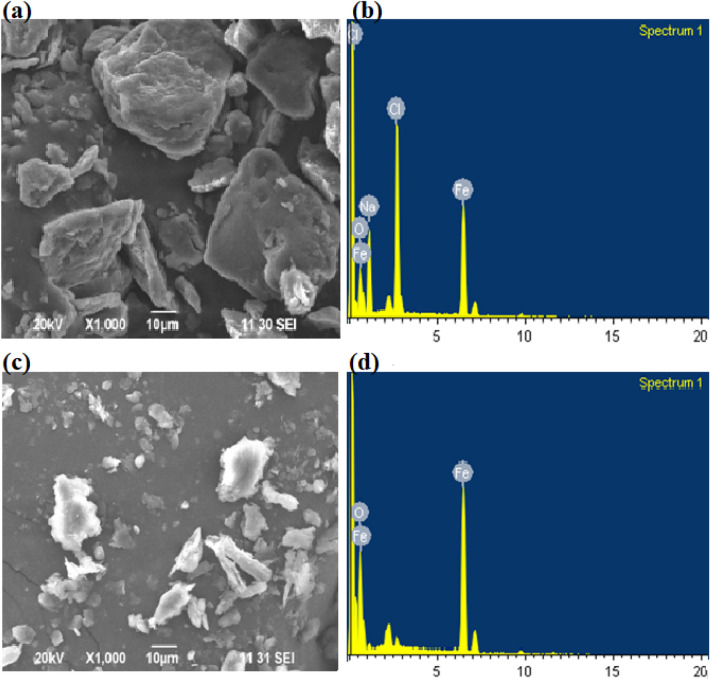
The morphologies of MNPs and laccase immobilized MNPs are shown in the SEM images (Fig. 2a–d). Figure 2a shows the undamaged structure of the MNPs, which consisted of homogeneous particles. The corresponding energy-dispersive spectroscopic (EDS) analysis confirmed the presence of Fe in their elemental composition (Fig. 2b). Figure 2c shows the etching appearance of MNPs in which the integrated particles (laccase) were visually damaged and the appearance of enlarged inter lamellar spaces. The corresponding EDS analysis confirmed the presence of Fe in the elemental composition of the nanoparticles (Fig. 2d). The results are consistent with a previous study reporting the immobilization of laccase on bentonite materials (Wen et al. 2019). The presence of elemental Fe in the spectra of enzyme immobilized magnetic iron particles confirmed the enzyme cross-linking over the nanoparticles. Similar observations were also reported by Das et al. (2020) and confirmed the contribution of Fe signals by the laccase enzymes.
Fig. 2.
SEM images of and related EDS of (a, b) MNPs before immobilization and (c, d) MNPs-Lac after immobilization
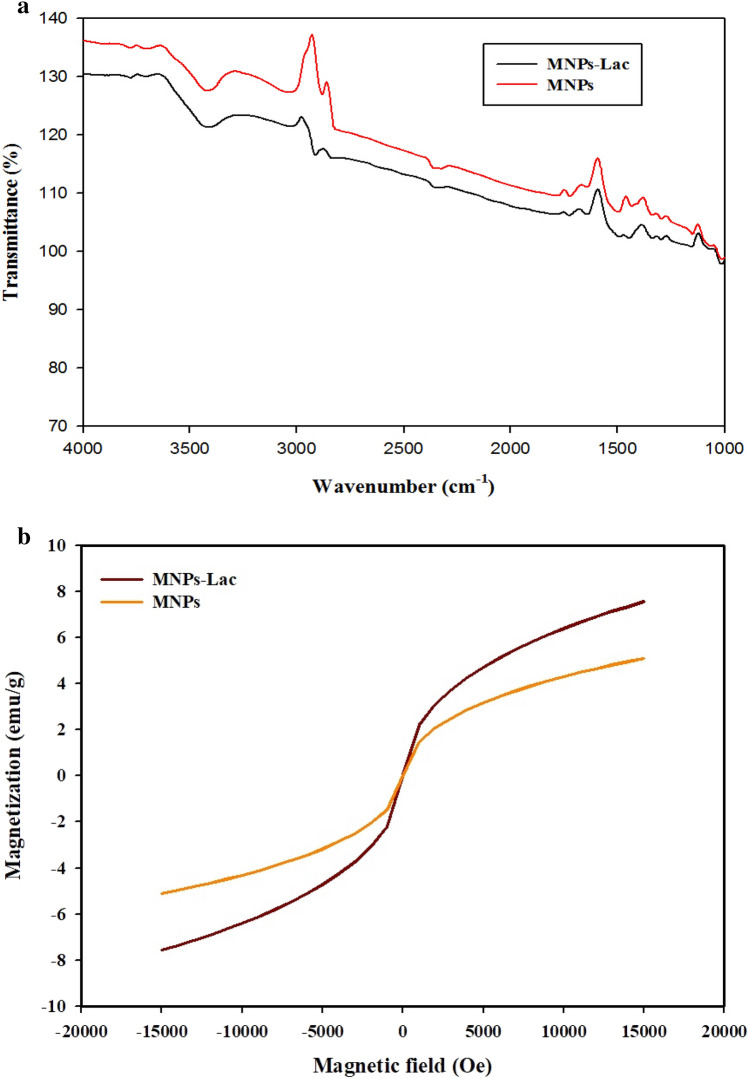
The FT-IR spectra of the MNPs and immobilized laccase are shown in Fig. 3a. The broad bands of the MNPs (3417.86/cm) and immobilized laccase (3035.96/cm and 2679.72/cm) can be attributed to the stretching vibration of the O–H groups. The bands at 2912.51, 1338, and 1012/cm of the MNPs and immobilized laccase are due to aromatic and aliphatic C–H stretching. The small peaks at 1498.69/cm and 804.32/cm are ascribed to the C=O-stretching vibration in aldehyde groups and to the formation of C=N in the immobilization reaction. The small bands at 1012/cm are attributed to the C–O–C absorption peaks. The FT-IR results are consistent with the previous studies that reported the immobilization of laccase using Fe and Kaolini nanoparticles (Jiang et al. 2018; Ztrk et al. 2008; Lin et al. 2007). The variations in the stretching vibrations of the MNPs and immobilized laccase indicate buffer interference which was used during the immobilization process. A decrease in vibration bands attributed to groups of the enzyme probably due to low amount of immobilized enzyme was observed; FT-IR could not detect it (Fortes et al. 2017).
Fig. 3.
a FT-IR spectra of MNPs before immobilization and MNPs-Lac after immobilization. b VSM image of MNPs before and after immobilization
The magnetic properties of MNPs and immobilized laccase were investigated by VSM measurements (Fig. 3b). The results clearly indicated that the magnetization of MNPs was about approximately 8 EMU/g and that of the immobilized laccase with MNPs was nearly 4 EMU/g. A reduction in magnetic strength by 50% might be due to the surface modification of MNPs by elemental Fe. The magnetic strength observed in the present study is high when compared to the results of Kalkan et al. (2011) and Mahdavi et al. (2013), who reported ten times higher magnetic strength. The oxidation of magnetite particles into maghemite could be the possible reason for the reduction in the magnetic strength of the material (Mahdavi et al. 2013). The observed results confirm that the MNPs are super-magnetic at room temperature owing to the lack of magnetic hysteresis and exhibit a high magnetic responsiveness for use in magnetic separation (Lin et al. 2017; Wang et al. 2013).
Effect of pH, temperature, and storage time on the stability of the immobilized laccase
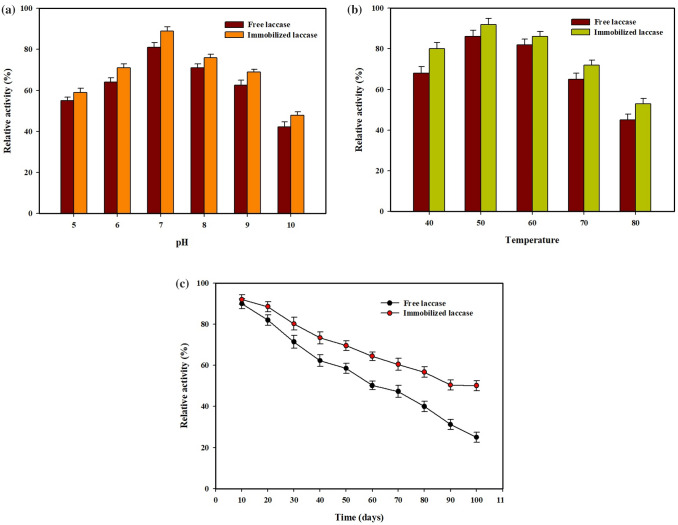
It has been reported that immobilized laccase shows enhanced enzymatic activity in comparison with free laccase (Wu et al. 2019). The optimum pH conditions (pH 5–10) of the free laccase and immobilized laccase are shown in Fig. 4a. The results indicate that the activity of both free and immobilized laccase is high at pH 7.0. However, when the pH of the solution reached basic conditions (pH 8–10), the immobilized laccase showed higher activity than the free laccase. It has been reported that the interaction of laccase molecules with the amino groups of MNPs causes more stable activity than the free laccase (Chen et al. 2016).
Fig. 4.
Effect of pH a temperature b and stability c of the immobilized laccase and free enzyme activity
Expectedly, the immobilized laccase showed activity at a wide range of temperature (40–80 °C) than the free laccase (Fig. 4b). Both free and immobilized laccase showed maximum activity at 50 °C. There was no significant difference in the activity of the enzymes at 50 °C. However, when the temperature was increased to 60 °C, the immobilized laccase showed a minor decrease in activity. However, at the same temperature, free laccase showed a marked decrease in activity. In addition, the immobilized laccase maintained over 55% of its activity in the range of 55–80 °C. Statistical analysis showed that the free enzyme and immobilized laccase activity significantly (p < 0.5) differed in pH, temperature, and storage time.
It has been observed that the immobilization increased the steadiness of the laccase enzyme to alterations in pH and temperature. The shift in pH might be due to the variations in the enzyme during covalent bond development leading to changes in the microenvironment of the laccase enzyme (Chiou and Wu 2004; Das et al. 2020). It has been reported that the occurrence of chemical transformation in the enzymes during temperature variations might be the reason for the shift in enzyme activity of free and immobilized enzymes (Kunamneni et al. 2008). Ramírez-Montoya et al. (2015) reported that the support material might have caused hindrances and diffusion limitations for the enzymes which could have also been the reason for the changes in the free and immobilized enzymes. The maximum activity range of immobilized enzyme than the free enzyme indicates its resistance to the alkaline changes that occurred in the medium (Quiroga et al. 2011).
The immobilized laccase retained 80% of its activity after storing for 100 h, while the activity of the free laccase was only 30% over the same period (Fig. 4c). These results showed that immobilized laccase presented a better storage stability compared to its free enzyme form. This drop in activity over time can be caused by conformational changes in the enzyme during the immobilization, which enhanced the stability of the immobilized laccase. It can also be due to conformational changes in the enzyme tertiary structure during the storage period (Fortes et al. 2017). Recent studies reported that the loss of immobilized laccase activity was smaller due to the relatively stable micro environment that prevented laccase in activation (Wu et al. 2019). Arsalan and Younus (2018) reported that the covalent bonding immobilization method provided greater stability than the adsorption methods.
Pesticide degradation studies
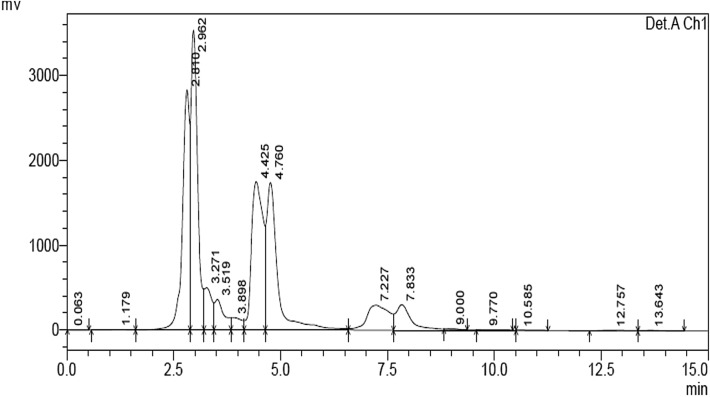
The chlorpyrifos degradation products were identified by the HPLC analysis. Our previous study reported the chlorpyrifos degrading ability of free laccase (Srinivasan et al. 2019). Hence, in this work, we tested the chlorpyrifos degradation ability of immobilized laccase, and the corresponding HPLC chromatogram is shown in Fig. 5. The immobilized laccase-mediated chlorpyrifos degradation showed dominant peak at a retention time of approximately 2.810 min, and the control showed a peak at 2.302 min (Srinivasan et al. 2019). The laccase-mediated degradation showed only two metabolic peaks. However, the immobilized laccase showed four major soluble peaks at retention times of approximately 2.810, 2.962, 4.425, and 4.760 min. The results clearly indicated that the immobilized laccase degrades chlorpyrifos more effectively than the free laccase. Based on these results, it is suggested that the immobilized laccase is capable of greater chlorpyrifos degradation.
Fig. 5.
HPLC chromatogram of chlorpyrifos degradation by MNPs-Lac
Conclusion
This study demonstrates that MNPs are a resourceful candidate for the immobilization of the enzyme laccase. The immobilization of laccase was confirmed by various structural characterization methods such as SEM and FT-IR. VSM analysis showed strong magnetic property of the immobilized laccase. The immobilized laccase displayed adequate enzymatic activity, including enhanced tolerance towards alkaline pH, high temperature, and storage stability up to 100 h. In addition, the immobilized laccase showed potential pesticide biodegradation activity. Hence, it is suggested that the immobilized laccase could be used as a potential candidate for biodegradation of pesticides in contaminated soil and/or water.
Acknowledgements
This research is partially supported by the PG & Research Department of Biotechnology, Mahendra Arts and Science College (Autonomous), and the Department of Science and Technology, Government of India (DST-FIST sponsored-Ref. No. SR/ FST/College-232/2014). This research was supported by Kyungpook National University Bokhyeon Research Fund, 2017. This study was supported by Researchers Supporting Project number (RSP-2020/144), King Saud University, Riyadh, Saudi Arabia.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
M. Govarthanan, Email: gova.muthu@gmail.com
Woong Kim, Email: elshine@knu.ac.kr.
K. Selvam, Email: ksk.selvam@gmail.com
References
- Ahmed I, Iqbal HM, Dhama K. Enzyme-based biodegradation of hazardous pollutants an overview. J Exp Biol Agric Sci. 2017;5:402–411. [Google Scholar]
- Amin F, Nawaz H, Muhammad B, Bilal M. Improvement of activity, thermo-stability and fruit juice clarification characteristics of fungal exo-polygalacturonase. Int J Biol Macromol. 2016 doi: 10.1016/j.ijbiomac.2016.10.086. [DOI] [PubMed] [Google Scholar]
- Antecka K, Zdarta J, Siwińska-Stefańska K, Sztuk G, Jankowska E, Oleskowicz-Popiel P, Jesionowski T. Synergistic degradation of dye wastewaters using binary or ternary oxide systems with immobilized laccase. Catalysts. 2018;8:402. [Google Scholar]
- Arsalan A, Younus H. Enzymes and nanoparticles: modulation of enzymatic activity via nanoparticles. Int J Biol Macromol. 2018;118:1833–1847. doi: 10.1016/j.ijbiomac.2018.07.030. [DOI] [PubMed] [Google Scholar]
- Bilal M, Asgher M, Hafiz MN, Hu H, Wang W, Zhang X. Bio-catalytic performance and dye-based industrial pollutants degradation potential of agarose-immobilized MnP using a Packed Bed Reactor System. Int J Biol Macromol. 2017;102:582–590. doi: 10.1016/j.ijbiomac.2017.04.065. [DOI] [PubMed] [Google Scholar]
- Bilal M, Asgher M, Parra-Saldivar R, Hu H, Wang W, Zhang X, Iqbal HM. Immobilized ligninolytic enzymes: an innovative and environmental responsive technology to tackle dye-based industrial pollutant a review. Sci Total Environ. 2017;576:646–659. doi: 10.1016/j.scitotenv.2016.10.137. [DOI] [PubMed] [Google Scholar]
- Bilal M, Rasheed T, Zhao Y, Iqbal HM. Agarose chitosan hydrogel immobilized horseradish peroxidase with sustainable bio-catalytic and dye degradation properties. Int J Biol Macromol. 2019;124:742–749. doi: 10.1016/j.ijbiomac.2018.11.220. [DOI] [PubMed] [Google Scholar]
- Chen YY, Stemple B, Kumar M, Wei N. Cell surface display fungal laccase as a renewable biocatalyst for degradation of persistent micro pollutants bisphenol A and sulfamethoxazole. Environ Sci Technol. 2016;50:8799–8808. doi: 10.1021/acs.est.6b01641. [DOI] [PubMed] [Google Scholar]
- Chiou SH, Wu WT. Immobilization of Candida rugosa lipase on chitosan with activation of the hydroxyl groups. Biomaterials. 2004;25:197–204. doi: 10.1016/s0142-9612(03)00482-4. [DOI] [PubMed] [Google Scholar]
- Das A, Singh J, Yogalakshmi KN. Laccase immobilized magnetic iron nanoparticles: fabrications and its performance evaluation in chlorpyrifos degradation. Int Biodeterior Biodegrad. 2017;117:183–189. [Google Scholar]
- Das A, Jaswal V, Yogalakshmi KN. Degradation of chlorpyrifos in soil using laccase immobilized iron oxide nanoparticles and their competent role in deterring the mobility of chlorpyrifos. Chemosphere. 2020;246:125676. doi: 10.1016/j.chemosphere.2019.125676. [DOI] [PubMed] [Google Scholar]
- Dyal A, Loos KM, Noto M, Chang SW, Spagnoli C, Shafi KVPM, Ulman A, Cowman M, Gross RA. Activity of Candida rugosalipase immobilized on g-Fe2O3 magnetic nanoparticles. J Am Chem Soc. 2003;125:1684–1685. doi: 10.1021/ja021223n. [DOI] [PubMed] [Google Scholar]
- Fortes C, Daniel-da-Silva AL, Xavier MRB, Tavares PM. Optimization of enzyme immobilization on functionalized magnetic nanoparticles for laccase biocatalytic reactions. Chem Eng Process. 2017;117:1–8. [Google Scholar]
- Govarthanan M, Jeon C-H, Jeon Y-H, Kwon J-H, Bae H, Kim W. Non-toxic nano approach for wastewater treatment using Chlorella vulgaris exopolysaccharides immobilized in iron-magnetic nanoparticles. Int J Biol Macromol. 2020;162:1241–1249. doi: 10.1016/j.ijbiomac.2020.06.227. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Gursharan S, Satinderpal K, Madhu K, Shailendramar K. Biobleaching for pulp and paper industry in India: emerging enzyme technology. Biocatal Agric Biotechnol. 2019;17:558–565. [Google Scholar]
- Halim SFA, Kamaruddin AH, Fernando WJN. Continuous biosynthesis of biodiesel from waste cooking palm oil in a packed bed reactor: optimization using response surface methodology (RSM) and mass transfer studies. Bioresour Technol. 2009;100:710–716. doi: 10.1016/j.biortech.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Huang D, Liu L, Zeng G, Xu P, Huang C, Deng L, Wang R, Wan J. The effects of rice straw biochar on indigenous microbial community and enzymes activity in heavy metal contaminated sediment. Chemosphere. 2017;174:545–553. doi: 10.1016/j.chemosphere.2017.01.130. [DOI] [PubMed] [Google Scholar]
- Jiang C, Yin L, Wen X, Du C, Wu L, Long Y, Liu Y, Ma Y, Yin Q, Zhou Z, Pan H. Micro plastics in sediment and surface water of west dongting lake and south dongting lake: abundance, source and composition. Int J Environ Res Public Health. 2018;15:2164. doi: 10.3390/ijerph15102164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jořenek M, Zajoncová L (2015) Immobilization of laccase on magnetic carriers and its use in decolorization of dyes. Chem. Biochem Eng 29:457–466
- Jordan C, Ahmed E, Cerasela Z. Industrial applications of enzymes: recent advances, techniques, and outlooks. Catalysts. 2018;8:238. [Google Scholar]
- Kalkan NA, Aksoy S, Aksoy EA, Hasirci N. Preparation of chitosan-coated magnetite nanoparticles and application for immobilization of laccase. J Appl Polym Sci. 2011;123:707–716. [Google Scholar]
- Krishna SH, Sattur AP, Karanth NG. Lipase catalyzed synthesis of isoamyl isobutyrate optimization using a central composite rotatable design. Process Biochem. 2001;37:9–16. [Google Scholar]
- Kumar S, Kaushik G, Dar M, Nimesh S, Opez-chuken U, Villarreal-chiu JF. Microbial degradation of organophosphate pesticides: a review. Pedosphere. 2018;28:190–208. [Google Scholar]
- Kunamneni A, Plou FJ, Ballesteros A, Alcalde M (2008) Laccases and their applications: a patent review. Recent Pat Biotechnol 2:10–24 [DOI] [PubMed]
- Kupski L, Salcedo GM, Caldas SS, Souza TD, Furlong EB, Primel EG. Optimization of a laccase-mediator system with natural redox-mediating compounds for pesticide removal. Environ Sci Pollut Res. 2019;26:5131–5139. doi: 10.1007/s11356-018-4010-y. [DOI] [PubMed] [Google Scholar]
- Lai C, Zhang M, Li B, Huang D, Zeng G, Qin L, Liu X, Yi H, Cheng M, Li L, Chen Z, Chen L. Fabrication of CuS/BiVO4 (0 4 0) binary hetero junction photo catalysts with enhanced photo catalytic activity for ciprofloxacin degradation and mechanism insight. Chem Eng J. 2019;358:891–902. [Google Scholar]
- Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- Li Z. Health risk characterization of maximum legal exposures for persistent organic pollutant (POP) pesticides in residential soil: an analysis. J Environ Manag. 2018;205:163–173. doi: 10.1016/j.jenvman.2017.09.070. [DOI] [PubMed] [Google Scholar]
- Lin D, Pan B, Zhu L, Xing B. Characterization and phenanthrene sorption of tea leaf powders. J Agric Food Chem. 2007;55:5718–5724. doi: 10.1021/jf0707031. [DOI] [PubMed] [Google Scholar]
- Lin J, Wen Q, Chen S, Le X, Zhou X, Huang L. Synthesis of amine-functionalized Fe3O4@C nanoparticles for laccase immobilization. Int J Biol Macromol. 2017;96:377–383. doi: 10.1016/j.ijbiomac.2016.12.051. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zeng Z, Zeng G, Tang L, Pang Y, Li Z, Liu C, Lei X, Wua M, Ren P, Liu Z, Chen M, Xie G. Immobilization of laccase on magnetic bimodal mesoporous carbon and the application in the removal of phenolic compounds. Bioresour Technol. 2012;115:21–26. doi: 10.1016/j.biortech.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Mahdavi M, Ahmad MB, Haron J, MdNamvar F, Nadi B, Rahman MZ, Amin J. Synthesis, surface modification and characterization of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules. 2013;18:7533–7548. doi: 10.3390/molecules18077533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqbool Z, Hussain S, Imran M, Mahmood F, Shahzad T, Ahmed Z, Muzammil S. Perspectives of using fungi as bioresource for bioremediation of pesticides in the environment: a critical review. Environ Sci Pollut Res. 2016;23:16904–16925. doi: 10.1007/s11356-016-7003-8. [DOI] [PubMed] [Google Scholar]
- Mir-Tutusaus JA, Baccar R, Caminal G, Sarrà M. Can white-rot fungi be a real wastewater treatment alternative for organic micro pollutants removal? A review. Water Res. 2018;138:137–151. doi: 10.1016/j.watres.2018.02.056. [DOI] [PubMed] [Google Scholar]
- Nandhini NT, Rajeshkumar S, Mythili R. The possible mechanism of eco-friendly synthesized nanoparticles on hazardous dyes degradation. Biocatal Agric Biotechnol. 2019;19:10113. [Google Scholar]
- Pereira LC, Souza AO, Bernardes MFF, Pazin M, Tasso MJ, Pereira PH, Dorta DJ. A perspective on the potential risks of emerging contaminants to human and environmental health. Environ Sci Pollut Res. 2015;22:13800–13823. doi: 10.1007/s11356-015-4896-6. [DOI] [PubMed] [Google Scholar]
- Quiroga E, Illanes CO, Ochoa NA, Barberis S. Performance improvement of araujia in, a cystein phytoprotease, by immobilization within calcium alginate beads. Process Biochem. 2011;46:1029–1034. [Google Scholar]
- Ramírez-Montoya LA, Hernandez-Montoya V, Montes Moran MA, Jauregui Rincon J, Cervantes FJ. Decolorization of dyes with different molecular properties using free and immobilized laccases from Trametes versicolor. J Mol Liq. 2015;212:3037. [Google Scholar]
- Selvam K, Govarthanan M, Senbagam D, Kamala Kannan S, Senthilkumar B, Selvankumar T. Activity and stability of bacterial cellulase immobilized on magnetic nanoparticles. Chin J Catal. 2016;37:1891–1898. [Google Scholar]
- Simonelli A, Basilicata P, Miraglia N, Castiglia L, Guadagni R, Acampora A, Sannolo N. Analytical method validation for the evaluation of cutaneous occupational exposure to different chemical classes of pesticides. J Chromatogr B. 2007;860:26–33. doi: 10.1016/j.jchromb.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Soozanipour A, Taheri kafrani A, LandaraniIsfahani A. Covalent attachment of xylanase on functionalized magnetic nanoparticles and determination of its activity and stability. Chem Eng J. 2015;270:235–243. [Google Scholar]
- Srinivasan P, Selvankumar T, Kamala-Kannan S, Mythili R, Sengottaiyan A, Govarthanan M, Senthilkumar B, Selvam K. Production and purification of laccase by Bacillus sp. using millet husks and its pesticide degradation application. 3 Biotech. 2019;9:396. doi: 10.1007/s13205-019-1900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera M, Nyanhongo GS, Pellis A, Rivas BL, Guebitz GM. Immobilization of Myceliophthora thermophila laccase on poly (glycidyl methacrylate) microspheres enhances the degradation of azinphos-methyl. J Appl Polym Sci. 2019;136:47417. [Google Scholar]
- Villarreal-Chiu JF, Acosta-Cortes AG, Kumar S, Kaushik G. Biological limitations on glyphosate biodegradation. In: Singh R, Kumar S, editors. Green technologies and environmental sustainability. 9. Cham: Springer; 2017. pp. 179–201. [Google Scholar]
- Wang J, Zhao G, Li Y, Liu X, Hou P. Reversible immobilization of glucoamylase onto magnetic chitosan nanocarriers. Appl Microbiol Biotechnol. 2013;97:681–692. doi: 10.1007/s00253-012-3979-2. [DOI] [PubMed] [Google Scholar]
- Wen X, Du C, Wan J, Zeng G, Huang D, Yin L, Deng R, Tan S, Zhang J. Immobilizing laccase on kaolinite and its application in treatment of malachite green effluent with the coexistence of Cd (П) Chemosphere. 2019;217:843–850. doi: 10.1016/j.chemosphere.2018.11.073. [DOI] [PubMed] [Google Scholar]
- Wu E, Li Y, Huang Q, Yang Z, Wei A, Qi Hu. Laccase immobilization on amino functionalized magnetic metal organic framework for phenolic compound removal. Chemosphere. 2019;233:327–335. doi: 10.1016/j.chemosphere.2019.05.150. [DOI] [PubMed] [Google Scholar]
- Zdarta J, Meyer AS, Jesionowski T, Pinelo M. Developments in support materials for immobilization of oxidoreductases: a comprehensive review. Adv Colloid Interface Sci. 2018;258:1–20. doi: 10.1016/j.cis.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Zeng S, Qin X, Xia L. Degradation of the herbicide isoproturon by laccase mediator systems. Biochem Eng J. 2017;119:92–100. [Google Scholar]
- Zheng F, Cui BK, Wu XJ, Meng G, Liu HX, Si J. Immobilization of laccase onto chitosan beads to enhance its capability to degrade synthetic dyes. Int Biodeterior Biodegrad. 2016;110:69–78. [Google Scholar]
- Ztrk N, Tabak A, Akgl S, Denizli A. Reversible immobilization of catalase by using a novel bentonite-cysteine (Bent-Cys) micro composites affinity sorbents. Colloid Surf Physicochem Eng Asp. 2008;322:148–154. [Google Scholar]