Jin et al. demonstrate that a recently defined T-cell population, known as virtual memory T cells, accumulate with human immunodeficiency virus (HIV), are inversely correlated with latent viral load and may be uniquely well suited to eliminate latently infected cells during chronic infections.
Infection with HIV presents many challenges to the immune system, but viral reservoirs are one of the most intractable. Treatment of HIV has been revolutionized by antiretroviral therapy (ART), which effectively ablates viral replication in patients. Unfortunately, a real cure remains elusive, as HIV will persist in latently infected cells. The identification of immune mechanisms that can reduce this latent reservoir may improve treatment and progress cure strategies for HIV. In this issue, Jin et al. demonstrate that the activity of an unconventional immune cell, the virtual memory T (TVM) cell, may play a role.1
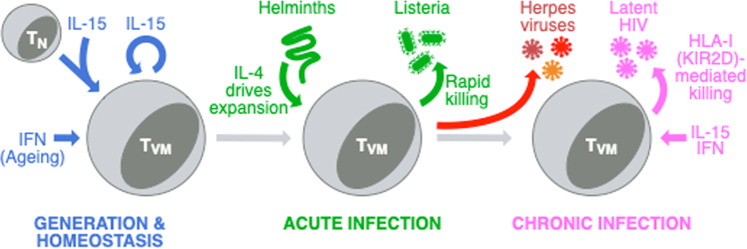
TVM cells are a population of semidifferentiated CD8 T cells that are antigen-naive but express some markers of memory cells. Their origin, features, and functions are well characterized in mice (Fig. 1). They arise in the periphery from naive CD8 T cells whose semidifferentiation is driven by cytokine signaling, particularly IL-15.2 They accumulate relative to true-naive CD8 T cells with age,3,4 and type I IFN signaling augments this accumulation.5 Since TVM cells are semidifferentiated, they respond rapidly to TCR stimulation, providing the first wave of responding cells during a primary immune response to infections, such as Listeria monocytogenes.6 They also respond in an innate-like manner to cytokines and express natural killer (NK) cell receptors that enable them to kill in an antigen-independent manner.7 While mouse TVM cells are well characterized, putative TVM cells have only been recently defined in humans on the basis of the expression of NK cell markers, including killer cell immunoglobulin-like receptors (KIRs) and NKG2A.7,8
Fig. 1.
TVM cell generation, homeostasis, and roles during acute and chronic infections. IL-15 is essential for the initial generation of TVM cells and their self-renewal in the periphery, while type I IFNs promote their accumulation with age. During acute infection, TVM cells can be expanded by IL-4 production during helminth infections, and expanded TVM cells can mediate rapid control of listerial infections. In chronic infections, helminth-expanded TVM cells can promote the control of gammaherpesvirus infection in mice. The work from Jin et al. suggests that increased IL-15 and type I IFN production during latent HIV infection increases the frequency of TVM cells, which can improve control of the viral reservoir. This control may be mediated by NK cell receptor-mediated killing, involving HLA-I and KIRs
TVM cells are highly sensitive to cytokines produced during infections. Studies in mice have shown that exogenous IL-152 or IL-4 generated during helminth infections9,10 can expand TVM cells. With helminth infections, this expanded population can improve the control of subsequent listerial infections9 or chronic viral infections with murid gammaherpesvirus 4 (MuHV-4).10 A major outstanding question in the field has therefore been whether TVM cells can also mediate the control of infections in humans, particularly chronic viral infections such as cytomegalovirus (CMV), Epstein–Barr virus (EBV), varicella virus, and HIV (Fig. 1).
In this regard, Jin et al.’s work is the first investigation of TVM cell function in a human infection model.1 A key finding of this study is that TVM cells increase in frequency and number in ART-treated patients, and this result also correlates with lower HIV DNA as a measure of the latent viral reservoir. A higher frequency of TVM cells also correlates with increased levels of IL-15 and IFN-α in ART-treated patients, indicating that patients with better control of HIV infection possess a cytokine environment conducive for generating and maintaining TVM cells.
Jin et al.’s work also validates that putative human TVM cells can exhibit innate-like immune function during an infection such as HIV. Previous studies have demonstrated innate-like functionality in mouse models11 and healthy humans,8 where TVM cells produce IFN-γ rapidly upon IL-12 and IL-18 stimulation. Jin et al. demonstrate that TVM cells from ART-treated patients also have a higher production of cytolytic molecules and cytokines than non-TVM cells upon stimulation with IL-12, -18, and -15.
Finally, Jin et al. directly assesses whether NK cell-like killing mechanisms contribute to the killing capacity of TVM cells in an in vitro viral suppression assay. Latently infected CD4 T cells from ART patients were used as targets to demonstrate that while total CD8 T cells can kill target cells, the killing capacity is greater in purified TVM cells. NK cells can mediate killing through the sensing of human leukocyte antigen I (HLA-I) expression on target cells by KIRs. When HLA-I or KIR2D are blocked in the viral suppression assay with TVM cells, the killing of targets increases.
While this suggests that TVM cells can borrow the NK cell-like mechanisms of cytotoxicity (Fig. 1), it is currently unclear precisely how blocking HLA-I or KIR2D increases viral suppression. HLA-I blockade induces a robust increase in viral suppression.1 Given that latent infection with HIV has been seen to downregulate HLA-I,12 HLA-I blockade presumably enhances this “missing-self” signal to promote the killing of target cells. KIR2D blockade induces a more subtle increase in viral suppression.1 This more subtle effect may reflect that KIR2D is only a subset of KIRs that can mediate the sensing of the “missing self” or that blockade simultaneously targets inhibiting (KIR2DL1/2/3) and activating (KIR2DS) the isoforms of KIR2D. This is complicated by the fact that it is not yet known which isoforms of KIR2D are expressed by TVM cells. However, the overall implication of this work is that the derepression of inhibitory KIRs leads to increased killing and HIV reservoir control in vivo.
Given that TVM cells seem to play a role in HIV infection, a key question then becomes whether they represent a universal mechanism for the control of viral reservoirs in chronic viral infections. TVM cells have already been shown to control early infection with MuHV-4 in mice,10 and CMV, EBV, and varicella virus are obvious next candidates in humans, as they would provide a strong evolutionary pressure for the emergence of such mechanisms. The ability of TVM cells to borrow NK cell receptor-mediated killing pathways may make them uniquely well suited to control latent infections in neurons and latency and transformation in leukocytes with herpesviruses.
One application of this work could be to rationalize TVM cells as an adjunct cellular or immunotherapy for HIV. For example, an IL-15 superagonist has been administered in nonhuman primate studies and clinical trials, with the aim of controlling HIV viral reservoirs. These studies have demonstrated a transient increase in viral control with IL-15,13 which may be due in part to an increase in TVM cells. TVM cells could theoretically be expanded in vitro with IL-15 and reinfused into ART patients to augment control. One limitation may be that TVM cells in mice have limited expression of chemokine receptors that permit access to the gut mucosa, and the gut is thought to be a major reservoir of viruses such as HIV.
Regardless, the complex nature of the HIV reservoir will likely require the targeting of multiple mechanisms simultaneously to eliminate this reservoir. As we discover these mechanisms, one by one, we become one step closer to a functional or even true cure for HIV.
Acknowledgements
K.M.Q. is supported by an RMIT University Vice-Chancellor’s Research Fellowship and the Rebecca L. Cooper Foundation. T.H. is supported by the Monash Graduate Scholarship and Monash International Postgraduate Research Scholarship.
Competing interests
The authors declare no competing interests.
References
- 1.Jin, J. H. et al. Cell Mol. Immunol. (2020).
- 2.Sosinowski T, et al. J. Immunol. 2013;190:1936–1947. doi: 10.4049/jimmunol.1203149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinn KM, et al. Cell Rep. 2018;23:3512–3524. doi: 10.1016/j.celrep.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 4.Renkema KR, Li G, Wu A, Smithey MJ, Nikolich-Zugich J. J. Immunol. 2014;192:151–159. doi: 10.4049/jimmunol.1301453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinet V, et al. Nat. Commun. 2015;6:7089. doi: 10.1038/ncomms8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JY, Hamilton SE, Akue AD, Hogquist KA, Jameson SC. Proc. Natl Acad. Sci. USA. 2013;110:13498–13503. doi: 10.1073/pnas.1307572110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White JT, et al. Nat. Commun. 2016;7:11291. doi: 10.1038/ncomms11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacomet F, et al. Eur. J. Immunol. 2015;45:1926–1933. doi: 10.1002/eji.201545539. [DOI] [PubMed] [Google Scholar]
- 9.Lin JS, et al. Mucosal Immunol. 2019;12:258–264. doi: 10.1038/s41385-018-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rolot M, et al. Nat. Commun. 2018;9:4516. doi: 10.1038/s41467-018-06978-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haluszczak C, et al. J. Exp. Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachtel ND, et al. PLoS Pathog. 2018;14:e1007257. doi: 10.1371/journal.ppat.1007257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb GM, et al. Blood Adv. 2018;2:76–84. doi: 10.1182/bloodadvances.2017012971. [DOI] [PMC free article] [PubMed] [Google Scholar]