The germinal center (GC) reaction is considered the cornerstone of our humoral immune response. Localized in specialized follicles inside secondary lymphoid organs, germinal center B cells (GCBCs) execute a pivotal task. Following an infection, the number of GCBCs is rapidly expanded, and subsequently GCBCs differentiate into specialized antibody-secreting factories. This intricate process is driven by the positive selection of GCBCs via the B-cell receptor with the highest affinity for the respective antigen. During this period of extensive proliferation, GCBCs are among the fastest dividing cells in our body. It has been established that cell proliferation requires tremendous amounts of energy and building blocks. However, there remains a paucity of evidence to explain how proliferating GCBCs meet these metabolic demands. In their latest publication in Nature Immunology, Weisel et al. provided new insights that may have a far-reaching impact on the evolution of the immunometabolism field.1
The current dogma states that proliferative cells predominantly employ (an)aerobic glycolysis.2 In this process, cells catabolize glucose into indispensable nucleotides and proteins, whilst producing limited amounts of energy. Because glycolysis does not require oxygen and the GC was recently shown to be hypoxic,3 GCBCs are thought to display a glycolysis-dependent phenotype. However, experiments with freshly isolated GCBCs have been technically challenging. Weisel et al. have overcome these limitations by utilizing an untouched bead-based purification approach. The validity of their approach was confirmed by the consistently observed phenotypic and transcriptomic characteristics of bona fide ex vivo GCBCs (Extended Data Figs. 1–3).
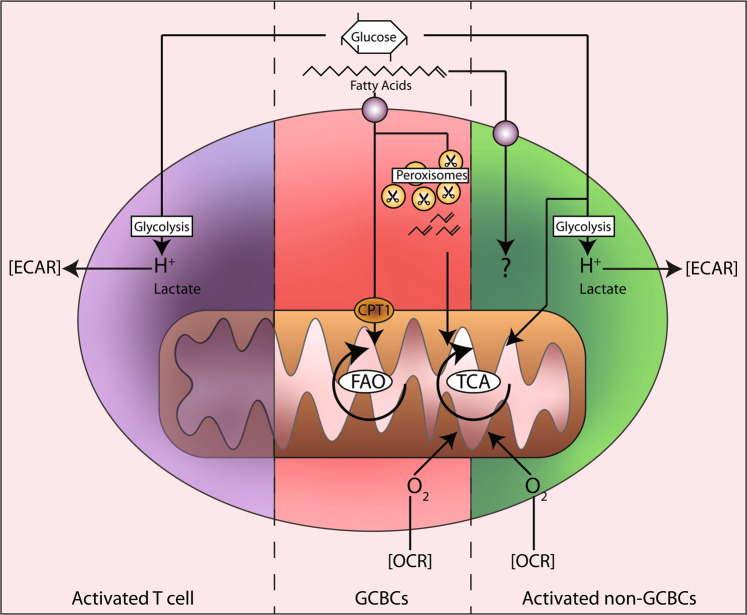
Strikingly, a functional analysis of these GCBCs showed minimal glycolytic activity compared to that of the activated non-GC B cells or T cells (Fig. 1). Under anaerobic conditions, cells can ferment the final product of glycolysis, pyruvate, into lactate. Protons formed during this process acidify the extracellular medium. Consequently, measuring the extracellular acidification rate (ECAR) directly reflects glycolytic activity. ECAR fluctuations in the presence of glucose or an glycolysis inhibitor (2DG) were not evident in the GCBCs, whereas the ECAR of the activated non-GC B cells and T cells was profoundly affected (Fig. 1b). Finally, the uptake of the fluorescent glucose analog 2-NDBG by the GCBCs was significantly lower than the uptake by other types of cells. This finding was in line with the transcriptomic analysis showing the downregulation of most glycolysis- and hypoxia-associated genes in GCBCs compared to the levels in activated non-GC B cells. Together, these findings indicate that GCBCs negligibly employ glycolysis, even under hypoxic conditions.
Fig. 1.
Summary of metabolic phenotypes of activated lymphocytes. Both activated T cells and activated non-germinal center B cells (non-GC B cells) efficiently take up glucose and catabolize it via glycolysis, as measured by the extracellular acidification rate (ECAR). Activated non-GC B cells partially catabolize glucose in mitochondria (through the TCA cycle), for which they require oxygen (measured by the oxygen consumption rate, OCR). In contrast, GCBCs import negligible amounts of glucose. Instead, they actively take up fatty acids from the environment. Through mitochondrial and peroxisomal fatty acid oxidation (FAO), they fuel the TCA cycle to generate energy.
Subsequently, the authors measured the mitochondrial respiration in GCBCs, activated non-GC B cells and T cells. In active mitochondria, oxygen functions as an acceptor for the electrons produced during the oxidation of NADH, the main product of the TCA (tricarboxylic acid or Krebs) cycle.4 Therefore, the oxygen consumption rate (OCR) correlates with mitochondrial activity. An OCR analysis under nutrient-deprived conditions revealed that the GCBCs consumed more oxygen than did the naïve B cells or activated T cells but less oxygen than the activated non-GC B cells (Fig. 1c). This finding is in line with previous studies on activated T cells demonstrating that they have a predominant glycolytic phenotype.5 Surprisingly, treatment with FCCP did not increase the OCR in any of the cell types studied (Fig. 1c). FCCP induces maximal nonphysiological mitochondrial respiration. The finding that the OCR was not increased by FCCP in the GCBCs implies that their mitochondria were already working at maximum capacity.
The importance of fatty acid (FA) metabolism for specific T-cell subset functions has recently been demonstrated by others.6,7 Weisel et al. investigated the contribution of FA metabolism to the OCR of GCBCs. FAs, hydrophobic chains of carbon atoms with a carboxyl group, are processed by enzymes in a cyclic process called β-oxidation or fatty acid oxidation (FAO). In this catabolic process, carbon atoms are split off in pairs, while simultaneously, acetyl-CoA, NADH and FADH2 moieties are generated. These moieties are oxidized by complexes of the electron transport chain to facilitate phosphorylation of ADP into ATP by ATP synthase (OXPHOS). FAO depends on the transport of long FAs into the mitochondria via carnitine palmitoyltransferase I (CPT1). Treatment with the CPT1 inhibitor etomoxir markedly decreased the OCR in the GCBCs and in naïve and activated non-GC B cells. Strikingly, supplementing these cell types with palmitate, a 16-carbon FA, increased the OCR only in the GCBCs. This finding implies that GCBCs, and not naïve or activated non-GC B cells, can take up and oxidize exogenous FAs. To confirm this observation, the authors measured the uptake of FAs using fluorescently labeled palmitate (BODIPY 16 C). In line with the observed OCR increase upon palmitate stimulation, the GCBCs were effective in their uptake of palmitate. However, the activated non-GC B cells also showed substantial palmitate uptake, raising the following question: why did these cells not show an increased OCR upon palmitate stimulation? Collectively, these findings show that both GCBCs and activated non-GC B cells are able to transport exogenous FAs, but only GCBCs use FAs for energy production.
To further characterize the fatty acid metabolism of GCBCs, the authors investigated peroxisomes, the only other organelle with FAO capacity. In these compartmentalized vesicles, longer and more complex FA variants are catabolized to acetyl-CoA, which can be subsequently transported into the mitochondria to fuel OXPHOS in a non-CPT1-dependent manner. Quantification of peroxisomal protein PMP70 demonstrated a larger peroxisomal pool in the activated non-GC B cells and GCBCs than in the naïve B cells. However, peroxisomal inhibition minimally affected the activated non-GC B cells, whereas the OCR in the GCBCs was markedly reduced. To verify these data in vivo, the authors inhibited peroxisomal and mitochondrial FAO in mice undergoing a GC reaction and observed reduced viability of the GCBCs. Interestingly, the GCBCs did not switch to glycolytic metabolism upon FAO inhibition, suggesting that GCBCs possess an inherent program to repress glycolysis. These data were further supported by 13C carbon tracing experiments, confirming that GCBCs specifically incorporate 13C derived from palmitate into acetyl-CoA and not from glucose. Finally, the abolition of mitochondrial FAO via genetic knockdown of the mitochondrial FA transporter CPT2 revealed a significant reduction in the GCBCs generated in vivo. In summary, these extensive studies identify FAO, and not glycolysis, as the driving force behind the GC reaction and humoral immune response (the model is shown in Fig. 1).
The authors convincingly challenge the dogma that GC B cells thrive on glycolysis. Notwithstanding, this valuable new insight leads to new questions. Rapid cell proliferation requires large amounts of lipids to facilitate new membrane synthesis. Precursors for lipid synthesis can be obtained by selectively using TCA cycle intermediates for cytosolic FA synthesis and not for fueling OXPHOS. The three main metabolites that fuel the TCA cycle are glucose, glutamine and fatty acids. The authors demonstrated that GCBCs import negligible amounts of glucose and that they completely catabolize glutamine (Fig. 1c) and exogenous fatty acids to fuel OXPHOS. Hence, the question arises: how do GCBCs obtain the lipids required for new membrane synthesis?
Furthermore, how exactly is the uptake of fatty acids modulated? The authors postulate that this uptake of FAs is facilitated via CD36. However, the expression of CD36 is highest on naïve B cells (Extended Data Fig. 4c), with minimal uptake of palmitate. Moreover, CD36 expression is similar between activated non-GC B cells and GCBCs, whereas the uptake is significantly different (Extended Data Fig. 4e). This discrepancy between CD36 expression and palmitate uptake indicates the importance of alternative FA transporters.
Moreover, it was shown by others that reactive oxygen species (ROS), such as H2O2, can induce the phosphorylation of membrane-proximal effectors of BCR signaling, independent of BCR engagement.8,9 The catabolism of fatty acids is associated with increased ROS production.10 Therefore, it can be speculated that fatty acid metabolism indirectly transduces survival signals to GCBCs by activating the downstream BCR signaling cascade. Since the total GCBC population was used to quantify fatty acid metabolism, there is no way to differentiate between cells that received survival signals from ROS and those that did not. Exploring GCBCs at the single-cell level may provide further insights into the selection process within GCs.
Finally, how does FA metabolism relate to the development of B-cell malignancies? The authors propose that the transcription factor BCL6 is involved in the metabolic switch of GCBCs. This supposition is interesting since aberrant BCL6 expression is also correlated with the prevalence of B-cell lymphomas.11 Although the relationship between lymphoma development and fatty acid metabolism needs further investigation, studies on leukemia indicate the potential clinical relevance for this question.12
In conclusion, the authors have provided valuable new insights into the importance of FA metabolism in GCBCs. Answering open questions in this exciting field will hopefully accelerate our understanding of B-cell biology in immune and disease contexts.
Acknowledgements
The authors acknowledge funding support by the European Research Council (Consolidator Grant 724281 to A.B.v.S.) and Sjoerd van Deventer and Vera Dunlock for critically reading the manuscript. The authors have no competing interest.
References
- 1.Weisel FJ, Shlomchik MJ. Germinal center B cells selectively oxidize fatty acids for energy while conducting minimal glycolysis. Nat. Immunol. 2020;21:331–342. doi: 10.1038/s41590-020-0598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 3.Jellusova J, et al. Gsk3 is a metabolic checkpoint regulator in B cells. Nat. Immunol. 2017;18:303–312. doi: 10.1038/ni.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waters LR, Ahsan FM, Wolf DM, Shirihai O, Teitell MA. Initial B Cell activation induces metabolic reprogramming and mitochondrial remodeling. iScience. 2018;5:99–109. doi: 10.1016/j.isci.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang CH, et al. XPosttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ecker C, et al. Differential Reliance on lipid metabolism as a salvage pathway underlies functional differences of t cell subsets in poor nutrient environments. Cell Rep. 2018;23:741–755. doi: 10.1016/j.celrep.2018.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howie D, et al. The role of lipid metabolism in T lymphocyte differentiation and survival. Front. Immunol. 2018;8:1949. doi: 10.3389/fimmu.2017.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wheeler ML, DeFranco AL. Prolonged production of reactive oxygen species in response to b cell receptor stimulation promotes b cell activation and proliferation. J. Immunol. 2012;189:4405–4416. doi: 10.4049/jimmunol.1201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diggins K, Huse JM, Irish HG, Polikowsky CE, Wogsland KE. Cutting Edge: Redox Signaling Hypersensitivity Distinguishes Human Germinal Center B Cells. J. Immunol. 2015;195:1364–1367. doi: 10.4049/jimmunol.1500904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quijano C, Trujillo M, Castro L, Trostchansky A. Interplay between oxidant species and energy metabolism. Redox Biol. 2016;8:28–42. doi: 10.1016/j.redox.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basso K, Dalla-Favera R. Roles of BCL6 in normal and transformed germinal center B cells. Immunol. Rev. 2012;247:172–183. doi: 10.1111/j.1600-065X.2012.01112.x. [DOI] [PubMed] [Google Scholar]
- 12.Ricciardi MR, et al. Targeting the leukemia cell metabolism by the CPT1a inhibition: functional preclinical effects in leukemias. Blood. 2015;126:1925–1929. doi: 10.1182/blood-2014-12-617498. [DOI] [PubMed] [Google Scholar]