Pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a newly recognized illness that has spread rapidly from Wuhan to other provinces in the People's Republic of China and around the world. Italy was one of the most affected countries. From February 2020, Lombardy (northern Italy) was the most affected region by the coronavirus disease 2019 (COVID-19) infection, with a total number of 93,990 positive cases and a total number of 16,302 deaths (data as of July 2, 2020). In particular, Brescia was 1 of the provinces of Lombardy most affected by the pandemic.
Data on the clinical characteristics and outcomes of patients having asthma with SARS-CoV-2 infection are scarce but are of paramount importance to evaluate the relationship between COVID-19 and asthma. Given the characteristics of the virus, one would expect to observe an increased prevalence of asthma and wheezing exacerbations in patients having COVID-19 with allergy and asthma comorbidity—a typical consequence in the case of a viral respiratory infection. 1 In this single-center, observational study, we investigated patients with asthma with confirmed SARS-CoV-2 pneumonia who were admitted to our hospital (Fondazione Poliambulanza Istituto Ospedaliero, Brescia, Italy), which is 1 of the designated hospitals to treat patients with SARS-CoV-2 pneumonia. We retrospectively evaluated the data of patients with asthma, from February 20, 2020 to April 20, 2020, who had been diagnosed as having SARS-CoV-2 pneumonia. Asthma was identified as diagnosed by physicians and by the standard criterion used for several real-life studies; in most of our cases, the diagnosis was supported by the results of previous historical standard spirometry with reversibility test with albuterol or methacholine challenge. We evaluated a total of 1043 hospitalized patients who received a positive test result for COVID-19 (men, 704; women, 339; age range, 14-91 years) with only 20 patients with asthma (men, 8; women, 12; age range, 41-77 years). None of the 20 patients were smokers or ex-smokers, and only 1 was an immigrant (woman, aged 47 years from Ghana; arrived in Italy 10 years before with no previous history of sensitization to aeroallergens and/or nasal polyposis).
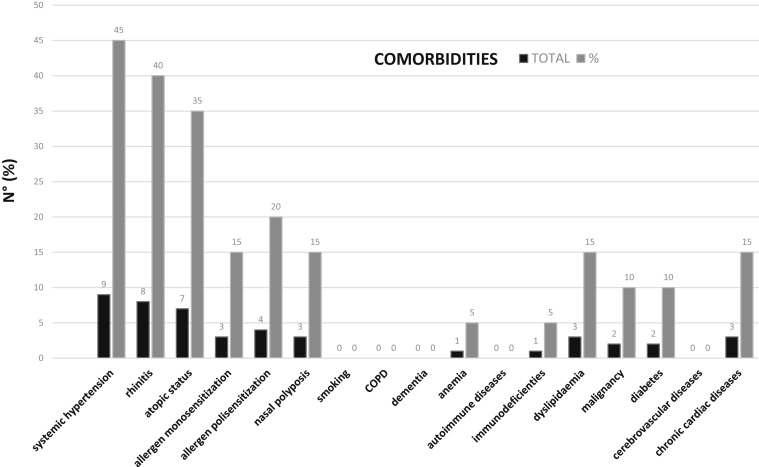
Of the 20 patients with asthma, 6 (30%) had no other comorbidities. The most frequent comorbidity was systemic hypertension (40% of the total cases) (Fig 1 ). The percentage distribution of lung parenchyma involvement derived from the computed tomography imaging data was as follows: (1) less than 25% (9 patients); (2) 25% to 50% (7 patients); (3) 50% to 75% (2 patients), and (4) greater than 75% (2 patients). None of the 20 patients had an evident exacerbation of asthma symptoms or objective evidence of bronchospasm on chest auscultation. With regard to antiasthmatic therapies taken by patients before admission to the hospital, we have documented the following treatments: (1) 15 patients (75%) took a preestablished association with long-acting β-agonists plus inhaled corticosteroids ICS; (2) 2 patients (10%) took inhaled steroid only and short-acting β-agonist on demand; (3) 4 patients (20%) took oral steroid at low doses (prednisone, 5-7.5 mg/d); and (4) 4 patients took (20%) montelukast (10 mg/d). No patients were taking tiotropium, biological agents, or allergen-specific immunotherapy. Of the 20 patients, 3 were transferred to the intensive care unit from the internal medicine area owing to worsening of the clinical picture and respiratory failure. A total of 2 fatal cases (2 women, aged 66 and 77 years, respectively) were recorded, both of whom were not related to the clinical condition of asthma. Both were moved to the intensive care unit and died from acute respiratory distress syndrome. Compared with the control group of patients without asthma hospitalized for COVID-19, the mortality in patients with asthma was much lower (10% vs 22.6%).
Figure 1.
Comorbidities of patients with asthma who were hospitalized for coronavirus disease 2019. COPD, chronic obstructive pulmonary disease.
The prevalence of asthma in our hospitalized patients with COVID-19 was particularly low (1.92%) when compared with the prevalence of asthma in the general population of Italy (6.1% men and 5.49% women, 6.64% total)2 or Europe (4%-7%).3 This observation is surprising if we consider the following: (1) the chronic inflammatory infiltration and the airway dysfunction characterizing asthma; (2) the close association between asthma and respiratory viral infections; (3) the increased frequency and severity of lower respiratory tract infections in patients with asthma; and (4) the influenza-related increased morbidity and community-acquired pneumonia. We can speculate that patients with a chronic respiratory disease such as asthma should be at increased risk of SARS-CoV-2 infection with worse clinical outcomes, but data, thus far, do not support this. In a study performed in Wuhan, electronic medical records including demographics, clinical manifestation, comorbidities, laboratory data, and radiology reports of 140 hospitalized patients with a confirmed result of SARS-CoV-2 viral infection were extracted and analyzed, and it was noted that asthma or other allergic diseases were not reported by any of the patients.4 Moreover, Yang et al5 conducted a systematic review of studies to evaluate the prevalence of comorbidities in patients diagnosed as having COVID-19 and the presence of underlying diseases in severe patients compared with nonsevere patients. A total of 46,248 participants were included in this meta-analysis. The most prevalent comorbidities were hypertension and diabetes, followed by cardiovascular diseases and respiratory system diseases (2%). Overall, given that the prevalence of asthma in the People's Republic of China is 4.2%, these early studies found that asthma is not a risk factor for SARS-CoV-2 infection. Furthermore, Richardson et al6 evaluated characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in New York City and found no increase in hospitalizations among patients with asthma; indeed, the reported percentage (9%) is in line with the annual American data.
Beyond the People's Republic of China and the United States, looking at the recent data from Italy, among the 355 patients who died because of SARS-CoV-2, asthma was not listed as a relevant comorbidity.7 Our data seem even more impressive if we consider that the COVID-19 pandemic in northern Italy coincided with the start of the new pollen season, and therefore, with the periodic annual increase in the cases of seasonal allergic asthma. An intriguing hypothesis is that having asthma protects against SARS-CoV-2, perhaps through a different pattern of immune response elicited by the chronic disease itself.8 More recently, Jackson et al9 reported that respiratory allergy and controlled allergen exposures are each associated with significant reductions in the angiotensin-converting enzyme 2 expression; it is noteworthy that nonatopic asthma was not associated with reduced angiotensin-converting enzyme 2 expression. Another possibility is that therapies used by patients with asthma can reduce the risk of infection. Recently, inhaled corticosteroids alone or in combination with bronchodilators have been reported to suppress coronavirus replication and cytokine production.10
In conclusion, according to our data, it seems that asthma is not a relevant risk factor in the development of COVID-19 infection. This study confirms that asthma is not in the top 10 comorbidities associated with COVID-19 fatalities, the more common of which were obesity, diabetes, and chronic heart disease.
Acknowledgments
The authors thank Paolo Matricardi (Department of Pediatrics, Division of Pulmonology, Immunology, and Critical Care Medicine, Charité Universitätsmedizin Berlin, Berlin, Germany), and Diego Bagnasco (Allergy and Respiratory Diseases, Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Martino, University Of Genoa, Genoa, Italy) for their valuable suggestions and contribution to the revision of this article.
Footnotes
Disclosures: The authors have no conflicts of interest to report.
Funding: The authors have no funding sources to report.
References
- 1.Jartti T., Bønnelykke K., Elenius V., Feleszko W. Role of viruses in asthma. Semin Immunopathol. 2020;42(1):61–74. doi: 10.1007/s00281-020-00781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cazzola M., Puxeddu E., Bettoncelli G. The prevalence of asthma and COPD in Italy: a practice-based study. Respir Med. 2011;105(3):386–391. doi: 10.1016/j.rmed.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Gibson G.J., Loddenkemper R., Lundbäck B., Sibille Y. Respiratory health and disease in Europe: the new European Lung White Book. Eur Respir J. 2013;42(3):559–563. doi: 10.1183/09031936.00105513. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J.-J., Dong X., Cao Y.-Y. Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 5.Yang J., Zheng Y., Gou X. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, andoutcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [e-pub ahead of print]. JAMA. https://doi.org/10.1001/jama.2020.4683, accessed march 23, 2020. [DOI] [PubMed]
- 8.Halpin D.M.G., Faner R., Sibila O., Badia J.R., Agusti A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir Med. 2020;8(5):436–438. doi: 10.1016/S2213-2600(20)30167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson D.J., Busse W.W., Bacharier L.B. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020;146(1):203–206. doi: 10.1016/j.jaci.2020.04.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaya M., Nishimura H., Deng X. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir Investig. 2020;58(3):155–168. doi: 10.1016/j.resinv.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]