Key Points
Question
What is the association of patients who are diagnosed with a postoperative neurocognitive disorder with total Medicare health care expenditures in the year after their surgical procedure?
Findings
In this retrospective cohort study including nearly 2.4 million Medicare patients, after adjusting for patient and hospital characteristics, the presence of a postoperative neurocognitive disorder was associated with an increase of $17 275 in payments in the 1 year after the index surgical procedure.
Meaning
These findings suggest that in older patients who underwent surgical treatment, a diagnosis of a postoperative neurocognitive disorder was associated with an increase in health care costs for up to 1 year after treatment, emphasizing the economic burden associated with this potentially modifiable complication.
This cohort study examined the association of postoperative neurocognitive disorders with health care expenditures among US Medicare patients.
Abstract
Importance
Postoperative neurocognitive disorders (PNDs) after surgical procedures are common and may be associated with increased health care expenditures.
Objective
To quantify the economic burden associated with a PND diagnosis in 1 year following surgical treatment among older patients in the United States.
Design, Setting, and Participants
This retrospective cohort study used claims data from the Bundled Payments for Care Improvement Advanced Model from 4285 hospitals that submitted Medicare Fee-for-service (FFS) claims between January 2013 and December 2016. All Medicare patients aged 65 years or older who underwent an inpatient hospital admission associated with a surgical procedure, did not experience a PND before index admission, and were not undergoing dialysis or concurrently enrolled in Medicaid were included. Data were analyzed from October 2019 and May 2020.
Exposures
PND, defined as an International Classification of Diseases, Ninth or Tenth Revision, diagnosis of delirium, mild cognitive impairment, or dementia within 1 year of discharge from the index surgical admission.
Main Outcomes and Measures
The primary outcome was total inflation-adjusted Medicare postacute care payments within 1 year after the index surgical procedure.
Results
A total of 2 380 473 patients (mean [SD] age, 75.36 (7.31) years; 1 336 736 [56.1%] women) who underwent surgical procedures were included, of whom 44 974 patients (1.9%) were diagnosed with a PND. Among all patients, most were White (2 142 157 patients [90.0%]), presenting for orthopedic surgery (1 523 782 patients [64.0%]) in urban medical centers (2 179 893 patients [91.6%]) that were private nonprofits (1 798 749 patients [75.6%]). Patients with a PND, compared with those without a PND, experienced a significantly longer hospital length of stay (mean [SD], 5.91 [6.01] days vs 4.29 [4.18] days; P < .001), were less likely to be discharged home (9947 patients [22.1%] vs 914 925 patients [39.2%]; P < .001), and had a higher incidence of mortality at 1 year after treatment (4580 patients [10.2%] vs 103 767 patients [4.4%]; P < .001). After adjusting for patient and hospital characteristics, the presence of a PND within 1 year of the index procedure was associated with an increase of $17 275 (95% CI, $17 058-$17 491) in cost in the 1-year postadmission period (P < .001).
Conclusions and Relevance
The findings of this cohort study suggest that among older Medicare patients undergoing surgical treatment, a diagnosis of a PND was associated with an increase in health care costs for up to 1 year following the surgical procedure. Given the magnitude of this cost burden, PNDs represent an appealing target for risk mitigation and improvement in value-based health care.
Introduction
Cognitive impairment is the most common complication experienced by older adults after surgical treatment.1 The incidence of cognitive impairment ranges from 10% to 65% and varies depending on a variety of factors, such as age, level of education, sex, comorbidities, surgery type, and assessment methods.2,3 Although delirium is often most apparent in the hospital setting and has been rigorously studied in the immediate postoperative phase, other distinct yet potentially connected forms of postoperative cognitive dysfunction continue through, and often past, discharge.4,5,6,7 Recently, an expert panel proposed the term perioperative neurocognitive disorders (PND) to realign the field with allied specialty nomenclature and presumptive pathophysiological characteristics.8 A diagnosis of PND describes the different types of cognitive disorders encountered in the perioperative setting from immediately postoperatively out to 1 year.9 Based on the diagnostic criteria from the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) (DSM-5),10 PND definitions are congruent with coding for postoperative delirium, delayed neurocognitive recovery, and mild or major neurocognitive disorders occurring from 1 to 12 months postoperatively.8 Collectively, these PNDs confer substantial morbidity and mortality among the 19 million surgical procedures performed annually in the US in adults aged 65 years or older.11,12,13,14
The short- and long-term consequences associated with PNDs may contribute to excessive health care costs.13,15 In previous studies, patients with PNDs have experienced not only increased hospital length of stay but also more frequent discharge to skilled nursing facilities.16 In particular, postoperative delirium has been shown to be associated with increased length of hospitalization, a 3-fold increase in the odds of discharge to a nursing facility, and a 17% increased risk of death at 1 year.16,17 Despite evidence supporting increased morbidity and mortality, the specific costs associated with PNDs in older adults has not been previously reported, to our knowledge.
To address these gaps, Medicare claims data were used to assess whether beneficiaries with a PND had higher health care expenditures following their index surgical procedure compared with those who did not experience a PND. Therefore, this study aimed to test the hypothesis that experiencing a PND was associated with increased inflation-adjusted total Medicare expenditures in the 1 year after surgical treatment.
Methods
This study was approved by the Dartmouth College Committee for the Protection of Human Subjects institutional review board. Informed consent was waived owing to the retrospective nature of the study, per institutional policy. The reporting of this study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Assembly of the Cohort
This retrospective cohort was assembled using claims data from the Bundled Payments for Care Improvement Advanced (BPCI-A) model from January 2013 through December 2016. The BPCI-A is an initiative from the Centers for Medicare & Medicaid Services that considers the aggregate payments made from the time period that begins with the index hospitalization (the anchor episode). In this analysis, patients were eligible for inclusion if they were enrolled in Medicare fee-for-service and underwent an inpatient hospital admission associated with a surgical procedure. Patients were excluded if they were dually eligible and enrolled in both Medicare and Medicaid, younger than 65 years, or were enrolled in the Medicare End Stage Renal Disease program prior to the surgical procedure. Patients who presented with pre-existing neurocognitive diagnoses, defined based on the International Classification of Diseases, Ninth Revision18 or Tenth Revision,19 (ICD-9/ICD-10) codes in the year preceding the index surgical procedure, were excluded from analysis (eTable 1 in the Supplement). These conditions included dementia, cognitive impairment, altered mental status, and cognitive deficits as a result of prior stroke or traumatic brain injury. Index surgical procedures were identified using the BPCI-A methods, which include the Medicare Severity-Diagnosis Related Group codes (eTable 2 in the Supplement). The BPCI-A episodes were later bundled into 3 surgical categories, cardiac, general, and orthopedic, to streamline the presentation and interpretation of the results.
Definition of PNDs
Several disorders associated with delayed or impaired cognitive recovery after surgical treatment were included under the definition of PND, including postoperative delirium, delayed neurocognitive recovery, and PNDs that can be classified as mild or major neurocognitive disorders occurring from 1 to 12 months postoperatively.8 In this study, PNDs, our primary exposure, were defined as an ICD-9 or ICD-10 diagnosis of delirium, mild cognitive impairment, or dementia within 1 year of discharge from the index surgical procedure (eTable 3 in the Supplement). These diagnoses were based on the DSM-5 criteria for neurocognitive disorders and are consistent with recent recommendations for a revised PND nomenclature.8
Healthcare Expenditures
The primary outcome was total inflation-adjusted Medicare postacute care payments within 1 year following the index surgical procedure. Postacute care payments represented the costs associated with readmissions, skilled nursing facilities, home health care, long-term care, physician office visits, and radiological or laboratory tests. Costs were adjusted for inflation using the Consumer Price Index and are reported in 2016 dollars.20
Statistical Analysis
Descriptive statistics of the data were assessed and reported. Categorical data are presented as frequencies and proportions and assessed with a χ2 test. Continuous data were reported as means and SDs or medians and interquartile ranges (IQRs), depending on the distribution of the data. Normality of continuous variables was assessed with a Shapiro-Wilk test. A complete case approach was used to address missing data in the primary analysis. No imputation for missing data was performed.
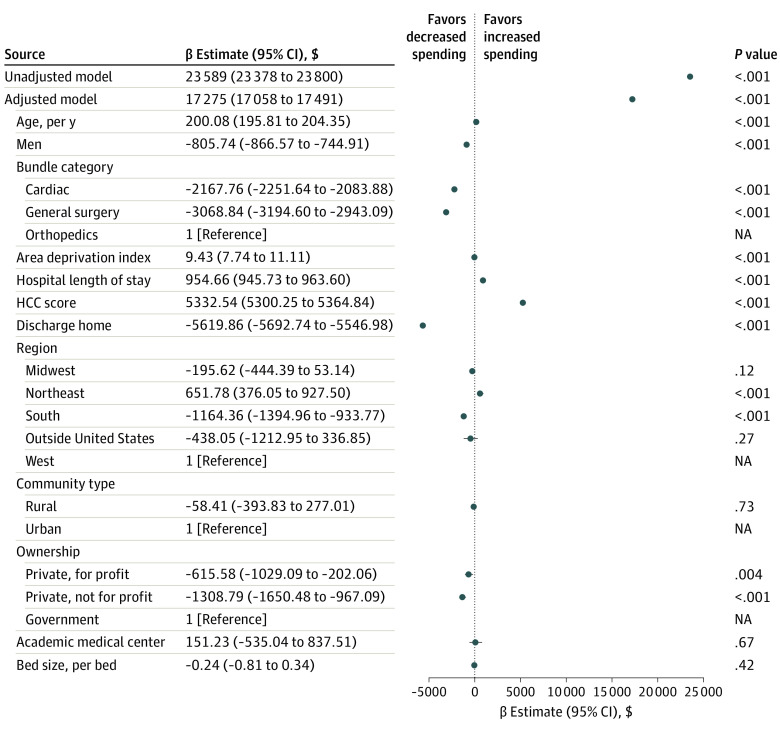
Mixed-effects linear hierarchal models were used to examine the association of PND with Medicare payments while adjusting for a priori defined patient and hospital characteristics. Patient and hospital characteristics were based on their biological plausibility and significance (P < .10) in unadjusted analyses. A model was first fit using full maximum likelihood estimation with no predictors to evaluate the variation in payments that could be attributed to the index admission hospital. From this model, the intraclass correlation coefficient is reported. Next, a model was fit using PNDs to estimate postacute care payments, in which the hospital was included as a random effect. A similar model was fit adjusting for the prespecified patient characteristics. Lastly, a multivariable model was fit in which the association of PND with payments was assessed, including hospital as a random effect and adjusting for patient and hospital characteristics (eAppendix in the Supplement). The final model adjusted for patient characteristics, including age, sex, surgical bundle (ie, cardiac, general surgery, or orthopedic), Area Deprivation Index, community Hierarchal Condition Category score, and discharged home status. Hospital characteristics included in the final model included region of the country, designation as rural or urban, ownership (ie, government, private for-profit, private nonprofit, or other), academic medical center designation, and number of beds. Results of the final model are presented as our primary analysis and are reported as β estimates and their associated 95% CIs.
All analyses were performed using SAS statistical software version 9.4 (SAS Institute) and R statistical software version 3.6 (R Project for Statistical Computing) with 2-sided P < .05 considered statistically significant. Because no adjustments were performed for multiple testing, prespecified secondary outcomes should be considered exploratory and interpreted with caution. Given the observational nature of this study using retrospective data, no a priori power calculation was performed. Statistical analysis was performed between October 2019 and May 2020.
Results
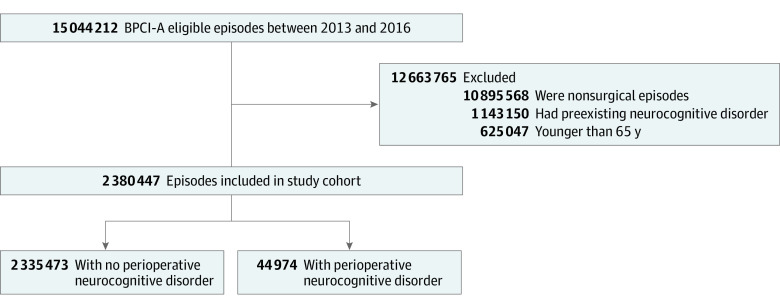
A total of 2 380 447 Medicare patients mean [SD] age, 75.36 (7.31) years; 1 336 736 [56.1%] women) who underwent surgical procedures were included, among whom 44 974 patients (1.9%) were diagnosed with a PND (Figure 1). Among all patients, most were White (2 142 157 patients [90.0%]), presenting for orthopedic surgery (1 523 782 patients [64.0%]) in urban medical centers (2 179 893 patients [91.6%]) that were private nonprofits (1 798 749 patients [75.6%]).
Figure 1. Assembly of the Cohort.
Data from the Bundled Payment for Care Improvement-Advanced (BPCI-A) data set were used to assess patients presenting for surgery between 2013 and 2016.
Baseline Comparison of Patents With and Without a PND
Patients with a PND, compared with those without a PND, were more likely to be older (mean [SD] age, 80.65 [8.18] years vs 75.26 [7.29] years; P < .001), women (26 523 [59.0%] women vs 1 310 213 [56.1%] women; P < .001), from the northeast (11 503 patients [25.6%] vs 581 055 patients [24.9%]; P < .001), have more baseline comorbidities (mean [SD] Hierarchal Condition Category community score, 1.69 [1.19] vs 1.17 [0.97]; P < .001), and have a higher mean (SD) area deprivation index (46.60 [25.23] vs 44.31 [24.80]; P < .001) (Table 1).
Table 1. Patient and Hospital Characteristics Stratified by Postoperative Neurocognitive Status.
| Characteristic | Patients, No (%) | P Value | |
|---|---|---|---|
| No PND (n = 2 335 473) | PND (n = 44 974) | ||
| Patient level | |||
| Age, mean (SD), y | 75.26 (7.29) | 80.65 (8.18) | <.001 |
| Women | 1 310 213 (56.1) | 26 523 (59.0) | <.001 |
| Race/ethnicity | |||
| White | 2 101 502 (90.0) | 40 655 (90.4) | <.001 |
| Black | 119 988 (5.1) | 2485 (5.5) | |
| Asian | 26 922 (1.2) | 425 (0.9) | |
| North American Native | 11 400 (0.5) | 313 (0.7) | |
| Hispanic | 22 901 (1.0) | 508 (1.2) | |
| Other | 28 244 (1.2) | 414 (0.9) | |
| Unknown | 24 516 (1.1) | 174 (0.4) | |
| HCC score, mean (SD), community | 1.17 (0.97) | 1.69 (1.19) | <.001 |
| Neurocognitive inpatient diagnosis | 0 | 696 (1.5) | <.001 |
| Area deprivation index, mean (SD) | 44.31(24.80) | 46.60 (25.35) | <.001 |
| Bundle category | |||
| Orthopedics | 1 496 142 (64.1) | 27 640 (61.5) | <.001 |
| Cardiac | 650 458 (27.9) | 13 751 (30.6) | |
| General surgery | 188 873 (8.1) | 3583 (8.0) | |
| Admission year | |||
| 2013 | 583 884 (25.0) | 5956 (13.2) | <.001 |
| 2014 | 556 705 (23.9) | 6146 (13.7) | |
| 2015 | 569 975 (24.4) | 13 498 (30.0) | |
| 2016 | 624 909 (26.8) | 19 374 (43.1) | |
| Hospital level | |||
| Region | |||
| Midwest | 418 214 (17.9) | 8326 (18.5) | <.001 |
| Northeast | 581 055 (24.9) | 11 503 (25.6) | |
| South | 767 255 (32.9) | 14 854 (33.0) | |
| West | 558 535 (23.9) | 10 121 (22.5) | |
| United States territories | 10 414 (0.5) | 170 (0.4) | |
| Community type | |||
| Rural | 195 819 (8.4) | 4735 (10.5) | <.001 |
| Urban | 2 139 654 (91.6) | 40 239 (89.5) | |
| Academic medical center | 113 992 (4.9) | 2281 (5.1) | .06 |
| Bed size, mean (SD) | 421.35 (374.2) | 423.25 (367.1) | .29 |
| Ownership | |||
| Government | 205 149 (8.8) | 4344 (9.7) | <.001 |
| Private for profit | 365 260 (15.6) | 6941 (15.4) | |
| Private not for profit | 1 765 060 (75.6) | 33 689 (74.9) | |
Abbreviations: HCC, Hierarchical Condition Category; PND, postoperative neurocognitive disorder.
Unadjusted Hospital and Payment Outcomes
Table 2 reports unadjusted outcomes stratified by PND status. Patients with a PND, compared with those without a PND, had longer acute inpatient hospital stays (mean [SD], 5.91 [6.01] days vs 4.29 [4.18] days; P < .001), were less likely to be discharged to home (9947 patients [22.1%] vs 914 925 [39.2%]; P < .001), and had a higher incidence of mortality at 1-year after index surgical treatment (4580 patients [10.2%] vs 103 767 [4.4%]; P < .001). Among patients who developed a PND, most experienced postoperative delirium (38 128 patients [84.8%]) followed by dementia (19 459 patients [43.3%]) and mild cognitive impairment (7999 patients [17.8%]).
Table 2. Patient Outcomes and Payment Characteristics.
| Characteristic | Patients, No. (%) | P value | |
|---|---|---|---|
| No PND (n = 2 335 473) | With PND (n = 44 974) | ||
| Index hospitalization outcomes | |||
| Discharged home | 914 925 (39.2) | 9947 (22.1) | <.001 |
| Hospital length of stay, mean (SD), d | 4.29 (4.18) | 5.91 (6.01) | <.001 |
| Mortality at 1 y | 103 767 (4.4) | 4580 (10.2) | <.001 |
| Time to death, mean (SD), d | 66.49 (79.98) | 123.61 (84.64) | <.001 |
| PND diagnosesa | |||
| Dementia | NA | 19 459 (43.3) | <.001 |
| Delirium | NA | 38 128 (84.8) | <.001 |
| Mild cognitive impairment | NA | 7999 (17.8) | <.001 |
| Episode payments | |||
| Episode payments, $ | 26 587.18 (17 445.65-43 448.52) | 50 631.71 (31 235.87-81 890.37) | <.001 |
| Inpatient acute payments, $ | 14 985.76 (11 838.94-23 530.12) | 17 484.16 (12 297.97-30 485.68) | <.001 |
| Total postacute care payments | |||
| Postacute care payments, $ | 7149.35 (3053.55-19 167.01) | 26 881.74 (10 544.44-54 497.84) | <.001 |
| Inpatient payments, mean (SD), $ | 11 451.16 (12 392.01) | 14 049.48 (13 746.48) | <.001 |
| Readmission payments, $ | 13 258.03 (8089.49-23 068.84) | 16 380.61 (8933.60-32 506.53) | <.001 |
| Long-term care payments, mean (SD), $ | 39 101.31 (29 270.33) | 40 752.25 (29 510.67) | .04 |
| Skilled nursing facility payments, $ | 10 453.28 (6085.24-17 566.25) | 16 819.52 (9677.44-27 943.74) | <.001 |
| Home health payments, $ | 3219.71 (2523.15-4371.75) | 3846.94 (2717.41-6000.17) | <.001 |
| Hospice payments, $ | 3149.89 (1378.83-8148.33) | 3418.10 (1457.38-7910.59) | .42 |
| Durable medical equipment, $ | 181.13 (69.22-528.39) | 179.39 (66.07-561.48) | .33 |
| Outpatient payments, $ | 878.60 (266.67-2217.12) | 1317.32 (422.31-3290.41) | <.001 |
| Part B payments, $ | 1736.34 (707.10-3616.89) | 3333.00 (1394.89-6726.03) | <.001 |
Abbreviations: NA, not applicable; PND, postoperative neurocognitive disorder.
Nonexclusive categories.
In unadjusted analyses, total inflation-adjusted 1-year payments after the initial visit were significantly higher among patients who experienced a PND (median [IQR], $26 881.74 [$10 554.44-$54 497.84]) compared with those who did not ($7149.35 [$3053.55-$19 167.01]; P < .001). This was especially pronounced in postacute skilled nursing facility payments for patients with PND vs those without PND (median [IQR] $16 819.52 [$9677.44-$27 943.74] vs $10 453.28 [$6085.24-$17 566.25]; P < .001). Episode and acute care payments for the inpatient stay tied to their index surgical admission were similarly associated with an increase. That is, total episode payments for the index inpatient hospital visit were significantly higher among those who experienced a PND compared with those who did not (median [IQR], $50 631.71 [$31 235.87-$81 890.37] vs $26 587.18 [$17 445.65-$43 448.52]; P < .001).
Subtypes of PNDs and Unadjusted Payments
To assess the costs associated with each disorder, patients were stratified into subtypes of PND. Given that these were not necessarily exclusive categories, some patients experienced them in combination. Of patients with a PND, a total of 6846 patients (15.2%) experienced mild cognitive impairment in isolation and 18 221 patients (40.5%) experienced delirium alone. Mild cognitive impairment and delirium occurred in combination in 448 patients (1.0%), delirium and dementia together occurred in 18 754 patients (41.7%), and 705 patients (1.6%) experienced all 3 PND subtypes.
Primary Analysis of Postacute Care Payments
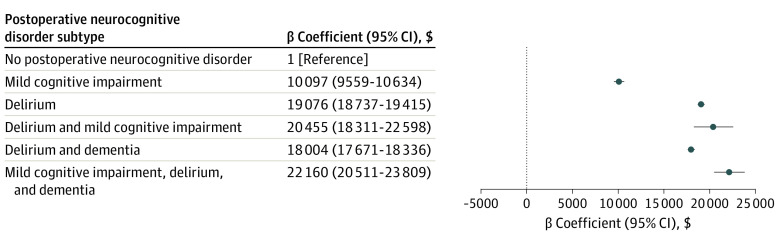
In our primary analysis, after adjusting for patient and hospital characteristics (Figure 2), presence of a PND within 1 year from the index procedure was associated with an increase in postacute care payments of $17 275 (95% CI, $17 058-$17 491) in the 1-year postadmission period (P < .001). In the unadjusted model, the intraclass correlation coefficient was 0.05, indicating that 5.0% of the variability in payment was attributed to the hospital. The adjusted total postacute care costs are illustrated in Figure 3. Patients with all PND subtypes had the highest postacute care payments ($22 160; 95% CI, $20 511-$23 809) followed by those with delirium and neurocognitive impairment ($20 455; 95% CI, $18 311-$22 598).
Figure 2. Unadjusted and Multivariable Models Predicting Health Care Costs.
HCC indicates Hierarchical Condition Category.
Figure 3. Adjusted Payments by Neurocognitive Disorder Subtype.
No patients were categorized as having both dementia and mild cognitive impairment in the first year following the index surgical admission and are thus not reported.
Discussion
This cohort study found that the diagnosis of PND was associated with a significant increase in annual Medicare payments. This association was driven largely by differences in skilled nursing care costs during the 1 year following the index surgical procedure. Payments made during the index hospital admission for patients with PND were higher than for those without PND.
It is important to note that our study was not designed to establish a causal link between surgical treatment and PND. While the association of the perioperative encounter with PND has been well described, the causal links, and therefore potentially modifiable targets, remain unclear. This is reflected in the relative paucity of successful preventative or therapeutic interventions available. Our study highlights the potential cost savings if we are able to identify at-risk surgical subgroups and use targeted measures to prevent or reduce the incidence, severity, or duration of PND.21
In this cohort study, the presence of a PND was associated with older age, lower socioeconomic status, and a higher comorbidity burden at baseline. These findings are consistent with previous studies that have demonstrated increasing risk of PND, particularly delirium, with advancing age.5 In this study, PND was defined within 12 months of surgical treatment based on the most recent guidelines,9 which now refer to both delirium and postoperative cognitive dysfunction as PNDs and define any neurocognitive disorder present up to 12 months after surgical treatment as postoperative in etiological origin. However, there must be a recognition of longitudinal decline and an acknowledgment that sustained PND later may not be entirely attributed to the surgical treatment itself.
Our findings are consistent with a large cohort study that included 841 hospitalized patients 70 years and older enrolled in a delirium prevention trial, and found that those developing delirium had mean costs more than 2.5-fold higher.22 Moreover, in that study, the 1-year costs observed for an individual episode of delirium contributed an additional cost ranging from $16 000 to $64 000 (in 2005 dollars).22 In our study, the adjusted 1-year payment associated with postoperative delirium alone was $19 076. In addition, we found that a diagnosis of mild cognitive impairment was associated with a significant increase in the median payment, and the highest payments were observed in patients with dual diagnoses.
Prior research has shown conflicting results with regard to the association of PNDs with an increased length of inpatient hospitalizations.23,24,25 In this study, presence of a PND was associated with a more than 1-day increase in the mean length of stay. Although it is possible that the length of stay was associated with the increased costs for the acute in-hospital payments for the index hospitalization that was observed, it is noteworthy that the difference, albeit statistically significant, was not substantially different clinically. This finding perhaps suggests that payments made for other complications incurred during the index hospitalization (eg, urinary tract infections) were not a primary driver of the cost difference between groups.
Although exploration of cost containment in patients with PND is necessary, of importance is the preoperative optimization of surgical candidates at higher risk of complications, such as PND, as well as a global assessment of appropriateness of surgical candidacy itself.26 Traditional approaches to clinical risk assessment use age as a factor for increased risk of stress. Relatively recent advancements in the study of aging have led to the concept of the frailty syndrome, a state of depleted physiologic reserve and clinical risk that is associated with, but variably present with, advancing age.17 Further validation of initiatives around frailty assessment to ascertain readiness for surgical treatment or guide perioperative treatment are ongoing.27 Presumably, mitigating the incidence and extent of PND through these initiatives in individuals deemed to be at higher risk may reduce expenditure and merits consideration.
Previous studies have examined a wide variety of preventative and treatment strategies to avoid or mitigate PND, with varying success,28 suggesting that PND is potentially modifiable and thus hypothetically cost saving.24 For instance, nonpharmacological interventions, such as the pivotal multicomponent Hospital Elder Life Program, have been shown to be effective in reducing the risk of developing delirium in hospitalized older patients by 40% to 60% compared with usual care,24,29 which translates to an annual savings of $9446 as a result of decreased long-term nursing home expenses.30 In a 2011 single-center study,31 a delirium prevention program was estimated to yield more than $1 million in cost savings annually. Fewer studies have reported on the economic consequences associated with PNDs other than delirium. Our findings provide robust evidence that helps to bridge this gap, in which we observe an association of increased postacute care costs in patients with other forms of PND.
Limitations
This study has some limitations, such as the use of administrative data to identify patients with a PND. Previous ICD validation studies for delirium and dementia have demonstrated low sensitivity and high specificity, which may explain why this cohort experienced much lower rates of PNDs than reported in prior research.32,33,34 However, if these are misclassified, these characteristics would likely underestimate the economic burden associated with PNDs, as false-negatives (ie, the patient had PND but was tagged as no PND) would be included in the no PND group, thus shrinking the effect size. Second, it is important to note that different modeling strategies could result in different absolute values of cost β estimates. We selected a linear model for analyzing costs because of the ease of interpreting the results. It is possible that this may have led to a model that did not have the most optimized fit for some variables. Other fields, particularly health economics, have identified other strategies to model cost data; however, the results of these models can often be hard to interpret. Thus, while it is possible that the alternative models could yield a smaller mean difference, it is unlikely that these strategies would change the conclusions we observed, namely that PNDs were associated with an increase in costs. Third, ICD coding transitioned from ICD-9 to ICD-10 during the study period. It is possible that this change resulted in miscoding, which would introduce misclassification bias, although studies suggest that the change to ICD-10 resulted in similar coding accuracy.33 Fourth, post-hoc payment adjustments used by the BPCI-A could influence our findings by removing payments for certain conditions, such as for trauma and cancer. For example, if a patient in our cohort was readmitted to the hospital with a femur fracture as a result of a fall, these payments would not be reflected in our study. Fifth, this study only examined Medicare payments to estimate the economic burden and is thus unable to evaluate or comment on other payment sources. A 2016 study by Kelley35 suggested that delirium may be associated with a significant out-of-pocket expenditure. Sixth, our study excluded patients with preexisting neurocognitive deficits; however, preexisting cognitive impairment is a strong risk factor for PND.28 Further studies are needed to both identify and quantify costs in this at-risk cohort. Lastly, in contrast to delirium, which can be diagnosed using a well-validated clinical assessment tools such as the Confusion Assessment Method, the diagnosis of other forms of PNDs using DSM-5 criteria10 have not been well studied.
Conclusion
The findings of our cohort study demonstrate that there were significant Medicare costs associated with PNDs occurring in the year following surgical treatment. As PNDs are often considered the most common postoperative complication in older patients, focusing on efforts to prevent or reduce duration of symptoms may result in significant cost savings.1,36 Future research is needed to determine whether measures to decrease the risk of PND are associated with cost savings.
eTable 1. Pre-existing Neurocognitive Diagnoses Definitions
eTable 2. Bundled Payments for Care Improvement Advanced Model Year 1-Episode Definitions
eTable 3. International Classification of Diseases, Ninth or Tenth Revision, Diagnosis Codes for Perioperative Neurocognitive Disorders
eAppendix. Statistical Methods
References
- 1.Mahanna-Gabrielli E, Schenning KJ, Eriksson LI, et al. . State of the clinical science of perioperative brain health: report from the American Society of Anesthesiologists Brain Health Initiative Summit 2018. Br J Anaesth. 2019;123(4):464-478. doi: 10.1016/j.bja.2019.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudolph JL, Marcantonio ER. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg. 2011;112(5):1202-1211. doi: 10.1213/ANE.0b013e3182147f6d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen LS, Larsen K, Houx P, Skovgaard LT, Hanning CD, Moller JT; The International Study of Postoperative Cognitive Dysfunction Group . The assessment of postoperative cognitive function. Acta Anaesthesiol Scand. 2001;45(3):275-289. doi: 10.1034/j.1399-6576.2001.045003275.x [DOI] [PubMed] [Google Scholar]
- 4.Abildstrom H, Rasmussen LS, Rentowl P, et al. ; International Study of Post-Operative Cognitive Dysfunction Group . Cognitive dysfunction 1-2 years after non-cardiac surgery in the elderly. Acta Anaesthesiol Scand. 2000;44(10):1246-1251. doi: 10.1034/j.1399-6576.2000.441010.x [DOI] [PubMed] [Google Scholar]
- 5.Moller JT, Cluitmans P, Rasmussen LS, et al. ; International Study of Post-Operative Cognitive Dysfunction Group . Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. Lancet. 1998;351(9106):857-861. doi: 10.1016/S0140-6736(97)07382-0 [DOI] [PubMed] [Google Scholar]
- 6.Daiello LA, Racine AM, Yun Gou R, et al. ; SAGES Study Group . Postoperative delirium and postoperative cognitive dysfunction: overlap and divergence. Anesthesiology. 2019;131(3):477-491. doi: 10.1097/ALN.0000000000002729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hshieh TT, Saczynski J, Gou RY, et al. ; SAGES Study Group . Trajectory of functional recovery after postoperative delirium in elective surgery. Ann Surg. 2017;265(4):647-653. doi: 10.1097/SLA.0000000000001952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckenhoff RG, Maze M, Xie Z, et al. . Perioperative neurocognitive disorder: state of the preclinical science. Anesthesiology. 2020;132(1):55-68. doi: 10.1097/ALN.0000000000002956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evered L, Silbert B, Knopman DS, et al. ; Nomenclature Consensus Working Group . Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth. 2018;121(5):1005-1012. doi: 10.1016/j.bja.2017.11.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed American Psychiatric Association; 2013. [Google Scholar]
- 11.Centers for Disease Control and Prevention Number of all listed procedures for discharges from short-stay hospitals, by procedure category and age. Accessed June 24, 2020. https://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf
- 12.Demeure MJ, Fain MJ. The elderly surgical patient and postoperative delirium. J Am Coll Surg. 2006;203(5):752-757. doi: 10.1016/j.jamcollsurg.2006.07.032 [DOI] [PubMed] [Google Scholar]
- 13.Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS; ISPOCD Group . Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110(3):548-555. doi: 10.1097/ALN.0b013e318195b569 [DOI] [PubMed] [Google Scholar]
- 14.Devore EE, Fong TG, Marcantonio ER, et al. . Prediction of long-term cognitive decline following postoperative delirium in older adults. J Gerontol A Biol Sci Med Sci. 2017;72(12):1697-1702. doi: 10.1093/gerona/glx030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fineberg SJ, Nandyala SV, Marquez-Lara A, Oglesby M, Patel AA, Singh K. Incidence and risk factors for postoperative delirium after lumbar spine surgery. Spine (Phila Pa 1976). 2013;38(20):1790-1796. doi: 10.1097/BRS.0b013e3182a0d507 [DOI] [PubMed] [Google Scholar]
- 16.Witlox J, Eurelings LSM, de Jonghe JFM, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451. doi: 10.1001/jama.2010.1013 [DOI] [PubMed] [Google Scholar]
- 17.Raats JW, van Eijsden WA, Crolla RMPH, Steyerberg EW, van der Laan L. Risk factors and outcomes for postoperative delirium after major surgery in elderly patients. PLoS One. 2015;10(8):e0136071. doi: 10.1371/journal.pone.0136071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization International Classification of Diseases, Ninth Revision (ICD-9). World Health Organization; 1977. [Google Scholar]
- 19.World Health Organization International Statistical Classification of Diseases, Tenth Revision (ICD-10). World Health Organization; 1992. [Google Scholar]
- 20.Health Resources and Services Administration CPI adjustment table. Accessed June 24, 2020. https://www.hrsa.gov/sites/default/files/hrsa/get-health-care/affordable/hill-burton/cpitables.pdf
- 21.Berian JR, Zhou L, Russell MM, et al. . Postoperative delirium as a target for surgical quality improvement. Ann Surg. 2018;268(1):93-99. doi: 10.1097/SLA.0000000000002436 [DOI] [PubMed] [Google Scholar]
- 22.Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32. doi: 10.1001/archinternmed.2007.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franco K, Litaker D, Locala J, Bronson D. The cost of delirium in the surgical patient. Psychosomatics. 2001;42(1):68-73. doi: 10.1176/appi.psy.42.1.68 [DOI] [PubMed] [Google Scholar]
- 24.Hshieh TT, Yue J, Oh E, et al. . Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520. doi: 10.1001/jamainternmed.2014.7779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potter BJ, Thompson C, Green P, et al. . Incremental cost and length of stay associated with postprocedure delirium in transcatheter and surgical aortic valve replacement patients in the United States. PLoS One. 2010;10(6):443-451. doi: 10.1002/ccd.28014 [DOI] [PubMed] [Google Scholar]
- 26.Makary MA, Segev DL, Pronovost PJ, et al. . Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901-908. doi: 10.1016/j.jamcollsurg.2010.01.028 [DOI] [PubMed] [Google Scholar]
- 27.Aranake-Chrisinger A, Cheng JZ, Muench MR, et al. . Ability of postoperative delirium to predict intermediate-term postoperative cognitive function in patients undergoing elective surgery at an academic medical centre: protocol for a prospective cohort study. BMJ Open. 2018;8(3):e017079. doi: 10.1136/bmjopen-2017-017079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berger M, Schenning KJ, Brown CH IV, et al. ; Perioperative Neurotoxicity Working Group . Best practices for postoperative brain health: recommendations from the fifth international perioperative neurotoxicity working group. Anesth Analg. 2018;127(6):1406-1413. doi: 10.1213/ANE.0000000000003841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inouye SK, Bogardus ST Jr, Charpentier PA, et al. . A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. doi: 10.1056/NEJM199903043400901 [DOI] [PubMed] [Google Scholar]
- 30.Leslie DL, Zhang Y, Bogardus ST, Holford TR, Leo-Summers LS, Inouye SK. Consequences of preventing delirium in hospitalized older adults on nursing home costs. J Am Geriatr Soc. 2005;53(3):405-409. doi: 10.1111/j.1532-5415.2005.53156.x [DOI] [PubMed] [Google Scholar]
- 31.Rubin FH, Neal K, Fenlon K, Hassan S, Inouye SK. Sustainability and scalability of the hospital elder life program at a community hospital. J Am Geriatr Soc. 2011;59(2):359-365. doi: 10.1111/j.1532-5415.2010.03243.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rizzuto D, Feldman AL, Karlsson IK, Dahl Aslan AK, Gatz M, Pedersen NL. Detection of dementia cases in two swedish health registers: a validation study. J Alzheimers Dis. 2018;61(4):1301-1310. doi: 10.3233/JAD-170572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim DH, Lee J, Kim CA, et al. . Evaluation of algorithms to identify delirium in administrative claims and drug utilization database. Pharmacoepidemiol Drug Saf. 2017;26(8):945-953. doi: 10.1002/pds.4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katznelson R, Djaiani G, Tait G, et al. . Hospital administrative database underestimates delirium rate after cardiac surgery. Can J Anaesth. 2010;57(10):898-902. doi: 10.1007/s12630-010-9355-8 [DOI] [PubMed] [Google Scholar]
- 35.Kelley A. The burden of healthcare costs in the last five years of life. J Pain Symptom Manage. 2016;51(2):352-353. doi: 10.1016/j.jpainsymman.2015.12.19926502320 [DOI] [Google Scholar]
- 36.Berger M, Nadler JW, Browndyke J, et al. . Postoperative cognitive dysfunction: minding the gaps in our knowledge of a common postoperative complication in the elderly. Anesthesiol Clin. 2015;33(3):517-550. doi: 10.1016/j.anclin.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Pre-existing Neurocognitive Diagnoses Definitions
eTable 2. Bundled Payments for Care Improvement Advanced Model Year 1-Episode Definitions
eTable 3. International Classification of Diseases, Ninth or Tenth Revision, Diagnosis Codes for Perioperative Neurocognitive Disorders
eAppendix. Statistical Methods