Abstract
Vitamin K-dependent carboxylation is a post-translational modification essential for the biological function of coagulation factors. Defects in carboxylation are mainly associated with bleeding disorders. With the discovery of new vitamin K-dependent proteins, the importance of carboxylation now encompasses vascular calcification, bone metabolism, and other important physiological processes. Our current knowledge of carboxylation, however, comes mainly from in vitro studies carried out under artificial conditions, which have a limited usefulness in understanding the carboxylation of vitamin K-dependent proteins in native conditions. Using a recently established mammalian cell-based assay, we studied the carboxylation of coagulation factors in a cellular environment. Our results show that the coagulation factor’s propeptide controls substrate binding and product releasing during carboxylation, and the propeptide of factor IX appears to have the optimal affinity for efficient carboxylation. Additionally, non-conserved residues in the propeptide play an important role in carboxylation. A cell-based functional study of naturally occurring mutations in the propeptide successfully interpreted the clinical phenotype of warfarin’s hypersensitivity during anticoagulation therapy in patients with these mutations. Unlike results obtained from in vitro studies, results from our cell-based study indicate that although the propeptide of osteocalcin cannot direct the carboxylation of the coagulation factor, it is required for the efficient carboxylation of osteocalcin. This suggests that the coagulation factors may have a different mechanism of carboxylation from osteocalcin. Together, results from this study provide insight into efficiently controlling one physiological process, such as coagulation without affecting the other, like bone metabolism.
Introduction
Vitamin K-dependent (VKD) carboxylation is a post-translational modification that converts specific glutamate residues (Glu) to gamma-carboxyglutamate residues (Gla) in VKD proteins. It is essential for the biological function of proteins that control blood coagulation, vascular calcification, bone metabolism, and other important physiological processes.1 Carboxylation has mostly been associated with coagulation, since it was originally observed in the clotting factor, prothrombin (PT).2 Defects of VKD carboxylation have long been known to cause bleeding disorders.3 There are two types of coagulation factors, one is procoagulant proteins which include PT, FVII, FIX, and FX. The other is anticoagulant proteins which include PC, PS, and PZ. The biological functions of these clotting factors require 9-13 Glu residues at the N-terminus of the mature protein (referred to as the Gla domain) to be properly modified by VKD carboxylation.
Carboxylation is catalyzed by an integral membrane protein gamma-glutamyl carboxylase (GGCX), which utilizes the reduced form of vitamin K, carbon dioxide, and oxygen as co-factors. This modification involves the subtraction of the gamma-hydrogen from the Glu residue followed by the addition of a carbon dioxide (carboxyl group). Simultaneously, reduced vitamin K is oxidized to vitamin K epoxide to provide the energy required for the carboxylation reaction. The enzymatic activity of GGCX was first discovered in the 1970s, showing that radioactive 14CO2 was incorporated into PT in rats, and that the amount incorporated was dependent upon the administration of vitamin K.4 Two decades later, the GGCX gene was cloned5 and the enzyme was purified6 by our laboratory, making it possible to study GGCX function at the molecular level.
Gamma-glutamyl carboxylase recognizes its protein substrate by binding tightly to the propeptide of the substrate, which tethers the substrate to the enzyme.7 The Glu residues within the Gla domain of the substrate protein are progressively modified so that multiple carboxylation reactions occur during a single enzyme and substrate binding event.8 In addition, binding of the propeptide to GGCX has been shown to significantly stimulate the activity of the enzyme toward non-covalently linked Glu-containing substrates.9,10 The propeptide of most VKD proteins is located at the N-terminus of the precursor protein that is proteolytically removed after carboxylation to form the mature protein. Notably, a propeptide can also be found at the C-terminus of the precursor protein11 or even within the mature VKD protein.12 Removal of the propeptide from the precursor of coagulation factors abolishes their carboxylation,7,13 suggesting the pivotal role of the propeptide for carboxylation. Nevertheless, the propeptide of osteocalcin (or referred to as bone Gla protein, BGP) appears to be unnecessary for its carboxylation.14 Moreover, high-affinity binding sites within the mature BGP were identified, which appeared to bind to GGCX through a different binding site to the propeptide binding site.15
Propeptides of coagulation factors are essential for the carboxylation of precursor proteins. These propeptide sequences are highly conserved, especially at residues −16, −10, −6, −4, and −1. It has been proposed that the N-terminal sequence of the propeptide is necessary for GGCX recognition, while the C-terminal sequence is required for propeptidase recognition.13 Despite the high sequence conservation, in an in vitro study, the apparent affinities of the coagulation factors’ propeptide for GGCX varied over 100-fold.16 Nevertheless, these coagulation factors appear to be fully carboxylated in physiological conditions. It has been shown that replacing FX propeptide with a reduced affinity propeptide (PT’s propeptide) enhanced the carboxylation of FX, which presumably increased substrate turnover.17 However, a similar strategy of replacing FIX propeptide failed to increase the carboxylation efficiency of FIX,18 although the reason for this discrepancy remains unclear.
It is worth noting that most of our knowledge of GGCX function and its interaction with natural protein substrates was obtained from in vitro studies carried out under artificial conditions using the pentapeptide FLEEL as the substrate.19 Consequently, we do not know how GGCX carboxylates natural VKD proteins in their native milieu.
Here, we studied the carboxylation of coagulation factors in a cellular environment with different chimeric reporter-proteins using our recently established cell-based assay.20 We compared the contribution of the propeptide, the Gla domain, and the C-terminal functional domain of the coagulation factor to its carboxylation. In addition, we examined the effect of naturally occurring mutations in the propeptide on the coagulation factor’s carboxylation to gain an insight into the corresponding phenotype of warfarin hypersensitivity. Our results confirmed the pivotal role of the propeptide in coagulation factor carboxylation and interpreted the clinical phenotype of the hypersensitivity of warfarin during anticoagulation therapy.
Methods
Materials and cell lines
The mammalian expression vector pcDNA3.1Hygro(+), mouse anti-carboxylated BGP monoclonal antibody, Alexa Fluor-488 conjugated donkey anti-mouse IgG, Alexa Fluor-568 conjugated donkey anti-sheep IgG, and Hoechst 33342 were from ThermoFisher Scientific (Waltham, MA, USA). The fluorescent protein-tagged marker proteins of the endoplasmic reticulum (ER) (mCherry-Sec61-N-18) and Golgi apparatus (pmScarlet_Giantin_C1) were gifts from Dr. Michael Davidson (Addgene plasmid # 55130) and Dr. Dorus Gadella (Addgene plasmid # 85048), respectively. The Ca2+-dependent monoclonal antibody to carboxylated Gla domain of PC (PCgla) was a gift from Dr. Paul Bajaj (University of California, Los Angeles, CA, USA).21 The horseradish peroxidase-conjugated goat anti-mouse IgG was from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA, USA). The monoclonal antibody against Gla residues was from Sekisui Diagnostics LLC (Stamford, CT, USA). The HEK293 and COS-7 cell lines were from ATCC (Manassas, VA, USA).
DNA manipulations and plasmid constructions
The pcDNA3.1Hygro(+) vector, with the cDNA of FIXgla-PC (PC with its Gla domain exchanged with that of FIX) cloned onto the XbaI site, was used as the cloning and expression vector, as previously described.20 All other chimeric reporter-proteins, with the different propeptides and/or Gla domains used in this study, were obtained by overlap polymerase chain reaction (PCR). Replacement of FIX epidermal growth factor (EGF) domain and the following domains with cell organelle marker proteins was performed by PCR. The nucleotide sequences of all constructs were verified by DNA sequencing at Eton Bioscience Inc. (RTP, NC, USA).
Reporter-protein carboxylation in HEK293 cells
The efficiency of reporter-protein (FIXgla-PC) carboxylation was determined in HEK293 cells, as previously described.20 For the warfarin resistance study, HEK293 cells stably expressing the corresponding mutant reporter-protein were cultured in complete medium containing 20 nM vitamin K with increasing concentrations of warfarin. The cell culture medium was collected 48 hours (h) later and used for the sandwich-based ELISA to determine the level of carboxylated reporter-proteins.20 For the BGP-PC reporter-protein (PC with its Gla domain replaced by BGP) detection, sheep anti-human PC IgG was used as the capture antibody, and mouse anti-carboxylated BGP antibody was used as the detection antibody. Experimental data were analyzed using GraphPad Prism.
To purify carboxylated chimeric reporter-proteins for use as a standard for ELISA, different chimeric proteins were stably expressed in HEK293/VKOR cells (HEK293 cells over-expressing VKOR). Carboxylated reporter-proteins were purified from the collected medium using 2-step chromatography, as previously described.17 Protein concentrations were quantified using the BCA protein assay kit.
Immunofluorescence confocal imaging
The subcellular localization of reporter-proteins were examined by immunofluorescence confocal imaging, as previously described.22 To examine the effect of the propeptide on reporter-protein carboxylation, different propeptide attached reporter-protein fusions were transiently expressed in COS-7 cells on cover-slips. For the localization of carboxylated reporter-proteins at different cell organelles, fluorescent protein-tagged cell organelle marker protein fusions, and the corresponding marker protein fused with FIXgla, were transiently co-expressed in COS-7 cells. Transfected cells were cultured with 11 μM vitamin K for 48 h, fixed with 4% paraformaldehyde, permeabilized with 0.20% Triton X-100, and immuno-stained with corresponding antibodies. The cell nuclei were stained with 2 μM Hoechst 33342.
Results
Contribution of the propeptide to coagulation factor carboxylation
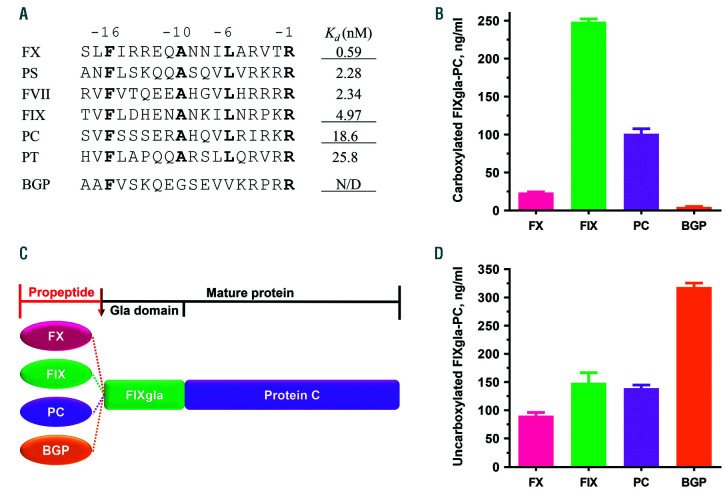
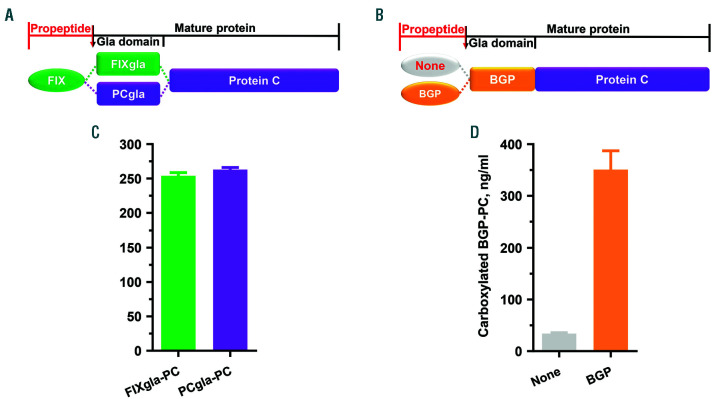
To explore the role of the propeptide on coagulation factor carboxylation in a cellular milieu, we used our recently established mammalian cell-based assay.20 Propeptides with different affinities for GGCX (Figure 1A) were fused to the N-terminus of the chimeric reporter-protein FIXgla-PC (Figure 1C). These chimeric fusion proteins were transiently expressed in HEK293 cells, and the efficiency of their carboxylation was determined by ELISA.20 We selected propeptides of FX, FIX, PC, and BGP for this study. Results from our cell-based study show that BGP propeptide cannot direct reporter-protein carboxylation (Figure 1B), which agrees with results from in vitro studies.14–16 In addition, FIX propeptide is the most efficient propeptide for reporter-protein carboxylation, which is approximately 10-fold higher than that of FX propeptide and 2.5-fold higher than PC propeptide. However, this cell-based result of carboxylation of propeptide attached protein substrate is different from the previous in vitro study showing that all of the propeptides stimulated carboxylation of the non-covalently linked substrate to a similar extent.23
Figure 1.
Effect of propeptides on coagulation factor carboxylation. (A) Sequence alignment of propeptides of vitamin K-dependent (VKD) coagulation factors and bone Gla protein (BGP), and their relative Kd values for gamma-glutamyl carboxylase (GGCX). Highly conserved residues are indicated by bold letters. The Kd values of different propeptides (adapted from Higgins-Gruber et al.23) are a relative measure of propeptides’ affinity for GGCX. The underlined Kd values correspond to the propeptides used in this study. (B) Carboxylation efficiency of the reporter-protein in HEK293 cells as directed by different propeptides. Reporter-proteins with different propeptides were transiently expressed in HEK293 cells, and the transfected cells were cultured in complete medium containing 11 μM vitamin K. The carboxylated reporter-protein in the cell culture medium was determined by ELISA.(C) Domain structure of the reporter-protein, factor IX gla-protein C (FIXgla-PC), with different propeptides. The propeptide is proteolytically removed after carboxylation to form the mature protein. (D) Expression of uncarboxylated reporter-proteins with different propeptides as in (B). Reporter-proteins were transiently expressed in HEK293 cells and the transfected cells were incubated in complete medium containing 5 μM warfarin. The total amount of uncarboxylated reporter-protein in the cell culture medium was determined by ELISA.
To confirm that the significantly decreased production of carboxylated reporter-proteins, directed by the propeptides of FX and BGP, was not due to the effect of the propeptide on reporter-protein expression, we determined the expression levels of the reporter-proteins in cell culture medium without carboxylation by feeding the cells with warfarin. The reporter-protein has similar expression levels when fused to different coagulation factor propeptides (FX, FIX, and PC) (Figure 1D). However, a significant amount of uncarboxylated reporter-protein was produced when BGP propeptide was used. Nevertheless, these results suggest that the significant difference in reporter-protein carboxylation, when fused to different propeptides (Figure 1B), is not due to the effect of the propeptide on reporter-protein expression, but rather to its carboxylation.
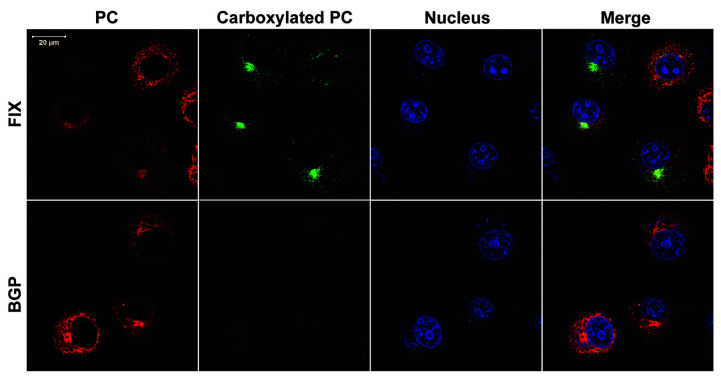
The unaffected expression and non-carboxylation characteristics of the reporter-protein, when fused to BGP propeptide, were confirmed further by immunofluorescence confocal imaging (Figure 2). The reporter-protein (fused with either a FIX or BGP propeptide) was properly synthesized and directed to the ER (Figure 2, red images, total reporter-protein), but only the fusion with FIX propeptide was properly carboxylated (Figure 2, green image, carboxylated reporter-protein). The location of the carboxylated reporter-protein appears to be in both the ER and Golgi apparatus, which is consistent with previous observations.24 Together, these results suggest that the propeptide plays an essential role in coagulation factor carboxylation.
Figure 2.
Localization of reporter-proteins by immunofluorescence confocal imaging. The reporter-protein factor IX gla-protein C (FIXgla-PC) with the propeptide of FIX or bone Gla protein (BGP) was transiently expressed in COS-7 cells. The transfected cells were cultured with complete medium containing 11 μM vitamin K. The carboxylated reporter-protein (carboxylated PC) was immuno-stained by the mouse anti-carboxylated FIXgla monoclonal antibody as the primary antibody, and Alexa Fluor-488 conjugated donkey anti-mouse IgG as the secondary antibody (green image). The total reporter-protein (PC) was probed by the sheep anti-PC polyclonal antibody as the primary antibody and the Alexa Fluor-568 conjugated donkey anti-sheep IgG as the secondary antibody (red image). The cell nucleus was stained by Hoechst 33342 (blue image).
The entire N-terminal sequence of the propeptide determines the efficiency of coagulation factor’s carboxylation
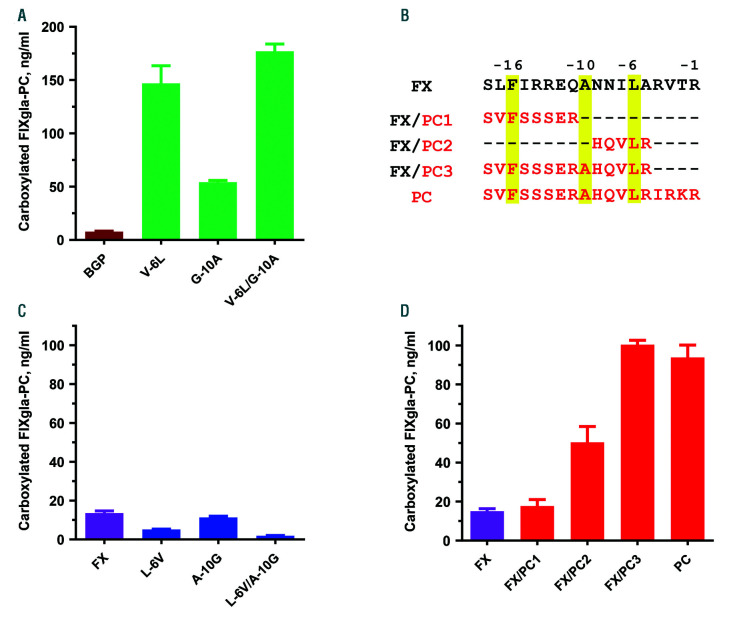
The alanine residue at −10 and leucine residue at −6 of the coagulation factor propeptide are highly conserved (Figure 1A). However, BGP propeptide has a glycine at −10 and valine at −6. The in vitro study shows that substituting alanine for glycine at −10 (G-10A) in BGP propeptide increased its affinity for GGCX 45-fold, and substituting leucine for valine at −6 (V-6L) increased its affinity approximately 100-fold.25 When both G-10A and V-6L are mutated in BGP propeptide, its apparent affinity for GGCX is similar to that of FIX propeptide. To examine how these mutations affect carboxylation efficiency in vivo, we made the same BGP propeptide substitutions in the chimeric reporter-protein of FIXgla-PC (Figure 1C) and examined their effect on reporter-protein carboxylation in HEK293 cells. The V-6L mutant increased reporter-protein carboxylation approximately 20-fold and the G-10A mutant increased reporter-protein carboxylation approximately 7-fold (Figure 3A). Mutating both residues increased reporter-protein carboxylation approximately 23-fold, a carboxylation efficiency close to that of FIX propeptide. These results, consistent with our previous in vitro study,25 suggest that the conserved residues at positions −6 and −10 in the propeptide are essential for its binding to GGCX and for substrate carboxylation.
Figure 3.
Contribution of propeptide’s conserved and non-conserved residues to reporter-protein carboxylation. Effect of conserved residues at −6 and −10 of the propeptide of bone Gla protein (BGP) (A) and factor X (FX) (C) on the carboxylation of reporter-proteins as evaluated by cell-based assay. Chimeric reporter-proteins with wild-type or mutant propeptides were transiently expressed in HEK293 cells and the carboxylated reporter-protein was determined by ELISA, as described in B. (B) Propeptide sequence of FX and the replacement of part of its sequence with that of protein C (PC) (highlighted in red). Highly conserved residues are highlighted in yellow. (D) Carboxylation of the reporter-protein directed by chimeric propeptide sequences of FX and PC as indicated in (B).
Factor X propeptide is the tightest binding propeptide of the coagulation factors, and carboxylation of FX has a slow turnover rate. Based on these observations and the above result (Figure 3A), we replaced the conserved residues of FX propeptide at −10 or −6 to that of the BGP in the chimeric reporter-protein of FIXgla-PC. We assumed these replacements would decrease the affinity of FX propeptide for GGCX and therefore increase the turnover rate of reporter-protein carboxylation. Unexpectedly, our results show that neither the single mutation (L-6V or A-10G) nor the double mutation (L-6V/A-10G) increased reporter-protein carboxylation (Figure 3C), suggesting that the non-conserved residues of the coagulation factor’s propeptide play a role in GGCX’s binding and substrate carboxylation.
To explore the contribution of the propeptide’s non-conserved residues to carboxylation, we replaced FX propeptide sequence between −11 and −18 with that of PC (FX/PC1) (Figure 3B), a propeptide that has approximately 90-fold lower affinity for GGCX.16 Results from our cell-based study show that this replacement does not show an obvious effect on reporter-protein carboxylation (Figure 3D). However, when we exchanged the propeptide sequence between −5 and −9 (FX/PC2) (Figure 3), reporter-protein carboxylation was increased approximately 3-fold. Further replacement of FX propeptide between −5 and −18 with that of PC (FX/PC3) (Figure 3), increased reporter-protein carboxylation approximately 6-fold, a level similar to the full-length propeptide of PC (Figure 3D). These results suggest that the entire N-terminal sequence of the propeptide (−5 to −18) determines the carboxylation efficiency of coagulation factors, which is consistent with previous observations.13
Effect of naturally occurring propeptide mutations on coagulation factor carboxylation
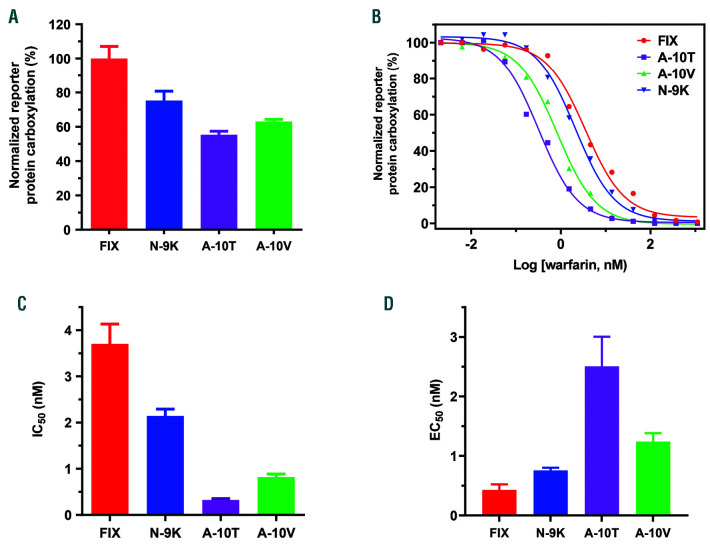
Naturally occurring mutations have been identified at positions −1, −4, −9 and −1025–30 in the propeptide of coagulation factors. Patients bearing mutations at positions −9 and −10 of FIX propeptide possess near-normal levels of active FIX; but, these levels are reduced to <1% of normal during anticoagulation therapy (referred to as warfarin hypersensitivity).27,28,31–33 Meanwhile, other VKD clotting factor levels only decreased to 30-40%. To examine the effect of these mutations on coagulation factor carboxylation in a cellular milieu, we introduced these mutations to FIX propeptide in our reporter-protein for cell-based functional study. Our results show that, despite significant differences in GGCX affinity in vitro,25 these mutations have only a moderate effect on reporter-protein carboxylation in a cellular environment (Figure 4A), which is consistent with clinical observations of patients bearing these mutations that have near-normal levels of active FIX.
Figure 4.
Effect of naturally occurring propeptide mutations on reporter-protein carboxylation. (A) Carboxylation efficiency of the chimeric reporter-protein with wild-type factor IX (FIX) propeptide or with its naturally occurring mutations N-9K, A-10T, and A-10V. The wild-type and corresponding mutant reporter-proteins were transiently expressed in HEK293 cells and the carboxylated reporter-protein was determined by ELISA, as described in the legend to Figure 1C. (B) Warfarin titration of reporter-protein carboxylation as directed by FIX propeptide with naturally occurring mutations at positions −9 and −10. The wild-type and corresponding mutant reporter-proteins were stably expressed in HEK293 cells. The corresponding reporter cells were cultured in complete medium containing 20 nM vitamin K with increasing concentrations of warfarin. (C) Inhibition efficiency of warfarin on the carboxylation of mutant reporter-proteins. The half-maximal inhibition concentration (IC50) of warfarin was determined from (B) using GraphPad software. (D) Effect of vitamin K concentration on reporter-protein carboxylation. HEK 293 cells stably expressing wild-type and corresponding mutant reporter-proteins were cultured with complete medium containing increasing concentrations of vitamin K. The efficiency of reporter-protein carboxylation was determined by ELISA and the half-maximal effective concentration (EC50) of vitamin K was determined using GraphPad software.
To clarify the warfarin hypersensitivity phenotype during anticoagulation therapy of patients carrying these mutations, we examined the response of the mutant reporter-proteins’ carboxylation to increasing concentrations of warfarin. We stably expressed the individual mutant or wild-type reporter-protein in HEK293 cells and examined their carboxylation efficiency under different warfarin or vitamin K concentrations. Results showed that, compared with the wild-type reporter-protein, the N-9K mutant has a moderate effect on warfarin inhibition, while the warfarin response curve of the A-10T and A-10V mutants significantly shifts to lower concentrations of warfarin (Figure 4B), suggesting that they are more sensitive to warfarin inhibition. The half-maximal inhibition concentration (IC50) of warfarin for the A-10T and A-10V mutants decreased 11.6-fold and 4.5-fold, respectively (Figure 4C), which is consistent with a recent similar cell-based study.26 As warfarin blocks the vitamin K recycling, we also examined the effect of vitamin K on the carboxylation of these mutant reporter-proteins. We determined the half-maximal effective concentration (EC50) of vitamin K for the carboxylation of these mutant reporter-proteins. Compared to the wild-type reporter-protein, the A-10T and A-10V mutants required a significantly higher concentration of vitamin K (5.8-fold and 2.9-fold, respectively) to achieve half-maximal carboxylation (Figure 4D). Together, these results suggest that mutations at −9 and −10 of the propeptide are more sensitive to warfarin inhibition.
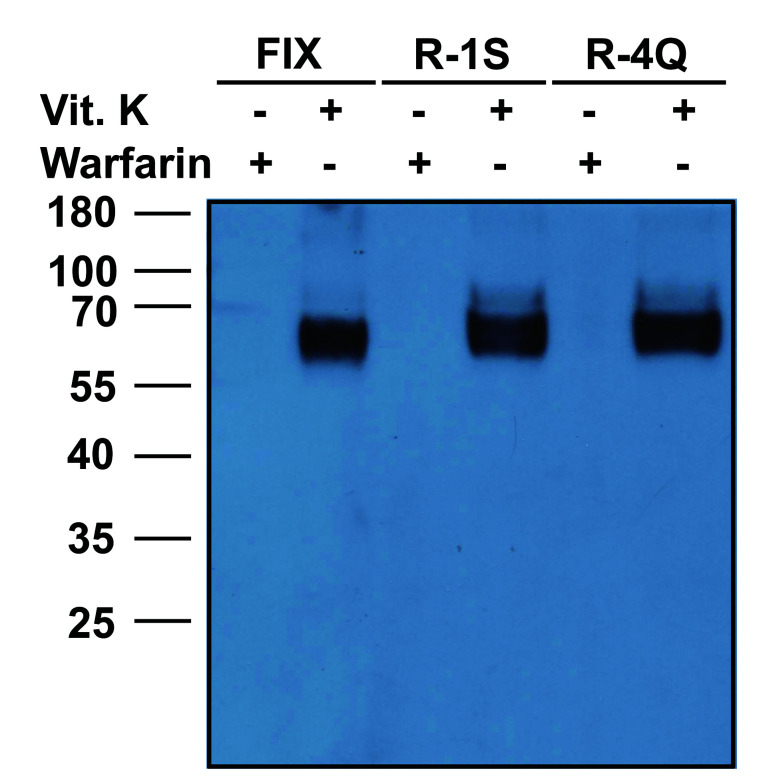
Naturally occurring mutations at position −4 and −1 of the propeptide preclude post-translational cleavage of the propeptide, resulting in secretion of the pro-coagulation factor with its propeptide still attached.29,30,34,35 To examine the effect of these mutations on carboxylation, we mutated R-1 (R-1S) or R-4 (R-4Q) of the propeptide in our reporter-protein and transiently expressed them in HEK293 cells. Transfected cells were cultured with vitamin K. However, we were unable to detect reporter-protein carboxylation using ELISA (data not shown). This could be due to the un-cleaved propeptide which prevents the recognition of the carboxylated Gla domain by the conformational specific antibody, as observed previously.29 To test this hypothesis, we examined reporter-protein carboxylation from cell culture medium using western blot analysis with an antibody that recognizes Gla residues. Our result shows that, compared to the wild-type reporter-protein, the R-1S and R-4Q mutants appear to be properly carboxylated, but migrate slower (Figure 5). This suggests that mutations at −1 and −4 do not affect reporter-protein carboxylation but prevent the cleavage of the propeptide, in agreement with previous observations.29,30,34
Figure 5.
Effect of naturally occurring propeptide mutations at positions −1 and −4 on reporter-protein carboxylation. Wild-type and −1 and −4 mutant reporter-proteins were transiently expressed in HEK293 cells; the transfected cells were cultured with a serum-free medium containing 11 μM vitamin K (Vit. K) or 5 μM warfarin (Warfarin). The cell culture medium was collected 48 hours post-transfection and loaded to SDS-PAGE for Western blot analysis. Carboxylated protein bands were probed by a mouse monoclonal antibody that recognizes Gla residues.
Contribution of other sequences of coagulation factor to vitamin K-dependent carboxylation
To test whether the Gla domain of the coagulation factor contributes to VKD carboxylation, we compared the carboxylation efficiency of the Gla domains of FIX (containing 12 Gla residues) and PC (containing 9 Gla residues) in our chimeric reporter-protein (Figure 6A). These reporter-proteins were transiently expressed in HEK293 cells, and their carboxylation efficiency was examined by ELISA using antibodies that specifically recognize the corresponding carboxylated Gla domains. Both reporter-proteins can be efficiently carboxylated to a similar level (Figure 6C). Together with previous observations,7,13 this suggests that the Gla domain of the coagulation factor contributes very little to GGCX binding and substrate carboxylation, and that the propeptide is sufficient to direct the following Gla domain of coagulation factors to GGCX for carboxylation.36
Figure 6.
Effect of coagulation factor’s Gla domain and bone Gla protein (BGP) propeptide on reporter-protein carboxylation. (A) Domain structure of the chimeric reporter-protein of protein C (PC) with different Gla domains. (B) Domain structure of BGP-PC chimeric reporter-protein with and without the BGP propeptide. (C) Carboxylation efficiency of the chimeric reporter-proteins factor IX gla-protein C (FIXgla-PC) and PCgla-PC. The corresponding reporter-protein was transiently expressed in HEK293 cells and the carboxylation efficiency of the reporter-protein was determined, as in B. (D) Carboxylation efficiency of the chimeric reporter-proteins in (B).
It should be noted that BGP propeptide has an undetectable affinity to GGCX in vitro,16 which has been suggested to be unnecessary for BGP carboxylation.14,15 To test this hypothesis, we removed BGP propeptide in the chimeric reporter-protein BGP-PC (Figure 6B), as previously described in the coagulation factor study.7 Our result from the cell-based study showed that the removal of BGP propeptide significantly decreased (10-fold) reporter-protein carboxylation (Figure 6D), suggesting that BGP propeptide plays an essential role in BGP carboxylation, which differs from in vitro studies.14,15
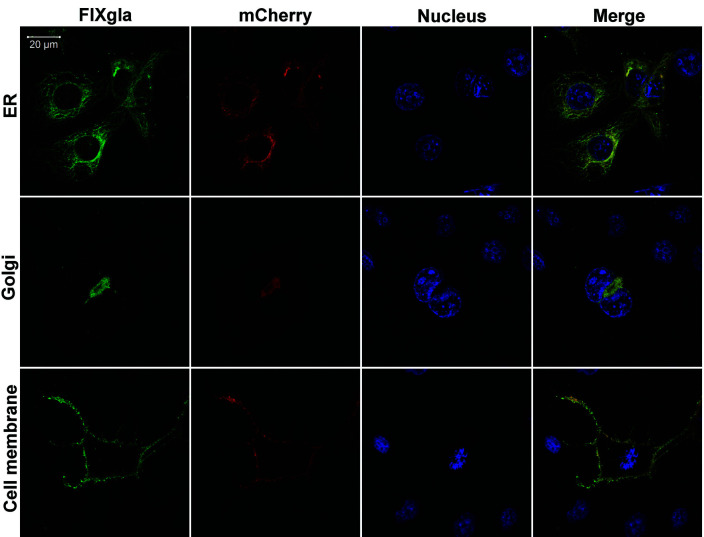
To examine the effect of coagulation factor’s remaining domains on its carboxylation, we used chimeric reporter-proteins of FIX with its EGF and following domains replaced by different cell organelle marker proteins, including Sec61B for ER, Giantin for Golgi, and tissue factor for the plasma membrane. As controls, cell organelle marker proteins were fused to the N-terminal of the mCherry fluorescent protein. The cell organelle specific FIXgla chimeric reporter-proteins and the corresponding controls were transiently co-expressed in COS-7 cells. The transfected cells were cultured with vitamin K, and the carboxylated reporter-protein was immuno-stained with an antibody that specifically recognizes the carboxylated Gla domain of FIX. These reporter-proteins were properly carboxylated (Figure 7, green image) and transported to the destined locations (Figure 7, red image), supporting the view that the remaining domains of FIX do not affect its carboxylation.
Figure 7.
Localization of carboxylated factor IX (FIX)gla fused chimeric cell organelle marker proteins. FIXgla and mCherry (control) fused cell organelle marker proteins (Sec61B for ER, Giantin for Golgi, and tissue factor for Cell membrane) were transiently co-expressed in COS-7 cells. The transfected cells were cultured with 11 μM vitamin K. Forty-eight hours post transfection, cells were fixed with 4% paraformaldehyde and permeabilized with 0.20% Triton X-100. Carboxylated reporter-proteins (FIXgla) were immuno-stained with a mouse anti-carboxylated FIXgla monoclonal antibody as the primary antibody, and Alexa Fluor-488 conjugated donkey anti-mouse IgG as the secondary antibody (green image). mCherry fusion proteins (mCherry) were directly visualized as a fluorescent protein (red image), and the cell nucleus was stained by Hoechst 33342 (blue image).
Discussion
The aim of this study was to explore carboxylation of coagulation factors in a cellular environment in order to explain the clinical phenotypes of naturally occurring mutations in coagulation factors, as related to their carboxylation modifications. Previous studies have shown that the propeptide is essential for directing coagulation factor carboxylation.7,13 Despite a significant variation in affinity, once the propeptide binds to GGCX, it has been proposed that it induces a conformational change in the GGCX active site that stimulates carboxylation of its substrate to a similar extent.23 The in vitro study shows that the carboxylation rate is much faster than the rate of product release,37 and the release of the carboxylated product from GGCX can be detected in coagulation factors with a lower affinity propeptide but not with a higher affinity propeptide.38 Therefore, it was hypothesized that exchanging the higher affinity propeptide with a reduced affinity propeptide would enhance coagulation factor carboxylation by allowing for a higher substrate turnover. For example, it has been shown that substituting FX propeptide with a lower affinity propeptide (PT propeptide) significantly increased FX carboxylation.17 However, it is not clear why this hypothesis applies to carboxylation of FX17 but not to that of FIX.18
Results from this study show that FIX propeptide is the most efficient propeptide for directing coagulation factor carboxylation and that the propeptide with either a higher (FX propeptide) or lower (PC propeptide) affinity has a reduced carboxylation efficiency (Figure 1). The affinity of FIX propeptide is approximately 8-fold lower than that of FX and is approximately 4-fold higher than that of PC.16,23 The efficiency of FX propeptide to direct reporter protein carboxylation is only approximately 10% of that of FIX. This result suggests that the affinity of FIX propeptide for GGCX is optimal, as it balances the rate of carboxylation and product releasing. This explains why the propeptide exchanging strategy increased carboxylation of FX but not that of FIX.17,18
It has been proposed that the propeptide contains two recognition elements: one for GGCX recognition (located towards the N-terminus) and one for propeptidase recognition (located near the C-terminus). For GGCX recognition, it appears that only a few conserved residues are essential for GGCX binding.25,39 Several naturally occurring mutations have been identified in the GGCX recognition region of FIX propeptide. These mutations are clinically silent in normal conditions, but selectively decrease FIX activity dramatically during warfarin therapy, which could cause life-threatening bleeding complications.27 To explore the role of the propeptide on coagulation factor carboxylation and the clinical consequence of mutations in the propeptide, we examined these questions using our recently established cell-based assays.40 Unlike previous in vitro studies, our results show that the entire N-terminal sequence of the propeptide, rather than a few conserved residues, determines the carboxylation efficiency of coagulation factors (Figure 3). This explains why the essential residues for GGCX binding in the propeptide of all coagulation factors are highly conserved, while the affinity of the propeptides for GGCX varies over 100-fold.16
Our results also show that mutations in FIX propeptide have a moderate effect on reporter-protein carboxylation at a higher vitamin K concentration (Figure 4A), but a significant effect on warfarin sensitivity (Figure 4B and C), which is consistent with the clinical phenotype of warfarin hypersensitivity in patients bearing these mutations during anticoagulation therapy.
The C-terminus of the propeptide is thought to be the propeptidase recognition site, essential for the propeptide cleavage to form mature coagulation factors.13 Naturally occurring mutations were found in this region at positions −4 and −1. Patients carrying these mutations in FIX have a bleeding diathesis and have a propeptide attached FIX (proFIX) detected in their plasma.30 This proFIX loses lipid binding ability and has no coagulation activity.41 Our results show that mutations at −4 and −1 do not affect reporter-protein carboxylation, although the propeptide is still attached to the carboxylated protein (Figure 5). These mutant proteins cannot be recognized by a calcium-dependent conformational specific antibody, suggesting a loss of function.41 However, contradictory results exist on whether these mutations affect FIX carboxylation. It has been shown that proFIX purified from hemophilia patients, carrying mutations at −4, is properly carboxylated,30,34 others have shown that proFIX with a mutation at −4 in a hemophilia patient is only partially carboxylated.35 This discrepancy may result from the different approaches used in determining the extent of carboxylation. Nevertheless, it has been confirmed that these mutations interfere with propeptide cleavage, and therefore affect the function of FIX (mainly by destabilizing the calcium-binding conformation),34 impair FIX binding to the lipid membrane, and affect FIX activity for coagulation.29,41
In contrast to numerous studies which indicate that the propeptide of BGP is not required for GGCX binding and its carboxylation,14–16 results from this study show that the removal of BGP propeptide dramatically decreased BGP carboxylation (Figure 6D). This result is consistent with previous studies showing that a non-covalently attached propeptide stimulates BGP carboxylation and a covalently attached propeptide directs complete carboxylation of BGP.42,43 In addition, consistent with previous studies, our results show that BGP propeptide cannot direct coagulation factor carboxylation (Figures 1B and 2). Together, these results suggest that BGP may have a different mechanism for carboxylation than coagulation factors. This supports observations that vitamin K intake affects the carboxylation of coagulation factors and of BGP in different ways.44–47 Further studies are ongoing to clarify the mechanistic differences in the carboxylation of a variety of VKD proteins. Results from these studies will continue to provide insights into efficiently controlling one physiological process without affecting the other.
Acknowledgments
The authors would like to thank Dr. Paul Bajaj from the University of California, Los Angeles for helpful discussions and for providing the Ca2+-dependent monoclonal antibody against carboxylated Gla domain of protein C.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/8/2164
Funding
This work is supported by grant HL131690 from the National Institutes of Health (to JKT and DWS).
References
- 1.Shearer MJ, Okano T. Key Pathways and Regulators of Vitamin K Function and Intermediary Metabolism. Annu Rev Nutr. 2018;38:127–151. [DOI] [PubMed] [Google Scholar]
- 2.Stenflo J, Fernlund P, Egan W, Roepstorff P. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proc Natl Acad Sci U S A. 1974;71(7):2730–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Napolitano M, Mariani G, Lapecorella M. Hereditary combined deficiency of the vitamin K-dependent clotting factors. Orphanet J Rare Dis. 2010;5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girardot JM, Delaney R, Johnson BC. Carboxylation, the completion step in prothrombin biosynthesis. Biochem Biophys Res Commun. 1974;59(4):1197–1203. [DOI] [PubMed] [Google Scholar]
- 5.Wu SM, Cheung WF, Frazier D, Stafford DW. Cloning and expression of the cDNA for human gamma-glutamyl carboxylase. Science. 1991;254(5038):1634–1636. [DOI] [PubMed] [Google Scholar]
- 6.Wu SM, Morris DP, Stafford DW. Identification and purification to near homogeneity of the vitamin K-dependent carboxylase. Proc Natl Acad Sci U S A. 1991;88(6):2236–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jorgensen MJ, Cantor AB, Furie BC, Brown CL, Shoemaker CB, Furie B. Recognition site directing vitamin K-dependent gamma-carboxylation resides on the propeptide of factor IX. Cell. 1987;48(2):185–191. [DOI] [PubMed] [Google Scholar]
- 8.Morris DP, Stevens RD, Wright DJ, Stafford DW. Processive post-translational modification. Vitamin K-dependent carboxylation of a peptide substrate. J Biol Chem. 1995;270(51):30491–30498. [DOI] [PubMed] [Google Scholar]
- 9.Cheung A, Engelke JA, Sanders C, Suttie JW. Vitamin K-dependent carboxylase: influence of the "propeptide" region on enzyme activity. Arch Biochem Biophys. 1989;274(2):574–581. [DOI] [PubMed] [Google Scholar]
- 10.Knobloch JE, Suttie JW. Vitamin K-dependent carboxylase. Control of enzyme activity by the "propeptide" region of factor X. J Biol Chem. 1987;262(32):15334–15337. [PubMed] [Google Scholar]
- 11.Brown MA, Begley GS, Czerwiec E, et al. Precursors of novel Gla-containing conotoxins contain a carboxy-terminal recognition site that directs gamma-carboxylation. Biochemistry. 2005;44(25):9150–9159. [DOI] [PubMed] [Google Scholar]
- 12.Price PA, Fraser JD, Metz-Virca G. Molecular cloning of matrix Gla protein: implications for substrate recognition by the vitamin K-dependent gamma-carboxylase. Proc Natl Acad Sci U S A. 1987; 84(23):8335–8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster DC, Rudinski MS, Schach BG, et al. Propeptide of human protein C is necessary for gamma-carboxylation. Biochemistry. 1987;26(22):7003–7011. [DOI] [PubMed] [Google Scholar]
- 14.Vermeer C, Soute BA, Hendrix H, de Boer-van den Berg MA. Decarboxylated bone Gla-protein as a substrate for hepatic vitamin K-dependent carboxylase. FEBS Lett. 1984;165(1):16–20. [DOI] [PubMed] [Google Scholar]
- 15.Houben RJ, Rijkers DT, Stanley TB, et al. Characteristics and composition of the vitamin K-dependent gamma-glutamyl carboxylase-binding domain on osteocalcin. Biochem J. 2002;364(Pt 1):323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanley TB, Jin DY, Lin PJ, Stafford DW. The propeptides of the vitamin K-dependent proteins possess different affinities for the vitamin K-dependent carboxylase. J Biol Chem. 1999;274(24):16940–16944. [DOI] [PubMed] [Google Scholar]
- 17.Camire RM, Larson PJ, Stafford DW, High KA. Enhanced gamma-carboxylation of recombinant factor X using a chimeric construct containing the prothrombin propeptide. Biochemistry. 2000;39(46):14322–14329. [DOI] [PubMed] [Google Scholar]
- 18.Blostein M, Cuerquis J, Landry S, Galipeau J. The carboxylation efficiency of the vitamin K-dependent clotting factors: studies with factor IX. Haemophilia. 2008; 14(5):1063–1068. [DOI] [PubMed] [Google Scholar]
- 19.Rishavy MA, Berkner KL. Vitamin K oxygenation, glutamate carboxylation, and processivity: defining the three critical facets of catalysis by the vitamin K-dependent carboxylase. Adv Nutr. 2012;3(2):135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tie JK, Jin DY, Straight DL, Stafford DW. Functional study of the vitamin K cycle in mammalian cells. Blood. 2011;117(10): 2967–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ndonwi M, Broze GJ, Jr, Agah S, Schmidt AE, Bajaj SP. Substitution of the Gla domain in factor X with that of protein C impairs its interaction with factor VIIa/tissue factor: lack of comparable effect by similar substitution in factor IX. J Biol Chem. 2007; 282(21):15632–15644. [DOI] [PubMed] [Google Scholar]
- 22.Wu S, Chen X, Jin DY, Stafford DW, Pedersen LG, Tie JK. Warfarin and vitamin K epoxide reductase: a molecular accounting for observed inhibition. Blood. 2018; 132(6):647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins-Gruber SL, Mutucumarana VP, Lin PJ, Jorgenson JW, Stafford DW, Straight DL. Effect of vitamin K-dependent protein precursor propeptide, vitamin K hydroquinone, and glutamate substrate binding on the structure and function of 42-glutamyl carboxylase. J Biol Chem. 2010;2 85(41):31502–31508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bristol JA, Ratcliffe JV, Roth DA, Jacobs MA, Furie BC, Furie B. Biosynthesis of pro-thrombin: intracellular localization of the vitamin K-dependent carboxylase and the sites of gamma-carboxylation. Blood. 1996; 88(7):2585–2593. [PubMed] [Google Scholar]
- 25.Stanley TB, Humphries J, High KA, Stafford DW. Amino acids responsible for reduced affinities of vitamin K-dependent propeptides for the carboxylase. Biochemistry. 1999;38(47):15681–15687. [DOI] [PubMed] [Google Scholar]
- 26.Pezeshkpoor B, Czogalla KJ, Caspers M, et al. Variants in FIX propeptide associated with vitamin K antagonist hypersensitivity: functional analysis and additional data confirming the common founder mutations. Ann Hematol. 2018;97(6):1061–1069. [DOI] [PubMed] [Google Scholar]
- 27.Sekhri A, Lisinschi A, Furqan M, et al. The Conundrum of "Warfarin Hypersensitivity": Prolonged Partial Thromboplastin Time From Factor IX Propeptide Mutation. Am J Ther. 2016; 23(3):e911–915. [DOI] [PubMed] [Google Scholar]
- 28.Chu K, Wu SM, Stanley T, Stafford DW, High KA. A mutation in the propeptide of Factor IX leads to warfarin sensitivity by a novel mechanism. J Clin Invest. 1996; 98(7):1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ware J, Diuguid DL, Liebman HA, et al. Factor IX San Dimas. Substitution of glutamine for Arg-4 in the propeptide leads to incomplete gamma-carboxylation and altered phospholipid binding properties. J Biol Chem. 1989;264(19):11401–11406. [PubMed] [Google Scholar]
- 30.Bentley AK, Rees DJ, Rizza C, Brownlee GG. Defective propeptide processing of blood clotting factor IX caused by mutation of arginine to glutamine at position -4. Cell. 1986;45(3):343–348. [DOI] [PubMed] [Google Scholar]
- 31.Ulrich S, Brand B, Speich R, Oldenburg J, Asmis L. Congenital hypersensitivity to vitamin K antagonists due to FIX propeptide mutation at locus −10: a (not so) rare cause of bleeding under oral anticoagulant therapy in Switzerland. Swiss Med Wkly. 2008;138(7-8):100–107. [DOI] [PubMed] [Google Scholar]
- 32.Aegerter C, Fontana S, Fux C, Demarmels Biasiutti F. Life threatening bleeding under adequate oral anticoagulation. Cases 4a, b. Hamostaseologie. 2003;23(3):113–116. [PubMed] [Google Scholar]
- 33.Baker P, Clarke K, Giangrande P, Keeling D. Ala-10 mutations in the factor IX propeptide and haemorrhage in a patient treated with warfarin. Br J Haematol. 2000;108(3): 663. [DOI] [PubMed] [Google Scholar]
- 34.Wojcik EG, Van Den Berg M, Poort SR, Bertina RM. Modification of the N-terminus of human factor IX by defective propeptide cleavage or acetylation results in a destabilized calcium-induced conformation: effects on phospholipid binding and activation by factor XIa. Biochem J. 1997;323(Pt 3):629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de la Salle C, Charmantier JL, Ravanat C, et al. The Arg-4 mutant factor IX Strasbourg 2 shows a delayed activation by factor XIa. Nouv Rev Fr Hematol. 1993;35(5):473–480. [PubMed] [Google Scholar]
- 36.Furie BC, Ratcliffe JV, Tward J, et al. The gamma-carboxylation recognition site is sufficient to direct vitamin K-dependent carboxylation on an adjacent glutamate-rich region of thrombin in a propeptide-thrombin chimera. J Biol Chem. 1997;272(45):28258–28262. [DOI] [PubMed] [Google Scholar]
- 37.Hallgren KW, Hommema EL, McNally BA, Berkner KL. Carboxylase overexpression effects full carboxylation but poor release and secretion of factor IX: implications for the release of vitamin K-dependent proteins. Biochemistry. 2002;41(50):15045–15055. [DOI] [PubMed] [Google Scholar]
- 38.Wallin R, Martin LF. Early processing of prothrombin and factor X by the vitamin K-dependent carboxylase. J Biol Chem. 1988;263(20):9994–10001. [PubMed] [Google Scholar]
- 39.Sanford DG, Kanagy C, Sudmeier JL, Furie BC, Furie B, Bachovchin WW. Structure of the propeptide of prothrombin containing the gamma-carboxylation recognition site determined by two-dimensional NMR spectroscopy. Biochemistry. 1991;30(41): 9835–9841. [DOI] [PubMed] [Google Scholar]
- 40.Tie JK, Stafford DW. Functional Study of the Vitamin K Cycle Enzymes in Live Cells. Methods Enzymol. 2017;584:349–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bristol JA, Freedman SJ, Furie BC, Furie B. Profactor IX: the propeptide inhibits binding to membrane surfaces and activation by factor XIa. Biochemistry. 1994;33(47): 14136–14143. [DOI] [PubMed] [Google Scholar]
- 42.Benton ME, Price PA, Suttie JW. Multi-site-specificity of the vitamin K-dependent carboxylase: in vitro carboxylation of des-gamma-carboxylated bone Gla protein and Des-gamma-carboxylated pro bone Gla protein. Biochemistry. 1995;34(29):9541–9551. [DOI] [PubMed] [Google Scholar]
- 43.Engelke JA, Hale JE, Suttie JW, Price PA. Vitamin K-dependent carboxylase: utilization of decarboxylated bone Gla protein and matrix Gla protein as substrates. Biochim Biophys Acta. 1991;1078(1):31–34. [DOI] [PubMed] [Google Scholar]
- 44.Theuwissen E, Cranenburg EC, Knapen MH, et al. Low-dose menaquinone-7 supplementation improved extra-hepatic vitamin K status, but had no effect on thrombin generation in healthy subjects. Br J Nutr. 2012;108(9):1652–1657. [DOI] [PubMed] [Google Scholar]
- 45.Kuwabara A, Fujii M, Kawai N, Tozawa K, Kido S, Tanaka K. Bone is more susceptible to vitamin K deficiency than liver in the institutionalized elderly. Asia Pac J Clin Nutr. 2011;20(1):50–55. [PubMed] [Google Scholar]
- 46.Rejnmark L, Vestergaard P, Charles P, et al. No effect of vitamin K1 intake on bone mineral density and fracture risk in perimenopausal women. Osteoporos Int. 2006;17(8):1122–1132. [DOI] [PubMed] [Google Scholar]
- 47.Price PA, Kaneda Y. Vitamin K counteracts the effect of warfarin in liver but not in bone. Thromb Res. 1987;46(1):121–131. [DOI] [PubMed] [Google Scholar]