Abstract
Inhibition of Janus kinases [JAKs] in Crohn’s disease [CD] patients has shown conflicting results in clinical trials. Tofacitinib, a pan-JAK inhibitor, showed efficacy in ulcerative colitis [UC] and has been approved for the treatment of patients with moderate to severe UC. In contrast, studies in CD patients were disappointing and the primary end point of clinical remission could not be met in the respective phase II induction and maintenance trials. Subsequently, the clinical development of tofacitinib was discontinued in CD. In contrast, efficacy of filgotinib, a selective JAK1 inhibitor, in CD patients was demonstrated in the randomized, double-blinded, placebo-controlled phase II FITZROY study. Upadacitinib also showed promising results in a phase II trial in moderate to severe CD. Subsequently, phase III programmes in CD have been initiated for both substances, which are still ongoing. Several newer molecules of this class of orally administrated immunosuppressants are being tested in clinical programmes. The concern of side effects of systemic JAK inhibition is addressed by either exclusively intestinal action or higher selectivity [Tyk2 inhibitors]. In general, JAK inhibitors constitute a new promising class of drugs for the treatment of CD.
Keywords: Crohn’s disease, Janus Kinase Inhibitors, treatment, induction of remission, maintenance of remission, clinical trials, side effects
1. Introduction
Many treatment strategies for patients with Crohn’s disease [CD] and ulcerative colitis [UC] are similar despite the fact that both diseases have different characteristics and probably also different pathophysiology. Steroids, thiopurines [for maintenance of remission], anti-tumour necrosis factor [TNF] antibodies, anti-alpha4beta7-integrin antibodies and anti-interleukin [IL]-23 antibodies have shown clinical efficacy in both subtypes of inflammatory bowel disease [IBD].1 However, a significant number of patients remain insufficiently treated, surgery is frequent in CD and 10–15% of patients with UC still undergo colectomy.
Subsequently, Janus kinase [JAK] inhibitors were considered for clinical trials in both CD and UC and clinical trials programmes for different compounds from this substance class have been established for both IBD entities.2–4 Tofacitinib, a pan-JAK inhibitor, in the meantime has been approved for the treatment of patients with moderate to severe UC.5–11 Reports on the clinical testing of three JAK inhibitors in patients with moderate to severe CD have been published: tofacitinib [pan-JAK inhibitor],10 filgotinib [JAK1 inhibitor]12 and upadacitinib [JAK1 inhibitor].12
Why did the inhibition of JAKs appear to be a promising new treatment strategy in IBD? JAKs phosphorylate activated cytokine receptors and are necessary for their signal transduction.13,14 They are located intracellularly and belong to the large family of tyrosine kinases, enzymes that bind a phosphate group to the amino acid tyrosine [tyrosine phosphorylation].2,15 Subsequently, these molecules are involved in the transduction of cytokine-transmitted signals from the cell surface to the cell nucleus to modify gene expression.2,15 Cytokines bind to their specific receptors on the surface of the target cells [e.g. lymphocytes or macrophages]. After binding of the cytokines such as IL-6 or IL-2 and IL12 and IL-23 to their receptors, the conformation of the receptor is changed and JAKs are activated to phosphorylate the receptor. This phosphorylation of the respective receptors by the tyrosine kinases of the JAK family allows members of the ‘signal transducer and activator of transcription’ [STAT] protein family to bind to the receptor. They also become phosphorylated and subsequently form dimers, which then dissociate from the receptor and move into the nucleus where they change the transcription of genes.2,15
Many cytokine receptors rely on JAK family proteins to transmit the signals after binding of a specific cytokine molecule. In contrast, this is not the case for example for TNF receptors: TNF receptors require other proteins that become bound to them to initiate intracellular signal transduction such as ‘Tumor necrosis factor receptor type 1-associated DEATH domain protein’ [TRADD], ‘TNF receptor associated factors’ [TRAFs], ‘Receptor-interacting protein [RIP] kinases’ and ‘Fas-associated protein with death domain’ [FADD].16–18 Nevertheless, there is a connection between TNF and the JAK/STAT pathways: after binding of TNF to its receptor, STAT proteins [which are involved in JAK-induced signal transduction] are up-regulated. The action of TNF can thereby amplify the signal transduction of JAK-dependent receptors, indicating that these pathways are not completely independent but influence each other.
Receptors that use JAK family members, however, are not only mediating pro-inflammatory signals. Among the cell surface receptors that depend on JAK signalling are also the erythropoietin receptor19 and ‘Granulocyte-macrophage colony-stimulating factor’ [GM-CSF]20–22 as well as ‘Granulocyte colony-stimulating factor’ [G-CSF].23 This explains why JAK inhibitors can be applied in myeloproliferative disorders. It also explains some side effects seen with certain compounds, such as anaemia [blockade of erythropoietin signalling].
Four different proteins belong to the JAK family of tyrosine kinases: JAK1, JAK2, JAK3 and ‘tyrosine-protein kinase 2’ [TYK2].2 In different combinations, usually two of these protein family members are associated with a certain receptor type.2
JAK inhibitors are under development for a variety of disease besides IBD, such as the treatment of rheumatoid arthritis, psoriasis, polycythemia vera, alopecia, thrombocythemia, myelofibrosis and vitiligo. Different compounds have different specificities for different JAKs or TYK. There is ongoing discussion regarding which profile of JAK inhibition would be optimal for which specific disease. The first approved JAK inhibitor (by the Food and Drug Administration [FDA] in 2011) was ruxolitinib [trade names Jakafi/Jakavi] against JAK1/JAK2 for psoriasis, myelofibrosis and rheumatoid arthritis.24–26 Tofacitinib [trade names Xeljanz/Jakvinus], a pan-JAK inhibitor, was first approved in November 2012 initially in rheumatoid arthritis in patients who had an inadequate response or intolerance to methotrexate.27–31 Peficitinib [ASP015K, JNJ-54781532; trade name Smyraf] was approved for the treatment of rheumatoid arthritis in Japan in 2019.32 Fedratinib [SAR302503; trade name Inrebic] was approved the FDA in August 2019 for treatment of myelofibrosis and essential thrombocythemia.33 Upadacitinib [trade name Rinvoq; ABT-494], mainly targeting JAK1, also was approved by the FDA for the treatment of rheumatoid arthritis in August 2019.34–38
Filgotinib [G-146034, GLPG-0634] also is relatively specific for JAK1. In spring 2019, Gilead and Galapagos announced that the Phase 3 FINCH 1 and FINCH 3 trials in rheumatoid arthritis patients met the primary and key secondary end points.39 Earlier, in 2018, it was reported that the TORTUGA Phase 2 trial of filgotinib in ankylosing spondylitis met the primary end point40 and well as the EQUATOR Phase 2 trial of filgotinib in psoriatic arthritis.41,42
Further JAK inhibitors in clinical development are Cerdulatinib [PRT062070] for haematological malignancies,43,44 Gandotinib [LY-2784544] for myeloproliferative neoplasms,45 Lestaurtinib [CEP-701] for acute myeloid leukaemia,46,47 Momelotinib [GS-0387, CYT-387] for myeloproliferative disorders,48,49 Pacritinib [SB1518] for relapsed lymphoma and advanced myeloid malignancies,50,51 and PF-04965842 for atopic dermatitis and moderate to severe psoriasis.52
2. Methods
For this review on the efficacy of JAK inhibitors in CD, PubMed, Embase and CENTRAL were systematically searched up to October 1, 2019. Randomized placebo‐controlled trials [RCTs] of JAK inhibitors in adult patients with CD were eligible. Additional information was retrieved from the Trials.gov database. MEDLINE was searched via PubMed, EMBASE via Ovid and The Cochrane Central Register of Controlled Trials [CENTRAL].
Furthermore, the reference lists of included studies and systematic reviews from the last 5 years on JAK therapy for the management of inflammatory bowel disease were searched for relevant studies.
The following search strategy was performed in the respective databases: (‘Crohn*[TIAB] OR IBD [TIAB] OR Inflammatory bowel disease*[TIAB] AND JAK [MeSH] OR janus kinase inhibitor[TIAB]) AND (randomized controlled trial [Publication Type] OR controlled clinical trial [Publication Type] OR randomized [TIAB] OR placebo [TIAB] OR drug therapy [Subheading] OR randomly [TIAB] OR trial [TIAB] OR groups [TIAB]). Non-English literature was excluded.
3. Lack of efficacy of tofacitinib in CD
As mentioned above Tofacitinib [Pfizer] has shown promising results for the treatment of UC and has been approved by the FDA and European Medicines Agency [EMA] as well as other regulatory agencies [e.g. SwissMedic].5,7,53–56 Tofacitinib is a rather broad JAK inhibitor that besides having a main activity for JAK1 and JAK3 also inhibits tyrosine kinases outside the JAK family.2,55 Therefore, it has always been a matter of discussion whether its action can solely be attributed to JAK inhibition.2 Tofacitinib has a functional half-life of ~3 h, making multiple dosing more promising than single dosing.57–59 Hepatic metabolism [70%] is more important than renal clearance [30%] for excretion of the drug.57–59 This explains why both liver disease and renal insufficiency have to be taken into account when oral dosing is decided.
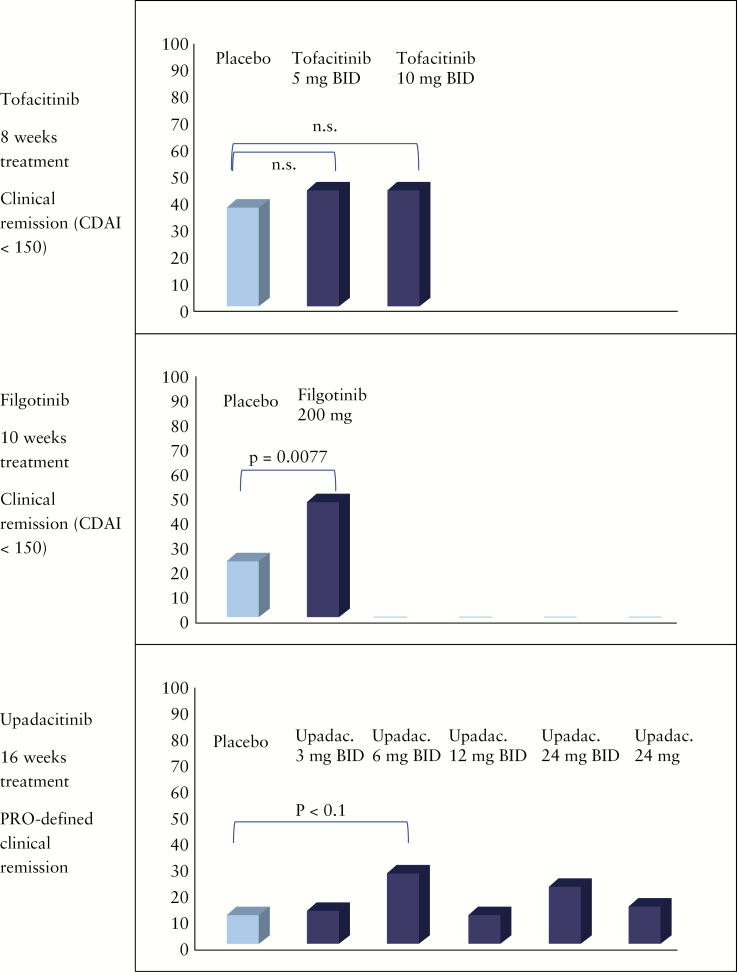
Tocatinib was tested in CD patients initially in a clinical phase IIa induction of remission design [NCT00615199].60 The results presented by Sandborn and co-workers of this 4-week induction study with moderate to severe CD were reported in 2014.60 In total, 139 CD patients were randomized to either placebo, tofacitinib 1 mg, 5 mg, or 15 mg twice daily [BID]. Forty-eight centres in 12 countries contributed to this placebo-controlled, randomized trial.60 The primary end point in this trial was not clinical remission but clinical response at week 4 (defined as a decrease from baseline in the Crohn’s Disease Activity Index [CDAI] score of >70 points [CR70]). A secondary end point was clinical remission [CDAI < 150] at week 4. In contrast to the findings for UC patients, disappointingly no differences in clinical response or remission between the placebo group and the treatment groups could be observed. A clinical response was seen in 47% of placebo-treated patients, 36% of patients receiving 1 mg tofacitinib BID, 58% in the 5-mg BID group and 46% in the 15-mg group.60 Clinical remission was reported in 21% of placebo-treated patients and in 31%, 24% and 14% of the 1-, 5- and 15-mg groups of tofacitinib-treated patients.60 In the 15-mg dose group reduced levels of C-reactive protein [CRP] and faecal calprotectin as compared to baseline were reported. Placebo response and remission rates in this trial were greater than expected and higher as in comparable studies on anti-TNF antibodies. The study was criticized because of the short time for induction therapy of only 4 weeks. This 4 week end point was initially chosen to reduce placebo response rates.60 In addition, the selected patients had a relatively mild disease course: only 21% of placebo patients previously had received thioguanines and only 2% previously had failed anti-TNF therapy.60 Furthermore, only 56% of patients had increased faecal calprotectin concentrations [>250 µg/g faeces].
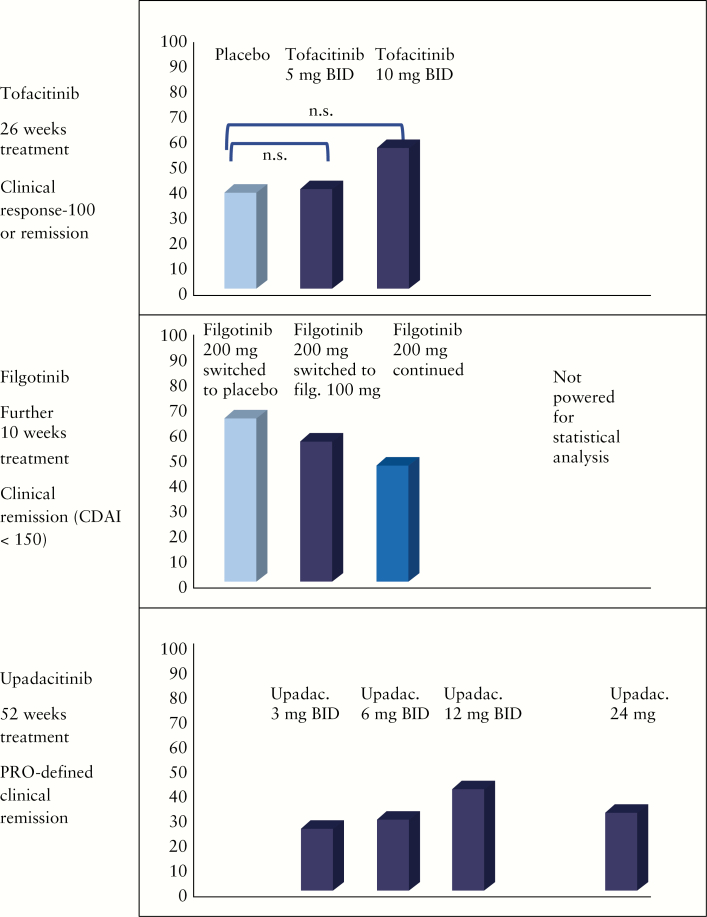
Subsequently, tofacitinib for the induction and maintenance of remission and clinical response in patients with CD was further investigated in two placebo-controlled, randomized, multicentre IIb trials [NCT013932626 and NCT01393899] reported by Panés et al. in 2017.61 Patients were enrolled into two sequential and integrated phase IIb, randomized, double-blind, placebo-controlled, parallel-group, dose-ranging, multicentre trials for induction [induction study] and maintenance [maintenance study] of remission.61 The studies were conducted at 80 sites in 18 countries. Adult patients with moderate to severe CD [CDAI between 220 and 450] were included. Endoscopic activity had to be confirmed, but not by central reading. Patients with an inadequate response or intolerance to steroids, thiopurines, methotrexate or anti-TNF antibodies were eligible. The study design intended a 3:2:2:4 randomization [placebo, 5, 10 or 15 mg tofacitinib BID]. Treatment duration in the induction trial was 8 weeks. The 15-mg BID treatment group was stopped during the trial and patients finally were randomized 1:1:1 [placebo, 5 or 10 mg tofacitinib BID].61 If patients had a significant reduction of disease activity [decrease of CDAI of >100 points] or achieved a clinical remission [usually defined as CDAI < 150] at week 8 they could be re-randomized 1:1:1 to receive either placebo or tofacitinib 5 or 10 mg BID for another 26 weeks [maintenance phase]. Steroid tapering during the maintenance study was mandatory with a reduction of corticosteroid doses of 5 mg prednisolone-equivalent weekly until 20 mg/day, and then by 2.5 mg/week until 10 mg/day.
In the induction study the primary end point was clinical remission [as mentioned, CDAI < 150 at week 8]. Secondary end points in the induction study were clinical response with a decrease in CDAI of either 70 points [CR70] or 100 points [CR100] as compared to baseline CDAI.61 In total, 280 CD patients were randomized in the induction study [92 placebo and 86 either 5 mg or 10 mg tofacitinib]. There was a high rate of patients pre-exposed to anti-TNF antibodies: 76.9% in the placebo arm and 77.9% treated with tofacitinib.61
At the end of the treatment phase at week 8 no significant differences between the placebo group and the treatment groups was observed [Figure 1]. Clinical remission was reported for 36.7% of placebo-treated patients, 43.5% of patients treated with 5 mg tofacitinib BID and 43.0% of patients treated with 10 mg tofacitinib BID.61 The high rate of patients achieving remission in the placebo arm again was surprising. With respect to the secondary end point, a significantly higher rate of CR100 [reduction of 100 points in CDAI] was seen with 5 mg tofacitinib BID as compared to placebo [70.6% vs 54.4%, p < 0.05]. Similarly, the reduction of 70 points in CDAI was significantly higher in the 5-mg tofacitinib group as compared to placebo-treated CD patients [76.5% vs 62.2%, p < 0.05]. However, again the high placebo response rates were surprising and unexpected.
Figure 1.
Induction of clinical remission.
Further, Panés et al. performed a post-hoc analysis.61 As patient-reported outcomes were regarded as being increasingly important by the regulators the investigators analysed the ‘patient reported outcome’ [PRO] remission: two PRO scores were evaluated. PRO2 [calculated as the sum of stool frequency and abdominal pain scores] for which remission is <75 points, and PRO3 [calculated as the sum of stool frequency, abdominal pain and general well-being scores] for which remission is defined <80 points.61 For the PROs Panés et al. found differences between the placebo and treatment groups: PRO2, 40.0% vs. 58.8% [p < 0.05]; and PRO3, 24.4 vs 38.8% [p < 0.05]. Furthermore, patients treated with tofacitinib had greater mean decreases in CRP over the course of the study as compared to placebo [p < 0.001].61 In contrast, there were surprisingly no significant differences for faecal calprotectin between the groups.
For the maintenance study [randomization at week 8 of the induction study] the primary end point was defined as clinical remission [CDAI < 150] or clinical response [CR100] at week 26.61 Secondary end points were changes in CRP and faecal calprotectin. In total, 180 patients could be re-randomized for this maintenance study. Fifty-nine patients received placebo, 60 patients were in the 5-mg tofacitinib BID group, and 61 patients were randomized to the 10-mg tofacitinib BID group. Similar to the induction study the primary end point of the maintenance trial was not achieved [Figure 2].61 There was no significant difference for the patients who were in clinical remission at week 26 and no difference for clinical response [CR100]. For the secondary end points, significantly lower CRP and faecal calprotectin was observed in the 10-mg tofacitinib group as compared to placebo.
Figure 2.
Maintenance of remission.
Whereas the primary end points of both studies could not be met, a modest effect of tofacitinib was seen for the secondary end points of CR70 and CR100 at week 8.61 Again the relatively high placebo rates raised doubts regarding the study design. The lack of a requirement for central reading was highlighted, although it remains questionable whether this had such a high influence. An important aspect is certainly that there was no protocol-defined threshold for an objective marker of disease activity, such as for CRP or faecal calprotectin levels at baseline.
The further development of tofacitinib in CD was stopped after these trials.
4. Filgotinib has demonstrated efficacy in phase II CD trials
Filgotinib [GLPG0634, GS-6034, Galapagos] has a 28-fold selectivity for JAK1 over JAK2 and is subsequently regarded as a JAK1-targeted JAK inhibitor. Filgotinib has a longer half life of ~6 h for the parent compound and ~23 h for the active metabolite as compared to tofacitinib.62 This allows a once daily dosing.
The efficacy of filgotinib for the induction of remission in moderate to severe CD patients was studied in the randomized, placebo-controlled, multicentre phase II FITZROY study.12 The inclusion criteria were targeted on adult CD patients with a CDAI between 220 and 450. Fifty-two centres in nine European countries contributed. In contrast to the tofacitinib studies, the FITZROY design included a central endoscopy reading. Patients could be included if the central reader agreed that there was an ulceration score of >1 in at least one ileocolonic segment and total Simple Endoscopic Score for Crohn’s Disease [SES-CD] > 7. Eligible patients were randomized 1:3 to placebo or filgotinib 200 mg once daily.12 Stratification was performed according to anti-TNF antibody exposure, baseline corticosteroid use and baseline CRP. The initial treatment period was 10 weeks.12 After 10 weeks, patients not responding to placebo were switched to filgotinib 100 mg daily for 10 weeks. Responders from the filgotinib group were re-randomized 1:2:2 to receive either placebo or filgotinib 100 mg daily or 200 mg daily. The complicated study design was completed by an arm for non-responders to filgotinib during the initial 10 weeks. They were randomized 1:3 to receive ether placebo or 200 mg filgotinib daily.
The primary end point of the FITZROY study was clinical remission [CDAI < 150] at week 10.12 Secondary end points were clinical response [as measured by CDAI and PRO2], endoscopic response [SES-CD reduction > 50%], endoscopic remission [SES-CD < 4 and ulcerated surface subscore < 1 in all segments], mucosal healing [SES-CD = 0], deep remission [CDAI < 150 and SES-CD < 4 and ulcerated surface subscore < 1 in all segments] as well as changes in CRP and faecal calprotectin.12 Endoscopic readouts were evaluated by central reading. In the FITZROY study, 174 patients were randomized. Of these patients, 44 received placebo and 130 received 200 mg filgotinib daily.
The primary end point was reached in the FITZROY study [Figure 1]. Clinical remission was found in 23% of CD patients treated with placebo as compared to 47% of patients who received 200 mg filgotinib daily [p = 0.0077] in the intention-to-treat population [Δ 24%]12 [Figure 1]. The difference was higher in anti-TNF naïve patients [13% vs 60%].12 With respect to the secondary end points, a significant difference was seen for CR100 [41% vs 59%, p < 0.05] and PRO2 [30% vs 50% [p < 0.03].12 No significant difference between placebo and filgotinib 200 mg was observed for the following secondary end points: SES-CD 50% response [14% vs 25, p = 0.16], endoscopic remission [7% vs 14%, p = 0.31], mucosal healing [2% vs 4%, p = 0.82] and deep remission [2% vs 8%, p = 0.31]. In particular, the low rates for endoscopic remission and mucosal healing were somewhat disappointing. For the second 10-week study period, 50% [200 mg] and 71% [100 mg] of initial filgotinib responders randomized were in clinical remission at week 20. However, this second study part was not powered for statistical analysis [Figure 2].
Despite the relatively disappointing rates for endoscopic end points and mucosal healing [only 4% of patients achieved mucosal healing], the overall positive data stimulated a large phase III induction and maintenance trial evaluating filgotinib in moderate to severe CD [Diversity1, NCT02914561]. Furthermore, a phase II trial evaluating the efficacy of filgotinib in fistulizing CD has been initiated [Divergence2, NCT03077412]. In addition, a phase II trial evaluating the efficacy of filgotinib for small bowel CD has been started [NCT03046056].
5. Upadacitinib does not reach its primary end points in phase II CD trials but meets some secondary end points
Upadacitinib [ABT-494, AbbVie], similar to filgotinib, is an oral JAK1 selective inhibitor with an even higher [74-fold] selectivity for JAK1 over JAK2.63 Upadacitinib has a half-life of ~4 h, which is shorter than filgotinib.63 Similar to tofacitinib, upadacitinib is eliminated to 80% via hepatic metabolization [CYP3A4 and CYP2D6] and 20% by urinary excretion [20%]. Subsequently, hepatic and renal functions need to be considered for dosing.
The efficacy of upadacitinib for the induction and maintenance of remission in moderate to severe CD patients was studied in a randomized, placebo-controlled multicentre phase II trial [CELEST], which so far has only been published in abstract form.64–67
The study design included a 16-week induction phase and a 36-week blinded extension phase. Similar to the above-mentioned trials, patients were eligible when they had moderate to severe CD with a CDAI of 220–450. Again, endoscopic activity was evaluated to include only truly inflamed and active CD patients [SES-CD > 6 or > 4 for isolated ileal disease]. Patients were randomized to receive either placebo or 3, 6, 12, 24 mg BID or 24 mg once upadacitinib.66–68 The number of patients who had previously received anti-TNF therapy was the highest of all trials reported here: 96% of patients in CELEST were anti-TNF experienced.66–68 The co-primary end points were clinical remission and endoscopic remission [SES-CD < 4 and a >2-point reduction from baseline with no subscore >1]. Secondary end points were modified clinical remission [stool frequency score < 2.8 and abdominal pain score < 1.0], clinical response [defined by a > 30% reduction scores] and endoscopic response [defined by a > 25% reduction in baseline SES-CD]. In total, 220 patients were randomized in the CELEST trial, 37 of whom received placebo, 39 received 3 mg upadacitinib BID, 37 received 6 mg upadacitinib BID, 36 received 12 mg upadacitinib BID, 36 received 24 mg upadacitinib BID and 35 received 24 mg upadacitinib once daily.66–68
At week 16 clinical remission was not significantly different for the upadacitinib groups as compared to placebo [Figure 1]. The highest difference was seen for 6 mg upadacitinib BID vs placebo for PRO-defined clinical remission [11% vs 27%, p < 0.10]. However, no clear dose response could be observed [Figure 1]. In subgroup analyses for patients receiving corticosteroids at baseline, clinical remission was significantly more frequent in patients treated with upadacitinib 24 mg BID [0% vs 33.3%, p < 0.05].65,68,69 For the secondary end point of endoscopic remission, a dose response could be found.65,68,69 Whereas no patients treated with placebo achieved endoscopic remission, 14% of CD patients treated with upadacitinib 24 mg daily and 22% of CD patients treated with upadacitinib 24 mg BID were in endoscopic remission at week 16 [p < 0.05 and p < 0.01, respectively]. Patients who received either 6 mg upadacitinib BID, 12 mg upadacitinib BID, 24 mg upadacitinib BID or 24 mg upadacitinib daily were more likely to achieve >25% and >50% reductions in SES-CD as compared to placebo [p < 0.05 for all comparisons].65,66,69–72
After the 16-week induction patients were randomized 1:1:1 to receive either 3 mg upadacitinib BID, 12 mg upadacitinib BID or 24 mg upadacitinib once daily for 36 weeks. The protocol was amended to drop the 24-mg once daily dose and instead a 6-mg upadacitinib BID treatment arm was added. In total, 180 patients were re-randomized. A certain dose dependency was observed: in patients who had achieved clinical response by week 16, clinical remission was observed in 25% [3 mg BID], 28.6% [6 mg BID], 41.4% [12 mg BID] and 31.6% [24 mg once daily] of patients receiving upadacitinib [Figure 2]. In patients who had achieved clinical and endoscopic response at week 16, endoscopic remission was observed in 25% [3 mg BID], 25% [6 mg BID], 37.5% [12 mg BID] and 10% [24 mg once daily] of patients receiving upadacitinib64
Based on the findings of the CELEST trial, two large phase III trials enrolling patients failing either biologic [NCT03345836] or conventional non-biologic [NCT03345849] therapies have been initiated.
6. New JAK-related molecules in deveopment in CD
Preliminary data suggest that besides the ‘classical’ JAKs, TYK2 may be a therapeutic target in CD. No TYK2-selective drug has so far been approved. TKY2 is involved in IL-12, IL-13 and interferon signalling. The relatively narrow range of cytokines dependent on TYK2 may reduce side effects of inhibition.73
Therefore, BMS-986165 [Bristol-Myers Squibb] is now being studied in IBD clinical trials. BMS-986165 selectively inhibits TYK2.74,75 It binds exclusively to the active catalytic site of TYK2, which irreversibly inhibits TYK2 activation.74 A phase II placebo-controlled, randomized, multicentre, multidosing interventional study [LATTICE] has been initiated to evaluate the safety and efficacy of BMS-986165 for the induction and maintenance of remission in patients with moderate to severe CD.
With respect to optimization of efficacy, PF-06700841 and PF-06651600 [Pfizer] are currently under study in IBD. JAK2 forms a homodimer important for erythropoietin signalling. To avoid JAK2 inhibition-mediated side effects, dual JAK1/TYK2 inhibition without an influence on JAK2 signalling could be of interest. PF-06700841 is a new selective JAK inhibitor also mainly targeting TYK2 but also JAK1.76,77
In contrast, PF-06651600 is a selective JAK3 inhibitor.78,79In vitro, PF-06651600 was shown to inhibit Th1 and Th17 cell differentiation. PF-06700841 and PF-06651600 are currently under study in phase II in patients with moderate to severe CD.
Another approach to avoid side effects of JAK inhibitors is a non-systemic but local application. TD-1473 [Theravance Biopharma] is a pan-JAK inhibitor [inhibiting JAK1, JAK2, JAK3 and TYK2] with high affinity. However, it is not absorbed and thus is distributed only in the intestinal tract, reducing systemic exposure.80,81 It is subsequently regarded to have a gut selective action. In a phase I trial in patients with UC, the safety, tolerability and pharmacodynamics of TD-1473 were evaluated.82,83 TD-1473 was well tolerated over 4 weeks, without serious or opportunistic infections. Low plasma levels and higher colonic tissue concentrations confirmed gut selectivity. Phase II and phase III trials have been initiated to study the efficacy of TD-1473 for the induction and maintenance of remission in patients with moderate to severe CD.
7. Safety profile of JAK inhibition in CD patients
JAK inhibitors have shown a pattern of safety signals in different patient groups. An about four-fold increased risk for herpes zoster was reported for tofacitinib in patients with rheumatoid arthritis or UC. Furthermore, FDA and European Medicines Evalution Agency [EMEA] warnings with respect to thromboembolic complications were released for tofacitinib in 2019. Patients with thromboembolic risk factors such as hormonal contraceptives should not receive the 10-mg BID dosage according to the EMEA warning.
In CD induction studies with tofacitinib, adverse events [AEs] were reported for 60.4%, 58.1% and 60.5% of patients receiving placebo, or tofacitinib 5 mg and 10 mg BID. In the maintenance study with tofacitinib, AEs were reported in 74.6%, 83.3% and 78.7% of patients receiving placebo, or tofacitinib 5 mg and 10 mg BID.60,61 Serious adverse events [SAEs] were more frequently reported in the maintenance study mainly in patients receiving 10 mg tofacitinib BID [11.6% in induction, 13.1% in maintenance] as compared to 5 mg BID [3.5% in induction, 10.0% in maintenance] or placebo [3.3% in induction, 11.9% in maintenance].61 In the maintenance study, three patients in the tofacitinib 5-mg BID group reported serious infections and two patients in the tofacitinib 10-mg BID group. No cases of opportunistic infections were reported. Similar to other studies, two cases of herpes zoster were reported in the tofacitinib 10-mg BID group. No thromboembolic complications were reported in the CD trials.
For the FITZROY study in both parts of the pooled safety analysis, AEs were not significantly different between the placebo group and the filgotinib groups [67% vs 75%].12 SAEs were also not statistically more frequent in the filgotinib groups albeit there was a numerical difference [4% for placebo, 9% for filgotinib]. This is relevant, as serious infections were only reported in filgotinib-treated patients [4/152].12 Again, there was a signal for herpes zoster, making a group effect for JAK inhibitor likely.
In the CELEST study for upadacitinib, the occurrence of AEs was not significantly different between the placebo group [73%] and the upadacitinib arms [82%].67,68 In contrast, SAEs occurred in 5% of patients in the placebo group compared with 15% of patients in the upadacitinib arms.67,68 Serious infections occurred in eight patients on upadacitinib. Among them were four cases of sepsis. Furthermore, two patients on upadacitinib had a myocardial infarction and two patients suffered from small bowel perforations.67,68
As mentioned, the EMA recently recommended that tofacitinib 10 mg BID should not be given to patients with thromboembolic risk factors such as current use of oral contraceptives or hormonal therapy, decompensated heart disease, history of previous thromboembolic events, hereditary coagulopathy, cancer and recent major surgical interventions.84 Pulmonary embolisms also occurred in the UC developments programme for tofacitinib.84
8. Discussion
JAK inhibitors represent an interesting class of molecules. A number of compounds have been approved for a variety of haematological and auto-immune diseases. The development of JAK inhibitors in the field of IBD has also shown progress in recent years.
Whereas data in UC are promising and have led to the approval of tofacitinib, treatment results in CD have in general been less impressive. The study design of the tofacitinib studies has been criticized and the high placebo rate found in the tofacitinib trials in CD patients certainly raises concerns. However, endoscopic end points in the FITZROY or CELEST studies also show no groundbreaking effect although treatment periods in the trials have been short [8–10 weeks].
Nevertheless, JAK inhibitors are an attractive therapeutic option also in CD patients as their oral bioavailability is high. The safety profile certainly needs to be investigated in more detail. The occurrence of thromboembolic complications, herpes zoster and serious infections raises concerns. Risk/benefit analyses should be performed for this class of compounds in CD. An appealing approach is the use of more specific [e.g. TYK2 inhibition] or gut selective compounds that are under development.
Conflict of Interest
G.R. has received consultancy fees from Abbvie, Augurix, BMS, Boehringer, Calypso, Celgene, FALK, Ferring, Fisher, Genentech, Gilead, Janssen, MSD, Novartis, Pfizer, Phadia, Roche, UCB, Takeda, Tillots, Vifor, Vital Solutions and Zeller; speaker’s honoraria from Astra Zeneca, Abbvie, FALK, Janssen, MSD, Pfizer, Phadia, Sandoz, Takeda, Tillots, UCB, Vifor and Zeller; educational grants and research grants from Abbvie, Ardeypharm, Augurix, Calypso, FALK, Flamentera, MSD, Novartis, Pfizer, Roche, Takeda, Tillots, UCB and Zeller. he is a co-founder of PharmaBiome.
References
- 1. Gomollón F, Dignass A, Annese V, et al.; ECCO 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 2. Danese S, Argollo M, Le Berre C, Peyrin-Biroulet L. JAK selectivity for inflammatory bowel disease treatment: does it clinically matter? Gut 2019;68:1893–9. [DOI] [PubMed] [Google Scholar]
- 3. D’Amico F, Fiorino G, Furfaro F, Allocca M, Danese S. Janus kinase inhibitors for the treatment of inflammatory bowel diseases: developments from phase I and phase II clinical trials. Expert Opin Investig Drugs 2018;27:595–9. [DOI] [PubMed] [Google Scholar]
- 4. Flamant M, Rigaill J, Paul S, Roblin X. Advances in the development of janus kinase inhibitors in inflammatory bowel disease: future prospects. Drugs 2017;77:1057–68. [DOI] [PubMed] [Google Scholar]
- 5. Lohan C, Diamantopoulos A, LeReun C, Wright E, Bohm N, Sawyer LM. Tofacitinib for the treatment of moderately to severely active ulcerative colitis: a systematic review, network meta-analysis and economic evaluation. BMJ Open Gastroenterol 2019;6:e000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanauer S, Panaccione R, Danese S, et al.. Tofacitinib induction therapy reduces symptoms within 3 days for patients with ulcerative colitis. Clin Gastroenterol Hepatol 2019;17:139–47. [DOI] [PubMed] [Google Scholar]
- 7. D’Amico F, Parigi TL, Fiorino G, Peyrin-Biroulet L, Danese S. Tofacitinib in the treatment of ulcerative colitis: efficacy and safety from clinical trials to real-world experience. Therap Adv Gastroenterol 2019;12:1756284819848631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paschos P, Katsoula A, Giouleme O, et al.. Tofacitinib for induction of remission in ulcerative colitis: systematic review and meta-analysis. Ann Gastroenterol 2018;31:572–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fernández-Clotet A, Castro-Poceiro J, Panés J. Tofacitinib for the treatment of ulcerative colitis. Expert Rev Clin Immunol 2018;14:881–92. [DOI] [PubMed] [Google Scholar]
- 10. Sandborn WJ, Su C, Sands BE, et al.; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–36. [DOI] [PubMed] [Google Scholar]
- 11. Izzo R, Bevivino G, Monteleone G. Tofacitinib for the treatment of ulcerative colitis. Expert Opin Investig Drugs 2016;25:991–7. [DOI] [PubMed] [Google Scholar]
- 12. Vermeire S, Schreiber S, Petryka R, et al.. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 2017;389:266–75. [DOI] [PubMed] [Google Scholar]
- 13. Gadina M, Le MT, Schwartz DM, et al.. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology (Oxford) 2019;58:i4–i16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galien R. Janus kinases in inflammatory bowel disease: Four kinases for multiple purposes. Pharmacol Rep 2016;68:789–96. [DOI] [PubMed] [Google Scholar]
- 15. Babon JJ, Lucet IS, Murphy JM, Nicola NA, Varghese LN. The molecular regulation of Janus kinase (JAK) activation. Biochem J 2014;462:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dostert C, Grusdat M, Letellier E, Brenner D. The TNF family of ligands and receptors: communication modules in the immune system and beyond. Physiol Rev 2019;99:115–60. [DOI] [PubMed] [Google Scholar]
- 17. Al-Lamki RS, Mayadas TN. TNF receptors: signaling pathways and contribution to renal dysfunction. Kidney Int 2015;87:281–96. [DOI] [PubMed] [Google Scholar]
- 18. Li J, Yin Q, Wu H. Structural basis of signal transduction in the TNF receptor superfamily. Adv Immunol 2013;119:135–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roskoski R., Jr Janus kinase (JAK) inhibitors in the treatment of inflammatory and neoplastic diseases. Pharmacol Res 2016;111:784–803. [DOI] [PubMed] [Google Scholar]
- 20. Choi JK, Kim KH, Park H, Park SR, Choi BH. Granulocyte macrophage-colony stimulating factor shows anti-apoptotic activity in neural progenitor cells via JAK/STAT5-Bcl-2 pathway. Apoptosis 2011;16:127–34. [DOI] [PubMed] [Google Scholar]
- 21. Fortin CF, Larbi A, Dupuis G, Lesur O, Fülöp T Jr. GM-CSF activates the Jak/STAT pathway to rescue polymorphonuclear neutrophils from spontaneous apoptosis in young but not elderly individuals. Biogerontology 2007;8:173–87. [DOI] [PubMed] [Google Scholar]
- 22. Watanabe S, Itoh T, Arai K. Roles of JAK kinase in human GM-CSF receptor signals. Leukemia 1997;11[Suppl 3]: 76–8. [PubMed] [Google Scholar]
- 23. Biethahn S, Alves F, Wilde S, Hiddemann W, Spiekermann K. Expression of granulocyte colony-stimulating factor- and granulocyte-macrophage colony-stimulating factor-associated signal transduction proteins of the JAK/STAT pathway in normal granulopoiesis and in blast cells of acute myelogenous leukemia. Exp Hematol 1999;27:885–94. [DOI] [PubMed] [Google Scholar]
- 24. Curto-Garcia N, Harrison CN. An updated review of the JAK1/2 inhibitor (ruxolitinib) in the Philadelphia-negative myeloproliferative neoplasms. Future Oncol 2018;14:137–50. [DOI] [PubMed] [Google Scholar]
- 25. Wade R, Hodgson R, Biswas M, Harden M, Woolacott N. A review of ruxolitinib for the treatment of myelofibrosis: a critique of the evidence. Pharmacoeconomics 2017;35:203–13. [DOI] [PubMed] [Google Scholar]
- 26. Wolfe L. Ruxolitinib in myelofibrosis and polycythemia vera. J Adv Pract Oncol 2016;7:436–44. [PMC free article] [PubMed] [Google Scholar]
- 27. Kawalec P, Mikrut A, Wiśniewska N, Pilc A. The effectiveness of tofacitinib, a novel Janus kinase inhibitor, in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Clin Rheumatol 2013;32:1415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ni H, Moe S, Myint KT, Htet A. Oral janus kinase inhibitor for the treatment of rheumatoid arthritis: tofacitinib. ISRN Rheumatol 2013;2013:357904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vyas D, O’Dell KM, Bandy JL, Boyce EG. Tofacitinib: the first Janus kinase (JAK) inhibitor for the treatment of rheumatoid arthritis. Ann Pharmacother 2013;47:1524–31. [DOI] [PubMed] [Google Scholar]
- 30. Dowty ME, Jesson MI, Ghosh S, et al.. Preclinical to clinical translation of tofacitinib, a Janus kinase inhibitor, in rheumatoid arthritis. J Pharmacol Exp Ther 2014;348:165–73. [DOI] [PubMed] [Google Scholar]
- 31. Wollenhaupt J, Silverfield J, Lee EB, et al.. Safety and efficacy of tofacitinib, an oral janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol 2014;41:837–52. [DOI] [PubMed] [Google Scholar]
- 32. Takeuchi T, Tanaka Y, Iwasaki M, Ishikura H, Saeki S, Kaneko Y. Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: a 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann Rheum Dis 2016;75:1057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harrison CN, Schaap N, Vannucchi AM, et al.. Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): a single-arm, open-label, non-randomised, phase 2, multicentre study. Lancet Haematol 2017;4:e317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burmester GR, Kremer JM, Van den Bosch F, et al.. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018;391:2503–12. [DOI] [PubMed] [Google Scholar]
- 35. Duggan S, Keam SJ. Upadacitinib: first approval. Drugs 2019;79:1819–28. [DOI] [PubMed] [Google Scholar]
- 36. Fleischmann R, Pangan AL, Song IH, et al.. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol 2019;71:1788–800. [DOI] [PubMed] [Google Scholar]
- 37. Fleischmann RM, Genovese MC, Enejosa JV, et al.. Safety and effectiveness of upadacitinib or adalimumab plus methotrexate in patients with rheumatoid arthritis over 48 weeks with switch to alternate therapy in patients with insufficient response. Ann Rheum Dis 2019;78:1454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Genovese MC, Fleischmann R, Combe B, et al.. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet 2018;391:2513–24. [DOI] [PubMed] [Google Scholar]
- 39. Genovese MC, Kalunian K, Gottenberg JE, et al.. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the FINCH 2 randomized clinical trial. JAMA 2019;322:315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van der Heijde D, Baraliakos X, Gensler LS, et al.. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active ankylosing spondylitis (TORTUGA): results from a randomised, placebo-controlled, phase 2 trial. Lancet 2018;392:2378–87. [DOI] [PubMed] [Google Scholar]
- 41. Orbai AM, Ogdie A, Gossec L, et al. Effect of filgotinib on health-related quality of life in active psoriatic arthritis: a randomized phase 2 trial (EQUATOR). Rheumatology [Oxford] 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mease P, Coates LC, Helliwell PS, et al.. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): results from a randomised, placebo-controlled, phase 2 trial. Lancet 2018;392:2367–77. [DOI] [PubMed] [Google Scholar]
- 43. Hamlin PA, Flinn IW, Wagner-Johnston N, et al.. Efficacy and safety of the dual SYK/JAK inhibitor cerdulatinib in patients with relapsed or refractory B-cell malignancies: results of a phase I study. Am J Hematol 2019;94:E90–3. [DOI] [PubMed] [Google Scholar]
- 44. Ishikawa C, Senba M, Mori N. Anti-adult T‑cell leukemia/lymphoma activity of cerdulatinib, a dual SYK/JAK kinase inhibitor. Int J Oncol 2018;53:1681–90. [DOI] [PubMed] [Google Scholar]
- 45. Verstovsek S, Mesa RA, Salama ME, et al.. A phase 1 study of the Janus kinase 2 (JAK2)V617F inhibitor, gandotinib (LY2784544), in patients with primary myelofibrosis, polycythemia vera, and essential thrombocythemia. Leuk Res 2017;61:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hexner EO, Mascarenhas J, Prchal J, et al.. Phase I dose escalation study of lestaurtinib in patients with myelofibrosis. Leuk Lymphoma 2015;56:2543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Diaz T, Navarro A, Ferrer G, et al.. Lestaurtinib inhibition of the Jak/STAT signaling pathway in Hodgkin lymphoma inhibits proliferation and induces apoptosis. PLoS One 2011;6:e18856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mesa RA, Kiladjian JJ, Catalano JV, et al.. SIMPLIFY-1: a phase III randomized trial of momelotinib versus ruxolitinib in Janus kinase inhibitor-naïve patients with myelofibrosis. J Clin Oncol 2017;35:3844–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ng K, Hendifar A, Starodub A, et al.. Phase 1 dose-escalation study of momelotinib, a Janus kinase 1/2 inhibitor, combined with gemcitabine and nab-paclitaxel in patients with previously untreated metastatic pancreatic ductal adenocarcinoma. Invest New Drugs 2019;37:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Singer JW, Al-Fayoumi S, Ma H, Komrokji RS, Mesa R, Verstovsek S. Comprehensive kinase profile of pacritinib, a nonmyelosuppressive Janus kinase 2 inhibitor. J Exp Pharmacol 2016;8:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang EG, Mustafa N, Tan EC, et al.. Design and synthesis of Janus Kinase 2 (JAK2) and Histone Deacetlyase (HDAC) bispecific inhibitors based on pacritinib and evidence of dual pathway inhibition in hematological cell lines. J Med Chem 2016;59:8233–62. [DOI] [PubMed] [Google Scholar]
- 52. Schmieder GJ, Draelos ZD, Pariser DM, et al.. Efficacy and safety of the Janus kinase 1 inhibitor PF-04965842 in patients with moderate-to-severe psoriasis: phase II, randomized, double-blind, placebo-controlled study. Br J Dermatol 2018;179:54–62. [DOI] [PubMed] [Google Scholar]
- 53. Tran V, Shammas RM, Sauk JS, Padua D. Evaluating tofacitinib citrate in the treatment of moderate-to-severe active ulcerative colitis: design, development and positioning of therapy. Clin Exp Gastroenterol 2019;12:179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Panés J, Gisbert JP. Efficacy of tofacitinib treatment in ulcerative colitis. Gastroenterol Hepatol 2019;42:403–12. [DOI] [PubMed] [Google Scholar]
- 55. Danese S, D’Amico F, Bonovas S, Peyrin-Biroulet L. Positioning tofacitinib in the treatment algorithm of moderate to severe ulcerative colitis. Inflamm Bowel Dis 2018;24:2106–12. [DOI] [PubMed] [Google Scholar]
- 56. Bonovas S, Lytras T, Nikolopoulos G, Peyrin-Biroulet L, Danese S. Systematic review with network meta-analysis: comparative assessment of tofacitinib and biological therapies for moderate-to-severe ulcerative colitis. Aliment Pharmacol Ther 2018;47:454–65. [DOI] [PubMed] [Google Scholar]
- 57. Krishnaswami S, Boy M, Chow V, Chan G. Safety, tolerability, and pharmacokinetics of single oral doses of tofacitinib, a Janus kinase inhibitor, in healthy volunteers. Clin Pharmacol Drug Dev 2015;4:83–8. [DOI] [PubMed] [Google Scholar]
- 58. Krishnaswami S, Chow V, Boy M, Wang C, Chan G. Pharmacokinetics of tofacitinib, a janus kinase inhibitor, in patients with impaired renal function and end-stage renal disease. J Clin Pharmacol 2014;54:46–52. [DOI] [PubMed] [Google Scholar]
- 59. Dowty ME, Lin J, Ryder TF, et al.. The pharmacokinetics, metabolism, and clearance mechanisms of tofacitinib, a janus kinase inhibitor, in humans. Drug Metab Dispos 2014;42:759–73. [DOI] [PubMed] [Google Scholar]
- 60. Sandborn WJ, Ghosh S, Panes J, Vranic I, Wang W, Niezychowski W; Study A3921043 Investigators A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014;12:1485–93.e2. [DOI] [PubMed] [Google Scholar]
- 61. Panés J, Sandborn WJ, Schreiber S, et al.. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut 2017;66:1049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Namour F, Diderichsen PM, Cox E, et al.. Pharmacokinetics and pharmacokinetic/pharmacodynamic modeling of filgotinib (GLPG0634), a selective JAK1 inhibitor, in support of phase IIB dose selection. Clin Pharmacokinet 2015;54:859–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mohamed MF, Camp HS, Jiang P, Padley RJ, Asatryan A, Othman AA. Pharmacokinetics, safety and tolerability of ABT-494, a novel selective JAK 1 inhibitor, in healthy volunteers and subjects with rheumatoid arthritis. Clin Pharmacokinet 2016;55:1547–58. [DOI] [PubMed] [Google Scholar]
- 64. Panes J, Sandborn WJ, Loftus EV, et al. Efficacy and safety of upadacitinib maintenance treatment for moderate to severe Crohn’s disease: results from the CELEST study. Gastroenterology 2018;154:S178–S9. [Google Scholar]
- 65. Panaccione R, Atreya R, Ferrante M, et al. Upadacitinib improves steroid-free clinical and endoscopic endpoints in patients with Crohn’s disease: data from the celest study. J Crohns Colitis 2018;12:S412–S3. [Google Scholar]
- 66. Sandborn WJ, Feagan B, Lewis JD, et al. Correlation of endoscopic and clinical endpoints during induction therapy in patients with moderate-to-severe Crohn’s disease: analysis from celest study. J Crohns Colitis 2018;12:S375–S6. [Google Scholar]
- 67. Sandborn WJ, Feagan BG, Panes J, et al. Safety and efficacy of abt-494 (upadacitinib), an oral JAK1 inhibitor, as induction therapy in patients with Crohn’s disease: results from celest. Gastroenterology 2017;152:S1308–S9. [Google Scholar]
- 68. Panes J, Sandborn WJ, Loftus EV, et al. Efficacy and safety of upadacitinib maintenance treatment for moderate to severe Crohn’s disease: results from the CELEST study. J Crohns Colitis 2018;12:S238–S9. [Google Scholar]
- 69. Panaccione R, Atreya R, Ferrante M, et al. Upadacitinib improves steroid-free clinical and endoscopic endpoints in patients with Crohn’s disease: data from the CELEST study. Gastroenterology 2018;154:S384–S. [Google Scholar]
- 70. Feagan BG, Sandborn WJ, Schreiber S, et al. Changes in simplified endoscopic score for Crohn’s disease (SES-CD) during a 16-week induction treatment with upadacitinib: analysis of the randomised controlled CELEST study. Gastroenterology 2019;156:S1103–S. [Google Scholar]
- 71. Feagan B, Sandborn W, Schreiber S, et al. Changes in simplified endoscopic score for Crohn’s disease (SES-CD) during a 16-week induction treatment with upadacitinib: analysis of the randomised controlled celest study. J Crohns Colitis 2019;13:S294–S5. [Google Scholar]
- 72. Sandborn WJ, Feagan BG, Lewis J, et al. Correlation of endoscopic and clinical endpoints during induction therapy in patients with moderate to severe Crohn’s disease: analysis from celest study. Gastroenterology 2018;154:S590–S. [Google Scholar]
- 73. Menet CJ. Toward selective TYK2 inhibitors as therapeutic agents for the treatment of inflammatory diseases. Pharm Pat Anal 2014;3:449–66. [DOI] [PubMed] [Google Scholar]
- 74. Wrobleski ST, Moslin R, Lin S, et al.. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem 2019;62:8973–95. [DOI] [PubMed] [Google Scholar]
- 75. Chimalakonda A, Aras U, Yao M, et al. Pharmacokinetics and adme characteristics of a selective tyk2 inhibitor, bms-986165, following oral administration in healthy subjects. Clin Pharmacol Ther 2019;105:S89–S. [Google Scholar]
- 76. Fensome A, Ambler CM, Arnold E, et al.. Dual inhibition of TYK2 and JAK1 for the treatment of autoimmune diseases: discovery of ((S)-2,2-difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl)amino)pyrimidin-4-yl)-3,8-diazabicyclo[3.2.1]octan-8-yl)methanone (PF-06700841). J Med Chem 2018;61:8597–612. [DOI] [PubMed] [Google Scholar]
- 77. Banfield C, Scaramozza M, Zhang W, et al.. The safety, tolerability, pharmacokinetics, and pharmacodynamics of a TYK2/JAK1 inhibitor (PF-06700841) in healthy subjects and patients with plaque psoriasis. J Clin Pharmacol 2018;58:434–47. [DOI] [PubMed] [Google Scholar]
- 78. Xu H, Jesson MI, Seneviratne UI, et al.. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol 2019;14:1235–42. [DOI] [PubMed] [Google Scholar]
- 79. Thorarensen A, Dowty ME, Banker ME, et al.. Design of a Janus kinase 3 (JAK3) specific inhibitor 1-((2S,5R)-5-((7H-Pyrrolo[2,3-d]pyrimidin-4-yl)amino)-2-methylpiperidin-1-yl)prop-2-en-1-one (PF-06651600) allowing for the interrogation of JAK3 signaling in humans. J Med Chem 2017;60:1971–93. [DOI] [PubMed] [Google Scholar]
- 80. Ferslew B, Sherman C, Nguyen D, Graham R. Safety, tolerability, and pharmacokinetics of the intestine-restricted oral pan-Janus kinase inhibitor Td-1473 after single and multiple oral doses in healthy subjects. J Crohns Colitis 2017;11:S317–S8. [Google Scholar]
- 81. Beattie D, Tsuruda P, Shen F, et al. Td-1473, a novel, potent, and orally administered, gi-targeted, pan-Janus kinase (JAK) inhibitor. J Crohns Colitis 2016;10:S123–S. [Google Scholar]
- 82. Sandborn W, Nguyen D, Ferslew B, et al. Clinical, endoscopic, histological and biomarker activity following treatment with the gut-selective, pan-jak inhibitor td-1473 in moderately to severely active ulcerative colitis. J Crohns Colitis 2019;13:S060–S1. [Google Scholar]
- 83. Sandborn WJ, Bhandari R, Leighton J, et al. The gut-selective, orally administered, pan-JAK inhibitor TD-1473 demonstrates favorable safety, tolerability, pharmacokinetic, and signal for clinical activity in subjects with moderately-to-severely active ulcerative colitis. Inflamm Bowel Dis 2019;25:S20–S1. [Google Scholar]
- 84. Sandborn WJ, Panés J, Sands BE, et al.. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther 2019;50:1068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]