Abstract
This review provides guidance in the decision-making process regarding when to choose a janus kinase [JAK] inhibitor as medical treatment strategy. The focus will be on ulcerative colitis, because the only yet available JAK inhibitor, tofacitinib, has approval for use in ulcerative colitis. The guidance path will include consideration of disease activity, previous treatment, comorbidities, family planning, patient preferences, pharmacology as well as concurrent chronic inflammatory diseases or extraintestinal manifestations. The suggested guidance path illustrates our daily difficulties in the decision-making process regarding best choice for the individual patient. However if predictive biomarkers are lacking, the named criteria can be applied to any other strategy and hence provide support in daily practice.
Keywords: Janus Kinases, inflammatory bowel disease, ulcerative colitis, Crohn’s disease, novel treatment
1. Introduction
The previous articles included in this issue of the journal have addressed the molecular structure and function, pharmacology, efficacy of janus kinase [JAK] inhibitors in ulcerative colitis [UC] and Crohn’s disease [CD], as well as safety in inflammatory bowel disease [IBD]. This review aims to position JAK inhibitors in current treatment algorithms, while considering the different aspects raised in the previous articles. To include JAK inhibitors in our current treatment paradigms, we toned to question the factors driving decision-making when we include a new strategy in our current treatment paradigm? Factors contributing to this decision include severity of disease and the time needed for a response, comorbidities, family planning, pharmacological considerations as well as extraintestinal manifestations and patient preferences. These factors are addressed in detail below, ultimately resulting in guidance for decision-making [Figures 1 and 2].
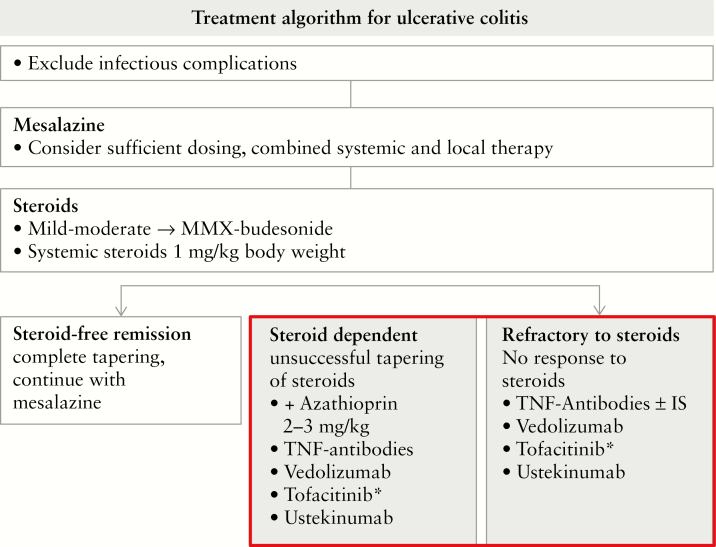
Figure 1.
General treatment algorithm in ulcerative colitis. In the case of frequent steroid use [>1/year], steroid-dependent or steroid refractory disease, an additional, long-term strategy is required. The picture illustrates the choices for moderate to severe ulcerative colitis, and thus excludes severe disease where the only studies available are for infliximab as well as ciclosporin.50,51 *See Figure 2 and the considerations when choosing tofacitinib as an exemplary JAK inhibitor. IS, immunosuppressant.
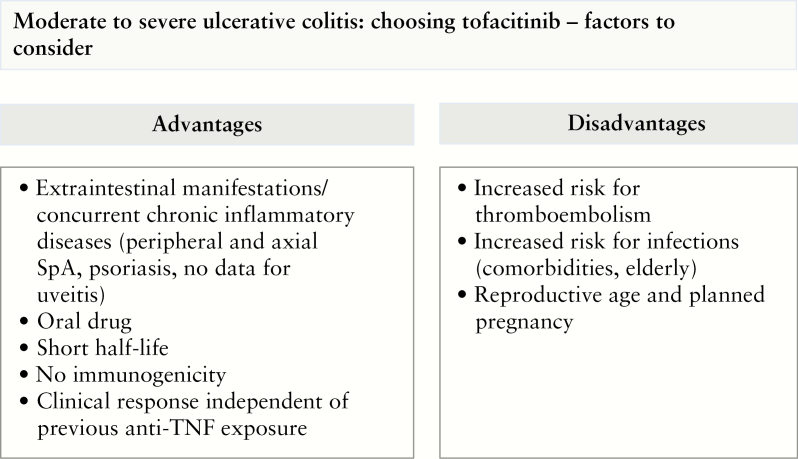
Figure 2.
Guidance for the decision-making process for tofacitinib. The scheme summarizes advantages and disadvantages as well as points to consider when choosing tofacitinib as an exemplary JAK inhibitor. SpA, spondyloarthropathy.
1.1. Clinical need
Over the past two decades our therapeutic options in IBD have been continuously increasing. This novel era started with the introduction of the class of anti-tumour necrosis factor [anti-TNF] antibodies, and was followed over a decade later by the first integrin-antagonist vedolizumab and only in the last years by the class of anti-p40 (interleukin-12 [IL-12]/IL-23] antibody.1–6 The latest addition has been the class of JAK inhibitors, namely tofacitinib as the only currently approved drug.7 Although the number of diverse strategies has been increasing, there is still a substantial proportion of patients who remain insufficiently treated. Over the years, it has been found that patients respond best to the first strategy introduced. Thus, it would be desirable to establish markers that allow for prediction for the best choice of strategy for the individual patient. Until now, we have only limited data that point to the direction that this might prospectively be possible. One example is the study by Atreya et al. where an increased expression of TNF in the intestinal mucosa could be associated with an increased likelihood for response to adalimumab treatment in CD patients.8 The other example stems from a phase 2 trial with etrolizumab, in which an increased intestinal expression of αE correlated with a clinical response.9 However, until a predictive analysis before the initiation of therapy is possible, selection of the therapeutic strategy depends on other factors. Can we specify these factors? The primary goal of treatment is predominantly to improve intestinal inflammation, and therefore we need to to ask: what is the disease severity, and how quickly do we need a response to therapy, or in other words, how much is the daily life of the individual patient affected? The second consideration is the individual patient: are there relevant comorbidities [e.g. diabetes mellitus, thrombotic events in the past], do we have elderly patients or is the patient young and planning a pregnancy? A final consideration should be coexisting extraintestinal manifestations or concurrent chronic inflammatory diseases that equally affect the patient’s quality of life and should ideally be treated with the same strategy.
These three points will be discussed below with regard to JAK inhibitors with a particular focus on tofacitinib. These considerations will then result in the inclusion of JAK inhibitors in the treatment paradigm [summarized in Figures 1 and Figure 2].
1.2. Disease activity and time to response
Clinical studies with regard to JAK inhibitors have been discussed in detail for UC by Marc Ferrante and for CD by Gerhard Rogler in this issue of the Journal of Crohn’s & Colitis. If we limit the discussion here to tofacitinib, the only as yet approved JAK inhibitor, the results of three phase 3, randomized, double-blind, placebo-controlled trials provide relevant data.7 The studies included patients with moderately to severely active UC. Thus, non-hospitalized patients with moderately to severely active UC are eligible for JAK inhibitor treatment. Remarkably, in both induction trials [OCTAVE 1 and 2] the treatment effect was independent of previous anti-TNF treatment.7 As alluded to above, in other treatment strategies previous exposure to anti-TNF therapy resulted in a decreased response rate.1,2,4,5 Hence, failure to an anti-TNF strategy might qualify for JAK inhibitor treatment.
As referred to above, the article by Rogler addresses the role of JAK inhibitors in CD. With regard to tofacitinib, the results of two phase 2b studies did not reveal clinical efficacy.10 Remarkably, the more specific JAK1 inhibitor filgotinib induced clinical remission in patients with active CD.11 Thus, the class of JAK inhibitors might prospectively provide an option in CD.
The next consideration is: when can I expect to observe the clinical response? To answer this question, post-hoc analyses of data from two phase 3 induction trials [OCTAVE 1 and 2] were performed.12 The analyses included patients on tofacitinib 10 mg bid [n = 905] or placebo [n = 234] for 8 weeks. As clinical parameters to evaluate response, Mayo stool frequency and rectal bleeding subscores were calculated by applying diary data from the first 15 days of therapy. Significant improvements were detected after 3 days, as indicated by a reduction of the stool frequency subscore [28.8% vs 17.9%] and the rectal bleeding subscore [32% vs 20.1%]. The authors conclude that tofacitinib at a dose of 10 mg bid shows a rapid onset in patients with moderate to severe UC.12 As a consequence, in patients with moderately to severely active UC, upon treatment with tofacitinib, the clinical response can be judged fairly quickly.
Is this limited to a clinical response or do the patients actually feel better as objectively assessed by quality of life measures? Within the OCTAVE induction 1 and 2 studies, quality of life was evaluated by applying different measures including the Inflammatory Bowel Disease Questionnaire [IBDQ] and the SF-36v2 Health Survey [SF-36v2].13 Changes in IBDQ [40.7 and 44.6 vs 21.0 and 25.0] and SF-36v2 with Physical and Mental Component Summaries [PCS/MCS] [PCS: 6.8 and 6.8; MCS: 6.8 and 7.6 vs placebo PCS: 2.5 and 4.6; MCS: 3.5 and 4.4] were significantly greater in patients on tofacitinib 10 mg bid. These changes were maintained in the OCTAVE Sustain trial at week 52 as expressed by the increased IBDQ [tofacitinib 5 mg: +3.7; tofacitinib 10 mg: +4.8; placebo: −26.5] and the maintained changes in SF-36v2 in either the tofacitinib 5 mg group [PCS: 0.0; MCS: −1.0] or the 10 mg group [PCS: 0.3; MCS: 0.1] vs placebo [PCS: −5.2; MCS: −6.7]. The authors conclude that tofacitinib improves the health-related quality of life throughout week 52.13
2. Comorbidities
This leads us to the second consideration, namely comorbidities. Which comorbidities influence our choice of strategy? The first concerns infectious complications and although the data are still limited and thus an effect on rare infections cannot be excluded, there is a signal for herpes zoster infections that will be outlined below. In addition, the effect on lipid metabolism is well described. Most important are the more recent signals on an increased risk of embolisms. All these points are discussed below.
The risk for developing herpes zoster infection was calculated based on the tofacitinib phase 2/3 and open-label, long-term extension trials in UC. A total of 1157 patients who were treated with tofacitinib within the named clinical trials were included in the analysis.14 Of these, 65 [5.6%] patients developed herpes zoster infection that manifested in 11 patients with multidermatomal involvement and one case of encephalitis. In five patients this led to treatment discontinuation. The hazard incidence ratio in the cohort was 4.07 [3.14–5.19]. Risk factors, as determined by highest incidence ratios, were age ≥65 years [9.55; 4.77–17.08], Asian race [6.49; 3.55–10.89], prior failure to TNF antibodies [5.38; 3.86–7.29] as well as patients on tofacitinib 10 mg bid [4.25; 3.18–5.56]. Hence, in the multivariate analysis older age and prior failure to TNF antibodies, Asian race, diabetes and concurrent steroids were identified as independent risk factors.14 A measure to illustrate the individual risk is the number needed to harm [NNH]. Over all studies [IBD, rheumatoid arthritis] tofacitinib at a dose of 10 mg bid revealed the highest risk for developing herpes zoster infection with an NNH of 22 patients, while for the 5 and 10 mg group together the risk was 36 patients.15 Consequently, the risk for developing herpes zoster infection is highest with tofacitinib as compared to other IBD therapies.16,17
Although patients with IBD overall have lower lipid concentrations in parallel with a lower body mass index [BMI], and lower prevalence of diabetes and hypertension,18–20 they have a slightly increased risk of cardiovascular morbidity.21 This increased risk of cardiovascular morbidity is equally true for other chronic inflammatory diseases, including in particular psoriasis and rheumatoid arthritis, underlining chronic inflammation as a cardiovascular risk factor.22,23 Thus, it is crucial to evaluate any new drug in the field of IBD for its potential effects on additional cardiovascular risk factors. A recent study included patients who received tofacitinib within the global study programme and evaluated inflammation, lipid concentrations and incidence rates of major adverse cardiovascular events [MACEs].24 The study comprised 1157 patients from 8-week induction studies, a maintenance study as well as an ongoing long-term extension study. Lipid concentrations were increased in patients on tofacitinib treatment in comparison with the placebo group through week 61. However, neither the ratio of low-/high-density lipoprotein cholesterol [LDL-c/HDL-c] nor of total cholesterol to HDL-c changed significantly. Four MACEs were documented, and three patients had four or more cardiovascular risk factors. The authors conclude that the observed changes in lipid ratio were not clinically relevant, although they did justify the label-indicated monitoring of blood lipids.24
The recently raised warning on the development of embolisms is discussed in detail by Colombel et al. Data on the incidence of deep vein thrombosis [DVT] and pulmonary embolism [PE] occurring within the tofacitinib UC programme were recently summarized.25 The analysis included data from a phase 2 study and two phase 3 induction studies as well as a phase 3 maintenance study in addition to the ongoing, open-label, long-term extension [OLE] study. The analysis included 1157 patients [2404 patient-years’ exposure]. This post-hoc analysis revealed one DVT and four PE cases, all patients were on 10 mg tofacitinib bid, all events occurred in the OLE study and all patients had venous thromboembolism risk factors.25 From this analysis the authors conclude that due to the small sample size and limited drug exposure, additional studies are required. Following the publication of these data, the Food and Drug Administration [FDA] released the last safety announcement in July 2019 stating that there is an ‘increased risk of blood clots and of death with the 10 mg twice daily dose of tofacitinib’. In addition, ‘the approved use of tofacitinib for ulcerative colitis will be limited to certain patients who are not treated effectively or who experience severe side effects with certain other medicines’.26 The recommendations released by the European Medicines Agency [EMA] in November 2019 differ slightly: tofacitinib ‘should be used with caution in all patients at high risk of blood clots. … the maintenance dose of 10 mg twice daily should not be used in patients with ulcerative colitis who are at high risk of blood clots unless there is no suitable alternative treatment’.27 Together with the FDA and EMA warning and the data derived from the rheumatoid arthritis studies, we can conclude that predisposing risk factors for venous thromboembolism at this point represent an exclusion criterium for the use of tofacitinib. This raises the interesting question of how we manage patients with an acute UC flare, which by itself has been shown to be a risk factor for DVT and PE.28 However, recent analysis of the tofacitinib programme revealed exclusively events within the OLE study and not in the induction phase. Nevertheless, more data are warranted to provide an evidence-based recommendation.
2.1. Family planning
The data available with regard to pregnancy are very limited at this point. Of 1157 patients included in UC interventional studies, 11 mothers and 14 fathers were exposed to tofacitinib either before or at the time of conception as well as during pregnancy. These pregnancies resulted in 15 healthy newborns, no fetal deaths, no neonatal death and no congenital malformations. Two spontaneous abortions and two medical terminations occurred.29 The data from tofacitinib studies in the rheumatoid arthritis and psoriasis trials also did not reveal a signal.30 However, this is a very limited number of pregnancies and the available mechanistic data support a more cautious approach. Tofacitinib, although not formally examined, is assumed to cross the placenta.29 Of note, in animal reproduction studies, tofacitinib was teratogenic and feticidal in rats at 146 times the 5 mg bid and 74 times the 10 mg bid human dose, respectively. In addition, this was equally true for rabbits at 13 times the 5 mg bid and 6.3 times the human 10 mg bid dose. Malformations included anasarca, membranous ventricular septal defects and skeletal abnormalities.31 Thus, until more data are available, one should follow the recommendations and use effective contraception during treatment and for 6 weeks after the last dose.31 Similarly, data for tofacitinib in lactation are lacking. Due to the small size, it can be assumed that tofacitinib is secreted in breast milk. Animal studies have revealed detectable tofacitinib levels in the milk of lactating rats.31 Due to this limited amount of data and complete lack of data in humans, a recommendation cannot be provided at this point.
2.2. Extraintestinal manifestations—coexisting non-intestinal chronic inflammatory diseases
The third consideration is extraintestinal manifestations or other coexisting chronic inflammatory diseases. There is a significant proportion of patients in whom not only intestinal inflammation is relevant for the decision-making but extraintestinal manifestations need to be considered. If we look objectively at the extraintestinal manifestations, where are JAK inhibitors effective? Regarding spondyloarthropathy with axial involvement, the phase 2 trial reported by Van der Heijde et al. indicates that tofacitinib 5 and 10 mg bid was more efficacious than placebo with regard to signs, symptoms and objective end points in ankylosing spondylitis patients.32 For axial involvement, the T helper 17 [Th17] cell pathway has been shown to be of importance.33 Th17 differentiation depends strongly on IL-23, which mediates its signal through the IL-23 receptor involving downstream JAK2 and Tyk2 as signal transducers. However, Jak2 is central for haematopoiesis and thus a specific inhibition of JAK2 in inflammatory disease is limiting whereas it might be desirable in haematological disorders.34 In parallel, the efficacy of tofacitinib has been shown for peripheral arthritis.35 Thus, for either axial or peripheral spondyloarthropathy, inhibition of JAKs (as exemplified here for tofacitinib) is an option. This is of particular interest, because anti-p40 antibodies are not effective for axial spondyloarthropathy.36 Tofacitinib is a pan-JAK inhibitor, and consequently the more specific JAK1 inhibitors such as filgotinib or upadacitinib will have to be evaluated for this indication. Moving from the joints to the skin there are two randomized phase 3 trials available indicating efficacy in psoriasis.37,38 Last, for uveitis the data are restricted to case reports that suggest that JAK inhibitors (in both publications tofacitinib was used) might present a therapeutic option, although controlled trials are lacking.39,40 In summary, tofacitinib as a pan-JAK inhibitor presents with a beneficial profile with regard to extraintestinal manifestations. However, these findings cannot be generalized to other JAK inhibitors due to different specificities.
2.3. Patient preferences
One would assume that patients prefer an oral drug over intravenous or subcutaneous administration. However, the data available are limited and the topic might include more aspects than initially considered. Previous data provide strong evidence that patients are willing to accept risks related to therapy escalation to avoid a future disease relapse.41 Remarkably, a previous questionnaire revealed that when asked for their preference of anti-TNF administration, patients showed a trend towards intravenous rather than subcutaneous treatment.42 The yet unexplored question is whether patients would prefer an oral drug or a subcutaneous/intravenous injection over a longer distance, thus allowing them to forget about the disease in the meantime. An additional consideration is adherence to therapy. In a recent single-centre study from the USA, a retrospective analysis revealed that 69% of patients were adherent to the self-injectable biologic.43 In a study evaluating adherence to an oral drug [5-ASA] in quiescent/mildly active UC, patients identified that baseline non-adherence was 52.4%. Even an educational effort was unable to increase the rate of adherence. Identified risk factors for non-adherence were young age, short disease duration and low education levels.44 Thus, caution is needed in stating which administration strategy might result in the highest adherence. Clearly, this field requires more data, including with regard to the novel highly efficient oral drugs.
2.3. Pharmacological considerations
The elegance of an oral small molecule drug is that it can be started and stopped as necessary without the danger of development drug-neutralizing antibodies. We have probably unconsciously acted in this way for decades in introducing and stopping classical immunosuppressants such as methotrexate, thiopurines and calcineurin inhibitors. However, methotrexate and in particular thiopurine are characterized by their long half-life, and cannot be used for fast-needed decisions and effects.45,46 Biologicals limit the possibility of intermittent treatment because this has been associated with an increased risk of developing anti-drug-antibodies [ADAs]. A recent analysis reviewed the incidence of ADA development in published adalimumab and infliximab trials. Irrespective of the assays applied, the incidence ADAs ranged widely among assays as well as inflammatory diseases [adalimumab 0–87% and infliximab 0–79%].47 In clinical practice the problem evolves during treatment and presents as a secondary loss of response. Although the incidence of ADAs in the newer biologicals vedolizumab and ustekinumab appears to be lower, they are still present.48,49 The introduction of a new class with small molecules and a very short half-life, in the absence of risk in developing ADAs, is highly intriguing. This will lead to novel concepts in treatment strategies.
3. Concluding Considerations
The elegance of an oral small molecule drug is that it can be started and stopped as necessary. Ultimately, this might prospectively even allow treatment strategies in which the JAK inhibitor is only included in daily treatment when needed. Prospective studies will need to demonstrate the feasibility of such theoretical considerations. Nevertheless, the option of starting and stopping a drug without immunogenicity concerns is appealing.
The detailed considerations discussed in this article indicate that there is a place for JAK inhibitors in the treatment of IBD, which is of particular interesting given the short time until a response can be judged as well as the effect of the majority of extraintestinal manifestations. Future studies will need to provide evidence of whether more specific JAK inhibitors reduce the named disadvantages and hence increase the potential of this class even further.
Funding
This work was supported by the German Research Foundation (TRR 241 and SFB 1340).
Conflicts of Interest
BS has served as Consultant for Abbvie, Boehringer, Celgene, Falk, Janssen, Lilly, Pfizer, Prometheus and Takeda and received speaker’s fees from Abbvie, CED Service GmbH, Falk, Ferring, Janssen, Novartis, Takeda [served as representative of the Charité]
References
- 1. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 2. Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016;375:1946–60. [DOI] [PubMed] [Google Scholar]
- 3. Rutgeerts P, Sandborn WJ, Feagan BG, et al.. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–76. [DOI] [PubMed] [Google Scholar]
- 4. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 5. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019;381:1201–14. [DOI] [PubMed] [Google Scholar]
- 6. Targan SR, Hanauer SB, van Deventer SJ, et al.. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med 1997;337:1029–35. [DOI] [PubMed] [Google Scholar]
- 7. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–36. [DOI] [PubMed] [Google Scholar]
- 8. Atreya R, Neumann H, Neufert C, et al.. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat Med 2014;20:313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vermeire S, O’Byrne S, Keir M, et al.. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet 2014;384:309–18. [DOI] [PubMed] [Google Scholar]
- 10. Panés J, Sandborn WJ, Schreiber S, et al.. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut 2017;66:1049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vermeire S, Schreiber S, Petryka R, et al.. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 2017;389:266–75. [DOI] [PubMed] [Google Scholar]
- 12. Hanauer S, Panaccione R, Danese S, et al.. Tofacitinib induction therapy reduces symptoms within 3 days for patients with ulcerative colitis. Clin Gastroenterol Hepatol 2019;17:139–47. [DOI] [PubMed] [Google Scholar]
- 13. Panés J, Vermeire S, Lindsay JO, et al.. Tofacitinib in patients with ulcerative colitis: health-related quality of life in phase 3 randomised controlled induction and maintenance studies. J Crohns Colitis 2018;12:145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Winthrop KL, Melmed GY, Vermeire S, et al.. Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis 2018;24:2258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caldera F, Hayney MS, Cross RK. Using number needed to harm to put the risk of herpes zoster from tofacitinib in perspective. Inflamm Bowel Dis 2019;25:955–7. [DOI] [PubMed] [Google Scholar]
- 16. Gupta G, Lautenbach E, Lewis JD. Incidence and risk factors for herpes zoster among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2006;4:1483–90. [DOI] [PubMed] [Google Scholar]
- 17. Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of herpes zoster among 108 604 patients with inflammatory bowel disease. Aliment Pharmacol Ther 2013;37:420–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geerling BJ, Badart-Smook A, Stockbrügger RW, Brummer RJ. Comprehensive nutritional status in recently diagnosed patients with inflammatory bowel disease compared with population controls. Eur J Clin Nutr 2000;54:514–21. [DOI] [PubMed] [Google Scholar]
- 19. Rungoe C, Nyboe Andersen N, Jess T. Inflammatory bowel disease and risk of coronary heart disease. Trends Cardiovasc Med 2015;25:699–704. [DOI] [PubMed] [Google Scholar]
- 20. Sridhar AR, Parasa S, Navaneethan U, Crowell MD, Olden K. Comprehensive study of cardiovascular morbidity in hospitalized inflammatory bowel disease patients. J Crohns Colitis 2011;5:287–94. [DOI] [PubMed] [Google Scholar]
- 21. Singh S, Singh H, Loftus EV Jr, Pardi DS. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014;12:382–93.e1: quiz e22. [DOI] [PubMed] [Google Scholar]
- 22. Mantel Ä, Holmqvist M, Nyberg F, et al.. Risk factors for the rapid increase in risk of acute coronary events in patients with new-onset rheumatoid arthritis: a nested case-control study. Arthritis Rheumatol 2015;67:2845–54. [DOI] [PubMed] [Google Scholar]
- 23. Solomon DH, Reed GW, Kremer JM, et al.. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol 2015;67:1449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sands BE, Taub PR, Armuzzi A, et al.. tofacitinib treatment is associated with modest and reversible increases in serum lipids in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2020;18:123–132.e3. [DOI] [PubMed] [Google Scholar]
- 25. Sandborn WJ, Panés J, Sands BE, et al.. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther 2019;50:1068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.FDA. Available at: https://www.Fda.Gov/drugs/drug-safety-and-availability/fda-approves-boxed-warning-about-increased-risk-blood-clots-and-death-higher-dose-arthritis-and., 2019.
- 27.EMA: Available at: https://www.Ema.Europa.Eu/en/news/ema-confirms-xeljanz-be-used-caution-patients-high-risk-blood-clots, 2019.
- 28. Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet 2010;375:657–63. [DOI] [PubMed] [Google Scholar]
- 29. Mahadevan U, Dubinsky MC, Su C, et al.. Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm Bowel Dis 2018;24:2494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clowse ME, Feldman SR, Isaacs JD, et al.. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf 2016;39:755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.FDA. Available at: https://www.Accessdata.Fda.Gov/drugsatfda_docs/label/2018/203214s018lbl.Pdf, 2018.
- 32. van der Heijde D, Deodhar A, Wei JC, et al.. Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis 2017;76:1340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paine A, Ritchlin CT. Targeting the interleukin-23/17 axis in axial spondyloarthritis. Curr Opin Rheumatol 2016;28:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Assi R, Verstovsek S, Daver N. ‘JAK-ing’ up the treatment of primary myelofibrosis: building better combination strategies. Curr Opin Hematol 2017;24:115–24. [DOI] [PubMed] [Google Scholar]
- 35. Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014;370:2377–86. [DOI] [PubMed] [Google Scholar]
- 36. Deodhar A, Gensler LS, Sieper J, et al.. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol 2019;71:258–70. [DOI] [PubMed] [Google Scholar]
- 37. Bachelez H, van de Kerkhof PC, Strohal R, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet 2015;386:552–61. [DOI] [PubMed] [Google Scholar]
- 38. Bissonnette R, Iversen L, Sofen H, et al.. Tofacitinib withdrawal and retreatment in moderate-to-severe chronic plaque psoriasis: a randomized controlled trial. Br J Dermatol 2015;172:1395–406. [DOI] [PubMed] [Google Scholar]
- 39. Bauermann P, Heiligenhaus A, Heinz C. Effect of janus kinase inhibitor treatment on anterior uveitis and associated macular edema in an adult patient with juvenile idiopathic arthritis. Ocul Immunol Inflamm 2019;27:1232–4. [DOI] [PubMed] [Google Scholar]
- 40. Paley MA, Karacal H, Rao PK, Margolis TP, Miner JJ. Tofacitinib for refractory uveitis and scleritis. Am J Ophthalmol Case Rep 2019; 13:53–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bewtra M, Fairchild AO, Gilroy E, et al.. Inflammatory bowel disease patients’ willingness to accept medication risk to avoid future disease relapse. Am J Gastroenterol 2015;110:1675–81. [DOI] [PubMed] [Google Scholar]
- 42. Allen PB, Lindsay H, Tham TC. How do patients with inflammatory bowel disease want their biological therapy administered? BMC Gastroenterol 2010;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shah NB, Haydek J, Slaughter J, et al. Risk factors for medication nonadherence to self-injectable biologic therapy in adult patients with inflammatory bowel disease. Inflamm Bowel Dis 2020;26:314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nikolaus S, Schreiber S, Siegmund B, et al.. Patient education in a 14-month randomised trial fails to improve adherence in ulcerative colitis: influence of demographic and clinical parameters on non-adherence. J Crohns Colitis 2017;11:1052–62. [DOI] [PubMed] [Google Scholar]
- 45. Ben-Horin S, Van Assche G, Chowers Y, et al.. Pharmacokinetics and immune reconstitution following discontinuation of thiopurine analogues: implications for drug withdrawal strategies. J Crohns Colitis 2018;12:1410–7. [DOI] [PubMed] [Google Scholar]
- 46. Dalrymple JM, Stamp LK, O’Donnell JL, et al. Pharmacokinetics of oral methotrexate in patients with rheumatoid arthritis. Arthritis Rheum 2008;58:3299–308. [DOI] [PubMed] [Google Scholar]
- 47. Gorovits B, Baltrukonis DJ, Bhattacharya I, et al.. Immunoassay methods used in clinical studies for the detection of anti-drug antibodies to adalimumab and infliximab. Clin Exp Immunol 2018;192:348–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Strand V, Balsa A, Al-Saleh J, et al.. Immunogenicity of biologics in chronic inflammatory diseases: a systematic review. BioDrugs 2017;31:299–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ward MG, Sparrow MP, Roblin X. Therapeutic drug monitoring of vedolizumab in inflammatory bowel disease: current data and future directions. Therap Adv Gastroenterol 2018;11:1756284818772786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet 2012;380:1909–15. [DOI] [PubMed] [Google Scholar]
- 51. Williams JG, Alam MF, Alrubaiy L, et al.. Infliximab versus ciclosporin for steroid-resistant acute severe ulcerative colitis (CONSTRUCT): a mixed methods, open-label, pragmatic randomised trial. Lancet Gastroenterol Hepatol 2016;1:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]