Abstract
Cytokines can trigger multiple signalling pathways, including Janus tyrosine kinases [JAK] and signal transducers and activators of transcription [STATS] pathways. JAKs are cytoplasmic proteins that, following the binding of cytokines to their receptors, transduce the signal by phosphorylating STAT proteins which enter the nuclei and rapidly target gene promoters to regulate gene transcription. Due to the critical involvement of JAK proteins in mediating innate and adaptive immune responses, these family of kinases have become desirable pharmacological targets in inflammatory diseases, including ulcerative colitis and Crohn’s disease. In this review we provide an overview of the main cytokines that signal through the JAK/STAT pathway and the available in vivo evidence on mutant or deleted JAK proteins, and discuss the implications of pharmacologically targeting this kinase family in the context of inflammatory diseases.
Keywords: Janus tyrosine kinase, cytokine signalling, inflammatory bowel disease
1. Introduction
Inflammatory bowel diseases [IBDs], including ulcerative colitis and Crohn’s disease, are thought to result from the interplay between genetic susceptibility and environmental factors that trigger an abnormal mucosal immune response. An impaired balance of pro- and anti-inflammatory mediators drives disease manifestation and hampers the resolution of inflammation, thereby perpetuating disease and increasing disease burden. Cytokines play a crucial role in all steps of the inflammatory cascade that occurs in IBD. Early studies identified cytokine deregulation in these patients.1–3 Furthermore, evidence in gene knockout [KO] animals revealed the crucial role of cytokine-driven immunoregulatory signals in maintaining mucosal homeostasis. Indeed, interleukin [IL]-2-KO4 and IL-10-KO5 animals have been described as spontaneous models of intestinal inflammation, underscoring the importance of these two cytokines in promoting regulatory responses at the mucosal barrier. Since then, innumerable studies have delineated patterns of cytokine regulation and their target cells, both in experimental models and in human disease.6 Remarkably, two of the currently approved therapies in IBD interfere with cytokine function by using antibodies against tumour necrosis factor alpha [TNFα] and p40[IL-12/IL-23]. These therapies block the extracellular function of cytokines, but an alternative and broader method for interfering with these mediators is to inhibit their intracellular signalling through cell-permeable small-molecule inhibitors.
In order to drive responses on target cells, cytokines need to bind to their specific receptors, which triggers a signalling pathway that will reach the cell nuclei. Although these intracellular signals vary among cytokines, they can be shared by different cytokine receptors. Specifically, a group of cytokines implicated in the pathogenesis of several diseases, including IBD, signal through the Janus tyrosine kinase [JAK] family.7,8 Thus, JAKs are currently desirable targets for the treatment of inflammatory disease.9,10 Specifically, tofacitinib, a potent pan-JAK inhibitor, has been approved to treat moderate to severe ulcerative colitis.11,12 Whereas the clinical potential of this antagonist is well proven, several questions remain unanswered, including: the specific cells and cytokine pathways these molecules act on in the context of IBD; the actual requirements for higher specificity in order to drive effective and safer JAK inhibition; the benefits of local versus systemic delivery; and so on.
Here we provide clinicians and translational researchers with an overview of the current understanding of JAKs’ function and their potential involvement in processes that could prove relevant to the treatment of intestinal inflammation.
2. Cytokines and Cytokine Receptors
The cytokine superfamily is a large group of structurally diverse low molecular weight soluble proteins that includes ILs, chemokines [CCL or CXCL], colony-stimulating factors [CSF], interferons [IFN], transforming growth factors [TGF], and TNF family members. A common way to categorise this large and diverse cytokine family is based on the class of receptors they bind to. These include the following: type I and type II receptors13 [Table 1A]; the TNF receptor superfamily [TNFR]; TGF-beta receptors; the immunoglobulin family, which includes the IL-1 receptor superfamily14,15; the enzyme-like receptor family, which encompasses the tyrosine kinases family [RPTKs]16,17; chemokine receptors [guanylate cyclase-coupled receptors]18; and tyrosine kinase class III receptors19 [Table 1B].
Table 1.
List of cytokine receptors and their main cytokine ligands.
| [A] | |
|---|---|
| JAK-dependent cytokine receptors and ligands | |
| Receptor family | Ligand |
| -Type I receptors | |
| Common ɣ chain [ɣc] | IL-2, IL-4, IL-7, IL-9, IL-15, IL-21 |
| TSLP receptor | TSLP |
| IL-6 family [gp-130] | IL-6, IL-11, IL-27, IL-35, LIF, OSM, CNTF, CT-1, CLC, NP, IL-31* |
| IL-12 family | IL-12, IL-23 |
| Common β chain | IL-3, IL-5, GM-CSF |
| Homodimer receptors | EPO, TPO, G-CSF, GH, PRL |
| -Type II receptors | |
| IL-13 receptor | IL-13, IL-4 |
| IFN type I | IFNα, IFNβ, |
| IFN type II | IFNɣ |
| IFN type III | IL28, IL28A, IL29 |
| IL-10 family | IL-10, IL-19, IL-20, IL-22, IL-24, IL26 |
| [B] | |
| JAK-independent cytokine receptors and ligands | |
| Receptor family | Ligand |
| -TNF receptor family | TNFα, TNFβ, LT, CD4, FasL, BAFF, Aprl, Ox40, GITR |
| -IL-17 receptor family | IL-17A, IL-17B, IL-17C, IL-17DIL-17E [IL-25], IL-17F |
| -TGF receptor family | TGFβs, Activin A, GDF1, GDF11, BMPs, Nodal |
| -Enzyme-like receptors | |
| Receptor tyrosine kinase family [RPTKs] | Ej. EGF, PDGF, VEGF, Insulin |
| Chemokine family [guanylate-cyclase-coupled receptors] | CCL, CXCL, XCL, CXC3L |
| Receptor tyrosine kinase class III | CSF-1, SCF, PDGFb, FLT3L |
| -Immunoglobulin-like family | |
| IL-1 receptor family | IL-1α, IL-1β, IL-18, IL-33, IL1F5, IL1F6, IL1F7, IL1F8, IL1F9, IL1F10 |
Cytokine receptors that depend on JAK signalling are shown in [A] and those that are JAK-independent are shown in[B].
IL, interleukin; TSLP, thymic stromal lymphopoietin; OSM, oncostatin M; LIF, leukaemia inhibitory factor; CNTF, cytokine ciliary neurotrophic factor; CT-1, cardiothropin 1; CLC, cardiothropin-like cytokine; NP, neuropoetin; EPO, erythropoietin; Tpo, thrombopoietin; G-CSF, granulocyte colony-stimulating factor; GH, growth hormone; PRL, prolactin; IFN, interferon; EGF, epidermal growth factor; PDGF, platelet-derived growth factor; VEGF, vascular endothelial growth factor; SCF, stem cell factor; M-CSF, macrophage colony-stimulating factor; FLT3L, FMS-like tyrosine kinase 3 ligand; PDGFb, platelet-derived growth factor subunit B; TNF, tumour necrosis factor. *The cytokine IL-31 does not signal through gp130 but shares the subunit [OSMRβ] with OSM, which belongs to the IL-6 receptor family.
Each receptor family uses different signalling molecules to reach the cell nucleus and initiate a cellular response. For instance, the G-protein coupled receptors that bind chemokines induce the activation of protein G [guanine nucleotide-binding protein] that hydrolyses GTP. Type I and type II receptors, on the other hand, rely on the catalytic activity of JAKs to phosphorylate and activate a group of transcriptional factors known as the signal transducer and activator of transcription [STAT] family. Within the group of cytokines that requires JAKs for their functionality, a few are essential to intestinal homeostasis and are involved in the pathophysiology of IBD; these include IL-12, IL-23, oncostatin [OSM], IFNɣ, IL-10, IL-9, and granulocyte-macrophage colony-stimulating factor [GM-CSF], among others.7 Nonetheless, it is important to note that several other key mucosal and IBD cytokines [i.e. TNF, IL-17A, chemokines, TGF-β, or IL-1] operate completely independently of the functions of JAKs. In other words, their activity would not be directly targeted by pharmacological inhibition of JAK activity.
3. Molecular Structure and Signal Transduction of Janus Tyrosine Kinases
In mammals, the JAK family is composed of four members: JAK1, JAK2, JAK3, and TYK2 [tyrosine kinase 2]. JAKs are non-receptor tyrosine kinase proteins constitutively associated with the intracellular domains of type I and type II cytokine receptors.20
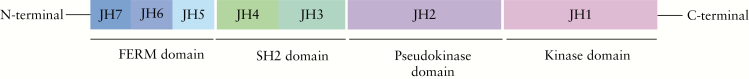
JAKs are large proteins with unique JAK homology [JH] domains numbered in a C-terminal to N-terminal direction [Figure 1]. From the primary structure, putative domains are known to be conserved between mammalian, avian, teleost, and insect JAKs.21 Seven JH [JH1–JH7] domains have been described and the C-terminal domain JH1, known also as the kinase domain, is the domain that presents catalytic activity.22 Adjacent to the kinase domain is the pseudokinase domain [JH2], a feature unique to JAKs. Although the pseudokinase domain lacks catalytic activity, it has an essential regulatory function since mutations within this domain can impact on kinase activity.23 The importance of this domain to JAK functionality is illustrated by a single-point mutation within the JH2 pseudokinase domain of JAK2, one that is present in the majority of patients with polycythaemia vera, as well as in a high percentages of patients with essential thrombocythaemia and idiopathic myelofibrosis.24–26 The N-terminal domain, known as the FERM [band-4.1 protein, ezrin, radixin, and moesin] domain [JH6-JH7], mediates interaction with the cytokine receptor subunits, since deletion of the N-terminal region abrogates binding.21 In addition, the FERM domain is thought to regulate catalytic activity of the C-terminal kinase domain, as mutations in this domain can impact JAK1 functionality.27 In between the FERM and the pseudokinase domain lies the Src homology 2 [SH2] domain [JH3-JH5], which also facilitates associations with the cytokine receptors that provide scaffolding.28 Recently completed crystal structures of JAK1, JAK2, and TYK2 revealed that the FERM and SH2 domains are closely associated to form a single receptor-binding module.29
Figure 1.
Schematic of Janus kinase proteins structure. Janus kinases comprise the FERdomain [JH6-JH7] and the SH2 domain [JH3-JH5], both mediating receptor interactions, the pseudokinase domain [JH2] with regulatory function, the catalytic domain [JH1], and the kinase domain.
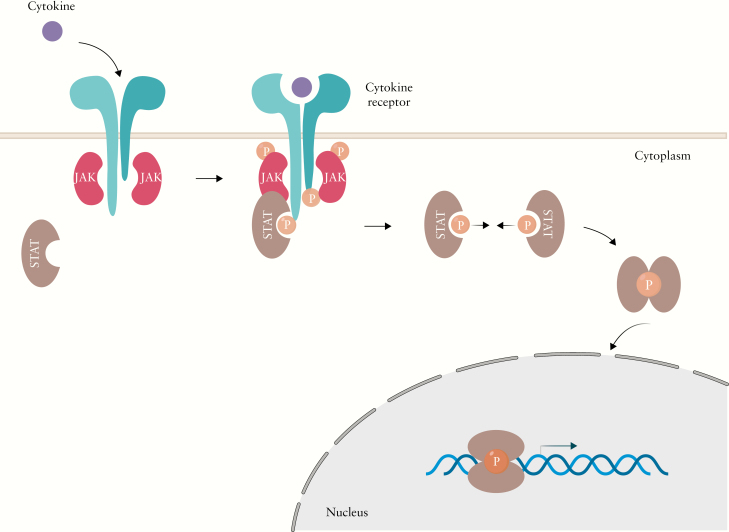
JAKs are located in the cytosol near the cell membrane. After ligand stimulation, receptors undergo conformational changes [dimerisation] that bring JAKs into proximity with each other [Figure 2]. JAKs through their N-terminal domain are constitutively associated with a proline-rich, membrane-proximal domain of these cytokine receptors. JAKs trans/auto-phosphorylate each other and subsequently phosphorylate the tyrosine residues within the intracellular tails of the receptor chains. These phosphorylated tyrosine residues then serve as docking sites for STAT proteins, which bind via their SH2 domains. At that point, JAKs phosphorylate the tyrosine residues of the C-termini of STATs. Once activated by phosphorylation, STATs dissociate from the receptor and homo/hetero-dimerise. The phosphorylated STAT dimer can subsequently translocate from the cytoplasm to the nucleus, where it binds to specific DNA sequences on target genes and induces or represses gene transcription.30
Figure 2.
Overview of cytokine signalling through the Janus kinase pathway. Cytokines bind to homodimeric or heterodimeric receptors, after ligand stimulation receptors undergo conformational changes and bring JAKs into proximity which each other. JAKs trans/auto phosphorylate each other and the receptor, allowing STATS to bind to the receptor. Subsequently JAKs phosphorylate STATS, allowing them to dimerise and translocate to the nucleus to regulate gene transcription.
In mammals, there are seven STAT proteins, STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6, that are involved in a wide variety of downstream signalling cascades. Consistent with the activation of JAK-dependent pathways, different STATs have been implicated in the pathophysiology of IBD.31,32 In contrast to JAK inhibitors, the development/utility of STAT inhibitors has been considered primarily in the context of cancer.33 Nevertheless, there have been no human studies to date examining STAT inhibition for the treatment of IBD.
4. JAK-dependent Receptors
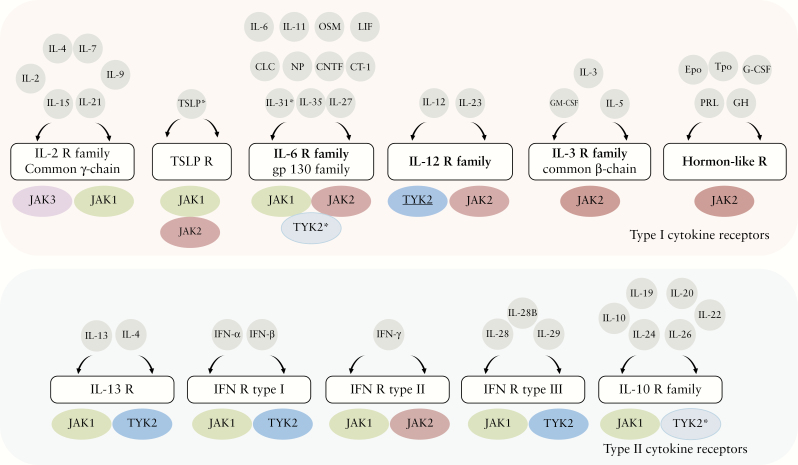
As discussed above, both type I and type II cytokine receptors require JAK activity to signal. Depending on the specific receptors, one or more different members of the JAK family will collaborate to mediate signal transduction [Figure 3]. Thus, each JAK participates in signalling downstream of multiple cytokine receptors, often in association with other JAK family members. In general, all type I and type II receptors rely on JAK1 and/or JAK2 for signalling. TYK2 can partner with both JAK1 and JAK2, whereas JAK3 is by far the less widely expressed JAK protein, being restricted to the common ɣ chain [ɣc]-containing receptors.
Figure 3.
Schematic representation of cytokine and receptor Janus kinase pathway. The four JAKs [JAK1, JAK2, JAK3, and TYK2] are selectively bound to and therefore mediate signalling for various cytokine and hormone receptors. Scheme representing all JAK pathway cytokines and with whom JAKs are associated. TYK2 [shown in dark blue] where there is evidence of its catalytic activity playing an essential role. TYK2 [in light blue] when it plays a scaffolding function. *The cytokine IL-31 does not signal through gp130 because of sharing the subunit [OSMRβ] with OSM, which belongs to the IL-6 receptor family.
4.1. Type I receptor-binding cytokines and their biological functions
IL-2 receptor family members share a common subunit, the common gamma chain ɣc [IL-2Rɣ]. Cytokines that signal through the γc include IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 [Table 1A]. Signalling through the IL-2 family of receptors requires the activation of both JAK3, which partners with the ɣc, and JAK1, which mediates signalling downstream of the corresponding α chain subunit: IL-2Rα, IL-4Rα, IL-7Rα, IL-9Rα, IL-15Rα, and IL-21R, respectively.34 Exceptionally, the IL-2 and IL-15 receptors include a third shared β chain,35 IL-2Rβ [CD122]. Besides using the γc receptor expressed by T cells, IL-4 can transduce its signal through a type II receptor comprising IL-4Rα and IL-13Rα1 [Figure 3].36 The type II IL-4 receptor is expressed primarily on B cells and non-lymphoid cells, and signals through JAK1 and TYK2.37–40 Remarkably, this same receptor complex is shared by another key Th2 cytokine, IL-13.41
Most cytokines in this group [i.e., IL-2, IL-4, IL-7, IL-15, and IL-21] are essential for lymphocyte survival, proliferation, and/or activation/differentiation. Indeed tofacitinib, a potent JAK1 and JAK3 inhibitor approved for psoriatic arthritis,ulcerative colitis, and rheumatoid arthritis, significantly reduces the number of circulating lymphocytes in patients.42,43 Although no data are yet available, other inhibitors under development which selectively target JAK3 [i.e., PF-06651600] or JAK1 [i.e., filgotinib and upadacitinib] may have this same effect based on the necessary role of both JAK3 and JAK1 for signalling downstream of the IL-2R family.
Thymic stromal lymphopoietin [TSLP] has a unique receptor complex that uses the IL-7Rα subunit, which partners with TSLPR [CRLF2] instead of ɣc. This cytokine thus relies on JAK1 and TYK2, but not on JAK3.44,45
Another group of type I cytokine receptors contains the gp130 [CD130] subunit, and is known as the IL-6 family of receptors. Cytokines using these receptors include IL-6, IL-11, IL-27, IL-35, leukocyte inhibitory factor [LIF], OSM, ciliary neurotrophic factor [CNTF], cardiotrophin-1 [CT-1], cardiotrophin-like cytokine [CLC], and neuropoietin [NP]46 [Table 1A].47 These cytokines use receptors composed of a unique α chain [IL-6R, IL-11R, IL27Rα [WSX-1], IL-12Rβ2, LIF receptor [LIFR], OSMRβ, and CNTFRα], which provides specificity and signals through JAK2, and the common gp130 chain, which mediates signalling through JAK1. TYK2 participates with these receptor complexes, but the role for TYK2-medited phosphorylation in driving responses remains unclear.
IL-6 and IL-11 are the only IL-6 type cytokines that can signal through gp130 homodimers and a third alpha subunit [IL-6R and IL-11R, respectively]. IL-27 and IL-35, which are heterodimeric proteins related to the IL-12 family of cytokines, signal [unlike IL-12 and IL-23] through the gp130 subunit. Gp130 partners with IL-27Rα [WSX-1] to deliver IL-27 signals,48,49 and the receptor for IL-35 uses IL-12Rβ2 [shared with the IL-12 receptor].50 IL-35 can also signal through homodimers of each of its receptor chains. Both IL-27 and IL-35 depend on JAK1- and JAK2-mediated phosphorylation.
LIF, OSM, and CT-1 use gp130 and the LIF receptor subunit [LIFR].51,52 In addition, a receptor formed by OSMRβ and gp130 can also bind to OSM to deliver signals in a JAK1- and JAK2-dependent manner. CNTF, CLC, and NP share their receptor, a tripartite signalling complex53 that includes CNTFRα, gp130, and LIFR, all of which use JAK1 and JAK2 for signal transduction.
IL-31 is also considered a member of the IL-6R family despite not using the gp130 subunit; instead, it relies on the IL-31RA and OSMRβ subunits, which partner with JAK1 and JAK2, respectively.54
The IL-12 receptor family, which used to be included within the IL-6 family of receptors, comprises the receptors for IL-12 and IL-23. The former binds a heterodimer formed by IL-12Rβ1 and IL-12-Rβ2, and the latter uses a heterodimer comprising IL-12Rβ1 and IL-23R. The shared IL-12-Rβ1 receptor signals through TYK2, and both IL-12Rβ2 and IL-23R associate with JAK2.
The IL-3 receptor family is also known as the common β-chain receptor family, as they all use a β-chain subunit for signalling. Cytokines that bind these receptors are IL-3, IL-5, and GM-CSF [Table 1A].55 β-chain receptors exclusively use JAK2 dimers to transduce their signals. Members of this cytokine family regulate the growth, differentiation, migration, and effector functions of many haematopoietic cells.
The hormone-like receptor family includes the receptors for erythropoietin [EPO], thrombopoietin [TPO], granulocyte colony stimulating factor [G-CSF], growth hormone [GH], and prolactin [PRL]. These are all homodimeric receptors that are exclusively dependent on JAK2.56 The crucial role played by most of these cytokines and growth factors in haematopoiesis has discouraged the development of selective JAK2 inhibitors in common immune-mediated diseases.
4.2. Type II receptor-binding cytokines and their biological functions
Type II receptors bind to a large group of cytokines, including type I [IFN-α, IFN-β],57 type II [IFN- ɣ], type III IFNs [IL-28, IL-29, IL28B], and IL-10 related cytokines [IL-10, IL-19, IL-20, IL-22, IL-24, IL-26]. Interestingly, they all rely on JAK1 and either TYK2 or JAK2 to deliver their intracellular signals. Furthermore, the IL-13 receptor is also considered a type II receptor and signals through JAK1 and TYK2. This receptor is formed by the subunits IL-4Rα and IL-13Rα1,58 the latter associated with TYK2. As mentioned above, IL-4 can also use this receptor complex to transduce signals.38
Type I IFN receptors are formed by IFNαR1, which is constitutively bound to TYK2, and IFNαR2, which recruits JAK1 to transduce signals.59
The IFN-ɣ receptor [type II cytokine receptor] is formed by IFNɣR1 and IFNɣR2, which recruit JAK1 and JAK2, respectively.
The type III IFN receptor 60 is constituted by two subunits: IL-28RA [IFNλRA], which recruits JAK1, and IL-10RB, which binds to TYK2. Both type I and type III IFNs are essential for antiviral responses.61–63 It is not surprising that both have similar biological functions, since their signal transduction cascades are very similar. Type II IFN is required for intracellular and extracellular bacteria killing.64 Both arms of the immune response may be compromised by the use of JAK1 inhibitors such as tofacitinib [a pan-JAK inhibitor], filgotinib, or upadacitinib.
The IL10 receptor family is formed by the receptors to IL-10, IL-19, IL-20, IL-22, IL-24, and IL-26.65 These have in common the IL-10RB subunit shared with type III IFNs, which recruits TYK2 for signal transduction. The IL10RB subunit assembles with IL-10RA, IL-20RA, and IL-22R to form the different cytokine receptors, all of which bind to JAK1.66,67 These groups of cytokines mediate diverse immune responses, including epithelial defence and regulatory immune responses.68 Monitoring the degree to which these responses may be affected by those JAK1 inhibitors in IBD would be relevant, especially during maintenance phases.
5. Establishing the Critical Role of JAKs In Vivo
JAKs orchestrate diverse functions of the innate and adaptive immune systems through their critical role in cytokine signal transduction. Shortly after their discovery, their essential role in cytokine signalling was established in experiments using mutagenised cell lines that were resistant to IFNs.57,59,69,70 The first in vivo evidence of the critical role played by JAKs was the identification of patients with a primary immunodeficiency that was linked to JAK3 functionality.71 In addition, data in knock-out mice have been crucial to unravelling the contribution of each of these kinases to biologically relevant processes.72–78
Based on all of this evidence, it is well established that loss of function of any of these four protein kinases entails biological consequences. Importantly, the only viable JAK deficiencies in humans and mice are described in TYK2 and JAK3, suggesting that the deletion of either JAK1 or JAK2 may be incompatible with life. Nonetheless, mutations in any of the four JAKs are linked with a variety of human diseases. For instance, somatic gain of function mutations in JAK2 are associated with myeloproliferative diseases, given the role of JAK2 in haematopoiesis.79 Other constitutive activating mutations in all four JAKs have been associated with a variety of haematological and solid organ malignancies.80–83
Below we discuss the available in vivo evidence on mutant or deleted JAK proteins which furthers our understanding of the roles these kinases play in biology. We also hypothesise on the implications of these data in pharmacologically targeting this kinase family.
6. TYK2
TYK2 was the first member of the JAK family to be isolated84 and was originally described as essential for type I IFN [IFNα and IFNβ] signalling in a human fibroblast cell line.57 A subsequent study confirmed that TYK2 was critical for type I IFNs signal transduction but was not required for IFNɣ [type II IFN] in human cells.69 Mice lacking TYK2 are viable and commonly studied today. Experiments on these mice have revealed a partial impairment of the response to IFNα/β. In contrast, the absence of TYK2 in murine cells leads to a complete lack of STAT3 activation in murine splenocytes activated with IL-12, thus establishing the absolute requirement of TYK2 for cellular responses to IL-12. In addition, TYK2 was shown to be dispensable for responses to IL-10, IL-6, or LIF murine fibroblasts.75 Overall TYK2 activity appears to be essential, at least in mice, for IL-12 signalling, with the partial contribution to type I IFN responses, though not essential for signalling through the IL-6R and IL-10R families, despite binding to these receptor chains.
Humans with TYK2 deficiency have been reported in the literature and their phenotypes vary. The first individual to be identified had a homozygous deletion of four nucleotides in the FERM domain of the TYK2 sequence, which abrogates its expression.85 This patient had been clinically diagnosed with hyper-IgE syndrome and atopic dermatitis, and presented a high susceptibility to infections from diverse microorganisms including viruses, fungi, and mycobacteria. Peripheral blood cells showed almost complete loss of type I IFNs and IL-12 signalling. Furthermore, impairment in IL-10 and IL-6 activity was shown, in apparent disagreement with murine data.75 Moreover, CD4+ T cells isolated from this patient’s blood failed to produce IFNɣ in response to IL-12 and IL-18, suggesting a defect in Th1 function and/or differentiation in this individual. However, when activated with PMA [phorbol 12-myristate 13-actetate] and ionomycin, JAK2-deficient T cells produced considerable amounts of IFNɣ, showing that type II IFN production was not impaired, but rather response to IL-12. In addition, T cells from this TYK2-deficient patient showed no response to IL-23, as measured by the lack of STAT3 phosphorylation upon stimulation with this cytokine. Overall, these data suggest that TYK2 plays an essential role in responses to IL-12 and IL-23, whereas IFNɣ activity remains as expected, TYK2-independent. Remarkably, in this individual the overall decrease in Th1 responses was accompanied by a bias towards Th2 responses including increased IL-5, IL-13, and IgE production, all of which may be involved in the disease manifestations described in this patient. This observation also agrees with studies performed in mice with a natural mutation in the TYK2 pseudokinase domain, which is associated with hyporesponsiveness to IL-12, IL-23, and type I IFNs.86 Therefore, although the essential role of TYK2 in response to IL-12 has been clearly established both in human and in murine cells, the requirement of this kinase in signalling downstream of other cytokine receptors that associate with TYK2 remains less clear.
More recently a new study has described seven additional patients, with increased susceptibility to mycobacterial infections, who turned out to carry mutations in TYK2 which led to a lack of protein expression. Furthermore, none of these patients developed hyper-IgE syndrome.87 They did however exhibit impaired responses to IL-12, IL-23, IFN-α, and IL-10, similarly to the first TYK2-deficient patient first described. Response to IL-6 however was not impaired in these seven patients, in contrast to the first reported TYK2-deficient individual. This observation would suggest that TYK2 may be dispensable for IL-6 signalling. Indeed, further data obtained from the first TYK2-deficient patient with the hyper-IgE syndrome showed that impaired response to IL-6 occurred independently of the TYK2 mutation87 in this patient, as restoring TYK2 expression rescued the response to IFN-α but not to IL-6.
Lack of response in TYK2-deficient cells could also be due to defective expression of cytokine receptors. Indeed, the role of TYK2 as a scaffolding protein to stabilise the IFNα receptor has been described in earlier studies.57,88 In agreement with that observation, TYK2-deficient patients showed a marked downregulation of IFN-αR1, as well as IL-10R2 and IL-12Rβ1, on the cell surface,87 strongly suggesting that TYK2 plays a crucial role in stabilising cell-surface receptor expression, which would explain the lack of response to type I IFNs, IL-10R and IL-12R binding cytokines. Thus, to dissect the scaffolding function of TYK2 from its catalytic activity [required for downstream signal transduction], one cannot rely on TYK2-deficient cells; instead, inhibitors of the enzymatic activity must be employed. Experiments using a panel of potent TYK2 antagonists with varying degrees of selectivity against other JAK kinases confirm that TYK2 is essential for IL-12 and IL-23 signalling, whereas it is not required for type I IFN, IL-6, and IL-10 induced STAT phosphorylation in human cells, which could instead be completely abrogated by JAK1-specific inhibitors.89 A recent paper using a TYK2 selective inhibitor [BMS-986165] appears to partially challenge this view.90 BMS-986165 is an allosteric inhibitor, thus highly specific, and was identified for its selectivity towards the TYK2 pseudokinase domain; nonetheless, it could also bind with lower affinity to the JAK1 pseudokinase domain. BMS-986165 competed with a fluorescent probe to bind to the adenosine 5′-triphosphate [ATP] binding site of the human recombinant TYK2 pseudokinase domain protein with a median inhibitory concentration [IC50] of 0.2 nM, and to the JAK1 pseudokinase domain with an IC50 of 1 nM. As expected, this compound effectively blocked IL-23 and IL-12 responses in human peripheral blood mononuclear cells [PBMCs] with an IC50 of 9 and 11 nM, respectively. In contrast to the previously described TYK2 selective inhibitors that presented 200-fold greater selectivity for TYK2 over JAK1,89 BMS-986165 inhibited responses to IFNα and IL-10 in human PBMCs with IC50 values ranging between 6 and 14.89 This effect could, however, be explained by the combined partial inhibition of JAK1 and the potent TYK2 impairment provided by the BMS compound. Regardless of the compound used, TYK2 catalytic activity does not appear essential for IL-6 responses.89,90
BMS-986165 was also administered as an inhibitor in two models of colitis that can be prevented by anti-p40 [IL-12/IL-23], and afforded complete protection as determined by decreased weight loss and colonic histological scores.90 In agreement with the potent inhibition of type I IFN responses, the BMS compound was also shown to protect mice from nephritis in a lupus-prone mouse model.
In summary, data from human and murine TYK2-deficient cells and selective [or partially selective] TYK2 inhibitors support the idea that the catalytic activity of TYK2 is required for signalling downstream of the IL-12R family, whereas it may be dispensable for responses to IL-6. Contradicting data are available on the essential role of TYK2 in mediating responses to type I IFNs and to cytokines binding to the IL-10R family. Nonetheless, minor allele homozygosity at the rs34536443 single nucleotide polymorphism [SNP] drives the near complete loss of TYK2 function and impairs type I IFN, IL-12, and IL-23 signalling, although responses to IL-6, IL-10 and IL-13 are unaffected.91 Remarkably, this SNP has been found to confer protection against psoriasis, rheumatoid arthritis, systemic lupus erythematosus, type 1 diabetes, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, multiple sclerosis, juvenile idiopathic arthritis, and primary biliary cirrhosis.92–98
Overall, all this evidence makes TYK2 a desirable target for multiple common autoimmune disorders, including IBD.
7. JAK1
Elucidating the non-redundant roles of JAK1 has been complicated by the fact that JAK1-knockdown in mice results in perinatal lethality,78 with no patients described to date with complete JAK1 deletion. Indeed, the role of JAK1 was initially discovered by generating cell lines resistant to IFN effects. Müller et al. generated a randomly mutagenised human fibrosarcoma cell line that lacked JAK1 and failed to respond to both IFNα/β and IFNɣ.59 Later, JAK1 was shown to be critical in mediating type III IFN-induced STAT phosphorylation.99
A 2016 report described a patient who presented an immunodeficiency with susceptibility to mycobacterial infections and who developed a fatal high-grade bladder cancer.100 This patient turned out to carry two homozygous missense germline mutations in the JAK1 pseudo-kinase domain whcih impaired JAK1 and STAT phosphorylation, resulting in a significantly reduced response to both type I and type II IFNs. Nonetheless, the impact of this mutation on other JAK1-dependent cytokines, including the IL-2, IL-6, and IL-10 family, was not explored in this study. However, impaired responses to IL-2, IL-7, and IL-15 [all using the ɣc that interacts with JAK1] may have contributed to the observed progressive T lymphopenia in this patient and impaired the development of NK and cytolytic T cells. In agreement with a role of JAK1 in lymphocyte development, JAK1-deficient mice die perinatally and have severely reduced numbers of thymocytes, pre-B cells, and mature T and B cells.78 Experiments using cells derived from these mice show the absolute requirement of JAK1 in mediating responses to all type II receptors [i.e., the receptors to IFNα/β, IFNɣ, and IL-10], as well as to ɣc [i.e., the receptors to IL-2, IL-4, IL-7, IL-9, and IL-15] and gp130-using receptors [i.e., the receptors to IL-6, IL-11, OSM, LIF, CNTF, and CT-1].
Overall the expression of JAK1 is shared by many cell types, as it associates with a large number of cytokine receptor chains. It is therefore not surprising that JAK1 has proven to be essential for embryonic development and that it is involved in many physiologically relevant pathways, including protection from infections and anti-tumour responses. Indeed, somatic mutations in JAK1 have been identified in multiple tumour types.101–104 Mutations predicted to cause loss of JAK1 function are associated with the reduced expression of IFN-associated genes in different tumour types,103 stressing the key role of JAK1-mediated responses in tumour surveillance. In addition, JAK1 is broadly involved in microbial responses [i.e,. IL-6, IFNs, OSM] and regulatory homeostatic signals [i.e. IL-10, IL-22], and interfering with its activity may pose risks in the long-term treatment of patients. Nonetheless, JAK1 inhibitors remain a potent and viable option to treat inflammatory diseases. Long-term treatment, however, should be carefully monitored as we learn more about these powerful inhibitors.
8. JAK2
Similar to JAK1, deletion of JAK2 is lethal.77,105 JAK2 deficiency causes embryonic death due to incomplete erythropoiesis-producing anaemia. Indeed, JAK2 is required for the transmission of signals downstream of the EPO receptor. The phenotype in JAK2-KO mice is more severe than that observed in embryos lacking the EPO receptor,106 which could be explained by defects in response to additional mediators such as TPO, which also contribute to the expansion of early erythroid lineage cells. Moreover, responses to other cytokines important for haematopoiesis [i.e. GM-CSF, G-CSF, IL-5, and IL-3] are also impaired in JAK2-KO mice. Whereas lack of function or deletion mutations have not been reported, gain-of-function mutations on JAK2 have been extensively documented and linked to myeloproliferative diseases. A single point mutation in the JH2 pseudo-kinase domain of JAK2V617F that leads to the constitutive tyrosine kinase activity of JAK2, is found in 80% of polycythaemia vera patients,24,107 an acquired myeloproliferative disorder associated with thrombocytosis, leukocytosis, and splenomegaly. Several fusion proteins comprising transcription factors and JAK2 have been recognised in lymphoproliferative and myeloproliferative disorders.108 Analogous to the activating JAK2V617F mutation, these fusion proteins are constitutively active kinases and promote cell survival and proliferation independently of signals received from cytokine binding. Indeed, the first inhibitor to be approved by the US Food and Drug Administration [FDA] was ruxolitinib [a JAK1/2 antagonist], indicated for the treatment of myeloproliferative neoplasms.109
In addition to JAK2’s role in signalling through hormone-like receptors and the IL3R family, this kinase is involved in the signalling mediated by IFNɣ and IL-12, the latter a key Th1-inducing cytokine. Indeed, fibroblasts from JAK2-KO mice were defective in IFNɣ signalling, whereas signalling via type I IFN receptors remained intact.110 In addition, gp130-using receptors have also been shown in mice embryonic stem cells to induce JAK2 and, to a lesser degree, JAK1 phosphorylation. Deletions or point mutations in the membrane-proximal cytoplasmic motifs in gp130 result in the loss of tyrosine phosphorylation of JAK2, which coincides with the lack of signal-transducing capability in gp130 mutants.111
In summary, despite contributing to the activation of pathways that are potentially involved in inflammatory diseases such as IBD, JAK2 has shown an ineluctable role in haematopoiesis that bars it from becoming a potential target for these diseases. Nonetheless, some molecules that block both JAK1 and JAK2 [i.e. baricitinib and ruxolitinib] have been approved in other autoimmune diseases such as rheumatoid arthritis112 and tested in lupus erythematosus,113 showing efficacy and acceptable safety patterns. In contrast, more selective JAK2 inhibitors [i.e., fedratinib] have been restricted to myeloproliferative disease such as myelofibrosis.114,115
9. JAK3
JAK3 was the fourth and last member of the JAK family to be discovered.116 Similar to TYK2, individuals lacking JAK3 protein expression have been reported.71,117,118 JAK3 associates exclusively with the ɣc [IL-2RG] and is required together with JAK1 for downstream signalling of the IL-2R family of receptors. In contrast to JAK1, which is activated by a large group of cytokine receptors, the role of JAK3 is rather restricted and it primarily regulates lymphocyte maturation, survival, activation, and differentiation. Thus, defects in JAK3 are the second most common form of a severe immunodeficiency in humans.71,117–119 This mutation shares the phenotype with IL2RG [ɣc] deficiency, known as X-linked severe combined immunodeficiency [X-SCID].119,120 SCID refers to a group of rare and inherited defects in primary immunity, resulting in the absence of lymphocyte development and significant deficits in host defence. This life-threatening condition is typically presented within the first few months of life by a combination of opportunistic infections. Currently, the only viable clinical therapy for SCID is haematopoietic stem cell transplantation [HSCT].121
JAK3-SCID is an autosomal recessive form of SCID characterised by the lack of peripheral T and NK lymphocytes with conserved numbers of B cells. Despite having normal B cell numbers, both JAK3- and X-SCID show compromised humoral responses, with impaired B cell activation, maturation, and antibody class switching. This can be explained in part by the lack of T-helper function, although it also stems from the intrinsic B cell dysfunction caused by defective responses to other ɣc/JAK3 cytokines [IL-4 and IL-21] which are important regulators of B cell proliferation and immunoglobulin class switching.
In agreement with human data, KO mouse models lacking JAK3 [JAK3-KO mice] or ɣc [IL2RG-KO mice] showed a characteristic SCID phenotype similar to that observed in humans.119,122–124 JAK3- and ɣc-KO mice have small thymi and lack lymph nodes. Both types of deficient mice showed a defect in T cell development and have functionally unresponsive peripheral T cells with an activated/memory cell phenotype.125 Moreover, JAK3- and ɣc-deficient mice developed marked B cell lymphopenia with residual functionally deficient B cells. The severely reduced numbers of bone marrow and peripheral B cells observed in ɣc- and JAK3-deficient mice differ from the common phenotype in human SCID, suggesting different requirements for B cell development between species. In JAK3 or IL-7R-KO mice, B cell development is blocked at the pre-B stage, likely due to the impaired IL-7R signalling in mice.126,127 Additionally, IL-7R has been found to promote rearrangement of the immunoglobulin heavy chain genes.127 Thus, the B cell maturation defect in KO mice its likely due to the inability of these early B cells to respond to IL-7 signals. In humans, IL-7 signalling is also essential for lymphoid development, survival, and differentiation, but not for B cell maturation.
The third most common mutations in SCID patients are in IL7R.119 IL7R-deficient patients show severe T lymphopenia with normal or increased B cells and, in contrast to JAK3- or ɣc-deficient individuals, normal NK cell development [T−B+NK+ SCID],128 supporting the importance of IL-7 in NK cell homeostasis in humans. Therefore, IL-7 appears to play different roles between species, being essential for B cell development in mice and for NK cell generation in humans.
Overall, JAK3 deficiency is associated in humans with a marked decrease in T and NK cell numbers, as well as a defect in mounting B cell responses. JAK3 inhibitors thus represent powerful tools to treat patients suffering from lymphocyte-dependent immune diseases, although they carry the risk of decreased immunosurveillance and thus require close monitoring. Besides mediating antimicrobial responses, at least four of the ɣc family cytokines [IL-2, IL-9, IL-15, and IL-21] have been reported to exhibit anti-cancer activities.34 On the other hand, IL-2 has also been considered in the treatment of autoimmunity, due to its critical role in Treg cell homeostasis.129
10. Translating Research Findings on Janus Kinase Biology to a Clinical Setting
JAKs receive signals from over 50 cytokines and growth factors with essential roles in the immune system, as evidenced by the dramatic phenotypes described in individuals bearing loss- or gain-of-function mutations in any of the four JAK members. Most of our current knowledge on JAKs derives from the study of these individuals, as well as from animal models that reproduce some of these mutations.
A significant success of these studies has been the development of JAK inhibitors—including broad inhibitors, ruxolitinib and tofacitinib—that have benefited thousands of patients suffering from chronic inflammatory diseases. The success of the first generation of JAK inhibitors has pushed research and the development of novel and more selective compounds. The benefits of inhibitors with higher specificity must still be established. Nonetheless, the in vivo and in vitro evidence discussed herein should guide the design of novel approaches and the interpretation of data that will be generated from testing these more specific inhibitors in patients.
In principle, molecules with increased JAK specificity may provide efficacy while potentially improving safety. Nonetheless, JAK antagonism represents a broad multiple cytokine-blocking approach to disease treatment. Even antagonists that, at a therapeutic dose range, bind exclusively to one of the four JAK proteins will inhibit an array of different cytokine pathways in contrast to the single-cytokine blocking antibodies commonly used in the clinics.
Beyond the difficulty of developing purely selective antagonists, another forthcoming challenge remains in defining which JAK would be the best target for each specific disease or disease phenotype.
Targeting JAK1, for example, inevitably interferes with the JAK3-dependent receptors that share signalling with JAK1. Supporting this argument, both tofacitinib [a JAK1 and JAK3 antagonist] and upadacitinib [a selective JAK1 inhibitor]130 are approved for the treatment of moderate to severe rheumatoid arthritis with inadequate response or intolerance to methotrexate.131–133 As discussed above, JAK1 inhibitors have a broad target range which may have contributed to the efficacy of tofacitinib, upadacitinib, or filgotinib, but which may have also impaired protective immune responses [i.e. viral responses].
On the other hand, JAK3-specific inhibitors currently under development [i.e., decernotinib and PF-06651600] exclusively target the ɣc receptor family, while sparing other JAK1-dependent IL-6R and IL-10R cytokines, IL-13, and types I, II, and III IFNs. Selective JAK3 antagonism, we argue, could prove beneficial to those disease phenotypes that rely primarily on T and/or B cells responses which are dependent on cytokine signals such as IL-2, IL-4, IL-7, IL-15, IL-21, and IL-9.134 The challenge ahead for selective JAK3 inhibitors remains proof of efficacy and a reduction of adverse effects.
Based on all the evidence available, JAK2 is essential for haematopoiesis and selective inhibition was initially limited to the treatment of myeloproliferative diseases. Nonetheless, an inhibitor to both JAK1 and JAK2, baricitinib, has been tested in immune-mediated diseases and recently approved for treatment of rheumatoid arthritis, although at the lower dose of 2 mg. Inhibition of both JAK1 and JAK2 catalytic activities should completely shut down all JAK-dependent cytokine receptors [Figure 3]. Indeed, baricitinib at the high dose of 4 mg was associated with an increased risk of herpes zoster and a higher incidence of malignancy, excluding non-melanoma skin cancer. The rate for the latter was higher in the 4-mg group. In addition, six patients in that group were diagnosed with lymphoma.135 The increased risk of thrombosis associated with the high dose led the FDA to approve baricitinib only at a dose of 2 mg.136
In contrast to the broad effects of JAK1 and/or JAK2, TYK2 appears to tightly control responses to IL-12 and IL-23 and is likely involved in type I IFN responses. Whereas TYK2 is essential for the cell surface expression of the IL-6 and the IL-10 receptor families, the signalling downstream of these receptors does not appear to require its catalytic activity. This profile makes it a highly desirable target in inflammatory diseases including IBD, psoriasis, and rheumatoid arthritis that respond to anti-p40 [IL-12/IL-23] antibodies. Indeed, a phase II study is currently under way for moderate to severe ulcerative colitis [NCT03934216].
Finally increasing our knowledge, as well as the pool of highly specific inhibitors available, could provide the basis for combination therapies. Treatment with both a TYK2 inhibitor and a JAK3 antagonist during induction may provide benefits to a broader number of patients, by simultaneously targeting the ɣc cytokines and the IL-12/IL-23 pathways. Furthermore, we would suggest that by combining a JAK3 anatagonist and a TYK2 inhibitor we could potentially reduce the required doses to achieve remission. As selectivity of most inhibitors, and indeed safety, can be negatively affected by increasing the administered doses, combining compounds that simultaneously target independent pathways may prove beneficial. Given the multifactorial and heterogeneous nature of immune mechanisms driving IBD, access to selective JAK inhibitors may support future therapeutic fine tuning of the relevant target for each individual needing personalised disease modulation.
Acknowledgements
We thank Joe Moore for English language editorial assistance. Figures were made with BioRender.
Funding
AG-T is funded by the Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas [CIBER-EHD], ISCII, Spain. AS is funded by the Ministerio de Ciencia, Innovación y Universidades [RTI2018-096946-B-I00], Spain.
Conflict of Interest
AS has received research funding from Pfizer. AG-T has no conflict of interest.
Author Contributions
AG-T and AS wrote the manuscript and designed the figures.
References
- 1. Fuss IJ, Neurath M, Boirivant M, et al. Disparate CD4+ lamina propria [LP] lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol 1996;157:1261–70. [PubMed] [Google Scholar]
- 2. Kusugami K, Matsuura T, West GA, Youngman KR, Rachmilewitz D, Fiocchi C. Loss of interleukin-2-producing intestinal CD4+ T cells in inflammatory bowel disease. Gastroenterology 1991;101:1594–605. [DOI] [PubMed] [Google Scholar]
- 3. Fiocchi C, Hilfiker ML, Youngman KR, Doerder NC, Finke JH. Interleukin 2 activity of human intestinal mucosa mononuclear cells. Decreased levels in inflammatory bowel disease. Gastroenterology 1984;86:734–42. [PubMed] [Google Scholar]
- 4. Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 1993;75:253–61. [DOI] [PubMed] [Google Scholar]
- 5. Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993;75:263–74. [DOI] [PubMed] [Google Scholar]
- 6. Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014;14:329–42. [DOI] [PubMed] [Google Scholar]
- 7. Coskun M, Salem M, Pedersen J, Nielsen OH. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol Res 2013;76:1–8. [DOI] [PubMed] [Google Scholar]
- 8. Soendergaard C, Bergenheim FH, Bjerrum JT, Nielsen OH. Targeting JAK-STAT signal transduction in IBD. Pharmacol Ther 2018;192:100–11. [DOI] [PubMed] [Google Scholar]
- 9. Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O’Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov 2017;16:843–62. [DOI] [PubMed] [Google Scholar]
- 10. Fernández-Clotet A, Castro-Poceiro J, Panés J. JAK inhibition: the most promising agents in the IBD pipeline? Curr Pharm Des 2019;25:32–40. [DOI] [PubMed] [Google Scholar]
- 11. Sandborn WJ, Ghosh S, Panes J, et al. ; Study A3921063 Investigators Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 2012;367:616–24. [DOI] [PubMed] [Google Scholar]
- 12. Sandborn WJ, Su C, Panes J. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;377:496–7. [DOI] [PubMed] [Google Scholar]
- 13. Schwartz DM, Bonelli M, Gadina M, O’Shea JJ. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol 2016;12:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boraschi D, Italiani P, Weil S, Martin MU. The family of the interleukin-1 receptors. Immunol Rev 2018;281:197–232. [DOI] [PubMed] [Google Scholar]
- 15. Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol 2010;10:89–102. [DOI] [PubMed] [Google Scholar]
- 16. Ségaliny AI, Tellez-Gabriel M, Heymann MF, Heymann D. Receptor tyrosine kinases: characterisation, mechanism of action and therapeutic interests for bone cancers. J Bone Oncol 2015;4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2010;141:1117–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horuk R. Molecular properties of the chemokine receptor family. Trends Pharmacol Sci 1994;15:159–65. [DOI] [PubMed] [Google Scholar]
- 19. Reilly JT. Class III receptor tyrosine kinases: role in leukaemogenesis. Br J Haematol 2002;116:744–57. [DOI] [PubMed] [Google Scholar]
- 20. Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene 2000;19:5662–79. [DOI] [PubMed] [Google Scholar]
- 21. Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol 1998;16:293–322. [DOI] [PubMed] [Google Scholar]
- 22. Yamaoka K, Saharinen P, Pesu M, Holt VE 3rd, Silvennoinen O, O’Shea JJ. The Janus kinases [Jaks]. Genome Biol 2004;5:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saharinen P, Takaluoma K, Silvennoinen O. Regulation of the Jak2 tyrosine kinase by its pseudokinase domain. Mol Cell Biol 2000;20:3387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005;434:1144–8. [DOI] [PubMed] [Google Scholar]
- 25. Baxter EJ, Scott LM, Campbell PJ, et al. ; Cancer Genome Project Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–61. [DOI] [PubMed] [Google Scholar]
- 26. Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 2005;352:1779–90. [DOI] [PubMed] [Google Scholar]
- 27. Haan S, Margue C, Engrand A, et al. Dual role of the Jak1 FERM and kinase domains in cytokine receptor binding and in stimulation-dependent Jak activation. J Immunol 2008;180:998–1007. [DOI] [PubMed] [Google Scholar]
- 28. Wallweber HJ, Tam C, Franke Y, Starovasnik MA, Lupardus PJ. Structural basis of recognition of interferon-α receptor by tyrosine kinase 2. Nat Struct Mol Biol 2014;21:443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferrao R, Lupardus PJ. The Janus kinase [JAK] FERM and SH2 domains: bringing specificity to JAK-receptor interactions. Front Endocrinol [Lausanne] 2017;8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O’Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell 2002;109[Suppl]:S121–31. [DOI] [PubMed] [Google Scholar]
- 31. Zundler S, Neurath MF. Integrating immunologic signaling networks: the JAK/STAT pathway in colitis and colitis-associated cancer. Vaccines [Basel] 2016, Feb 29. doi: 10.3390/vaccines4010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salas A, Hernandez-Rocha C, Duijvestein M, et al. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2020. in press. [DOI] [PubMed] [Google Scholar]
- 33. Yu H, Jove R. The STATs of cancer – new molecular targets come of age. Nat Rev Cancer 2004;4:97–105. [DOI] [PubMed] [Google Scholar]
- 34. Leonard WJ, Lin JX, O’Shea JJ. The γc family of cytokines: basic biology to therapeutic ramifications. Immunity 2019;50:832–50. [DOI] [PubMed] [Google Scholar]
- 35. Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev 2006;17:259–80. [DOI] [PubMed] [Google Scholar]
- 36. Junttila IS. Tuning the cytokine responses: an update on interleukin [IL]-4 and IL-13 receptor complexes. Front Immunol 2018;9:888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miloux B, Laurent P, Bonnin O, et al. Cloning of the human IL-13R alpha1 chain and reconstitution with the IL4R alpha of a functional IL-4/IL-13 receptor complex. FEBS Lett 1997;401:163–6. [DOI] [PubMed] [Google Scholar]
- 38. Hilton DJ, Zhang JG, Metcalf D, Alexander WS, Nicola NA, Willson TA. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc Natl Acad Sci U S A 1996;93:497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol 2003;111:677–90; quiz 691. [DOI] [PubMed] [Google Scholar]
- 40. McCormick SM, Heller NM. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine 2015;75:38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. LaPorte SL, Juo ZS, Vaclavikova J, et al. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell 2008;132:259–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Paniagua R, Si MS, Flores MG, et al. Effects of JAK3 inhibition with CP-690,550 on immune cell populations and their functions in nonhuman primate recipients of kidney allografts. Transplantation 2005;80:1283–92. [DOI] [PubMed] [Google Scholar]
- 43. Piscianz E, Valencic E, Cuzzoni E, et al. Fate of lymphocytes after withdrawal of tofacitinib treatment. PLoS One 2014;9:e85463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rochman Y, Kashyap M, Robinson GW, et al. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci U S A 2010;107:19455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ziegler SF, Roan F, Bell BD, Stoklasek TA, Kitajima M, Han H. The biology of thymic stromal lymphopoietin [TSLP]. Adv Pharmacol 2013;66:129–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Derouet D, Rousseau F, Alfonsi F, et al. Neuropoietin, a new IL-6-related cytokine signaling through the ciliary neurotrophic factor receptor. Proc Natl Acad Sci U S A 2004;101:4827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin [IL]-6-type cytokine signalling and its regulation. Biochem J 2003;374:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annu Rev Immunol 2015;33:417–43. [DOI] [PubMed] [Google Scholar]
- 49. Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol 2012;13:722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Collison LW, Delgoffe GM, Guy CS, et al. The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol 2012;13:290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Onishi K, Zandstra PW. LIF signaling in stem cells and development. Development 2015;142:2230–6. [DOI] [PubMed] [Google Scholar]
- 52. Lowe C, Gillespie GA, Pike JW. Leukemia inhibitory factor as a mediator of JAK/STAT activation in murine osteoblasts. J Bone Miner Res 1995;10:1644–50. [DOI] [PubMed] [Google Scholar]
- 53. White UA, Stephens JM. The gp130 receptor cytokine family: regulators of adipocyte development and function. Curr Pharm Des 2011;17:340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Auguste P, Guillet C, Fourcin M, et al. Signaling of type II oncostatin M receptor. J Biol Chem 1997;272:15760–4. [DOI] [PubMed] [Google Scholar]
- 55. Broughton SE, Dhagat U, Hercus TR, et al. The GM-CSF/IL-3/IL-5 cytokine receptor family: from ligand recognition to initiation of signaling. Immunol Rev 2012;250:277–302. [DOI] [PubMed] [Google Scholar]
- 56. Waters MJ, Brooks AJ. JAK2 activation by growth hormone and other cytokines. Biochem J 2015;466:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Velazquez L, Fellous M, Stark GR, Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell 1992;70:313–22. [DOI] [PubMed] [Google Scholar]
- 58. Aman MJ, Tayebi N, Obiri NI, Puri RK, Modi WS, Leonard WJ. cDNA cloning and characterization of the human interleukin 13 receptor alpha chain. J Biol Chem 1996;271:29265–70. [DOI] [PubMed] [Google Scholar]
- 59. Müller M, Briscoe J, Laxton C, et al. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature 1993;366:129–35. [DOI] [PubMed] [Google Scholar]
- 60. Donnelly RP, Kotenko SV. Interferon-lambda: a new addition to an old family. J Interferon Cytokine Res 2010;30:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Welsh RM, Bahl K, Marshall HD, Urban SL. Type 1 interferons and antiviral CD8 T-cell responses. PLoS Pathog 2012;8:e1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Egli A, Levin A, Santer DM, et al. Immunomodulatory function of interleukin 28B during primary infection with cytomegalovirus. J Infect Dis 2014;210:717–27. [DOI] [PubMed] [Google Scholar]
- 63. Robek MD, Boyd BS, Chisari FV. Lambda interferon inhibits hepatitis B and C virus replication. J Virol 2005;79:3851–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tau G, Rothman P. Biologic functions of the IFN-gamma receptors. Allergy 1999;54:1233–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ouyang W, O’Garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity 2019;50:871–91. [DOI] [PubMed] [Google Scholar]
- 66. Kotenko SV, Izotova LS, Mirochnitchenko OV, et al. Identification of the functional interleukin-22 [IL-22] receptor complex: the IL-10R2 chain [IL-10Rbeta] is a common chain of both the IL-10 and IL-22 [IL-10-related T cell-derived inducible factor, IL-TIF] receptor complexes. J Biol Chem 2001;276:2725–32. [DOI] [PubMed] [Google Scholar]
- 67. Donnelly RP, Sheikh F, Kotenko SV, Dickensheets H. The expanded family of class II cytokines that share the IL-10 receptor-2 [IL-10R2] chain. J Leukoc Biol 2004;76:314–21. [DOI] [PubMed] [Google Scholar]
- 68. Sabat R. IL-10 family of cytokines. Cytokine Growth Factor Rev 2010;21:315–24. [DOI] [PubMed] [Google Scholar]
- 69. Darnell JE Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994;264:1415–21. [DOI] [PubMed] [Google Scholar]
- 70. Watling D, Guschin D, Müller M, et al. Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon-gamma signal transduction pathway. Nature 1993;366:166–70. [DOI] [PubMed] [Google Scholar]
- 71. Russell SM, Tayebi N, Nakajima H, et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science 1995;270:797–800. [DOI] [PubMed] [Google Scholar]
- 72. Suzuki K, Nakajima H, Saito Y, Saito T, Leonard WJ, Iwamoto I. Janus kinase 3 [Jak3] is essential for common cytokine receptor gamma chain [gamma©]-dependent signaling: comparative analysis of gamma©, Jak3, and gamma© and Jak3 double-deficient mice. Int Immunol 2000;12:123–32. [DOI] [PubMed] [Google Scholar]
- 73. Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O’Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev 2004;202:139–56. [DOI] [PubMed] [Google Scholar]
- 74. Shimoda K, Kato K, Aoki K, et al. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity 2000;13:561–71. [DOI] [PubMed] [Google Scholar]
- 75. Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity 2000;13:549–60. [DOI] [PubMed] [Google Scholar]
- 76. Witthuhn BA, Quelle FW, Silvennoinen O, et al. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell 1993;74:227–36. [DOI] [PubMed] [Google Scholar]
- 77. Neubauer H, Cumano A, Müller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell 1998;93:397–409. [DOI] [PubMed] [Google Scholar]
- 78. Rodig SJ, Meraz MA, White JM, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell 1998;93:373–83. [DOI] [PubMed] [Google Scholar]
- 79. Casanova JL, Holland SM, Notarangelo LD. Inborn errors of human JAKs and STATs. Immunity 2012;36:515–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stark GR, Cheon H, Wang Y. Responses to cytokines and interferons that depend upon JAKs and STATs. Cold Spring Harb Perspect Biol 2018, Jan 2. doi: 10.1101/cshperspect.a028555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Elliott NE, Cleveland SM, Grann V, Janik J, Waldmann TA, Davé UP. FERM domain mutations induce gain of function in JAK3 in adult T-cell leukemia/lymphoma. Blood 2011;118:3911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chen E, Staudt LM, Green AR. Janus kinase deregulation in leukemia and lymphoma. Immunity 2012;36:529–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mullighan CG, Zhang J, Harvey RC, et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A 2009;106:9414–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Firmbach-Kraft I, Byers M, Shows T, Dalla-Favera R, Krolewski JJ. tyk2, prototype of a novel class of non-receptor tyrosine kinase genes. Oncogene 1990;5:1329–36. [PubMed] [Google Scholar]
- 85. Minegishi Y, Saito M, Morio T, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity 2006;25:745–55. [DOI] [PubMed] [Google Scholar]
- 86. Shaw MH, Boyartchuk V, Wong S, et al. A natural mutation in the Tyk2 pseudokinase domain underlies altered susceptibility of B10.Q/J mice to infection and autoimmunity. Proc Natl Acad Sci U S A 2003;100:11594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kreins AY, Ciancanelli MJ, Okada S, et al. Human TYK2 deficiency: mycobacterial and viral infections without hyper-IgE syndrome. J Exp Med 2015;212:1641–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pellegrini S, John J, Shearer M, Kerr IM, Stark GR. Use of a selectable marker regulated by alpha interferon to obtain mutations in the signaling pathway. Mol Cell Biol 1989;9:4605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sohn SJ, Barrett K, Van Abbema A, et al. A restricted role for TYK2 catalytic activity in human cytokine responses revealed by novel TYK2-selective inhibitors. J Immunol 2013;191:2205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Burke JR, Cheng L, GilloolyKM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci Transl Med 2019, Jul 24. doi: 10.1126/scitranslmed.aaw1736 [DOI] [PubMed] [Google Scholar]
- 91. Dendrou CA, Cortes A, Shipman L, et al. Resolving TYK2 locus genotype-to-phenotype differences in autoimmunity. Sci Transl Med 2016;8:363ra149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cortes A, Hadler J, Pointon JP; International Genetics of Ankylosing Spondylitis Consortium Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet 2013;45:730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jostins L, Ripke S, Weersma RK, et al. ; International IBD Genetics Consortium [IIBDGC] Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Franke A, McGovern DP, Barrett JC, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet 2010;42:1118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Beecham AH, Patsopoulos NA, Xifara DK, et al. ; International Multiple Sclerosis Genetics et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet 2013;45:1353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tsoi LC, Spain SL, Knight J, et al. ; Collaborative Association Study of Psoriasis [CASP]; Genetic Analysis of Psoriasis Consortium; Psoriasis Association Genetics Extension; Wellcome Trust Case Control Consortium 2 Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet 2012;44:1341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Diogo D, Bastarache L, Liao KP, et al. TYK2 protein-coding variants protect against rheumatoid arthritis and autoimmunity, with no evidence of major pleiotropic effects on non-autoimmune complex traits. PLoS One 2015;10:e0122271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Onengut-Gumuscu S, Chen WM, Burren O, et al. ; Type 1 Diabetes Genetics Consortium Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet 2015;47:381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dumoutier L, Lejeune D, Hor S, Fickenscher H, Renauld JC. Cloning of a new type II cytokine receptor activating signal transducer and activator of transcription [STAT]1, STAT2 and STAT3. Biochem J 2003;370:391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Eletto D, Burns SO, Angulo I, et al. Biallelic JAK1 mutations in immunodeficient patient with mycobacterial infection. Nat Commun 2016;7:13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ren Y, Zhang Y, Liu RZ, et al. JAK1 truncating mutations in gynecologic cancer define new role of cancer-associated protein tyrosine kinase aberrations. Sci Rep 2013;3:3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Longo T, McGinley KF, Freedman JA, et al. Targeted exome sequencing of the cancer genome in patients with very high-risk bladder cancer. Eur Urol 2016;70:714–7. [DOI] [PubMed] [Google Scholar]
- 103. Albacker LA, Wu J, Smith P, et al. Loss of function JAK1 mutations occur at high frequency in cancers with microsatellite instability and are suggestive of immune evasion. PLoS One 2017;12:e0176181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Flex E, Petrangeli V, Stella L, et al. Somatically acquired JAK1 mutations in adult acute lymphoblastic leukemia. J Exp Med 2008;205:751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Parganas E, Wang D, Stravopodis D, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell 1998;93:385–95. [DOI] [PubMed] [Google Scholar]
- 106. Wu H, Liu X, Jaenisch R, Lodish HF. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell 1995;83:59–67. [DOI] [PubMed] [Google Scholar]
- 107. Wolanskyj AP, Lasho TL, Schwager SM, et al. JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol 2005;131:208–13. [DOI] [PubMed] [Google Scholar]
- 108. Lacronique V, Boureux A, Valle VD, et al. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science 1997;278:1309–12. [DOI] [PubMed] [Google Scholar]
- 109. Geyer HL, Mesa RA. Therapy for myeloproliferative neoplasms: when, which agent, and how? Blood 2014;124:3529–37. [DOI] [PubMed] [Google Scholar]
- 110. Bacon CM, McVicar DW, Ortaldo JR, Rees RC, O’Shea JJ, Johnston JA. Interleukin 12 [IL-12] induces tyrosine phosphorylation of JAK2 and TYK2: differential use of Janus family tyrosine kinases by IL-2 and IL-12. J Exp Med 1995;181:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Narazaki M, Witthuhn BA, Yoshida K, et al. Activation of JAK2 kinase mediated by the interleukin 6 signal transducer gp130. Proc Natl Acad Sci U S A 1994;91:2285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med 2016;374:1243–52. [DOI] [PubMed] [Google Scholar]
- 113. Wallace DJ, Furie RA, Tanaka Y, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2018;392:222–31. [DOI] [PubMed] [Google Scholar]
- 114. Pardanani A, Harrison C, Cortes JE, et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol 2015;1:643–51. [DOI] [PubMed] [Google Scholar]
- 115. Harrison CN, Schaap N, Vannucchi AM, et al. Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib [JAKARTA-2]: a single-arm, open-label, non-randomised, phase 2, multicentre study. Lancet Haematol 2017;4:e317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science 2002;298:1912–34. [DOI] [PubMed] [Google Scholar]
- 117. Macchi P, Villa A, Giliani S, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency [SCID]. Nature 1995;377:65–8. [DOI] [PubMed] [Google Scholar]
- 118. Notarangelo LD, Giliani S, Mazza C, et al. Of genes and phenotypes: the immunological and molecular spectrum of combined immune deficiency. Defects of the gamma©-JAK3 signaling pathway as a model. Immunol Rev 2000;178:39–48. [DOI] [PubMed] [Google Scholar]
- 119. O’Shea JJ, Husa M, Li D, et al. Jak3 and the pathogenesis of severe combined immunodeficiency. Mol Immunol 2004;41:727–37. [DOI] [PubMed] [Google Scholar]
- 120. Leonard WJ, Noguchi M, Russell SM, McBride OW. The molecular basis of X-linked severe combined immunodeficiency: the role of the interleukin-2 receptor gamma chain as a common gamma chain, gamma c. Immunol Rev 1994;138:61–86. [DOI] [PubMed] [Google Scholar]
- 121. Castagnoli R, Delmonte OM, Calzoni E, Notarangelo LD. Hematopoietic stem cell transplantation in primary immunodeficiency diseases: current status and future perspectives. Front Pediatr 2019;7:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Nosaka T, van Deursen JM, Tripp RA, et al. Defective lymphoid development in mice lacking Jak3. Science 1995;270:800–2. [DOI] [PubMed] [Google Scholar]
- 123. Cao X, Shores EW, Hu-Li J, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity 1995;2:223–38. [DOI] [PubMed] [Google Scholar]
- 124. DiSanto JP, Müller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc Natl Acad Sci U S A 1995;92:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Baird AM, Thomis DC, Berg LJ. T cell development and activation in Jak3-deficient mice. J Leukoc Biol 1998;63:669–77. [DOI] [PubMed] [Google Scholar]
- 126. Thomis DC, Gurniak CB, Tivol E, Sharpe AH, Berg LJ. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science 1995;270:794–7. [DOI] [PubMed] [Google Scholar]
- 127. Corcoran AE, Smart FM, Cowling RJ, Crompton T, Owen MJ, Venkitaraman AR. The interleukin-7 receptor alpha chain transmits distinct signals for proliferation and differentiation during B lymphopoiesis. EMBO J 1996;15:1924–32. [PMC free article] [PubMed] [Google Scholar]
- 128. Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T[-]B[+]NK[+] severe combined immunodeficiency. Nat Genet 1998;20:394–7. [DOI] [PubMed] [Google Scholar]
- 129. Sharabi A, Tsokos MG, Ding Y, Malek TR, Klatzmann D, Tsokos GC. Regulatory T cells in the treatment of disease. Nat Rev Drug Discov 2018;17:823–44. [DOI] [PubMed] [Google Scholar]
- 130. Mohamed MF, Beck D, Camp HS, Othman AN. Preferential inhibition of JAK1 relative to JAK3 by upadacitinib: exposure-response analyses of ex vivo data from 2 phase 1 clinical trials and comparison to tofacitinib. J Clin Pharmacol 2019, Aug 25. doi: 10.1002/jcph.1513. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Fleischmann R, Mysler E, Hall S, et al. ; ORAL Strategy investigators Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis [ORAL Strategy]: a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 2017;390:457–68. [DOI] [PubMed] [Google Scholar]
- 132. Duggan S, Keam SJ. Upadacitinib: first approval. Drugs 2019;79:1819–28. [DOI] [PubMed] [Google Scholar]
- 133. Smolen JS, Pangan AL, Emery P, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate [SELECT-MONOTHERAPY]: a randomised, placebo-controlled, double-blind phase 3 study. Lancet 2019;393:2303–11. [DOI] [PubMed] [Google Scholar]
- 134. Gadina M, Schwartz DM, O’Shea JJ. Decernotinib: a next-generation Jakinib. Arthritis Rheumatol 2016;68:31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Smolen JS, Genovese MC, Takeuchi T, et al. Safety profile of baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J Rheumatol 2019;46:7–18. [DOI] [PubMed] [Google Scholar]
- 136. Mogul A, Corsi K, McAuliffe L. Baricitinib: the second FDA-approved JAK inhibitor for the treatment of rheumatoid arthritis. Ann Pharmacother 2019;53:947–53. [DOI] [PubMed] [Google Scholar]