Abstract
Background/Objectives
The paediatric reference range of fecal calprotectin (FC) has not been decisively established and previous studies show a wide within-age variability, suggesting that other factors like anthropometric data or type of feeding can influence FC. Our aims were to establish the normal levels of FC in healthy children grouped by age and analyze whether sex, gestational age, birth weight, type of delivery, type of feeding, or anthropometric data influence FC values.
Methods
This multicentre, cross-sectional, and observational study enrolled healthy donors under 18 years of age who attended their Primary Health Care Centre for their routine Healthy Child Program visits. The exclusion criteria were: (i) immunodeficiency, (ii) autoimmune or (iii) gastrointestinal disease; (iv) medication usage; (v) gastrointestinal symptoms; or (vi) positive finding in the microbiological study.
Results
We enrolled 395 subjects, mean age was 4.2 years (range 3 days to 16.9 years), and 204 were male. The median FC was 77.0 mcg/g (interquartile range 246). A negative correlation between age and FC was observed (Spearman’s rho = −0.603, P<0.01), and none of the other factors analyzed were found to influence FC levels.
Conclusions
Normal FC values in healthy children (particularly in infants) are higher than those considered to be altered in adults and show a negative correlation with age. It is necessary to reconsider the upper limits of FC levels for paediatric patients according to age, with further studies required to determine other factors that influence FC during infancy.
Keywords: Children, Fecal calprotectin, Healthy
Improved biomarkers for suspected paediatric inflammatory bowel disease (IBD) are a clinical need, since none of the currently available surrogate inflammatory markers are completely reliable (1). Calprotectin is a 36.5 kDa calcium binding protein that accounts for as much as 60% of the total protein in the cytosol of neutrophilic granulocytes and macrophages. When bound to calcium, it is remarkably resistant to proteolytic enzymes and heat, and is stable in feces for 3 to 4 days at room temperature (2). Its fecal concentration correlates with neutrophil infiltration of the intestinal mucosa and disease activity in IBD and could theoretically be used as a noninvasive screening test for bowel inflammation in children (3–12).
However, fecal calprotectin (FC) is a nonspecific inflammation marker that also rises in other acute and chronic gastrointestinal disorders (13–15) and can additionally be influenced by factors such as drug intake, diet, weight, gestational age, type of delivery, and especially age (16–24). Previous studies report that children tend to have higher FC levels than adults (17,20,21,25–29), but the paediatric reference range has not been decisively established. Some of these studies analyzed small samples and/or were recruited from populations with socioeconomic disparities (21,25,26,30–32) and show a wide within-age variability. Our primary objective was to establish the normal levels of FC in a healthy population of children grouped by age. Our secondary aim was to analyze whether sex, anthropometric data, gestational age, birth weight, type of delivery, or type of feeding during the first months of life influence FC values.
MATERIALS AND METHODS
A multicentre, cross-sectional, and observational study was conducted for 2 years, from January 2015 to December 2016.
Children included
The samples were obtained from healthy voluntary donors from 0 to 18 years of age who attended one of the four participating Primary Health Care Centres located in Madrid (Spain) for the routine follow-up visits recommended in the Healthy Child Program of this region. The absolute exclusion criteria were: previous diagnosis of (i) immunodeficiency, (ii) autoimmune disease, or (iii) gastrointestinal diseases such as cystic fibrosis, celiac disease, inflammatory bowel disease, eosinophilic esophagitis and/or gastroenteritis, cow’s milk protein allergy, allergic gastroenteropathy, or gastroesophageal reflux disease. The relative exclusion criteria were: (i) intake of immunomodulators, antibiotics, corticosteroids, proton pump inhibitors, probiotics, ibuprofen, or montelukast in the previous 15 days; (ii) gastrointestinal symptoms such as abdominal pain, diarrhea (defined as an increase in the number of daily stools or change in their consistency to types 6 to 7 of the Bristol scale), constipation, anal fissure or vomiting in the previous 30 days; or (iii) positive finding in the accompanying microbiological study. Those subjects meeting the relative exclusion criteria were allowed to participate in the study at a later date, 1 to 3 months after these conditions were resolved. To determine the effect of age on FC, the children were divided into eight age groups. For practical reasons, these groups were based on the routine follow-up visits recommended in the Healthy Child Program of this region: 0 to 1 month, 1 to 5 months, 6 to 11 months, 12 to 23 months, 2 to 3 years, 4 to 7 years, 8 to 11 years, and 12 to 18 years.
Those children fulfilling the mentioned criteria were enrolled after obtaining a written informed consent from the next of kin, caretakers, or guardians on behalf of the children (Figure 1). We recorded demographic, perinatal, and anthropometric data. The dietary patterns were recorded in subjects under 6 months. This information was restricted to this age group due to the limited variation with only three options: exclusive breastfeeding, mixed feeding, and formula feeding. Once the sample was collected, it was stored in a domestic refrigerator (between 3 and 5°C) until its delivery within 24 hours to their primary health care centre. The samples were then brought to the Hospital Infantil Universitario Niño Jesús (Clinical Analysis Department) and also stored in a refrigerator (4°C) for a 24 to 48 hours period before being processed.
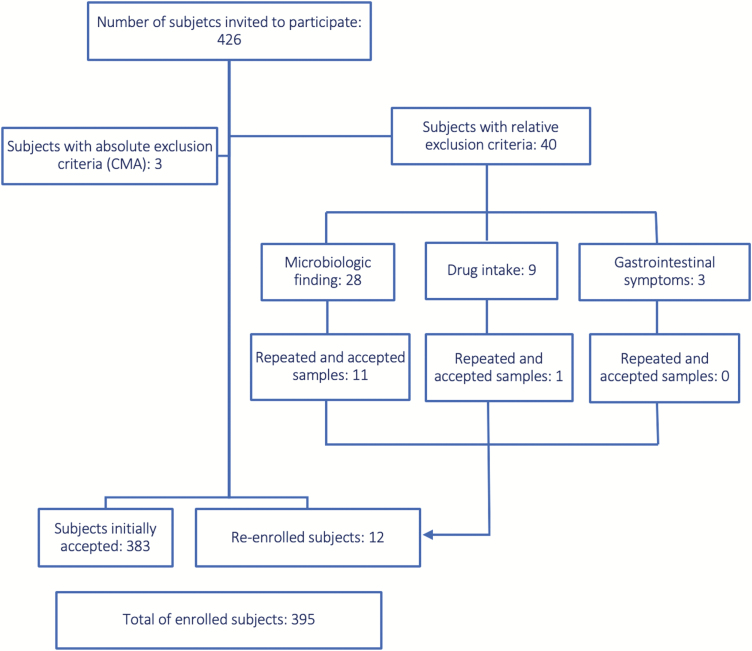
Figure 1.
Diagram showing the number of enrolled patients. CMA Cow’s milk allergy. Those subjects meeting the relative exclusion criteria were allowed to participate in the study at a later date, 1 to 3 months after these conditions were resolved. A total of 426 children were invited to participate; 43 potential subjects were excluded according to the described exclusion criteria. Of these, 12 subjects were re-enrolled. Ultimately, 395 healthy children were recruited for our study.
Measurement of FC
The FC concentration was determined within 48 hours, after being homogenized, using a commercial enzyme linked immunoassay (Quantum Blue fCAL Extended test, BÜHLMANN Laboratories AG, Schönenbuch, Switzerland) with a sensitivity of <10 mcg/g FC in fecal sample and acceptable imprecision levels for both low and high values (coefficient of variation < 10% for intra-assay and <15% for interassay) (33). The results are expressed as mcg/g stool. The upper reference limit in healthy adults supplied by the manufacturer was 50 mcg/g stool (34). Stool culture, parasites, rotavirus, and adenovirus detection were performed. The two latter tests were only performed in those subjects under 5 years of age. If any of these tests yielded positive results, the patient was excluded from the study and both the family and the corresponding General Paediatrician were informed. Those participants whose FC concentrations were higher than 50 mcg/g received a follow-up call in the following weeks to ascertain the absence of symptoms or drug intake. Subjects reporting symptoms or drug intake were asked to deliver a second sample 1 to 3 months after these conditions were resolved.
Statistical analysis
The sample size was estimated for a reference population of 1,200,000 subjects under 18 years old in the Community of Madrid. A standard deviation (SD) of 140 mcg/g (29) with 95% confidence interval was expected. These SD had to be based on a narrower study, due to the scarce data available on this issue. The Epidat statistical software package 4.2, 2016 (Xunta de Galicia, Spain; Organización Panamericana de saúde OPS-OMS; Univerdidade CES, Colombia) estimated that 383 subjects were needed for a 14 mcg/g precision. The statistical analysis was performed using the SPSS statistical software package 20 (IBM). The quantitative variable ‘CF level’ (mcg/g) in both the total sample and each age group was described by median with interquartile range. The normality of the sample was analyzed via Kolmogorov-Smirnov test. Data followed a non-normal distribution, so nonparametric tests were used for the rest of the analysis. We used the Mann–Whitney, Kruskall–Wallis or Spearman’s rho correlation tests as appropriate. All the tests were two-tailed, and statistical significance was set at P<0.05.
The study was approved by the Ethics and Clinical Research Committee of Hospital Infantil Universitario Niño Jesús and the Central Research Commission of the Madrid Health Service and conducted in accordance with the revised Declaration of Helsinki. Prior written informed consent was obtained from the parents or legal guardians of all participants and from those volunteers that were 12 years and older.
RESULTS
A total of 426 children were invited to participate in our study. Ultimately, 395 healthy children were recruited, as shown in Figure 1.
Up to 239 participants had a FC level >50 mcg/g (60.5%), which has been considered as the upper limit for adults. We were able to contact 219 of them (91.6%) for a telephonic follow-up, and none reported to be taking drugs or suffering from gastrointestinal symptoms or respiratory infections.
The median age was 2 years (IQR 6.5 years) with a range from 3 days to 16.9 years old. There were 204 boys (51.6%) and 191 girls (48.4%). The median FC was 77.0 mcg/g (IQR 246). The sample demographic and anthropometric data, and FC levels are shown in Table 1 and Figure 2.
Table 1.
Demographic and anthropometric data of each age group
| Age group (number of subjects) | Boys:girls VD:CS Median GA in weeks (IQR) |
Mean weight SD (95% CI) (number of subjects measured) |
Mean height SD (95% CI) (number of subjects measured) | Mean BMI SD (95% CI) (number of subjects measured) | FC 50thP (μg/g) (IQR) |
|---|---|---|---|---|---|
| <1 month (43) | 27:16 37:6 39 weeks (2) BF:MF:FF 33:5:5 |
−0.56 SD (−1.00 to −0.13) (43) | −0.35 SD (−0.67 to −0.03) (43) | −0.46 SD (−0.96 to 0.03) (43) | 303 (202) |
| 1–5 months (64) | 35:29 51:13 39 weeks (2) BF:MF:FF 36:21:4 |
−0.39 SD (−0.69 to −0.09) (63) | −0.33 SD (−0.66 to −0.01) (62) | −0.26 SD (−0.54 to 0.01) (61) | 325.5 (375) |
| 6–11 months (46) | 24:22 34:12 40 weeks (2) |
−0.37 SD (−0.69 to −0.06) (46) | −0.31 SD (−0.68 to 0.05) (46) | −0.26 SD (−0.52 to 0.00) (46) | 63 (126) |
| 12–23 months (42) | 22:20 29:13 39 weeks (2) |
−0.52 SD (−0.84 to −0.20) (42) | −0.55 SD (−1.07 to −0.04) (41) | −0.23 SD (−0.55 to 0.08) (41) | 97 (275) |
| 2–3 years (45) | 23:22 32:13 39 weeks (2) |
−0.40 SD (−0.68 to −0.11) (41) | −0.46 SD (−0.85 to −0.07) (40) | −0.18 SD (−0.48 to 0.11) (39) | 71 (130) |
| 4–7 years (64) | 33:31 41:22 39 weeks (3) |
−0.18 SD (−0.48 to 0.12) (62) | −0.25 SD (−0.60 to 0.09) (61) | −0.12 SD (−0.41 to 0.17) (61) | 46 (89) |
| 8–11 years (46) | 22:24 33:11 39.5 weeks (2) |
−0.22 SD (−0.45 to 0.01) (46) | 0.06 SD (−0.33 to 0.44) (44) | −0.23 SD (−0.44 to −0.03) (44) | 34.5 (48) |
| 12–18 years (45) | 18:27 36:8 39 weeks (2) |
−0.20 SD (−0.47 to 0.07) (45) | −0.03 SD (−0.37 to 0.32) (45) | −0.28 SD (−0.52 to −0.03) (45) | 30 (19) |
| Total (0–18 years) (395) | 204:191 293:98 39 weeks (2) |
−0.35 SD (−0.45 to −0.24) (388) | −0.28 SD (−0.40 to −0.15) (382) | −0.25 SD (−0.35 to −0.14) (380) | 77 (246) |
BF Breast-fed; CI Confidence interval; CS Caesarean section; FC Fecal calprotectin; FF Formula-fed; GA Gestational age; IQR Interquartile range; MF Mixed-fed; 50thP 50th percentile (median); SD Standard deviation; VD Vaginal delivery.
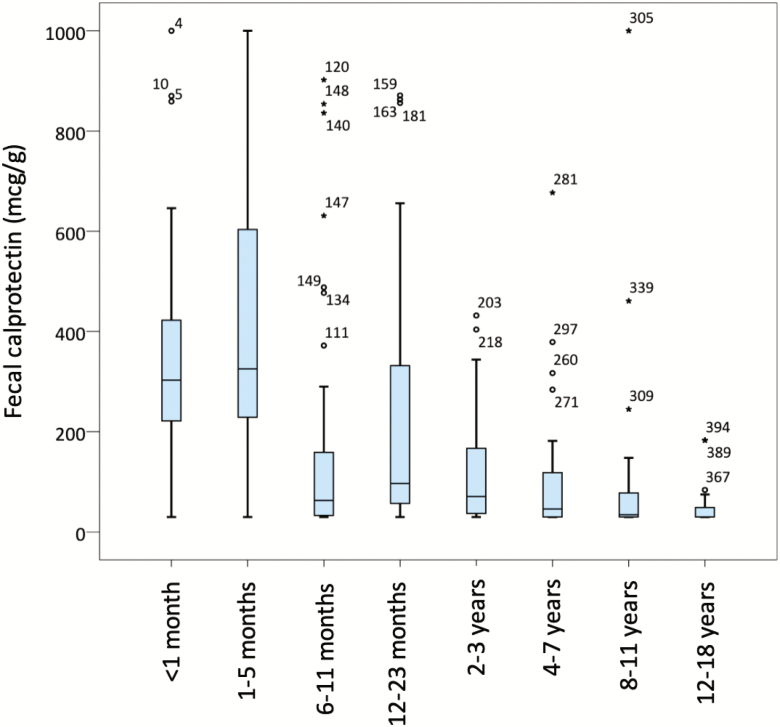
Figure 2.
Box-and-whiskers plots showing fecal calprotectin (FC) values for each age group. The line in the middle of the boxes represents the median FC. The bottom and top of the box indicate the 25th and 75th percentile. The lower and upper whiskers show the least and greatest value excluding outliers. The points and stars represent outliers and extreme outliers.
FC Levels In Different Age Groups of Healthy Children
FC is higher than in adults and decreases with age
The FC values obtained did not show a normal distribution, with higher values in children under 6 months old. The FC concentrations showed a negative trend from newborns (median FC 303 mcg/g, interquartile range [IQR] 202) to 6 to 11 months of age (median FC 63 mcg/g, IQR 126) followed by an increase in the group aged 12 to 23 months (median FC 97 mcg/g, IQR 275), and a further decrease with age, as shown in Figure 2.
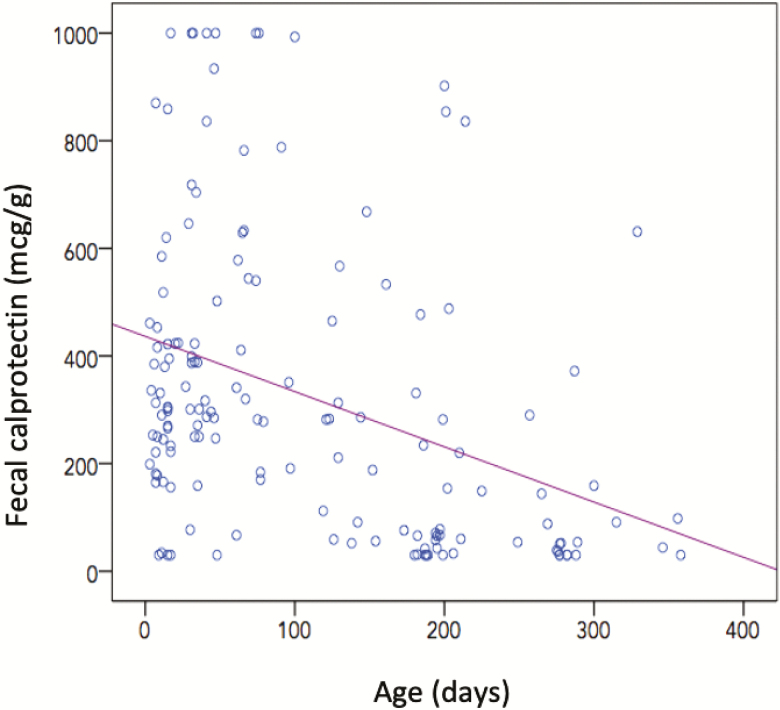
The correlation between age (days) and FC (mcg/g) was analyzed using the Spearman’s rho test, which yielded a coefficient of −0.603 (95% confidence interval [CI] −0.662 to −0.536) with a bilateral significance (P=0.00) and therefore suggested a negative linear relationship between both variables. Figure 3 shows the scatter plot in which this relationship is observed in those under 12 months of age.
Figure 3.
Scatter plot that show the negative linear relationship between age and fecal calprotectin levels in those subjects <12 months of age.
Correlation between sex, type of birth, gestational age or feeding, and levels of FC
FC is not different between sexes or influenced by type of birth, gestational age or feeding
Regarding the correlation with sex, male subjects had a median FC of 86.0 mcg/g (IQR 251) and female participants showed a median FC of 71.0 mcg/g (IQR 223). There were no significant differences between sexes with a bilateral significance (Mann–Whitney test 18,785.5, P=0.536).
The type of birth and gestational age was recorded in 391 volunteers (99% of the total sample). A total of 293 subjects (74.9%) were born by vaginal delivery and 98 subjects (25.1%) were born by Caesarean section. There were 344 (88.0%) term births (between 37 and 41 + 6 weeks of gestation), 34 (8.7%) preterm births (<37 weeks), and 13 (3%) post-term births (> 42 weeks). The mean gestational age was 38.7 weeks (95% CI of 38.4 to 39.0), with a median of 39 weeks. In the term birth group, median FC was 81.0 mcg/g (IQR 249). The birth weight was registered in 392 participants (99.2%). The mean was 3.2 kg (95% CI of 3.1 to 3.3 kg), and the median was 3.2 kg (minimum 750 gr, maximum 4.9 kg, range 4.2 kg).
There was no correlation between the type of delivery (Mann–Whitney test 14,080.0, P=0.773), weeks of gestation (Spearman’s rho test 0.016, P=0,748), or birth weight (Spearman’s rho test 0.047, P=0,356) and FC values in the whole sample nor in the youngest subgroups of patients (<1 month and 1 to 5 months of age).
The type of feeding received at the time of enrolment was analyzed in the group of infants younger than 6 months (N=107 subjects). These data were collected in 104 subjects of the group (97.2%). A total of 69 infants (66.4%) received exclusive breastfeeding (median FC 343 mcg/g, IQR 392), nine infants (8.7%) received mixed feeding (median FC 331 mcg/g, IQR 261), and 26 (25%) were formula-fed infants (median FC 274 mcg/g, IQR 210). There were no statistically significant differences between the 3 groups (Kruskal–Wallis test 4.083, P=0.130).
Correlation between weight, height, and BMI and levels of FC
Weight and BMI did not show a correlation with FC levels. Height showed a negative correlation with FC
Weight, height, and body mass index (BMI) were recorded in 383, 389, and 382 volunteers respectively. The correlation of these absolute values with FC levels was analyzed using the Spearman’s test. In all three cases, it showed a negative value with a bilateral significance (P=0.000), suggesting a negative correlation between each of the three variables and the FC. In order to determine the role of age as a possible confounding factor, we also analyzed the correlation between the sex and age-specific Z-scores for the anthropometric data. We found a negative correlation trend with no statistical significance between both weight SD (Spearman’s test −0.067, P=0.190) and BMI SD (Spearman’s test −0.029, P=0.569) and FC levels. Nevertheless, we found a negative correlation between height SD (Spearman’s test −0.1, P=0.015) and FC.
Finally, we performed a post-hoc multivariate analysis to further control the variable age. This analysis showed no correlation of weight and BMI with FC. However, it confirmed a negative correlation between height and FC.
DISCUSSION
To the best of our knowledge, this is the first study which describes the FC in a population of healthy children of all ages. We found higher FC concentrations than those considered as the upper reference in healthy adults (50 mcg/g) (17,34). This was particularly clear in healthy children under 1 month and from 1 to 6 months of age, whose mean FC values (303 mcg/g and 325.5 mcg/g, respectively) were higher to those described in IBD patients. In fact, a recent meta-analysis showed that FC values of 250 mcg/g could distinguish adult IBD patients in remission compared with active disease with a sensitivity and specificity of 80 and 82%, respectively (35). Although there is no ideal paediatric cut-off value to reflect mucosal inflammation and predict disease outcome in paediatric IBD patients, 250 mcg/g has also been suggested in the evidence-based guidelines (36). Interestingly, our FC values showed a clear negative correlation with age.
Since the FC could potentially be used by clinicians to make relevant management decisions such as the need to perform other diagnostic tests or refer to a gastroenterologist (37,38), the availability of trustworthy reference values becomes highly desirable. In addition, a better understanding of the factors other than bowel inflammation that affect FC levels would help to make a more accurate interpretation of this surrogate marker. Our study found no correlation between sex or perinatal data and FC levels in healthy infants.
The negative correlation found with weight and BMI was not confirmed by the post-hoc multivariate analysis. This probably indicates that age acts as a confounding factor since it is associated with anthropometric data. However, height showed a negative correlation with FC levels in the post-hoc analysis. This finding has not been previously described and would need to be analyzed in further studies.
The influence of the type of feeding was only studied in those participants under 6 months of age. We found no statistically significant differences between the three groups (exclusive breastfeeding, mixed feeding and formula-fed infants). However, the fact that the median FC in mixed feeding infants (331 mcg/g, IQR 261) was in between the breast (343 mcg/g, IQR 392) and formula-fed ones (274 mcg/g, IQR 210) suggests a possible dose–response. Additional specifically designed studies with a larger sample of infants should help to analyze this effect.
In contrast, and as our principal finding, we observed a negative correlation of FC with age. High FC values have been previously described, especially in infants (39). The ‘immune theory’ has been the most accepted hypothesis concerning this difference with adults. The FC may reflect an increased transepithelial trafficking of neutrophils in a young gut, also influenced by the intestinal colonization process or dietary diversification. The role of infant nutrition has been previously analyzed. The FC is reported to be lower in breast-fed infants than formula-fed infants, even after weaning began. The main hypothesis for this finding is that breastfeeding plays a role in the development of the gut immune system (40,41). However, some other studies do not support these results, and show higher levels in exclusively breast-fed infants (42–44).
Based on our data, we are unable to confirm this ‘immune theory’, but some of our results could possibly be explained in this way. In our group of children under 6 months, the FC pattern was not significantly influenced by the type of feeding. Interestingly, the FC concentrations showed a negative trend from newborns to 11 months of age followed by an increase in the group aged 12 to 23 months. This peak could have several explanations. This age group (12 to 23 months) is exposed to significant environmental and nutritional changes, including increased contact with other children and increased exposition to viral and bacterial infections. Moreover, this is when the toddler’s nutritional patterns become more varied and similar to an adult’s diet. It is known that all these factors affect the development of the gut immune system and the establishment of a normal bacterial colonization (27). However, the lack of dietary patterns data of the subjects over 6 months of age bounds the ability of our study to explore this possibility. Further studies are necessary to confirm this hypothesis.
There are several limitations to our study. We made a cross-sectional study, so the FC levels were not dynamically monitored. Also, the laboratory test used had a FC detection range between 30 and 1,000 mcg/g (34), the levels above 1,000 mcg/g could not be precisely determined and were analyzed as 1,000 mcg/g. This could imply that the real FC mean and median values were higher than those presented here. However, only eight cases over the total sample suffered from this ceiling effect; so, our main results should not be particularly affected. Additionally, it is impossible to guarantee that the participant children were completely healthy since no other examinations, such as blood tests, upper or lower gastrointestinal endoscopy were performed to exclude any significant gastrointestinal condition. However, performing any of these invasive tests to asymptomatic volunteer children would have been ethically questionable. Nevertheless, all the children with FC concentrations > 50 mcg/g received a follow-up phone call, and none of them reported any significant gastrointestinal symptoms. Finally, our study was developed in a potentially monocultural and socioeconomically homogeneous setting. These factors can have an influence through environmental exposures, diet, etc. and limit the extrapolation to other populations. Similar studies should be replicated by other study groups in order to clarify the role of these variables.
In conclusion, FC values in healthy children were higher than those that are considered as altered in adults and showed a negative correlation with age. The reference levels currently used should be particularly questioned in younger children, whose higher FC levels are possibly related to increased mucosal permeability and immature adaptive immunity in infancy. Based on this, it is necessary to reassess the levels of FC considered as upper limits in children by age group and to further study other factors that could influence FC levels in infancy. The data we present could be useful when evaluating FC concentrations in paediatric patients and could help to better interpret the FC levels obtained.
Acknowledgements
We would like to express our sincerest appreciation to the many people—both from the participating Primary Health Care Centres and from the Medical Laboratory Department (Hospital Infantil Universirtario Niño Jesús, Madrid)—who have so generously contributed by donating their time, resources, and precious energy to participate in the project.
Funding: There are no funders to report for this submission.
Potential Conflicts of Interest: The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Institution where the work originated: Hospital Infantil Universitario Niño Jesús, Madrid, Spain.
Ethics boards that approved the study: Ethics and Clinical Research Committee of Hospital Infantil Universitario Niño Jesús (code R-0063/14) and Central Research Commission of the Madrid Health Service (code 05/15).
References
- 1. Deeke SA, Starr AE, Ning Z, et al. Mucosal–luminal interface proteomics reveals biomarkers of pediatric inflammatory bowel disease-associated colitis. Am J Gastroenterol 2018;113(5): 713–24. [DOI] [PubMed] [Google Scholar]
- 2. Acevedo D, Salvador MP, Girbes J, Estan N. Fecal calprotectin: A comparison of two commercial enzymoimmunoassays and study of fecal extract stability at room temperature. J Clin Med Res 2018;10(5):396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caccaro R, D’Incá R, Sturniolo GC. Clinical utility of calprotectin and lactoferrin as markers of inflammation in patients with inflammatory bowel disease. Expert Rev Clin Immunol 2010;6(4):551–8. [DOI] [PubMed] [Google Scholar]
- 4. Carroccio A, Iacono G, Cottone M, et al. Diagnostic accuracy of fecal calprotectin assay in distinguishing organic causes of chronic diarrhea from irritable bowel syndrome: A prospective study in adults and children. Clin Chem 2003;49(6 Pt 1):861–7. [DOI] [PubMed] [Google Scholar]
- 5. Joishy M, Davies I, Ahmed M, et al. Fecal calprotectin and lactoferrin as noninvasive markers of pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2009;48(1):48–54. [DOI] [PubMed] [Google Scholar]
- 6. Pang T, Leach ST, Katz T, Day AS, Ooi CY. Fecal biomarkers of intestinal health and disease in children. Front Pediatr 2014;2(January):1–12. Available from: http://journal.frontiersin.org/article/10.3389/fped.2014.00006/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gisbert JP, McNicholl AG, Gomollon F. Questions and answers on the role of fecal lactoferrin as a biological marker in inflammatory bowel disease. Inflamm Bowel Dis 2009;15(11):1746–54. [DOI] [PubMed] [Google Scholar]
- 8. Van de Vijver E, Schreuder AB, Cnossen WR, Muller Kobold AC, van Rheenen PF, North Netherlands Pediatric IBD Consortium. Safely ruling out inflammatory bowel disease in children and teenagers without referral for endoscopy. Arch Dis Child 2012;97(12):1014–8. [DOI] [PubMed] [Google Scholar]
- 9. van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: Diagnostic meta-analysis. Bmj 2010;341:c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holtman GA, Lisman-van Leeuwen Y, Day AS, et al. Use of laboratory markers in addition to symptoms for diagnosis of inflammatory bowel disease in children: A meta-analysis of individual patient data. JAMA Pediatr 2017;171(10):984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh H, Ee L. Recurrent abdominal pain in children: Is colonoscopy indicated? J Pediatr Gastroenterol Nutr 2019;68(2):214–17. [DOI] [PubMed] [Google Scholar]
- 12. Heida A, Park KT, van Rheenen PF. Clinical utility of fecal calprotectin monitoring in asymptomatic patients with inflammatory bowel disease: A systematic review and practical guide. Inflamm Bowel Dis 2017;23(6):894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ertekin V, Selimoğlu MA, Turgut A, Bakan N. Fecal calprotectin concentration in celiac disease. J Clin Gastroenterol 2010;44(8):544–6. [DOI] [PubMed] [Google Scholar]
- 14. Vaos G, Kostakis ID, Zavras N, Chatzemichael A. The role of calprotectin in pediatric disease. Biomed Res Int 2013;2013:542363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rumman N, Sultan M, El-Chammas K, et al. Calprotectin in cystic fibrosis. BMC Pediatr 2014;14:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Josefsson S, Bunn SK, Domellof M. Fecal calprotectin in very low birth weight infants. J Pediatr Gastroenterol Nutr 2007;44(4; ): 407–13. [DOI] [PubMed] [Google Scholar]
- 17. Campeotto F, Butel MJ, Kalach N, et al. High faecal calprotectin concentrations in newborn infants. Arch Dis Child Fetal Neonatal Ed 2004;89(4):F353–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kapel N, Campeotto F, Kalach N, Baldassare M, Butel MJ, Dupont C. Faecal calprotectin in term and preterm neonates. J Pediatr Gastroenterol Nutr 2010;51(5):542–7. [DOI] [PubMed] [Google Scholar]
- 19. Mendall MA, Chan D, Patel R, Kumar D. Faecal calprotectin: Factors affecting levels and its potential role as a surrogate marker for risk of development of Crohn’s disease. BMC Gastroenterol 2016;16(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grondona A, Silvestri T, Deleonardi G, et al. Evaluation of fecal calprotectin levels in children aged between 1 and 12 months. Dig Liver Dis 2014;46:e122. [Google Scholar]
- 21. Zhu Q, Li F, Wang J, Shen L, Sheng X. Fecal calprotectin in healthy children aged 1–4 years. PLoS One 2016;11(3):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakayuenyongsuk W, Christofferson M, Stevenson DK, Sylvester K, Lee HC, Park KT. Point-of-care fecal calprotectin monitoring in preterm infants at risk for necrotizing enterocolitis. J Pediatr 2018;196:98–103.e1. [DOI] [PubMed] [Google Scholar]
- 23. Zoppelli L, Güttel C, Bittrich HJ, Andrée C, Wirth S, Jenke A. Fecal calprotectin concentrations in premature infants have a lower limit and show postnatal and gestational age dependence. Neonatology 2012;102(1):68–74. [DOI] [PubMed] [Google Scholar]
- 24. Zhang M, Zhang X, Zhang J. Diagnostic value of fecal calprotectin in preterm infants with necrotizing enterocolitis. Clin Lab 2016;62(5):863–9. [DOI] [PubMed] [Google Scholar]
- 25. Oord T, Hornung N. Fecal calprotectin in healthy children. Scand J Clin Lab Invest 2014;74(3):254–8. [DOI] [PubMed] [Google Scholar]
- 26. Hestvik E, Tumwine JK, Tylleskar T, et al. Faecal calprotectin concentrations in apparently healthy children aged 0-12 years in urban Kampala, Uganda: A community-based survey. BMC Pediatr 2011;11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rugtveit J, Fagerhol MK. Age-dependent variations in fecal calprotectin concentrations in children. J Pediatr Gastroenterol Nutr 2002;34(3):323–4; author reply 324–5. [DOI] [PubMed] [Google Scholar]
- 28. Herrera OR, Christensen ML, Helms RA. Review article calprotectin : Clinical applications in pediatrics. J Pediatr Pharmacol Ther 2016;21(4):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Savino F, Castagno E, Calabrese R, Viola S, Oggero R, Miniero R. High faecal calprotectin levels in healthy, exclusively breast-fed infants. Neonatology 2010;97(4):299–304. [DOI] [PubMed] [Google Scholar]
- 30. Davidson F, Lock RJ. Paediatric reference ranges for faecal calprotectin : A UK study. Ann Clin Biochem 2017;54(2):214–218. [DOI] [PubMed] [Google Scholar]
- 31. Fagerberg UL, Lööf L, Merzoug RD, Hansson LO, Finkel Y. Fecal calprotectin levels in healthy children studied with an improved assay. J Pediatr Gastroenterol Nutr 2003;37(4):468–72. [DOI] [PubMed] [Google Scholar]
- 32. Roca M, Rodriguez Varela A, Donat E, et al. Fecal calprotectin and eosinophil-derived neurotoxin in healthy children between 0 and 12 years. J Pediatr Gastroenterol Nutr 2017;65(4):394–8. [DOI] [PubMed] [Google Scholar]
- 33. Kittanakom S, Shajib MS, Garvie K, et al. Comparison of fecal calprotectin methods for predicting relapse of pediatric inflammatory bowel disease. Can J Gastroenterol Hepatol 2017;2017:1450970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. NICE. National Institute for Health and Care Excellence. Faecal calprotectin diagnostic tests for inflammatory diseases of the bowel. Diagnostics guidance. 2013. Available from: https://www.nice.org.uk/guidance/dg11 [Google Scholar]
- 35. Lin JF, Chen JM, Zuo JH, et al. Meta-analysis: Fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis 2014;20(8):1407–15. [DOI] [PubMed] [Google Scholar]
- 36. Turner D, Ruemmele FM, Orlanski-Meyer E, et al. Management of paediatric ulcerative colitis, Part 1: Ambulatory care- an evidence-based guideline from ECCO and ESPGHAN. J Pediatr Gastroenterol Nutr 2018;67(2):257–91. Available from: https://journals.lww.com/jpgn/fulltext/2018/08000/Management_of_Paediatric_Ulcerative_Colitis,_Part.24.aspx#pdf-link [DOI] [PubMed] [Google Scholar]
- 37. Akobeng A. Clinical usefulness of the faecal calprotectin test in suspected paediatric inflammatory bowel disease. Acta Paediatr 2018;107(11):2019–2023. [DOI] [PubMed] [Google Scholar]
- 38. Fagerberg UL, Lööf L, Myrdal U, Hansson LO, Finkel Y. Colorectal inflammation is well predicted by fecal calprotectin in children with gastrointestinal symptoms. J Pediatr Gastroenterol Nutr 2005;40(4):450–5. [DOI] [PubMed] [Google Scholar]
- 39. Olafsdottir E, Aksnes L, Fluge G, Berstad A. Faecal calprotectin levels in infants with infantile colic, healthy infants, children with inflammatory bowel disease, children with recurrent abdominal pain and healthy children. Acta Paediatr 2002;91(1):45–50. [DOI] [PubMed] [Google Scholar]
- 40. Golden B, Bunn S, Main M. Age-Dependent variations in fecal calprotectin concentrations in children. J Pediatr Gastroenterol Nutr. 2002;34(3):324 Available from: https://journals.lww.com/jpgn/Fulltext/2002/03000/Age_Dependent_Variations_in_Fecal_Calprotectin.23.aspx [DOI] [PubMed] [Google Scholar]
- 41. Cacho NT, Lawrence RM, Bliss JM, Cacho NT. Innate immunity and breast milk. Front Immunol. 2017;8:8–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dorosko SM, Mackenzie T, Connor RI. Fecal calprotectin concentrations are higher in exclusively breastfed infants compared to those who are mixed-fed. Breastfeed Med 2008;3(2):117–9. [DOI] [PubMed] [Google Scholar]
- 43. Asgarshirazi M, Shariat M, Nayeri F, Dalili H, Abdollahi A. Comparison of fecal calprotectin in exclusively breastfed and formula or mixed fed infants in the first six months of life. Acta Med Iran. 2017;55(1):53–8. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L620842203%0A 10.5812/compreped.59992 [DOI] [PubMed] [Google Scholar]
- 44. Li F, Ma J, Geng S, Wang J, Ren F, Sheng X. Comparison of the different kinds of feeding on the level of fecal calprotectin. Early Hum Dev 2014;90(9):471–5. [DOI] [PubMed] [Google Scholar]