Abstract
Background
Vaso-occlusive crisis (VOC) is one of the most frequent causes of emergency visits and admission in children with sickle cell disease (SCD).
Objectives
This study aims to evaluate whether the use of a new pain management pathway using intranasal (IN) fentanyl from triage leads to improved care, translated by a decrease in time to first opiate dose.
Methods
We performed a retrospective chart review of patients with SCD who presented to the emergency department (ED) with VOC, in the period pre- (52 patients) and post- (44 patients) implementation period of the protocol. Time to first opiate was the primary outcome and was evaluated pre- and postimplementation. Patients received a first opiate dose within 52.3 minutes of registration (interquantile range [IQR] 30.6, 74.6), corresponding to a 41.4-minute reduction in the opiate administration time (95% confidence interval [CI] −56.1, −27.9). There was also a 43% increase in the number of patients treated with a nonintravenous (IV) opiate as first opiate dose (95% CI 26, 57). In patients who were discharged from the ED, there was a 49% decrease in the number of IV line insertions (95% CI −67, −22). There was no difference in the hospitalization rates (difference of 6 [95% CI −13, 25]).
Conclusions
This study validates the use of our protocol using IN fentanyl as first treatment of VOC in the ED by significantly reducing the time to first opiate dose and the number of IVs.
Keywords: Hemoglobinopathies, Paediatric hematology, Pain, Sickle cell disease
Sickle cell vaso-occlusive crisis (VOC) is the most frequent cause of emergency room visits and hospitalizations in children with sickle cell disease (SCD) (1–3). Although SCD mortality in children has significantly decreased over the past 20 years (4), VOC is still associated with significant morbidity (5–7).
Many treatment protocols (8–10) are currently used for the treatment of VOCs. Several quality of care indicators have also been proposed. For example, the Canadian consensus statement encourages a rapid evaluation of pain, using a graded pain scale, and the administration of tailored pain medications which should be given as soon as possible in order to achieve adequate pain control (11). Adjunct therapies should also be initiated if not already done at home or on a chronic basis (12). Disposition of the patient should be decided within 2 to 8 hours (13). The 2014 NIH recommendations state that pain management should include parenteral opioids for severe pain, guided by an individualized or an institutional SCD-specific protocol. Moreover, multiple protocols only use the oral (PO) or intranasal (IN) routes as a bridge to IV medications while others have IV medications as standard therapy for all (8).
In 2012, a set of paediatric hematologists, paediatric emergency physicians, paediatricians, pharmacists, and nurses with a dedicated interest in the care of SCD pain crisis started a group named ‘DrepaNoPain’ at our centre. This group reviewed the treatment protocols for VOC management in SCD patients and proposed a new standardized preprinted order based upon a review of recent literature. This new standardized preprinted order favoured the use of a non-IV opiate as first intervention for acute pain episodes. A recent study demonstrated that the use of this standardized protocol at our centre led to a reduction in hospitalization rates, an increase in the use of PO opiates as first-line pain medication along with a nonsteroidal anti-inflammatory drug and a significant reduction in the need to use IV therapies (14). However, this initial protocol failed to improve the time to first opiate administration, which fell outside of the recommended 30-minute window from triage and 1 hour from registration (15).
Recently, the IN route has gained interest for the administration of medications (16). The dense nasal mucosal vascularization renders transmucosal absorption rapid and effective (17). Lipid soluble drugs such as fentanyl are especially well absorbed (18). Fentanyl, a selective opioid mu receptor agonist, has an 80- to 100-fold potency compared with morphine (19). Its onset of action via intranasal route is rapid, with onset of action in less than 5 minutes and reaching a maximal effect at 15 minutes. Side effects and safety profile are comparable to those of morphine, but duration of action is much shorter (about 60 minutes) (20,21). The use of IN fentanyl has been studied in paediatric emergency medicine with interesting results for pain management of musculoskeletal pathologies in children (22–29). Its ease of use was also of great interest using a mucosal atomization device (30). Moreover, avoiding painful and unpleasant IV insertion in children is also an undeniable advantage for patients and for nursing staff alike.
In the last 4 years, our emergency department (ED) has successfully been using IN fentanyl with a standardized prewritten order for pain associated with musculoskeletal injuries and certain invasive procedures such as burn debridement. Both physicians and nursing staff have gained more experience with IN fentanyl. More importantly, nurses are willing to use this medication promptly due to its numerous advantages such as rapid onset of action, robust pain reduction and ease of use (30). Some centres in the USA, as well as certain staff in our ED have also started using IN fentanyl for the treatment of sickle cell VOC (8,31). Studies have demonstrated that its use at triage could reduce time to first opiate dose, lead to faster pain relief, and even reduce hospitalization rates (8,31). However, its use does not appear to reduce the need for securing IV access. Given these advantages, the DrepaNoPain group chose to introduce the use of IN Fentanyl into our pain management pathway as quicker first-line treatment.
We hypothesized that the use of a VOC management protocol using IN fentanyl from triage would lead to improved care of SCD, translated by a decrease in time to the first opiate dose. Moreover, we aimed to evaluate if this new strategy would result in a reduced need for IV procedures and therapies, lower hospitalization rates, shorten the length of stay in the ED, and lastly, decrease return visits.
METHODS
Study design
This is a single-centre retrospective study of patients with SCD seen in the ED for VOC, following the implementation of a new VOC management protocol. The institution’s ethics review board approved the study.
Study setting and population
The study includes patients with SCD who presented to the ED with VOC requiring treatment in the period pre- (January to June 2014) and post- (January to June 2016) implementation of the new protocol including a standardized preprinted order and treatment algorithm, which was initiated in July 2015. Dates were chosen outside of the implementation period (summer 2015) to correctly assess both pre and post periods (washout period). The algorithm implied the use of pain scales for pain evaluation pre and post opiate doses, using the Evendol (32) scale for children under the age of 4 years old, and the Oucher (33,34) scale for children aged 4 years and older.
The setting is an urban, tertiary paediatric academic centre in Montreal with more than 80,000 ED and 6,500 hematology outpatient clinic visits per year. As of June 2016, a cohort of 340 patients with SCD was regularly followed by the hematology service, through a dedicated SCD program. Patients can be seen at our hematology day centre during opening hours on weekdays. All patients seen urgently for VOC in the ED were included in the study. Patients who presented with both fever and VOC were excluded from our study, as these patients are treated with IV antibiotics. Patients were included if they were between the ages of 2 and 18 years old. Exclusion criteria were presence of acute chest syndrome, as well as contraindications to IN fentanyl including known allergy, acute or chronic nasal problems (e.g., acute epistaxis, rhinitis treated with vasoconstrictive medications), hemodynamic instability, associated head trauma, or altered level of consciousness.
Study protocol
All identified charts from ED databases were evaluated by a data abstractor and a paediatric emergency resident who was not blinded to the study objectives. A structured chart review was used to abstract all data from the ED medical record, including the following outcome variables: time of registration, time of triage, time of physician assessment, time of discharge from the ED, time and route of administration of the first opiate dose, as well as all subsequent doses, and disposition of the patient. Time to the first opiate dose was calculated by subtracting the time of first opiate dose from the time of registration. It is important to note that all patients with SCD and pain are triaged in our ED as Canadian Triage Acuity Scale (CTAS) category 2 (35), giving them the highest priority outside of patients brought to the crash room with life-threatening conditions. The chart reviewer, a paediatric emergency resident, was formally trained by one of the study authors. A kappa score of ≥0.80 was needed for data abstraction for the first 10% of charts.
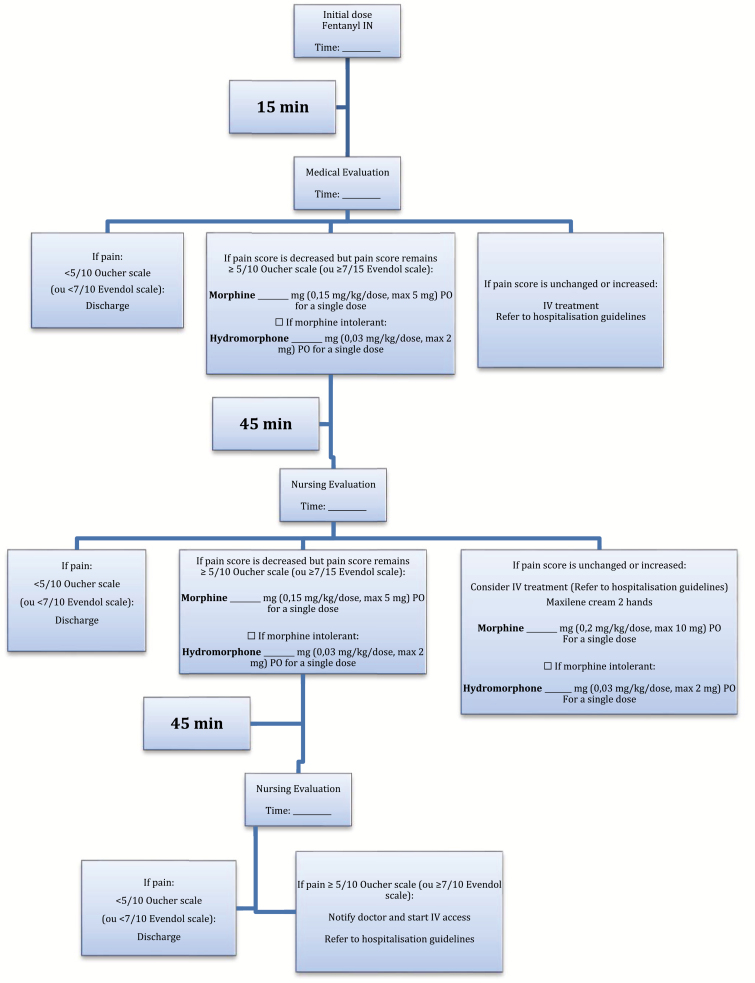
Our new protocol employs a dose of 2 mcg/kg/dose of IN Fentanyl (maximum 100 mcg/dose), and subsequent doses of 0.3 mg/kg/dose of PO morphine (maximum 15 mg/dose) or 0.1 mg/kg/dose of IV morphine (maximum 10 mg/dose). The dosages used in our protocol were in line with current recommendations and equivalent protocols in other centres. Hydromorphone was the substitute drug if the patient was known to be morphine intolerant. As stated in our algorithm, for a patient to receive opiates, he/she must report pain ≥ 5/10 on the Oucher pain scale, or ≥ 7/15 on the Evendol scale. When assessing a patient meeting these criteria, the triage nurse only administers the IN fentanyl after having asked the attending physician to sign the protocol standing order. The physician may decide otherwise as per the clinical presentation. If not received at home, a dose of PO acetaminophen and ibuprofen are also given. Application of lidocaine liposomal cream on both hands was considered for possible securing of IV access. Following the first opiate dose, patients were treated with subsequent opiate doses upon reassessment if their pain remained over the above-mentioned threshold for each scale. Subsequent doses were given orally if the patients’ pain had improved, but were given IV if their pain had remained unchanged or had increased. Pre- and postprotocol implementation admission criteria were identical, and included IN and/or oral treatment failure, need for IV opiates, chest pain, tachypnea, and neurological symptoms (Figure 1).
Figure 1.
Guideline algorithm for treatment of VOC in the ED.
Other variables abstracted included demographics (age, sex, sickle cell phenotype), and use of hydroxyurea. Return to ED or outpatient clinic at 72 hours was also noted, either for ongoing VOC or a scheduled follow-up appointment. If a patient visited the ED multiple times during the study period, each visit was recorded as a new event and analyzed separately.
Data analysis
Information was recorded on an excel data spreadsheet. Each variable’s normality distribution was tested using the D’Agostino-Pearson test using Med-Calc (v 13.1.2). Proportions were compared by chi-square, and medians were compared by the Mann-Whitney test using SPSS version 20. Confidence intervals for the difference were reported. A P value of <0.05 was defined as significant.
Sample size calculation
We estimated that 50 visits per arm would be sufficiently powered to detect at least a 40% increase in patients meeting quality of care indicators, with a power of 80% and a significance of 0.05.
RESULTS
A total of 96 visits of patients with VOC without fever were seen in the ED during the study period and all were included in our study: 52 visits preprotocol implementation (pre) and 44 visits postprotocol implementation (post). While we do note that our sample size was not reached in the postprotocol period after removing patients who presented with both fever and VOC, we did reach statistical significance. Individual patient characteristics are presented by period in Table 1 and ED visit characteristics are presented in Table 2.
Table 1.
Patient characteristics per study period
| Period pre | Period post | |
|---|---|---|
| Number of visits | 52 | 44 |
| Number of patients | 36 | 37 |
| Age, years (IQR) | 9 (5, 12.5) | 10.5 (7.6, 14.1) |
| Male, n (%) | 28 (54) | 21 (48) |
| Phenotype, n (%) | ||
| SS | 35 (67) | 27 (61) |
| SC | 15 (29) | 14 (32) |
| SB°Thal | 1 (2) | 2 (5) |
| SB+Thal | 1 (2) | 1 (2) |
| Hydroxyurea, n (%) | 22 (42) | 30 (68) |
| Opiates received, n (%) | 44 (85) | 43 (98) |
Data presented represent individual patients and are organized by study period.
IQR: Interquantile range n Number; SS Hemoglobin SS; SC Hemoglobin SC; SB°Thal Hemoglobin SB°Thal; SB+Thal Hemoglobin SB+Thal.
Table 2.
ED visit characteristics
| Period pre | Period post | |
|---|---|---|
| Time reg. to triage (min) | 13.5 (8, 19) | 16.8 (9.9, 21.6) |
| Time triage to MD (min) | 14.5 (8, 27) | 11.8 (6.7, 24) |
| Time MD to first dose (min) | 63.5 (41, 93) | 20.4 (4, 37.4) |
| Time reg. to first dose (min) | 94.5 (70.5, 121.5) | 52.3 (30.6, 74.6) |
| LOS (min) | 281 (213.5, 351) | 302 (196, 429) |
Data presented represent individual ED visits and are organized by study periods.
All reported times represent the median ± IQR.
ED Emergency department; LOS Length of stay; MD Medical doctor; Reg Registration to emergency department.
Time to first opiate dose was 94.5 minutes (70.5, 121.5) in pre and 52.3 minutes (30.6, 74.6) in post. Our new pathway using IN Fentanyl therefore led to a significant difference of −41.4 minutes (95% CI −56.1, −27.9) in the opiate administration time. 38.5% of the patients received an opiate within the recommended 60 minutes in pre compared to 61.4% in post, a per cent increase of 59%. The use of our protocol also significantly decreased the overall number of IV treatments. Furthermore, and more importantly, the percentage of patients discharged home without having had an IV line placed was markedly increased (Table 3). There were less avoidable IV insertions in post, with only 4 of 44 (9%) patients with IVs inserted despite not receiving IV opiates, compared with 17 of 52 (33%) in pre. A total of 48 of 52 (92%) patients received co-analgesia (ibuprofen, acetaminophen) in pre compared with 41 of 44 (93%) in post. The protocol using IN fentanyl at triage also increased the use of pain scales at triage evaluation and increased the use of non-IV opiates as the first opiate dose (Table 3). In the postperiod, median pain score at triage evaluation was 8 (6.5, 9), at first reassessment 5 (4, 7.3) and at second reassessment, it was 5 (3, 7), with no statistically significant differences.
Table 3.
Comparatives results for study objectives
| Period pre (52 visits) |
Period post (44 visits) |
Δ (95% CI) | |
|---|---|---|---|
| Hospitalization rates, n (%) | 25 (48) | 24 (54.5) | 6 (−13, 25) |
| Non-IV opiate for first opiate dose, n (%) | 26 (50) | 41 (93) | 43 (26, 57) |
| Time reg. to first opiate dose, min (IQR) | 94.5 (70.5, 121.5) | 52.3 (30.6, 74.6) | −41.4 (−56.1, −27.9) |
| Patients treated in <60 min, n (%) | 20 (38.5) | 27 (61.4) | 22.9 (2.9, 40.5) |
| Number of patients with no IV inserted, n (%) | 12 (23) | 19 (43) | 20 (1, 37) |
| Number of discharged patients with IV inserted, n (% of discharged patients) | 16 (59) | 2 (10) | −49 (−67, −22) |
| Pain scale use pre-first opiate dose, n (%) | 28 (54) | 41 (93) | 39 (22, 53) |
| Return to ER within 72 h, n (%) | 3 (6) | 5 (11) | 6 (−6, 19) |
| Discharged patients, n (%) | 27 (52) | 20 (45.5) | −6.5 (−25.4, 13.2) |
Data presented represent individual patients and are organized by study period.
IQR Interquantile range; IV Intravenous; reg Registration.
There was no difference in the hospitalization rate and return visits, with 3 of 27 (11%) of the patients discharged in pre returning within 72 hours of VOC, compared with 5 of 20 (25%) in the postimplementation period, a difference of 13.9 (95% CI −8.0, 36.9). Of those, two of three patients finally required hospitalizations in pre, as opposed to five of five in post (Table 3). We had no significant adverse events following the administration of IN fentanyl.
DISCUSSION
Our study demonstrates an improvement in the care of patients with SCD presenting to the ED with VOC as demonstrated by a significant decrease in the time to first opiate dose, now meeting quality of care indicators. Indeed, we were able to show that using IN fentanyl at triage as part of a VOC treatment protocol is possible and led to a shorter lag in opiate administration. This is supported by a study by Ender et al. showing that the use of a clinical pathway improved management of sickle cell VOC in the ED, by decreasing the time interval to first analgesic (36). While we acknowledge that the NIH recommendations of 15 to 20 minutes to first analgesic may be very difficult to achieve in our ED, we thought that the introduction of our protocol would allow for more rapid administration of opiates. A more realistic quality of care indicator for treatment of VOC was suggested by Wang et al., recommending administration of an opiate within no longer than 30 minutes from triage or 1 hour from registration (15). Our protocol led to the administration of opiate (IN Fentanyl) at a median time of 52 minutes from registration, and also increased the percentage of patients receiving their first opiate dose within the recommended time.
Our previous protocol had already shown that PO medications and therapies may be sufficient in a subset of patients with VOC, and led to decreased rates of hospitalization (14). Treatment goals had indeed shifted from pain eradication to pain control, which allowed for those whose pain was stable or significantly improved on PO medications to be treated as outpatients. With the addition of IN fentanyl at triage, our current pathway now addresses these patients’ pain more rapidly and robustly. A similar decrease in opiate administration time has been demonstrated in a mixed adult and paediatric ED, although the study in question did not comment on subsequent opiate doses and IV use (18). The most likely potential explanation for a faster administration of IN fentanyl compared to PO morphine comes from the enthusiasm of nurses with the use of this medication, who as per their feedback, see its rapid effect and thus believe in its efficacy, as well as appreciate that it can be given without an IV. Furthermore, the fact that the new protocol mandates a pain score at triage reinforces the need for rapid administration of pain medications, all of which was encouraged by nursing supervisors who were responsible for training nurses to use the protocol. While opiate administration time was faster, this however did not lead to a decrease in the hospitalization rate.
Following the implementation of our new protocol, pain scores pre- and postopiate administration were increasingly recorded, showing that a standardized protocol increased the use of pain scales. Indeed, since pain scales were required to follow the treatment algorithm of our protocol, an increase in pain score documentation was not only seen at first evaluation, but also at each re-evaluation. We were not able to compare differences in pain scores at each reassessment given the low number of recorded pain scores in the pre period. Our study encourages us to continue our efforts to promote the use of pain scales and adequately document them, which will allow us to study the impact of protocols targeting pain management and contribute to better pain management for patients in our centre.
Since 2014, our protocol has favoured the use of non-IV opiate administration for pain control. Our first study using oral morphine clearly demonstrated that it could significantly reduce the percentage of hospitalizations. The use of IN Fentanyl also favours an avoidance of the IV route in a significant percentage of patients. A study by Jacobson, cited in the Cochrane review on pain management for SCD, showed no significant difference between PO and IV morphine in the mean overall pain scores, frequency of rescue analgesia, and of adverse effects (37,38). Favouring PO versus IV opiates was also found to decrease the admission rates (39). Given IN fentanyl’s high potency, rapid onset of action and avoidance of the IV route, we favoured the use of alternative routes for opiate administration to provide rapid analgesia for these patients.
We did find an overall decrease in IV line placements and we also showed a decreased number of IV therapies in patients who were discharged from the ED. This is of particular importance in patients with SCD who frequently require blood tests and IV therapies, possibly leading to chronic pain and difficult IV placement secondary to scarring (40). Our better understanding of their pain and our growing comfort with non-IV opiates allowed for a significant number of patients to be treated without the use of IV medications and IV hydration, through achieving adequate pain control. Whenever possible and guided by the physician’s judgement, our experience clearly demonstrates that a non-IV opiate is an appropriate choice.
Limitations of our study include the use of a retrospective chart review. Specific to our study, we found that recording of opiate route and time of administration was very well documented and present for all our patients. Moreover, time of registration, triage, MD assessment, and time of discharge from ED are all electronically entered through our ED system, which made data abstraction efficient and reliable. Also, the lack of consistent data on home opiate intake prior to visit did not allow us to analyze its effect on pain treatment in the ED and need for hospitalization. We did note that our sample size did not meet the 50 stated patients in each arm after removing patients who presented with both fever and VOC. However, given that we found a statistically significant difference, the sample size is of less importance. The role of access to the hematology day centre was also not assessed as a possible factor in decreasing hospitalization rates, although the capacity of the hematology clinic, including its opening hours and the composition of the hematology team did not change over both periods. Regarding delays in therapy, we were also unable to measure the contribution of other factors such as concomitant high acuity cases, ED overcrowding or opiate distribution delays by pharmacy in prolonging the time to initial opiate.
CONCLUSIONS
This study validates the use of our new protocol using IN fentanyl as first treatment of VOC in the ED by significantly reducing the time to first opiate dose. Our protocol did not decrease the hospitalization rate but it did decrease the total number of painful IV procedures as well as the number of unnecessary IV insertions in the subgroup of patients who could be discharged home. Having an algorithm that mandates a pain score also increased the use of pain scales at triage and at reassessment.
Compliance with Ethical Standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants (or their caregivers) included in the study.
Potential Conflicts of Interest: All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Source of support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The CHU Ste-Justine ethics board approved the study.
Contributors’ Statements: All authors conceptualized and designed the study. HP designed the data collection instruments, coordinated and supervised data collection, and drafted the initial manuscript. BB carried out the statistical analyses. All authors reviewed and revised the manuscript, and approved the final manuscript as submitted.
References
- 1. Panepinto JA, Brousseau DC, Hillery CA, Scott JP. Variation in hospitalizations and hospital length of stay in children with vaso-occlusive crises in sickle cell disease. Pediatr Blood Cancer 2005;44(2):182–6. [DOI] [PubMed] [Google Scholar]
- 2. Steinberg MH, Forget BG, Higgs DR, Weatherall DJ.. Disorders of hemoglobin: genetics, pathophysiology, and clinical management. 2nd ed Cambridge, UK: Cambridge University Press, 2011. 1268 pages. [Google Scholar]
- 3. Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood 2010;115(22):4331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999-2009). Pediatr Blood Cancer 2013;60(9):1482–6. [DOI] [PubMed] [Google Scholar]
- 5. McClish DK, Penberthy LT, Bovbjerg VE, et al. Health related quality of life in sickle cell patients: The PiSCES project. Health Qual Life Outcomes 2005; 3:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood 2010;115(17):3447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wright J, Ahmedzai SH. The management of painful crisis in sickle cell disease. Curr Opin Support Palliat Care 2010;4(2):97–106. [DOI] [PubMed] [Google Scholar]
- 8. Kavanagh PL, Sprinz PG, Wolfgang TL, et al. Improving the management of vaso-occlusive episodes in the pediatric emergency department. Pediatrics 2015;136(4):e1016–25. [DOI] [PubMed] [Google Scholar]
- 9. Yang YM, Shah AK, Watson M, Mankad VN. Comparison of costs to the health sector of comprehensive and episodic health care for sickle cell disease patients. Public Health Rep 1995;110(1):80–6. [PMC free article] [PubMed] [Google Scholar]
- 10. Zempsky WT, Loiselle KA, McKay K, et al. Retrospective evaluation of pain assessment and treatment for acute vasoocclusive episodes in children with sickle cell disease. Pediatr Blood Cancer 2008;51(2):265–8. [DOI] [PubMed] [Google Scholar]
- 11. Canadian Haemoglobinopathy Association. Consensus Statement on the Care of Patients with Sickle Cell Disease in Canada. [Internet] 2015. <http://canhaem.org/the-consensus-statement-on-the-care-of-patients-with-sickle-cell-disease-in-canada/> (Accessed April 2015).
- 12. Ender KL, Krajewski JA, Babineau J, et al. Use of a clinical pathway to improve the acute management of vaso-occlusive crisis pain in pediatric sickle cell disease. Pediatr Blood Cancer 2014;61(4):693–6. [DOI] [PubMed] [Google Scholar]
- 13. Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312(10):1033–48. [DOI] [PubMed] [Google Scholar]
- 14. Paquin H, Trottier ED, Robitaille N, Pastore Y, Dore Bergeron M-J, Bailey B. Oral morphine protocol evaluation for the treatment of vaso-occlusive crisis in paediatric sickle cell patients Paediatr Child Health. 2018. doi:10.1093/pch/pxy074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang CJ, Kavanagh PL, Little AA, Holliman JB, Sprinz PG. Quality-of-care indicators for children with sickle cell disease. Pediatrics 2011;128(3): 484–93. [DOI] [PubMed] [Google Scholar]
- 16. Grassin-Delyle S, Buenestado A, Naline E, et al. Intranasal drug delivery: An efficient and non-invasive route for systemic administration: Focus on opioids. Pharmacol Ther 2012;134(3):366–79. [DOI] [PubMed] [Google Scholar]
- 17. Wolfe TR, Braude DA. Intranasal medication delivery for children: A brief review and update. Pediatrics 2010;126(3):532–7. [DOI] [PubMed] [Google Scholar]
- 18. Holdgate A, Cao A, Lo KM. The implementation of intranasal fentanyl for children in a mixed adult and pediatric emergency department reduces time to analgesic administration. Acad Emerg Med 2010;17(2):214–7. [DOI] [PubMed] [Google Scholar]
- 19. Sahyoun C, Krauss B. Clinical implications of pharmacokinetics and pharmacodynamics of procedural sedation agents in children. Curr Opin Pediatr 2012;24(2):225–32. [DOI] [PubMed] [Google Scholar]
- 20. Fleischman RJ, Frazer DG, Daya M, Jui J, Newgard CD. Effectiveness and safety of fentanyl compared with morphine for out-of-hospital analgesia. Prehosp Emerg Care 2010;14(2):167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nave R, Schmitt H, Popper L. Faster absorption and higher systemic bioavailability of intranasal fentanyl spray compared to oral transmucosal fentanyl citrate in healthy subjects. Drug Deliv 2013;20(5):216–23. [DOI] [PubMed] [Google Scholar]
- 22. Mudd S. Intranasal fentanyl for pain management in children: A systematic review of the literature. J Pediatr Health Care 2011;25(5):316–22. [DOI] [PubMed] [Google Scholar]
- 23. Murphy A, O’Sullivan R, Wakai A, Grant TS, Barrett MJ, Cronin J, et al. Intranasal fentanyl for the management of acute pain in children. Eur J Emerg Med 2017;24( 6):450–4. [DOI] [PubMed] [Google Scholar]
- 24. Borland ML, Clark LJ, Esson A. Comparative review of the clinical use of intranasal fentanyl versus morphine in a paediatric emergency department. Emerg Med Australas 2008;20(6):515–20. [DOI] [PubMed] [Google Scholar]
- 25. Christrup LL, Foster D, Popper LD, Troen T, Upton R. Pharmacokinetics, efficacy, and tolerability of fentanyl following intranasal versus intravenous administration in adults undergoing third-molar extraction: A randomized, double-blind, double-dummy, two-way, crossover study. Clin Ther 2008;30(3):469–81. [DOI] [PubMed] [Google Scholar]
- 26. Crellin D, Ling RX, Babl FE. Does the standard intravenous solution of fentanyl (50 µg/mL) administered intranasally have analgesic efficacy? Emerg Med Australas 2010;22(1):62–7. [DOI] [PubMed] [Google Scholar]
- 27. Hansen MS, Mathiesen O, Trautner S, Dahl JB. Intranasal fentanyl in the treatment of acute pain–a systematic review. Acta Anaesthesiol Scand 2012;56(4):407–19. [DOI] [PubMed] [Google Scholar]
- 28. Saunders M, Adelgais K, Nelson D. Use of intranasal fentanyl for the relief of pediatric orthopedic trauma pain. Acad Emerg Med 2010;17(11):1155–61. [DOI] [PubMed] [Google Scholar]
- 29. Shelley K, Paech MJ. The clinical applications of intranasal opioids. Curr Drug Deliv 2008;5(1):55–8. [DOI] [PubMed] [Google Scholar]
- 30. Rech MA, Barbas B, Chaney W, Greenhalgh E, Turck C. When to pick the nose: Out-of-hospital and emergency department intranasal administration of medications. Ann Emerg Med 2017;70(2):203–11. [DOI] [PubMed] [Google Scholar]
- 31. Fein DM, Avner JR, Scharbach K, Manwani D, Khine H. Intranasal fentanyl for initial treatment of vaso-occlusive crisis in sickle cell disease. Pediatr Blood Cancer 2017;64(6). [DOI] [PubMed] [Google Scholar]
- 32. Fournier-Charrière E, Tourniaire B, Carbajal R, et al. EVENDOL, a new behavioral pain scale for children ages 0 to 7 years in the emergency department: Design and validation. Pain 2012;153(8):1573–82. [DOI] [PubMed] [Google Scholar]
- 33. Beyer JE, Denyes MJ, Villarruel AM. The creation, validation, and continuing development of the oucher: A measure of pain intensity in children. J Pediatr Nurs 1992;7(5):335–46. [PubMed] [Google Scholar]
- 34. Tomlinson D, von Baeyer CL, Stinson JN, Sung L. A systematic review of faces scales for the self-report of pain intensity in children. Pediatrics 2010;126(5):e1168–98. [DOI] [PubMed] [Google Scholar]
- 35. Gravel J, Gouin S, Goldman RD, et al. The Canadian triage and acuity scale for children: A prospective multicenter evaluation. Ann Emerg Med 2012;60(1):71–7.e3. [DOI] [PubMed] [Google Scholar]
- 36. Baker M, Hafner JW. What is the best pharmacologic treatment for sickle cell disease pain crises? Ann Emerg Med 2012;59(6):515–6. [DOI] [PubMed] [Google Scholar]
- 37. Dunlop RJ, Bennett KCLB. Pain management for sickle cell disease. Cochrane Database Syst Rev 2006;( 2):CD003350. [DOI] [PubMed] [Google Scholar]
- 38. Jacobson SJ, Kopecky EA, Joshi P, Babul N. Randomised trial of oral morphine for painful episodes of sickle-cell disease in children. Lancet 1997;350(9088):1358–61. [DOI] [PubMed] [Google Scholar]
- 39. Campos J, Lobo C, Queiroz AM, et al. Treatment of the acute sickle cell vaso-occlusive crisis in the emergency department: A Brazilian method of switching from intravenous to oral morphine. Eur J Haematol 2014;93(1):34–40. [DOI] [PubMed] [Google Scholar]
- 40. Telfer P, Bahal N, Lo A, Challands J. Management of the acute painful crisis in sickle cell disease- a re-evaluation of the use of opioids in adult patients. Br J Haematol 2014;166(2):157–64. [DOI] [PubMed] [Google Scholar]