Abstract
Aims
We sought to perform a head-to-head comparison of contemporary 30-day outcomes and readmissions between valve-in-valve transcatheter aortic valve replacement (VIV-TAVR) patients and a matched cohort of high-risk reoperative surgical aortic valve replacement (re-SAVR) patients using a large, multicentre, national database.
Methods and results
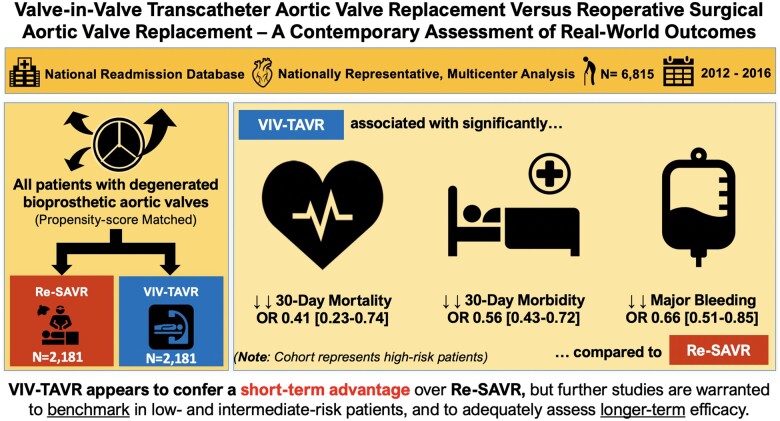
We utilized the nationally weighted 2012–16 National Readmission Database claims to identify all US adult patients with degenerated bioprosthetic aortic valves who underwent either VIV-TAVR (n = 3443) or isolated re-SAVR (n = 3372). Thirty-day outcomes were compared using multivariate analysis and propensity score matching (1:1). Unadjusted, VIV-TAVR patients had significantly lower 30-day mortality (2.7% vs. 5.0%), 30-day morbidity (66.4% vs. 79%), and rates of major bleeding (35.8% vs. 50%). On multivariable analysis, re-SAVR was a significant risk factor for both 30-day mortality [adjusted odds ratio (aOR) of VIV-SAVR (vs. re-SAVR) 0.48, 95% confidence interval (CI) 0.28–0.81] and 30-day morbidity [aOR for VIV-TAVR (vs. re-SAVR) 0.54, 95% CI 0.43–0.68]. After matching (n = 2181 matched pairs), VIV-TAVR was associated with lower odds of 30-day mortality (OR 0.41, 95% CI 0.23–0.74), 30-day morbidity (OR 0.53, 95% CI 0.43–0.72), and major bleeding (OR 0.66, 95% CI 0.51–0.85). Valve-in-valve TAVR was also associated with shorter length of stay (median savings of 2 days, 95% CI 1.3–2.7) and higher odds of routine home discharges (OR 2.11, 95% CI 1.61–2.78) compared to re-SAVR.
Conclusion
In this large, nationwide study of matched high-risk patients with degenerated bioprosthetic aortic valves, VIV-TAVR appears to confer an advantage over re-SAVR in terms of 30-day mortality, morbidity, and bleeding complications. Further studies are warranted to benchmark in low- and intermediate-risk patients and to adequately assess longer-term efficacy.
Keywords: Valve-in-valve TAVR, Reoperative surgical aortic valve replacement, Failed bioprostheses
See page 2756 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa441)
Introduction
Over the last decade, we have witnessed a momentum shift in the utilization of transcatheter aortic valve replacement (TAVR) among patients with symptomatic aortic stenosis to commensurate with the US Food and Drug Administration approval starting with high- and extreme-risk patients to the most recent low-risk patients.1–5 Technical refinements in device technologies and improved patient selection via the use of multidisciplinary structural heart teams have further contributed towards improved patient outcomes.6 , 7 These changes have brought a substantial interest in valve-in-valve TAVR (VIV-TAVR) for patients with degenerated aortic bioprostheses. Conventional reoperative surgical aortic valve replacement (re-SAVR) is regarded as the gold standard approach for these patients because long-term outcomes are well-established.8–10 On the other hand, a number of studies have demonstrated the feasibility and safety of VIV-TAVR in appropriately selected patients.9 , 11–14 Mid-term outcomes have further demonstrated improved haemodynamic status and excellent functional outcomes.15 , 16
These reports lead to the FDA approval of VIV-TAVR in March of 2015. The use of VIV-TAVR is increasing, and this increasing trend can be attributed to its less invasive nature, which is more appealing to patients, and given that re-SAVR has a reported operative mortality ranging from 4% to as high as 9%,8 , 10 and high overall morbidity, such as stroke, vascular complications, and permanent pacemaker implantation.8 , 10 Although head-to-head assessment of contemporary outcomes between the two procedures have been previously reported, they are limited by either their small sample sizes or single-centre design.9 , 11 , 12 , 17 Understanding contemporary outcomes of VIV-TAVR vs. re-SAVR are key for several reasons. First, to provide us with a framework to establish comparative benchmarks to help in the design of future clinical trials, and second, to provide real-world data to help in decision-making, patient counselling, and risk-stratification. Because VIV-TAVR is only approved as the alternative therapy as opposed to re-SAVR in patients who are at high risk for complications related to reoperation, in this large, multicentre, nationwide study, we perform a head-to-head comparison of 30-day outcomes and readmissions between VIV-TAVR patients and a comparable cohort of high-risk re-SAVR patients.
Methods
Data source
This population-based, nationally representative study retrospectively analysed the National Readmissions Database (NRD). This is a unique and powerful database to allow for a national assessment of hospital inpatient stays and readmissions among patients of all ages and across all payer types inclusive of private and government insurance and the uninsured. While the NRD contains verified patient identifiers to track individuals across hospital admissions within and across a state’s hospitals,18 this database contains completely de-identified data (i.e. no social security numbers or patient-specific identifiers) using unique patient keys that are tracked by the state. The NRD is drawn from the Agency for Healthcare Research and Quality’s (AHRQ) state inpatient databases and contains data from approximately 17 million discharges each year, representing 36 million discharges when weighted to yield national estimates of inpatient stays. This NRD is closely mandated and managed by AHRQ and is a collaborative effort between state data organizations, hospital associations, private data organizations, and the federal government. National weights are provided by AHRQ to account for available data derived from individual state inpatient claims. Because the NRD is a publicly available deidentified database, this study was exempt from review by our Institutional Review Board.
Patient selection
We utilized nationally weighted 2012–16 NRD claims to identify all US adult patients aged ≥18 years with degenerated bioprosthetic aortic valves who underwent either VIV-TAVR or isolated re-SAVR. We utilized the following International Classification of Disease, Clinical Modifications codes to isolate patients with failed or degenerated bioprostheses: ICD-9-CM (424.1 and 996.02) and ICD-10 codes (I35.x and either T82.01XA or T82.02XA or T82.03XA or T82.09XA or T82.221A or T82.222A or T82.223A or T82.228A or Z45.09 or Z95.2 or T82.857A). Respective codes for TAVR and SAVR are highlighted in Supplementary material online, Table e1. Patients with endocarditis, concomitant percutaneous coronary intervention or coronary artery bypass grafting (CABG), or other valve surgery were excluded to ensure clinically comparable groups. Patients were also excluded if they underwent both re-SAVR and VIV-TAVR in same hospitalization or if there were missing data on sex or mortality during hospitalization. Among otherwise eligible patients, zero was missing information on sex and only n = 3 were missing information on mortality.
Variables and outcomes of interest
We utilized relevant ICD-9-CM and ICD-10-CM codes to identify patient baseline characteristics, in-hospital procedures, and outcomes (Supplementary material online, Table e1). The primary outcomes were 30-day mortality, 30-day readmissions, and 30-day morbidity which was defined as a composite outcome of pneumonia, pulmonary embolism, renal failure, cerebrovascular accident, myocardial infarction, cardiac arrest, adult respiratory distress syndrome, sepsis, and septic shock.19 Secondary outcomes included post-operative complications [stroke, renal failure, permanent pacemaker placement (PPM), complete heart block, and major bleeding], hospitalization length of stay (LOS), routine home discharges, and total index hospitalization costs. Costs were obtained from reported total hospital charges and converted to costs using cost: charge ratios and adjusted for inflation to be reported in 2019 USD. Causes of readmissions were classified as cardiac (e.g. heart failure, arrhythmias/conduction disorders) and non-cardiac (e.g. respiratory, infectious, bleeding, trauma). Routine home discharge was defined as any discharge to home following the index admission. Relevant ICD codes are highlighted in Supplementary material online, Table e2. Potential confounders calculated/abstracted from the data included age gender, dyslipidaemia, hypertension, diabetes mellitus, peripheral vascular disease, cerebrovascular disease, stroke/transient ischaemic attack (TIA), chronic obstructive pulmonary disorder, chronic kidney disease without dialysis, coronary artery disease, atrial fibrillation, previous myocardial infarction, congestive heart failure, prior percutaneous coronary intervention, and prior CABG.
Statistical analysis
Patient characteristics and comorbidities between both groups were first compared prior to matching using descriptive statistics: one-way analysis of variance for normally distributed continuous variables and χ2 tests for categorical variables (Table 1). Differences in outcomes were compared using unadjusted logistic regression for dichotomous outcomes (mortality, readmission, complications, discharge destination) and quantile/median regression for non-normally distributed LOS and total index hospital costs in 2019 USD. The latter approach was chosen since LOS and cost are inherently skewed precluding the ability to run linear regression. Ln-transforming does not resolve the issue and while more complicated methods (gamma or ln-binomial modelling) can be used, quantile regression (aka median regression) is an efficient and understandable means of modelling non-normal continuous data. Odds ratios (ORs) are presented with 95% confidence intervals (CIs) (median differences and 95% CI for quantile regression).
Table 1.
Baseline patient characteristics and comorbidities before and after propensity-score matching between re-SAVR and VIV-TAVR
| Before matching |
After matching |
|||||||
|---|---|---|---|---|---|---|---|---|
| Re-SAVR (N = 3372) | VIV-TAVR (N = 3443) | P-value | Std. diff. | Re-SAVR (N = 2181) | VIV-TAVR (N = 2181) | P-value | Std. diff. | |
| Age (years), mean ± SD | 70.0 ± 14.0 | 75.2 ± 11.8 | <0.001 | 0.397 | 72.9 ± 12.2 | 72.5 ± 12.0 | 0.316 | −0.042 |
| Age (years), median (IQR) | 72 (62–80) | 78 (69–84) | — | — | 75 (66–82) | 74 (66–81) | — | — |
| Female | 1271 (37.7%) | 1446 (42.0%) | 0.075 | 0.076 | 833 (38.2%) | 848 (38.9%) | 0.709 | 0.016 |
| Comorbidities | ||||||||
| Dyslipidaemia | 1888 (56.0%) | 2069 (60.1%) | 0.064 | 0.096 | 1244 (57.0%) | 1265 (58.0%) | 0.641 | 0.020 |
| Hypertension | 1875 (55.6%) | 1901 (55.2%) | 0.869 | −0.019 | 1214 (55.7%) | 1228 (56.3%) | 0.761 | 0.013 |
| Diabetes mellitus | 867 (25.7%) | 854 (24.8%) | 0.682 | −0.043 | 548 (25.1%) | 604 (27.7%) | 0.161 | 0.059 |
| Peripheral vascular disease | 958 (28.4%) | 895 (26.0%) | 0.260 | −0.039 | 575 (26.4%) | 560 (25.7%) | 0.714 | −0.015 |
| Cerebrovascular disease | 152 (4.5%) | 96 (2.8%) | 0.067 | −0.057 | 86 (3.9%) | 77 (3.5%) | 0.615 | −0.021 |
| Stroke/transient ischaemic attack | 263 (7.8%) | 234 (6.8%) | 0.372 | −0.017 | 156 (7.2%) | 140 (6.4%) | 0.506 | −0.028 |
| COPD | 985 (29.2%) | 1078 (31.3%) | 0.339 | 0.064 | 674 (30.9%) | 683 (31.3%) | 0.833 | 0.009 |
| CKD without dialysis | 900 (26.7%) | 1157 (33.6%) | 0.008 | −0.080 | 135 (6.2%) | 135 (6.2%) | 0.991 | 0.001 |
| Coronary artery disease | 236 (7.0%) | 182 (5.3%) | 0.115 | −0.144 | 770 (35.3%) | 743 (34.1%) | 0.540 | −0.026 |
| Atrial fibrillation | 1197 (35.5%) | 967 (28.1%) | 0.002 | 0.158 | 655 (30.0%) | 645 (29.6%) | 0.812 | −0.010 |
| Previous myocardial infarction | 287 (8.5%) | 382 (11.1%) | 0.066 | 0.109 | 205 (9.4%) | 231 (10.6%) | 0.339 | 0.040 |
| Congestive heart failure | 1976 (58.6%) | 2579 (74.9%) | <0.001 | 0.367 | 1458 (66.9%) | 1471 (67.4%) | 0.767 | 0.012 |
| Prior PCI | 175 (5.2%) | 155 (4.5%) | 0.525 | −0.008 | 115 (5.3%) | 119 (5.5%) | 0.834 | 0.009 |
| Prior CABG | 486 (14.4%) | 799 (23.2%) | <0.001 | 0.246 | 361 (16.5%) | 337 (15.5%) | 0.482 | −0.030 |
Two-sided P-values were taken from χ2 tests for categorical variables, and one-way ANOVA for age. Boldface values denote statistical significance.
1:1 propensity score matching was performed with an allowed caliber on the ln-odds of 0.005 (logistic model) and no replacement over common support (overlapping P-scores in ln-odds range).
CABG, coronary artery bypass grafting; CKD, chronic kidney disease; IQR, interquartile range; PCI, percutaneous coronary intervention; SD, standard deviation.
In order to address potential confounding related to the study outcomes and treatment assignment, differences in outcomes were further assessed using risk-adjusted logistic/quantile regression and 1:1 propensity score matching. Multivariable logistic regression (quantile/median regression for LOS and cost) models were risk-adjusted for: age (continuous), gender, dyslipidaemia, hypertension, diabetes mellitus, peripheral vascular disease, cerebrovascular disease, stroke/TIA, chronic obstructive pulmonary disorder, chronic kidney disease without dialysis, coronary artery disease, atrial fibrillation, previous myocardial infarction, congestive heart failure, prior percutaneous coronary intervention, and prior CABG. 1:1 matching was performed without replacement using the same variables with an allowable caliber for the underlying logistic regression model of a 0.005 unit difference in the propensity score ln-odds between neighbouring pairs. All matches were required to be ‘on support’ falling within the overlap of both groups’ baseline distributions prior to matching. Distributions of scores (Supplementary material online, Figure e1) and potential confounders (Table 1) were virtually identical after the match (Hosmer–Lemeshow for the underlying logistic model assuming 10 groups: χ2 = 23.65, P = 0.003).
Factors that were present on primary admission that were associated with 30-day morbidity after each procedure were also examined using multivariable logistic regression. Additional statistical analysis was limited to 30-day morbidity given power constraints on 30-day mortality and 30-day readmission events, which occurred less commonly. To account for changes in coding from ICD-9 to ICD-10 from October 2015, the learning curve as well as changes in device technology, we performed an additional sensitivity analysis using a contemporary cohort of patients based who were isolated based on ICD-10 codes only (unadjusted and multivariable regression results are reported). Survey data analysis tools were utilized to generate weighted national estimates and variances that accounted for design weights in NRD and clustering of patients within hospitals; all data were analysed using robust standard errors. Variables with cell sizes <10 were not reported given NRD reporting guidelines. In total, less than 0.5% of eligible patients were removed, combining any missing of ≥1 variable together. All analyses were conducted using STATA Version 16.0 (StataCorp LP, College Station, TX, USA) with two-sided P-values <0.05 as the criterion for significance. The study was reported in accordance with the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) recommendations, and its checklist is included in the Supplementary material online.
Results
Baseline patient characteristics in the overall cohort
Patient selection is outlined in the study flow diagram (Figure 1). The final cohort comprised of 6815 procedures (3443 VIV-TAVR and 3372 re-SAVR). Distributions of demographic parameters before matching are presented in Table 1. Before matching, the baseline mean ages in the VIV-TAVR and re-SAVR groups were 75.2 and 70 years, respectively (P < 0.05). Patients between the ages of 75–84 years accounted for the majority of patients (Supplementary material online, Table e3). While the VIV-TAVR cohort included more patients with chronic kidney disease (33.6% vs. 26.7%), congestive heart failure (74.9% vs. 58.6%), and prior CABG (23.2% vs. 14.4%; all P < 0.001).
Figure 1.
Study flow diagram.
Unadjusted outcomes in the overall cohort
Compared to re-SAVR patients, VIV-TAVR patients had significantly lower 30-day mortality (2.8% vs. 5.0%; OR 0.55, 95% CI 0.33–1.91), 30-day morbidity (66.4% vs. 79%; OR 0.52, 95% CI 0.41–0.66), and rates of major bleeding complications (35.8% vs. 49.9%; OR 0.56, 95% CI 0.44–0.71; Table 2). Valve-in-valve TAVR patients also had shorter hospital LOS (7 vs. 9 days, mean savings of 2 days, 95% CI 1.4–2.6) and were more likely to be discharged to home (45.9% vs. 34.2%; OR 1.57, 95% CI 1.28–1.93). However, there were no significant differences in the rates of post-operative stroke, renal failure, PPM, and complete heart block, as well as total index hospitalization costs between the two procedures (all P > 0.05). The 30-day readmission rates were similar between VIV-TAVR and re-SAVR (10.6% vs. 10.5%; OR 0.99, 95% CI 0.75–1.32), and the majority of the readmissions were largely due to non-cardiac causes (75.7% and 75.3%, respectively; Supplementary material online, Table e4).
Table 2.
Bivariate comparison in-hospital outcomes between re-SAVR and VIV-TAVR
| Outcomes | Bivariate comparison |
||
|---|---|---|---|
| Re-SAVR | VIV-TAVR | P-value | |
| 30-day mortality | 5.0% | 2.8% | 0.018 |
| 30-day readmission | 10.6% | 10.5% | 0.959 |
| 30-day morbidity | 79.0% | 66.4% | <0.001 |
| In-hospital outcome(s) | |||
| Stoke/TIA | 0.9% | 0.6% | 0.487 |
| Renal failure | 22.7% | 20.7% | 0.341 |
| PPM placement | 8.5% | 10.9% | 0.089 |
| Complete heart block | 11.2% | 12.4% | 0.432 |
| Major bleeding | 49.9% | 35.8% | <0.001 |
| Routine discharge (home) | 34.2% | 45.9% | <0.001 |
| Length of stay (days), median (IQR) | 9 (5–17) | 7 (3–13) | <0.001 |
| Total index hospital costs (2019 USD), median (IQR) | 59 862 (44 649–83 274) | 58 997 (44 265–82 521) | 0.584 |
Two-sided P-values were taken from χ2 tests for categorical variables. Boldface values denote statistical significance.
Non-normally distributed continuous outcomes reported as median and interquartile range.
Two-sided P-values for continuous outcomes taken from comparison of median values using quantile regression.
TIA, transient ischaemic attack.
Multivariable regression analysis
On multivariable analysis, re-SAVR was a significant risk factor for both 30-day mortality [adjusted odds ratio (aOR) of VIV-SAVR (vs. re-SAVR) 0.48, 95% CI 0.28–0.81] and 30-day morbidity [aOR for VIV-TAVR (vs. re-SAVR) 0.54, 95% CI 0.43–0.68; Table 3]. In addition, VIV-TAVR patients had a 41% lower odds of major bleeding and 86% higher odds of routine home discharges. A similar finding was observed for hospital LOS with median savings of 2.4 days for VIV-TAVR patients. In our subgroup analysis, the presence of preoperative heart failure was more likely to be associated with higher 30-day morbidity among the re-SAVR cohort (Figure 2). Although not statistically significant, there also appeared to be a clinical trend towards higher 30-day morbidity in patients with advanced age (ages >84 years: aOR 3.09; 75–84 years: aOR 1.33; reference group: 65–74 years).
Table 3.
Comparison of unadjusted and risk-adjusted in-hospital outcomes using multivariable regression and propensity score matching between VIV-TAVR and re-SAVR
| Outcomes | Unadjusted (Ref: re-SAVR) |
Multivariable Regression |
Propensity-score matched |
|||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| 30-Day mortality | 0.55 | 0.33–1.91 | 0.48 | 0.28–0.81 | 0.41 | 0.23–0.74 |
| 30-Day readmission | 0.99 | 0.75–1.32 | 0.95 | 0.71–1.29 | 0.94 | 0.67–1.31 |
| 30-Day morbidity | 0.52 | 0.41–0.66 | 0.54 | 0.43–0.68 | 0.56 | 0.43–0.72 |
| In-hospital outcome(s) | ||||||
| Stoke/TIA | 0.71 | 0.26–1.89 | 1.08 | 0.31–3.78 | 1.25 | 0.43–3.64 |
| Renal failure | 0.89 | 0.70–1.13 | 0.80 | 0.61–1.05 | 0.79 | 0.58–1.07 |
| PPM placement | 1.33 | 0.95–1.83 | 1.29 | 0.93–1.80 | 1.13 | 0.76–1.69 |
| Complete heart block | 1.12 | 0.84–1.49 | 1.08 | 0.80–1.47 | 1.08 | 0.76–1.55 |
| Major bleeding | 0.56 | 0.44–0.71 | 0.59 | 0.47–0.75 | 0.66 | 0.51–0.85 |
| Routine discharge (to home) | 1.57 | 1.28–1.93 | 1.86 | 1.47–2.33 | 2.11 | 1.61–2.78 |
| Index Length of stay (days) | (median diff.) −2.0 | −2.6 to −1.4 | −2.4 | −3.0 to −1.7 | −2.0 | −2.7 to −1.3 |
| Total index hospital costs (2019 USD) | (median dif.) −962 | −4537 to 2613 | 2500 | −1161 to 6162 | −904 | −5118 to 3310 |
Results of unadjusted and multivariable regression are based on the entire cohort. There were a total of 2181 matched-pairs. Boldface values denote statistical significance.
Quantile/median regression was used to account for the non-normal nature of the continuous outcome data (LOS, cost).
Multivariable logistic regression (quantile/median regression for LOS and cost) models were risk-adjusted for: age (continuous), gender, dyslipidaemia, hypertension, diabetes mellitus, peripheral vascular disease, cerebrovascular disease, stroke/TIA, chronic obstructive pulmonary disorder, chronic kidney disease without dialysis, coronary artery disease, atrial fibrillation, previous myocardial infarction, congestive heart failure, prior percutaneous coronary intervention, and prior coronary artery bypass grafting.
1:1 propensity score matching was performed using the same variables with an allowed caliber on the ln-odds of 0.005 (logistic model) and no replacement over common support (overlapping p-scores in ln-odds range). This resulted in n = 1126 matched pairs (n = 2181 when nationally weighted).
Models were weighted to account for National Readmission Database sampling stratum, design weights, and clustering of patients within hospitals. They were analysed using robust standard errors.
TIA, transient ischaemic attack.
Figure 2.
Forest plot demonstrating the independent predictors of 30-day morbidity following re-surgical aortic valve replacement and valve-in-valve transcatheter aortic valve replacement.
Take home figure.
Visual Abstract summarizing key findings of this study.
Propensity-matched analysis
There are a total of 2181 matched pairs analysed. There were no significant differences in baseline characteristics between the two groups after matching (Table 1). Even after matching, our finds remained robust: VIV-TAVR was associated with lower odds of 30-day mortality (OR 0.41, 95% CI 0.23–0.74), 30-day morbidity (OR 0.53, 95% CI 0.43–0.72), and major bleeding (OR 0.66, 95% CI 0.51–0.85). Valve-in-valve TAVR was also associated with shorter LOS (median savings of 2 days, 95% CI 1.3–2.7) and higher odds of routine home discharges (OR 2.11, 95% CI 1.61–2.78) compared to re-SAVR (Table 3).
Sensitivity analysis
For our sensitivity analysis, we separately analysed a cohort of 4566 patients (2441 VIV-TAVR and 2125 re-SAVR) who were isolated based on ICD-10 coding only. Our overall findings persisted even after multivariable risk-adjustment. For instance, re-SAVR patients had a 59% higher odds of 30-day mortality, 63% higher odds of 30-day morbidity, and 71% higher odds of major bleeding (Supplementary material online, Table e5). Valve-in-valve TAVR patients had a 3.9-fold higher odds of routine home discharges.
Discussion
This large, nationally representative analysis of 2181 matched pairs, which is the largest series to date directly comparing VIV-TAVR and re-SAVR, has several key findings: first, we demonstrate that compared to re-SAVR patients, VIV-TAVR patients had significantly lower 30-day mortality, 30-day morbidity, and rates of major bleeding. Second, VIV-TAVR patients had shorter hospital LOS and increased the likelihood of home discharges but there were no significant differences in other post-operative complications as well as 30-day readmissions. Importantly, our findings remained robust in both the multivariate analysis and propensity-score matched analysis as well as in our sensitivity analysis which accounted for changes in ICD coding and reflected a more contemporary cohort.
In recent years, interest in VIV-TAVR has grown in light of the success of TAVR in native valves. According to a recent analysis of the The Society of Thoracic Surgeon's (STS)/American College of Cardiology (ACC) registry, the 30-day mortality rate was 2.9%.13 Previously reported 30-day mortality rates, such as those in the Global VIV registry were much higher (8.4%), likely explained by differences in patient characteristics and valve technologies.14 In our study, the overall VIV-TAVR 30-day mortality was 2.8%, in line with these studies. Likewise, Kaneko et al.8 reported the STS database outcomes in a series of 3380 re-SAVR patients from 2011 to 2013. The operative mortality was 4.6%. which is consistent with our findings of 5%. In terms of PPM, our series reported rates of 10.9% for VIV-TAVR and 8.5% for re-SAVR (P = 0.089), which are similar to previous studies.8 , 9 We anticipate that these rates will improve with further advances in procedural techniques, post-operative management, and patient selection.
Importantly, our study adds to the growing number of small comparative effectiveness studies of VIV-TAVR and re-SAVR, albeit with a larger cohort and a more nationally representative, multi-institutional sample.9 , 12 A recent meta-analysis of five observational studies (n = 342 patients) demonstrated no significant differences in procedural mortality (relative risk (RR) 0.74, 95% CI 0.18–2.97) and 30-day mortality (RR 1.29, 95% CI 0.44–3.78) although VIV-TAVR was associated with shorter intensive care unit stay and the hospital say (P = 0.02).12 Our study corroborates these findings by demonstrating significantly shorter hospital LOS (median savings of 2 days) with VIV-TAVR. In contrast, VIV-TAVR was associated with lower 30-day mortality (absolute difference of −2.1%; 59% lower odds) and 30-day morbidity (absolute difference of −13%, 44% lower odds). The lack of significance in the smaller studies is likely due to low power. The finding of higher bleeding rates with re-SAVR is not surprising since reoperative chest surgery is high risk in itself.
In lieu of a randomized control trial comparing VIV-TAVR and re-SAVR, this present head-to-head comparative study provides the best available benchmarking evidence on the current practices and favourable short-term outcomes with VIV-TAVR, in line with existing guideline recommendations.20 According to the 2017 European Society of Cardiology EACTS - European European Association for Cardio-Thoracic Surgery (ESC/EACTS) guidelines, the choice of intervention must be individualized based on careful assessment of clinical characteristics and anatomic/technical aspects by the multidisciplinary Heart Team (Class I).20 For instance, TAVR may be favourable in patients with prior cardiac surgery and those with severe comorbidities, whereas SAVR may be more suitable in patients with unfavourable anatomy. Furthermore, VIV-TAVR should be considered depending on the risk of reoperation and the type and size of prosthesis (Class IIa).20 However, this data must be interpreted with several cautions. The FDA approved the self-expandable Medtronic CoreValve (Medtronic, Inc., MN, USA) for VIV-TAVR in March 2015 and later the balloon-expandable Edwards Sapien XT (Edwards Lifesciences Ltd, Irvine, CA, USA) in October 2015, Since all the procedures done prior to these approvals were off-label procedures, they were likely done in high-risk patients. Hence, the findings of this matched study are only applicable to high-risk patients.
Importantly, this analysis does not include clinical and anatomical data on several issues that are closely linked to the clinical efficacy of VIV-TAVR. First, is the issue of clinical valve thrombosis and structural valve deterioration. Clinical valve thrombosis, defined as either a combination of new valve dysfunction and imaging evidence of leaflet thrombosis, is a significant complication following bioprosthetic valve implantation and is associated with increased risk of cerebrovascular complications.21 The incidence of this entity has previously been thought to be low, but increasing research in the TAVR era has shown that the rate of thrombus formation (in particular subclinical thrombus) has been underestimated.22 The issue of TAVR valve durability remains another central concern.23 While surgical bioprostheses have been extensively studied, long-term structural valve deterioration data in the TAVR and VIV-TAVR population is lacking. Further prospective research including echocardiographic follow-up is needed to comment on these above entities.
The second concern with VIV-TAVR is that of residual gradient and patient–prosthesis mismatch, which likely stems from the underexpansion of TAVR valve limited by the surgical rings.13 , 24 Bioprosthetic valve fracture with either a balloon-expandable or self-expanding valves has been described, however this is not performed routinely.25 The third and major concern for VIV-TAVR is that of coronary obstruction, and the risk of coronary obstruction markedly differs according to the type of initial bioprosthesis. The risk is markedly higher for VIV-TAVR in a stentless prosthesis or stented prosthesis with externally mounted leaflets.26 The BASILICA procedure may provide an effective and safe option in patients who are at high risk for coronary obstruction.27 Likewise, valve commissure alignment during initial TAVR deployment may help facilitate leaflet splitting and mitigate the risk of future coronary obstruction.28 This cannot be analysed in the present study due to the lack of information on the type of prosthesis but should be addressed in the discussion. Given the high mortality of coronary obstruction (around 50%), this complication is likely to influence the short-term outcome.14 The NRD does not provide information on the anatomy or the valve-type used, therefore, these questions cannot be answered. Larger series with detailed computed tomography information and prosthesis type is needed to answer these extremely important questions.
Other limitations of this study are the following: first, NRD is an administrative database and there is potential for miscoding, mis-coded events and missing observations within the database. However, the AHRQ has quality control measures to ensure best practices for coding, ensure linkages to state-level data are verified and reliable, and also ensure internal validation of diagnosis codes through continuous audits.18 The NRD lacks information on access type, echocardiographic variables, individual patient risk scores, medication use, type of anaesthesia, and post-procedural paravalvular leaks. Second, although matching was used to adjust for differences in baseline characteristics, it does not address anatomic bias in the study. ICD coding does not differentiate between previous TAVR or SAVR. Thus, given the nature of the database, the type, or size of initial prosthesis was not identifiable, and may create bias but we suspect the numbers for initial TAVR are likely small based on our clinical experience and lack of existing published data. In addition, we could not determine the timing of operation between biosprosthesis failure and subsequent procedure. Information on STS PROM scores was also not available. Causes of readmission were based on ICD coding and may be subject to misattribution. Nonetheless, NRD is robust in evaluating cardiac and non-cardiac causes and cost-analysis, as previously described.8 Finally, we could not examine mid-term and long-term outcomes in these patients. Likewise, the sampling design of NRD precludes robust and complete analysis of hospital-level procedure volume–outcome relationships in the context of VIV-TAVR. Both these aspects warrants an additional study.
Conclusion
In this large, nationwide study of matched high-risk patients with degenerated bioprosthetic aortic valves, VIV-TAVR appears to confer an advantage over re-SAVR in terms of 30-day mortality, morbidity, and bleeding complications. Further studies are warranted to benchmark in low- and intermediate-risk patients and to adequate assess longer-term efficacy.
Funding
C.Z. is supported by the National Institute of Health Medical Scientist Training Program Training [T32GM007205]. She is the Principal investigator of an F30 award through the National Institute on Aging F30AG066371 entitled ‘The ED.TRAUMA Study: Evaluating the Discordance of Trauma Readmission and Unanticipated Mortality in the Assessment of hospital quality’.
Conflict of interest: T.K. is a speaker for Edwards Life Sciences, Medtronic, Abbott, and Baylis Medical and is a consultant for 4C Medical. All others have declared no conflict of interest.
Supplementary Material
Contributor Information
Sameer A Hirji, Division of Cardiac Surgery, Brigham and Women’s Hospital, Harvard Medical School, 15 Francis Street, Boston, MA 02115, USA.
Edward D Percy, Division of Cardiac Surgery, Brigham and Women’s Hospital, Harvard Medical School, 15 Francis Street, Boston, MA 02115, USA.
Cheryl K Zogg, Yale School of Medicine, New Haven, 67 Cedar Street, New Haven, CT 06510, USA.
Alexandra Malarczyk, Division of Cardiac Surgery, Brigham and Women’s Hospital, Harvard Medical School, 15 Francis Street, Boston, MA 02115, USA.
Morgan T Harloff, Division of Cardiac Surgery, Brigham and Women’s Hospital, Harvard Medical School, 15 Francis Street, Boston, MA 02115, USA.
Farhang Yazdchi, Division of Cardiac Surgery, Brigham and Women’s Hospital, Harvard Medical School, 15 Francis Street, Boston, MA 02115, USA.
Tsuyoshi Kaneko, Division of Cardiac Surgery, Brigham and Women’s Hospital, Harvard Medical School, 15 Francis Street, Boston, MA 02115, USA.
References
- 1. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 2. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, Askew J, Sorajja P, Rovin J, Chetcuti SJ, Adams DH, Teirstein PS, Zorn GL 3rd, Forrest JK, Tchetche D, Resar J, Walton A, Piazza N, Ramlawi B, Robinson N, Petrossian G, Gleason TG, Oh JK, Boulware MJ, Qiao H, Mugglin AS, Reardon MJ; Evolut Low Risk Trial Investigators. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019;380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 3. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR; PARTNER 3 Investigators. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 4. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG; PARTNER 2 Investigators. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 5. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 6. Bavaria JE, Tommaso CL, Brindis RG, Carroll JD, Deeb GM, Feldman TE, Gleason TG, Horlick EM, Kavinsky CJ, Kumbhani DJ, Miller DC, Seals AA, Shahian DM, Shemin RJ, Sundt TM 3rd, Thourani VH. 2018 AATS/ACC/SCAI/STS expert consensus systems of care document: operator and institutional recommendations and requirements for transcatheter aortic valve replacement: a joint report of the American Association for Thoracic Surgery, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2019;73:340–374. [DOI] [PubMed] [Google Scholar]
- 7. Rezq A, Godino C, Montorfano M, Covello D, Colombo A. Comprehensive multidisciplinary patient assessment and selection before TAVI procedure. Minerva Cardioangiol 2014;62:177–191. [PubMed] [Google Scholar]
- 8. Kaneko T, Vassileva CM, Englum B, Kim S, Yammine M, Brennan M, Suri RM, Thourani VH, Jacobs JP, Aranki S. Contemporary outcomes of repeat aortic valve replacement: a benchmark for transcatheter valve-in-valve procedures. Ann Thorac Surg 2015;100:1298–1304; discussion 304. [DOI] [PubMed] [Google Scholar]
- 9. Ejiofor JI, Yammine M, Harloff MT, McGurk S, Muehlschlegel JD, Shekar PS, Cohn LH, Shah P, Kaneko T. Reoperative surgical aortic valve replacement versus transcatheter valve-in-valve replacement for degenerated bioprosthetic aortic valves. Ann Thorac Surg 2016;102:1452–1458. [DOI] [PubMed] [Google Scholar]
- 10. Kalra A, Raza S, Hussain M, Shorbaji K, Delozier S, Deo SV, Khera S, Kleiman NS, Reardon MJ, Kolte D, Gupta T, Mustafa R, Bhatt DL, Sabik JF. Aortic valve replacement in bioprosthetic failure: insights from the Society of Thoracic Surgeons National Database. Ann Thorac Surg 2019; doi:10.1016/j.athoracsur.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 11. Erlebach M, Wottke M, Deutsch MA, Krane M, Piazza N, Lange R, Bleiziffer S. Redo aortic valve surgery versus transcatheter valve-in-valve implantation for failing surgical bioprosthetic valves: consecutive patients in a single-center setting. J Thorac Dis 2015;7:1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gozdek M, Raffa GM, Suwalski P, Kołodziejczak M, Anisimowicz L, Kubica J, Navarese EP, Kowalewski M;SIRIO-TAVI group. Comparative performance of transcatheter aortic valve-in-valve implantation versus conventional surgical redo aortic valve replacement in patients with degenerated aortic valve bioprostheses: systematic review and meta-analysis. Eur J Cardiothorac Surg 2018;53:495–504. [DOI] [PubMed] [Google Scholar]
- 13. Tuzcu EM, Kapadia SR, Vemulapalli S, Carroll JD, Holmes DR Jr, Mack MJ, Thourani VH, Grover FL, Brennan JM, Suri RM, Dai D, Svensson LG. Transcatheter aortic valve replacement of failed surgically implanted bioprostheses: the STS/ACC registry. J Am Coll Cardiol 2018;72:370–382. [DOI] [PubMed] [Google Scholar]
- 14. Dvir D, Webb J, Brecker S, Bleiziffer S, Hildick-Smith D, Colombo A, Descoutures F, Hengstenberg C, Moat NE, Bekeredjian R, Napodano M, Testa L, Lefevre T, Guetta V, Nissen H, Hernandez JM, Roy D, Teles RC, Segev A, Dumonteil N, Fiorina C, Gotzmann M, Tchetche D, Abdel-Wahab M, De Marco F, Baumbach A, Laborde JC, Kornowski R. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation 2012;126:2335–2344. [DOI] [PubMed] [Google Scholar]
- 15. Webb JG, Murdoch DJ, Alu MC, Cheung A, Crowley A, Dvir D, Herrmann HC, Kodali SK, Leipsic J, Miller DC, Pibarot P, Suri RM, Wood D, Leon MB, Mack MJ. 3-Year outcomes after valve-in-valve transcatheter aortic valve replacement for degenerated bioprostheses: the PARTNER 2 registry. J Am Coll Cardiol 2019;73:2647–2655. [DOI] [PubMed] [Google Scholar]
- 16. Phan K, Zhao DF, Wang N, Huo YR, Di Eusanio M, Td Y. Transcatheter valve-in-valve implantation versus reoperative conventional aortic valve replacement: a systematic review. J Thorac Dis 2016;8:E83–E93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choi CH, Cheng V, Malaver D, Kon N, Kincaid EH, Gandhi SK, Applegate RJ, Zhao D. A comparison of valve-in-valve transcatheter aortic valve replacement in failed stentless versus stented surgical bioprosthetic aortic valves. Catheter Cardiovasc Interv 2019;93:1106–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). Introduction to the HCUP Nationwide Readmissions Database (NRD), 2013. 2015. https://www.hcup-us.ahrq.gov/db/nation/nrd/NRD_Introduction_2013.jsp (20 Jan 2020)
- 19. Zogg CK, Olufajo OA, Jiang W, Bystricky A, Scott JW, Shafi S, Havens JM, Salim A, Schoenfeld AJ, Haider AH. The need to consider longer-term outcomes of care: racial/ethnic disparities among adult and older adult emergency general surgery patients at 30, 90, and 180 days. Ann Surg 2017;266:66–75. [DOI] [PubMed] [Google Scholar]
- 20. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 21. Chakravarty T, Søndergaard L, Friedman J, De Backer O, Berman D, Kofoed KF, Jilaihawi H, Shiota T, Abramowitz Y, Jørgensen TH, Rami T, Israr S, Fontana G, de Knegt M, Fuchs A, Lyden P, Trento A, Bhatt DL, Leon MB, Makkar RR, Ramzy D, Cheng W, Siegel RJ, Thomson LM, Mangat G, Hariri B, Sawaya FJ, Iversen HK; RESOLVE; SAVORY Investigators. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet 2017;389:2383–2392. [DOI] [PubMed] [Google Scholar]
- 22. Abdel-Wahab M, Simonato M, Latib A, Goleski PJ, Allali A, Kaur J, Azadani AN, Horlick E, Testa L, Orvin K, Kornowski R, Kass M, Don CW, Richardt G, Webb JG, Dvir D. Clinical valve thrombosis after transcatheter aortic valve-in-valve implantation. Circ Cardiovasc Interv 2018;11:e006730. [DOI] [PubMed] [Google Scholar]
- 23. Sondergaard L, Ihlemann N, Capodanno D, Jorgensen TH, Nissen H, Kjeldsen BJ, Chang Y, Steinbruchel DA, Olsen PS, Petronio AS, Thyregod H. Durability of transcatheter and surgical bioprosthetic aortic valves in patients at lower surgical risk. J Am Coll Cardiol 2019;73:546–553. [DOI] [PubMed] [Google Scholar]
- 24. Ziccardi MR, Groves EM. Bioprosthetic valve fracture for valve-in-valve transcatheter aortic valve replacement: rationale, patient selection, technique, and outcomes. Interv Cardiol Clin 2019;8:373–382. [DOI] [PubMed] [Google Scholar]
- 25. Chhatriwalla AK, Allen KB, Saxon JT, Cohen DJ, Aggarwal S, Hart AJ, Baron SJ, Dvir D, Borkon AM. Bioprosthetic valve fracture improves the hemodynamic results of valve-in-valve transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2017;10:e005216. [DOI] [PubMed] [Google Scholar]
- 26. Ribeiro HB, Webb JG, Makkar RR, Cohen MG, Kapadia SR, Kodali S, Tamburino C, Barbanti M, Chakravarty T, Jilaihawi H, Paradis J-M, de Brito FS, Cánovas SJ, Cheema AN, de Jaegere PP, del Valle R, Chiam PTL, Moreno R, Pradas G, Ruel M, Salgado-Fernández J, Sarmento-Leite R, Toeg HD, Velianou JL, Zajarias A, Babaliaros V, Cura F, Dager AE, Manoharan G, Lerakis S, Pichard AD, Radhakrishnan S, Perin MA, Dumont E, Larose E, Pasian SG, Nombela-Franco L, Urena M, Tuzcu EM, Leon MB, Amat-Santos IJ, Leipsic J, Rodés-Cabau J. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol 2013;62:1552–1562. [DOI] [PubMed] [Google Scholar]
- 27. Khan JM, Greenbaum AB, Babaliaros VC, Rogers T, Eng MH, Paone G, Leshnower BG, Reisman M, Satler L, Waksman R, Chen MY, Stine AM, Tian X, Dvir D, Lederman RJ. The BASILICA trial: prospective multicenter investigation of intentional leaflet laceration to prevent TAVR coronary obstruction. JACC Cardiovasc Interv 2019;12:1240–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tang GHL, Zaid S, Gupta E, Ahmad H, Patel N, Khan M, Khan A, Kovacic JC, Lansman SL, Dangas GD, Sharma SK, Kini A. Impact of initial Evolut transcatheter aortic valve replacement deployment orientation on final valve orientation and coronary reaccess. Circ Cardiovasc Interv 2019;12:e008044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.