Abstract
Childhood maltreatment has long lasting impacts on neural development of the hippocampus, which is important for learning and memory. The present study aimed to assess the effects of a mindfulness based intervention on hippocampal morphometry and episodic memory in this population. We administered MRI, psychological questionnaires and an episodic memory task to 21 participants (5 males) before and after a mindfulness-based behavioral intervention, compared to 21 participants (7 males) on the waiting list. Changes in Gray Matter Volume (GMV) in bilateral hippocampi were analyzed with Voxel-Based Morphometry (VBM). One cluster was identified in the right hippocampus with a group by time interaction effect that consisted of 130 contiguous voxels but fell short of significance with full FDR correction (p = 0.077). GMV in this cluster increased by 0.76% in the mindfulness group and decreased by 0.78% in the control group. Within the mindfulness group, changes in hippocampal GMV were negatively associated with changes in perceived stress and depression severity and positively associated with enhancement in performance accuracy on the episodic memory task. Findings from this pilot study suggest that a mindfulness-based intervention may lead to an increase in partial hippocampal GMV with associated symptom reduction and improvement in episodic memory.
Keywords: stress, depression, anxiety, episodic memory, childhood trauma, neural plasticity
1. Introduction
Childhood maltreatment is associated with increased risks for various medical and psychiatric problems throughout the entire lifespan (Pietrek et al., 2013). Adverse childhood experiences, such as physical and emotional abuse or neglect, have been repeatedly reported to have long lasting impact on neural development (Teicher et al., 2016), which may underlie their enhanced risk of psychopathology (McCrory et al., 2012).
In particular, prior research showed that childhood maltreatment was associated with reduced Gray Matter Volumes (GMV) of the hippocampus (Dannlowski et al., 2012; Woon and Hedges, 2008), particularly the volumes of hippocampal subfields CA3, dentate gyrus, and subiculum (Teicher et al., 2012). The hippocampus, an important brain structure for learning and memory (Burgess et al., 2002), also plays an important role in stress regulation with its projections to the hypothalamus and its corticosteroid receptors (Jacobson and Sapolsky, 1991). Studies with animal models have revealed various kinds of stress, including prenatal stress (Marrocco et al., 2012), neonatal stress (Andersen and Teicher, 2004), chronic stress as well as acute stress (Murakami et al., 2005), all negatively impact the morphometry of the hippocampus. Human studies also identified stress as a primary predictor for hippocampal changes among maltreated children (Carrion et al., 2007).
Mindfulness based clinical interventions have beneficial effects on stress related psychological and medical symptoms, many of which are commonly found among childhood maltreatment victims, including depression (Williams et al., 2014) and anxiety (Goldin and Gross, 2010). Several studies further revealed neurobiological changes associated with mindfulness practices (Gotink et al., 2016). In particular, with respect to the hippocampus, long term meditators were shown to have higher GMV than controls in the left (Luders and Kurth, 2018) and right hippocampus (Hölzel et al., 2008) as well as bilateral hippocampal subiculum (Luders and Kurth, 2018). Increases in left hippocampal GMV were reported among healthy adults after they went through an eight-week mindfulness-based stress reduction intervention program (Hölzel et al., 2011). Furthermore, studies also suggest mindfulness practices enhance episodic memory (Brown et al., 2016), which is an important function of the hippocampus (Burgess et al., 2002).
In the present study we sought to assess the effects of a mindfulness intervention on hippocampal GMV as well as performance on an episodic memory task among young adults with self-reported histories of childhood maltreatment. We hypothesized that hippocampal GMV would increase after the mindfulness-based intervention and would be associated with symptom reduction and improvement in performance on an episodic memory task.
2. Methods
2.1. Subject Enrollment
Detailed information on subject recruitment and enrollment was presented in a previous publication (Joss et al., 2019). There were three waves of recruitment, during each wave, subjects were assigned to either the mindfulness group or waiting list control depending on their projected availability to attend the majority of intervention sessions, and subjects on the waiting list were offered the mindfulness intervention in the following wave. A total of 43 subjects were enrolled, 4 of which dropped out, and 3 subjects did not yield research data due to logistic issues. Among the remaining 36 subjects, 21 completed the mindfulness intervention and the research data collection before and after the intervention, while another 21 on the waiting list completed research data collection at the corresponding time, among which 6 subjects completed the mindfulness intervention after the waiting period (Figure 1.A).
Figure 1:
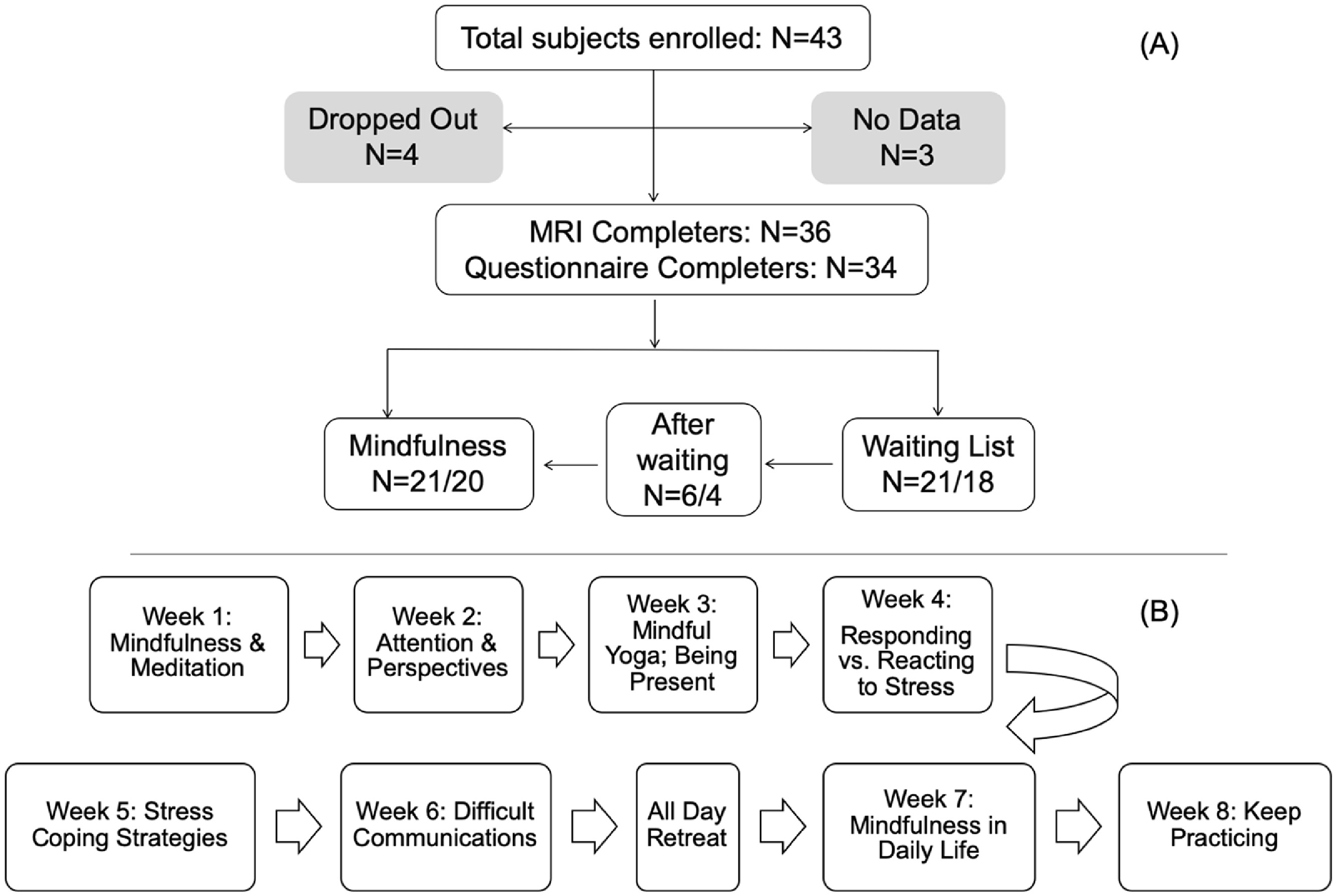
(A)Flow chart of subject enrolment and retention. One subject in the mindfulness group and 3 subjects in the control group did not complete the required questionnaires. Six subjects completed the mindfulness intervention program after their waiting period. All subjects that completed the MRI also completed the episodic memory task. (B) Flow chart of the primary topics for each week of the eight-week mindfulness- based intervention.
2.2. Subject Assessment
Comprehensive standardized clinical assessment on childhood experiences and psychiatric history were completed in the previous study where the subjects were recruited from (Khan et al., 2015). When subjects were enrolled for the present study, clinicians administered the Longitudinal Interview Follow-up Evaluation - Psychiatric Status Ratings (LIFE) (Keller et al., 1987) to assess their current mental health condition to determine whether they meet the inclusion/exclusion criteria (Joss et al., 2020, 2019). A battery of online questionnaires (Joss et al., 2020, 2019) were administered with the REDCap electronic data capture tool (Harris et al., 2009), among which three questionnaires were used in the present study: Perceived Stress Scale (PSS, (Cohen et al., 1994)), State-Trait Anxiety Inventory (STAI, (Spielberger and Sydeman, 1994)), and Beck Depression Inventory (BDI,(Beck et al., 1996)).
2.3. Research Procedures
Overview
Participants first went through a clinical interview to determine their eligibility, then eligible participants were instructed to fill out online questionnaires and complete an MRI visit which typically took place within a month before the start of the mindfulness intervention program or waiting period. At the MRI visit, all subjects underwent a urine drug test and female subjects also completed a urine pregnancy test. The MRI procedures included a 6-minute anatomical scan, and a 7-minute resting state fMRI scan that is not included in this manuscript. Subjects also completed a computerized episodic memory task outside the MRI scanner. Then subjects attended the mindfulness-based intervention program for eight continuous weeks. This consisted of eight 2.5 hour long weekly weekday evening sessions plus one six-hour weekend session. Within a month after the intervention program was finished, subjects came back for a second MRI visit during which they repeated the same MRI and computer test procedures and completed the same online questionnaires. Subjects on the waiting list were administered the same research procedures around the same time as subjects participating in the intervention program.
MRI parameters
MRIs were acquired on a Siemens 3T magnetom Skyra system at the Martinos Center for Biomedical Imaging of the Massachusetts General Hospital. MRIs of all subjects were acquired on the same scanner with exactly the same parameters without software upgrades during the course of the study. A 32-channel head coil was used to acquire all MRI images. High resolution anatomical image was acquired using a T1-weighted multi-echo MPRAGE (MEMPRAGE) sequence (van der Kouwe et al., 2008), which acquires 4 separate structural scans with different TE values ranging from 1.5 to 7 ms, but in the same time span as a conventional scan, and then the 4 separate images were averaged to increase the signal to noise ratio. Voxel size was 1.0×1.0×1.0 mm, Field of View (FOV) read was 256mm, base resolution was 256, and there were 176 slices per slab. Phase encoding direction was A>>P. TR=2530ms, TI=1100ms, TE 1=1.69ms. Flip angle =7.0 degrees.
Mindfulness based intervention program
The mindfulness based intervention program was modeled after the Mindfulness Based Stress Reduction program (Kabat-Zinn, 1990; Santorelli et al., 2017), which covered topics such as mindfulness meditation, attention and awareness, mindful yoga, responding vs. reacting to stress, stress coping strategies, handling difficult emotions and communications, as well as applying mindfulness in everyday life (Figure 1.B). Several modifications were adapted to increase the program’s trauma sensitivity (Joss et al., 2020), such as providing audio instructions for meditation practice of various lengths (2, 10, 20 or 30 minutes, as opposed to the original 45 minutes), inclusion of the 3-minute breathing space practice (King et al., 2013), incorporation of more mindful movement components, as well as cultivating a sense of empowerment by providing choices and flexibility throughout program instructions.
Episodic memory task
An episodic memory task (Stark et al., 2013) was administered on the computer outside the MRI scanner at each MRI visit. During the task, participants viewed color photographs of common objects, and were instructed to indicate whether they were viewing an object they had seen before (“old”), a slight variation of an object they had previously seen (“similar”), or an object they had not previously seen (“new”). Participants responded among the 3-alternative forced choices: “old”, “similar” and “new”. The whole task included 768 trials that were equally divided into 8 blocks. Each stimulus was presented for 2000ms with a 500ms inter-stimulus-interval. Presentation of the three conditions were fully randomized throughout the whole task. Presentation of the task was implemented with the Psychophysics Toolbox extensions (Pelli, 1997) on the MATLAB (MathWorks, Natick, MA) 2009 platform on a Windows 8 operating system (Microsoft, Redmond, WA).
2.4. Data analyses
A total of 36 participants were able to provide research data from at least two time points, either pre- and post-intervention or the two corresponding time points as a waiting list control. There were 6 subjects that completed the mindfulness intervention program after their waiting period, thus contributing data to both the control group and the mindfulness group. As a result, there were 21 pairs of MRI data for each group. One subject in the mindfulness group and 3 subjects in the control group did not complete the required questionnaires. All subjects that completed MRIs also completed the episodic memory task, although one subject during the post-waiting period testing could not complete the last block of the task due to technical issues, and performance accuracy of this subject was calculated based on the available data.
Questionnaire data and task performance accuracy was first analyzed with ANOVA with repeated measures to specifically evaluate the group by time interaction effect, effect size was evaluated with partial eta squared (η2), with a value above 0.06 indicates medium effect size and above 0.14 indicates large effect size. Then for each group, paired t-test was used to assess changes between the two time points (post- vs. pre-intervention/waiting period), effect size was evaluated with Cohen’s d (d), with a value above 0.5 indicates medium effect size and above 0.8 indicates a large effect size.
We used the CAT12 toolbox (Gaser and Dahnke, 2016) and SPM12 (Friston et al., 1995) for Voxel-Based Morphometry (VBM) analysis (Ashburner, 2007). The anatomical MRIs from all scans of each subject were segmented into gray matter, white matter and cerebrospinal fluid using the longitudinal module of CAT12. The mean image of all time points was created for each subject after realignment and bias correction; then based on the segmentation of the mean image, CAT12 estimated the spatial normalization parameters with the help of a Dartel normalization (Ashburner, 2007) for each image (i.e., the image from each time point). Finally, these normalization parameters were applied to each of the segmented images and then modulated and co-registered to the Montreal Neurological Institute (MNI) template. The estimated GMV map was then smoothed with Gaussian kernel full width at half maximum 8 by 8 by 8 mm3. Flexible factorial design was used for testing the group by time interaction effect. Total Intracranial Volumes (TIV) of each scan of each subject were used as covariates to control for individual variability in brain sizes.
Difference maps of GMV estimates were calculated (post minus pre) from the smoothed GMV maps of each subject with the “ImCalc” function in SPM12. Regression analyses were conducted within each group with the difference maps and the changes of questionnaire scores or episodic memory task performance accuracy. Average TIVs across two MRI data from two time-points for each subject were used as covariates in each regression analysis. Global scaling with individual TIV values was performed by adapting the “global calculation”, “global normalization”, and “proportional normalization” processing steps to reduce the issues of collinearity and increase orthogonality between TIV and independent variables.
Statistical results from all the above analyses were conducted within the anatomical masks of the left and right hippocampus obtained from the neuromorphometrics label template in SPM12. We used the 3dClustSim function in the AFNI (afni.nimh.nih.gov) package to determine the appropriate threshold for multiple comparison corrections within the hippocampal masks. There was a total of 1359 voxels in the left hippocampus and 1457 voxels in the right, setting the uncorrected p-threshold at p < 0.05, to reach a corrected p-value of p < 0.05, a cluster must have a minimum of 141 voxels in the left hippocampus or 152 voxels in the right hippocampus.
For illustrative purposes, after each regression analysis, we used the MarsBar (Brett et al., 2002) toolbox of SPM12 to extract the average GMV per voxel of each individual subject from the clusters of interest , and plotted them with the variable of interest, i.e., score changes of PSS, BDI and episodic memory task performance accuracy, with strength of association represented with Pearson correlation values.
3. Results
3.1. Results from research questionnaires and the episodic memory task
Detailed information on subject demographics and research questionnaires were reported in a previous publication (Joss et al., 2019). There were no group differences in age, sex, or ethnicity distribution. The mindfulness group (N = 21) had an average age of 26.05 years (SD = 2.25, range 22–29), with 76% female and 57% Caucasian. The control group (N = 21) had an average age of 25.19 years (SD = 2.69, range 22–29), with 67% female and 71% Caucasian. The two groups had 6 overlapping subjects who completed the mindfulness-based intervention after their waiting period (Figure 1.A).
There were significant group by time interaction effects with the scores of the PSS (F(1,34) = 9.276, p = 0.004, η2 = 0.214) and the STAI-t (F(1,33)= 6.785, p = 0.014, η2 = 0.171), but not the BDI (F(1,33) = 0.034, p = 0.855, η2 = 0.001). The mindfulness group had significantly reduced scores on PSS (t(18) =−3.529, p = 0.002, d = 0.809) and STAI-t (t(19) = −2.945, p = 0.008, d = 0.659), while the control group had no significant change in any of the symptom scores (p > 0.429) (Table 1).
Table 1:
Symptom questionnaires scores and episodic memory task performance accuracy.
| Mindfulness Group | Waiting List Control | ANOVA (time X group interaction) | |||||
|---|---|---|---|---|---|---|---|
| mean (SD) | Paired t-test | mean (SD) | paired t-test | ||||
| Pre | Post | Pre | Post | ||||
| PSS | 22.421 (8.375) | 16.895 (8.055) | t=3.529 p=0.002 | 19.176 (6.013) | 20.176 (7.860) | t=−0.696 p= 0.497 | F(1,34) = 9.276, p = 0.004, η2=0.214 |
| STAI-t | 45.850 (13.019) | 41.000 (12.998) | t=2.945 p=0.008 | 44.333 (11.902) | 45.667 (13.069) | t=−0.814 p=0.429 | F (1,33) = 6.785, p = 0.014, η2=0.171 |
| BDI | 18.211 (11.835) | 17.000 (15.790) | t=0.389 p=0.702 | 16.688 (2.981) | 16.188 (3.304) | t=0.249 p=0.807 | F(1,33) = 0.034, p = 0.855, η2=0.001 |
| Performance Accuracy | 0.858 (0.053) | 0.874 (0.034) | t=−1.5971 p=0.126 | 0.850 (0.072) | 0.887 (0.041) | t=−3.241 p=0.004 | F(1,40) =1.341, p = 0.254, η2= 0.032 |
The post-intervention change of episodic memory task performance accuracy of the mindfulness group did not reach significance because 6 subjects who completed the intervention after their waiting period completed the task for the third time, thus there could be ceiling effect with their performance improvement; after removing the 6 subjects, the mindfulness group did have significant increase in performance accuracy (average (SE) pre-intervention accuracy =0.841(0.059), post-intervention accuracy = 0.874 (0.037), t(14)=2.734, p = 0.016, d = 1.038).
There was no significant group by time interaction with performance accuracy on the episodic memory task (F(1,40) =1.341, p = 0.254, η2= 0.032). Both groups had increased accuracy at post-intervention/waiting period testing, but only the control group reached significance (t(20)=3.259, p=0.004, d=0.904). After removing the 6 overlapped subjects from the mindfulness group, the mindfulness group had a significant increase as well (t(14) =2.734, p = 0.016, d= 1.038)(Table 1).
3.3. MRI results: group by time interaction effect
The largest cluster identified for the group by time interaction effect was located in the right hippocampus and consisted of 130 contiguous voxels. The size of this cluster did not reach the threshold of 152 voxels required for an FDR-corrected significance (p < 0.05) level (Table 2). The FDR corrected p value for this cluster was p = 0.077. We extracted average GMV values of each subject at each time point from this cluster and plotted the values in Figure 2 for illustrative purposes. There was a 0.76% increase in GMV among the mindfulness group, and a 0.78% decrease in the control group, with a significant group difference from two sample t-test (t(40)=−3.19, p < 0.01).
Table 2:
Clusters within bilateral hippocampi for different statistical effects.
| Statistical effect | Cluster size | F or t value2 | Z value | p-value (uncorrected) | side | Peak voxel MNI coordinates (x, y, z) | ||
|---|---|---|---|---|---|---|---|---|
| Group by time interaction | 130 | 8.784 | 2.566 | 0.005 | R3 | 30 | −18 | −21 |
| Negative association with BDI score changes in the mindfulness group | 197*4 | 5.411 | 4.022 | 0.00003 | R | 15 | −10.5 | −20 |
| Negative association with PSS score changes in the mindfulness group | 160* | 2.748 | 2.451 | 0.007 | L | −24 | −10.5 | −27 |
| Negative association with STAIt score changes in the mindfulness group | 94 | 3.184 | 2.781 | 0.003 | R | 16.5 | −7.5 | −21 |
| Negative association with BDI score changes in the control group | 92 | 3.819 | 3.072 | 0.001 | R | 28.5 | −18 | −18 |
| Negative association with PSS score changes in the control group | 52 | 2.748 | 2.417 | 0.008 | L | −27 | −39 | 1.5 |
| Negative association with STAIt score changes in the control group | 49 | 3.067 | 2.585 | 0.005 | R | 27 | −19.5 | −16.5 |
| Positive correlation with task performance accuracy changes in the mindfulness group | 80 | 2.847 | 2.553 | 0.005 | L | −25.5 | −19.5 | −16.5 |
F value for group by time interaction effect; t value for all the other statistical effects; F or t values, Z values and p values are of the peak voxels; p values are uncorrected.
R: right hippocampus; L: left hippocampus.
Cluster size passed the minimum threshold for FDR correction (p < 0.05). For statistical effects that did not have any cluster passing the minimum size threshold, only the largest cluster is presented in this table.
Figure 2:
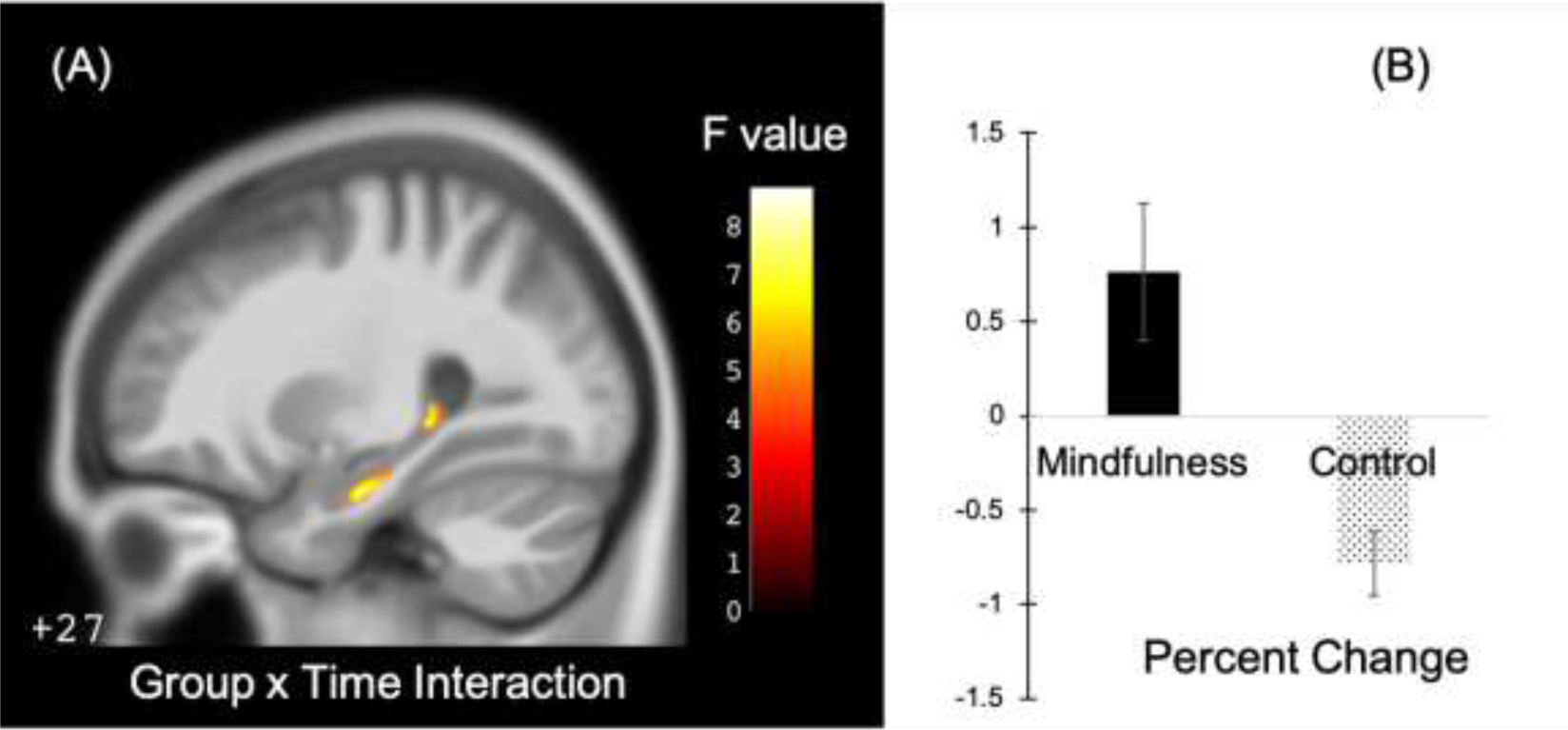
Group by time interaction effect and changes in each group within the right hippocampus. (A)Illustration of the location of the cluster with group by time interaction effect (note: the number (“+27”) on the lower left corner of the MRI image indicates the location within the MNI atlas of the slice being displayed). Color bar indicates F-values of the group by time interaction effect. (B)Plot of average percent change of GMV within this cluster for each group.
3.4. MRI results: correlations with changes of symptom severity and task performance accuracy
We used regression analyses in SPM to assess the association in each group between unilateral hippocampal GMV and symptom reduction. Within the mindfulness group, there was one cluster in the right hippocampus that showed significant negative association with BDI score changes, and one cluster in the left hippocampus that showed significant negative association with PSS score changes, both of which reached the minimum cluster size threshold to be considered as significant clusters (FDR corrected, p < 0.05) (Table 2, Figure 3). Hippocampal clusters from regression analyses of the STAI-t did not reach the minimum size threshold.
Figure 3:
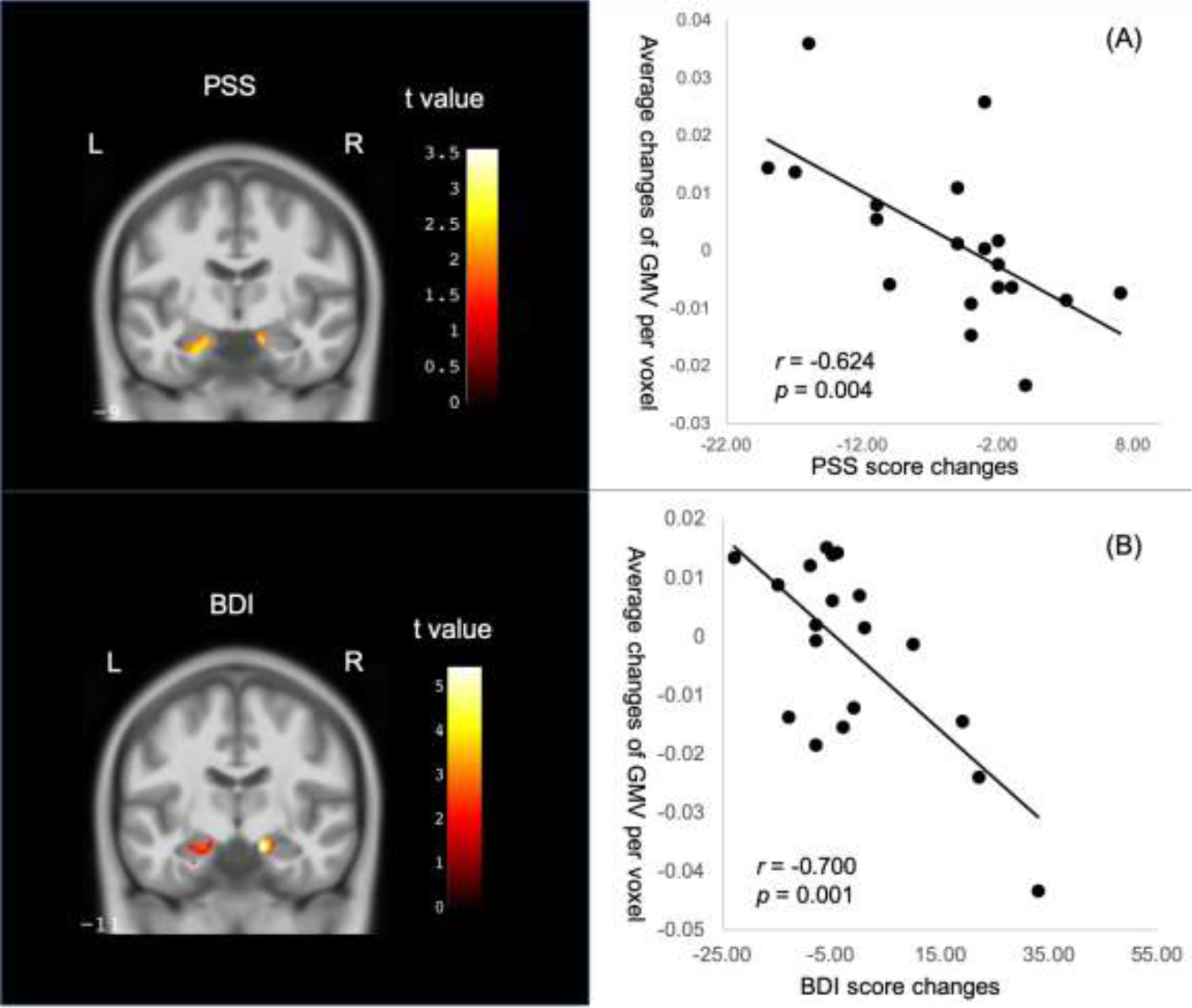
Negative associations within the mindfulness group between changes of hippocampal GMV and changes of scores on the (A)Perceived Stress Scale (PSS) and (B)Beck Depression Inventory (BDI). L: left side, R: right side. Scatter plots demonstrate correlations between symptom changes and the averages of modulated GMV changes among all voxels in the significant clusters (see Table 2). Color bar indicates the absolute t-values of the regression model.
The same analyses within the control group revealed some clusters of voxels that also showed negative correlations with changes of PSS, BDI and STAI-t scores (p < 0.05, uncorrected), but the cluster sizes did not reach the minimum threshold for FDR corrected significance level of p < 0.05. The largest clusters with negative correlation with symptom score changes in the control group were located in the same side of the hippocampus as those in the mindfulness group (Table 2).
Regression analyses within the mindfulness group revealed two small clusters of 80 and 53 voxels respectively in the left hippocampus that showed positive association (p < 0.05, uncorrected) between changes in GMV and changes in performance accuracy on the episodic memory task (Figure 4); neither cluster reached the minimum size threshold for FDR corrected significance level of p < 0.05. The same analysis within the control group did not reveal any significant (p < 0.05, uncorrected) voxels within bilateral hippocampi that had positive associations between changes of GMV and task performance accuracy.
Figure 4:
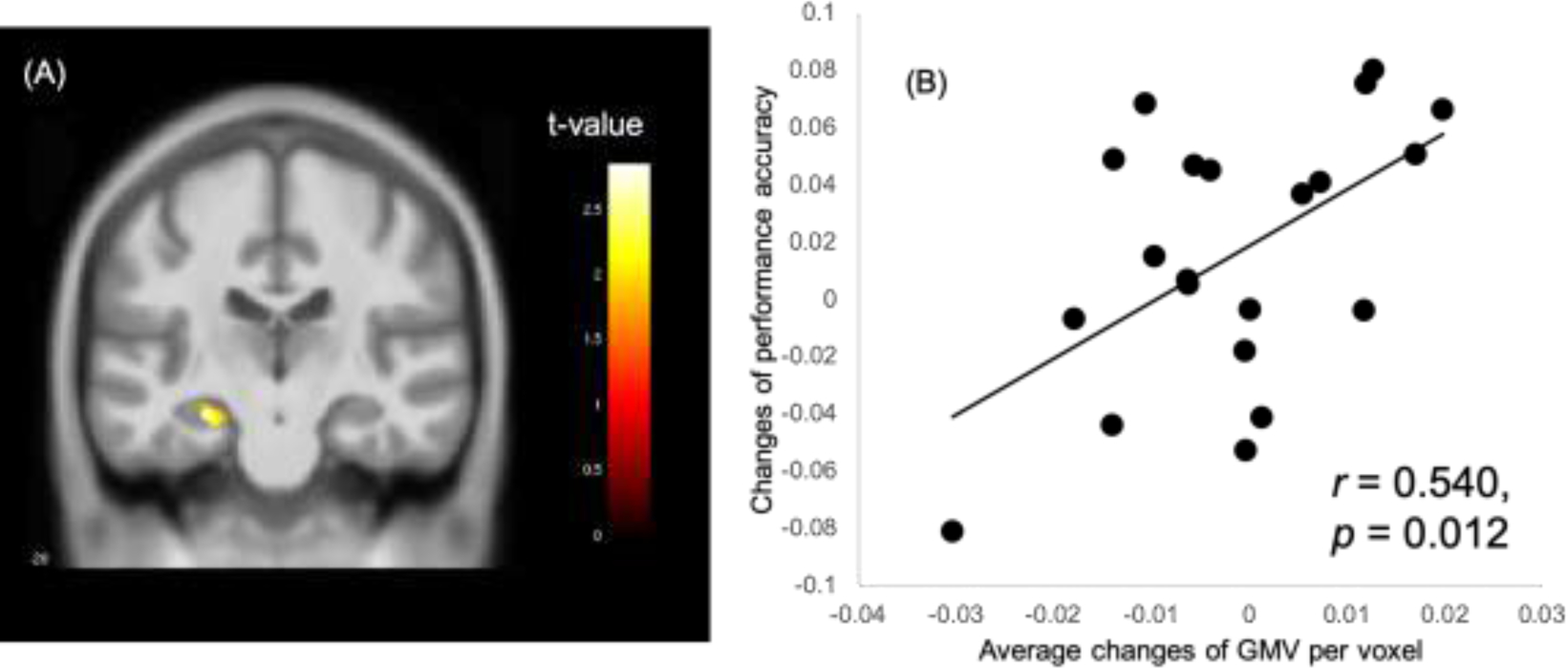
Positive association within the mindfulness group between changes in hippocampal GMV and changes in performance accuracy on the episodic memory task. (A)Illustration of the cluster of significant hippocampal voxels (p < 0.05 uncorrected). (B) Scatter plot for the correlation between changes in task performance accuracy and the average change of modulated GMV among all significant voxels.
4. Discussion
This pilot study investigated changes in hippocampal GMV after a mindfulness based behavioral intervention among young adults with childhood adversity. We found a 130-voxel cluster in the right hippocampus in which the mindfulness group showed an increase in GMV after the intervention as opposed to a decrease observed in the control group after the waiting period. Furthermore, there were two hippocampal clusters that showed significant associations with stress and depression symptom reduction. A subthreshold cluster was also observed with positive association with performance accuracy changes on an episodic memory task. The implications of these findings are discussed below.
It’s well-known that stress has adverse impacts on the hippocampus (McEwen et al., 2012). Stress impacts the hippocampus by inducing “debranching and shortening of dendrites and suppression of neurogenesis” (Czéh et al., 2001), which are reflected as morphometric changes in human neuroimaging studies. Our findings of treatment related morphometric changes over eight weeks are similar to prior reports. For example, a significant correlation was reported between numbers of stressful life events and reduction of right hippocampal GMV over a 3-months interval (Papagni et al., 2011). Another study found increased hippocampal gray matter among patients with unipolar depression after an 8-week antidepressant treatment (Arnone et al., 2013). The decreased GMV after the waiting periods in the control group likely reflects the effect of stress and anxiety on hippocampal morphometry (Czéh et al., 2001; McEwen et al., 2012). Unlike the mindfulness group which had significantly reduced stress and anxiety after the intervention, these metrics remained high in the control group and trended slightly upward.
Our findings of a subthreshold cluster of increased hippocampal GMV after mindfulness intervention is consistent with previous reports of higher hippocampal GMV among long term meditators (Luders and Kurth, 2018) and increased hippocampal GMV after a mindfulness based stress reduction program (Hölzel et al., 2011), which was attributed to possible neurogenesis (Gage, 2002) upon stress alleviation (Hölzel et al., 2011). However, both previous studies reported significant findings in the left hippocampus, whereas in the present study the identified cluster was located in the right hippocampus. Such difference in laterality might be related to the effect of childhood maltreatment on hippocampal development. A meta-analysis found that trauma exposure was associated with hippocampal volume reduction, and the impact was more prominent in the right hippocampus among subjects that developed PTSD (Woon et al., 2010). Overall, the laterality of reported effects in maltreated individuals is mixed with the majority reporting bilateral effects (43%) followed by left only (30%) and right only (25%) (Teicher et al., 2016). Despite the mixed findings on laterality in previous reports, the adverse impact of childhood maltreatment on hippocampal development could have caused the hippocampus to respond differently to the mindfulness intervention compared to previous studies in nontraumatized populations.
The episodic memory task indirectly reflects hippocampal functionality. In previous functional MRI (fMRI) studies, the episodic memory task was used to observe hippocampal activations (Bakker et al., 2012; Stark et al., 2013). Prior longitudinal studies have reported associations between changes of hippocampal morphometry and episodic memory performance (Engvig et al., 2014; Gorbach et al., 2017). In the present study, both groups had improved performance accuracy, but only the mindfulness group showed positive association with hippocampal GMV changes, while the same analyses did not reveal any significant hippocampal voxels with such association in the control group. Therefore, unlike the hippocampus-associated performance enhancement in the mindfulness group, the accuracy improvement of the control group might have recruited different neural resources (Kramer et al., 2005).
Existing research suggests that stress related hippocampal impairments are reversible under appropriate conditions. Several mechanisms have been found to increase hippocampal volumes. One animal study found that antidepressant treatment prevented stress-induced hippocampal volume reduction (Czéh et al., 2001) while human studies found antidepressant medication treatment led to increased hippocampal GMV among PTSD patients (Vermetten et al., 2003). Environmental enrichment and voluntary exercise were also found to increase adult hippocampal neurogenesis (Kim et al., 2010; Olson et al., 2006). Mindfulness based interventions seem to have similar effects on hippocampal morphometry (Hölzel et al., 2011), although the exact cellular mechanism is still unclear. In the present study, we found that changes in GMV were significantly associated with reduction in perceived stress and depression, suggesting that hippocampal changes may be accompanied by clinical improvement.
This pilot study has several limitations, such as a small sample size, use of a waiting list control as opposed to an active control condition, lack of long term follow-up, as well as the inability to account for other factors that can affect neural changes such as hormonal fluctuations over the menstrual cycle (Protopopescu et al., 2008). Nevertheless, findings from this pilot study suggest that mindfulness-based interventions can be helpful for promoting hippocampal neural plasticity with associated improvements in clinical symptoms and episodic memory.
Highlights:
Mindfulness based intervention for young adults with childhood maltreatment history
Increased gray matter volumes at the right hippocampus in the mindfulness group
Hippocampal changes negatively associated with depression and stress level changes in the mindfulness group
Hippocampal changes positively associated with improvement in performance accuracy on an episodic memory task in the mindfulness group
Acknowledgment
This work was supported by the Mind and Life Institute, the Martinos Center for Biomedical Imaging, and NIH (grant number: 5K01AT009085). We thank Zayda Vallejo, Lauri J Klein and David Schouela for the intervention program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Competing Interest
None.
References
- Andersen SL, Teicher MH, 2004. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology 29, 1988–1993. [DOI] [PubMed] [Google Scholar]
- Arnone D, McKie S, Elliott R, Juhász G, Thomas EJ, Downey D, Williams S, Deakin JFW, Anderson IM, 2013. State-dependent changes in hippocampal grey matter in depression. Mol. Psychiatry 18, 1265–1272. [DOI] [PubMed] [Google Scholar]
- Ashburner J, 2007. A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113. [DOI] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M, 2012. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 74, 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF, 1996. Comparison of Beck Depression Inventories-IA and-II in psychiatric outpatients. J. Pers. Assess 67, 588–597. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B, 2002. Region of interest analysis using an SPM toolbox, in: 8th International Conference on Functional Mapping of the Human Brain. Sendai, Japan, p. 497. [Google Scholar]
- Brown KW, Goodman RJ, Ryan RM, Anālayo B, 2016. Mindfulness enhances episodic memory performance: Evidence from a multimethod investigation. PLoS One 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J, 2002. The human hippocampus and spatial and episodic memory. Neuron 35, 625–641. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Reiss AL, 2007. Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics 119, 509–516. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1994. Perceived stress scale. Meas. Stress A Guid. Heal. Soc. Sci 235–283. [Google Scholar]
- Czéh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E, 2001. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci 98, 12796–12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, 2012. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol. Psychiatry 71, 286–293. [DOI] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Skaane NV, Dale AM, Holland D, Due-Tønnessen P, Sundseth Ø, Walhovd KB, 2014. Effects of cognitive training on gray matter volumes in memory clinic patients with subjective memory impairment. J. Alzheimer’s Dis 41, 779–791. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SCR, Frackowiak RSJ, Turner R, 1995. Analysis of fMRI time-series revisited. Neuroimage 2, 45–53. [DOI] [PubMed] [Google Scholar]
- Gage FH, 2002. Neurogenesis in the adult brain. J. Neurosci 22, 612–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Dahnke R, 2016. CAT-a computational anatomy toolbox for the analysis of structural MRI data. HBM 2016, 336–348. [Google Scholar]
- Goldin PR, Gross JJ, 2010. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion 10, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach T, Pudas S, Lundquist A, Orädd G, Josefsson M, Salami A, de Luna X, Nyberg L, 2017. Longitudinal association between hippocampus atrophy and episodic-memory decline. Neurobiol. Aging 51, 167–176. [DOI] [PubMed] [Google Scholar]
- Gotink RA, Meijboom R, Vernooij MW, Smits M, Hunink MGM, 2016. 8-week mindfulness based stress reduction induces brain changes similar to traditional long-term meditation practice–a systematic review. Brain Cogn. 108, 32–41. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, 2009. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform 42, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Carmody J, Vangel M, Congleton C, Yerramsetti SM, Gard T, Lazar SW, 2011. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. Neuroimaging 191, 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Ott U, Gard T, Hempel H, Weygandt M, Morgen K, Vaitl D, 2008. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Soc. Cogn. Affect. Neurosci 3, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R, 1991. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev 12, 118–134. [DOI] [PubMed] [Google Scholar]
- Joss D, Khan A, Teicher MH, Lazar SW, 2019. Effects of a mindfulness-based intervention on self-compassion and psychological health among young adults with a history of childhood maltreatment. Front. Psychol. accepted [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joss D, Lazar SW, Teicher MH, 2020. Nonattachment Predicts Empathy, Rejection Sensitivity, and Symptom Reduction After a Mindfulness-Based Intervention Among Young Adults with a History of Childhood Maltreatment. Mindfulness (N. Y) 11, 975–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J, 1990. Full catastrophe living: Using the wisdom of your body and mind in everyday life. New York Delacorte. [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC, 1987. The Longitudinal Interval Follow-up Evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch. Gen. Psychiatry 44, 540–548. [DOI] [PubMed] [Google Scholar]
- Khan A, McCormack HC, Bolger EA, McGreenery CE, Vitaliano G, Polcari A, Teicher MH, 2015. Childhood maltreatment, depression, and suicidal ideation: critical importance of parental and peer emotional abuse during developmental sensitive periods in males and females. Front. psychiatry 6, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-E, Ko I-G, Kim B-K, Shin M-S, Cho S, Kim C-J, Kim S-H, Baek S-S, Lee E-K, Jee Y-S, 2010. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp. Gerontol 45, 357–365. [DOI] [PubMed] [Google Scholar]
- King AP, Erickson TM, Giardino ND, Favorite T, Rauch SAM, Robinson E, Kulkarni M, Liberzon I, 2013. A pilot study of group mindfulness‐ based cognitive therapy (MBCT) for combat veterans with posttraumatic stress disorder (PTSD). Depress. Anxiety 30, 638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Rosen HJ, Du A-T, Schuff N, Hollnagel C, Weiner MW, Miller BL, Delis DC, 2005. Dissociations in hippocampal and frontal contributions to episodic memory performance. Neuropsychology 19, 799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Kurth F, 2018. The Neuroanatomy of Long-term Meditators. Curr. Opin. Psychol 28, 172–178. [DOI] [PubMed] [Google Scholar]
- Marrocco J, Mairesse J, Ngomba RT, Silletti V, Van Camp G, Bouwalerh H, Summa M, Pittaluga A, Nicoletti F, Maccari S, 2012. Anxiety-like behavior of prenatally stressed rats is associated with a selective reduction of glutamate release in the ventral hippocampus. J. Neurosci 32, 17143–17154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E, 2012. The link between child abuse and psychopathology: a review of neurobiological and genetic research. J. R. Soc. Med 105, 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter RG, Miller MM, 2012. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology 62, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E, 2005. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci. Res 53, 129–139. [DOI] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR, 2006. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus 16, 250–260. [DOI] [PubMed] [Google Scholar]
- Papagni SA, Benetti S, Arulanantham S, McCrory E, McGuire P, Mechelli A, 2011. Effects of stressful life events on human brain structure: a longitudinal voxel-based morphometry study. Stress 14, 227–232. [DOI] [PubMed] [Google Scholar]
- Pelli DG, 1997. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat. Vis 10, 437–442. [PubMed] [Google Scholar]
- Pietrek C, Elbert T, Weierstall R, Müller O, Rockstroh B, 2013. Childhood adversities in relation to psychiatric disorders. Psychiatry Res. 206, 103–110. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M, McEwen B, Silbersweig D, Stern E, 2008. Hippocampal structural changes across the menstrual cycle. Hippocampus 18, 985–988. [DOI] [PubMed] [Google Scholar]
- Santorelli SF, Kabat-Zinn J, Blacker M, Meleo-Meyer F, Koerbel L, 2017. Mindfulness-based stress reduction (MBSR) authorized curriculum guide. Cent. Mindfulness Med. Heal. Care, Soc. (CFM). Univ. Massachusetts Med. Sch [Google Scholar]
- Spielberger CD, Sydeman SJ, 1994. State-Trait Anxiety Inventory and State-Trait Anger Expression Inventory, in: Maruish ME (Ed.), The Use of Psychological Testing for Treatment Planning and Outcome Assessment. Lawrence Erlbaum Associates, Inc, Hillsdale, NJ. [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CEL, 2013. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia 51, 2442–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A, 2012. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci 109, E563–E572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, Ohashi K, 2016. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci 17, 652–666. [DOI] [PubMed] [Google Scholar]
- van der Kouwe AJW, Benner T, Salat DH, Fischl B, 2008. Brain morphometry with multiecho MPRAGE. Neuroimage 40, 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD, 2003. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol. Psychiatry 54, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JMG, Crane C, Barnhofer T, Brennan K, Duggan DS, Fennell MJV, Hackmann A, Krusche A, Muse K, Von Rohr IR, 2014. Mindfulness-based cognitive therapy for preventing relapse in recurrent depression: a randomized dismantling trial. J. Consult. Clin. Psychol 82, 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon FL, Hedges DW, 2008. Hippocampal and amygdala volumes in children and adults with childhood maltreatment- related posttraumatic stress disorder: A meta‐ analysis. Hippocampus 18, 729–736. [DOI] [PubMed] [Google Scholar]
- Woon FL, Sood S, Hedges DW, 2010. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog. Neuro-Psychopharmacology Biol. Psychiatry 34, 1181–1188. [DOI] [PubMed] [Google Scholar]


