Significance
Reptiles of the Mesozoic Era are known for their remarkable size: dinosaurs include the largest known land animals, and their relatives, the pterosaurs, include the largest creatures to ever fly. The origins of these groups are poorly understood, however. Here, we present a species (Kongonaphon kely) from the Triassic of Madagascar close to the ancestry of dinosaurs and pterosaurs, providing insight into the early evolution of those groups. Kongonaphon is a surprisingly small animal (estimated height, ∼10 cm). Analysis of ancestral body size indicates that there was a pronounced miniaturization event near the common ancestor of dinosaurs and pterosaurs. Tiny ancestral body size may help explain the origins of flight in pterosaurs and fuzzy integument in both groups.
Keywords: body size, evolution, Dinosauria, Triassic, phylogeny
Abstract
Early members of the dinosaur–pterosaur clade Ornithodira are very rare in the fossil record, obscuring our understanding of the origins of this important group. Here, we describe an early ornithodiran (Kongonaphon kely gen. et sp. nov.) from the Mid-to-Upper Triassic of Madagascar that represents one of the smallest nonavian ornithodirans. Although dinosaurs and gigantism are practically synonymous, an analysis of body size evolution in dinosaurs and other archosaurs in the context of this taxon and related forms demonstrates that the earliest-diverging members of the group may have been smaller than previously thought, and that a profound miniaturization event occurred near the base of the avian stem lineage. In phylogenetic analysis, Kongonaphon is recovered as a member of the Triassic ornithodiran clade Lagerpetidae, expanding the range of this group into Africa and providing data on the craniodental morphology of lagerpetids. The conical teeth of Kongonaphon exhibit pitted microwear consistent with a diet of hard-shelled insects, indicating a shift in trophic ecology to insectivory associated with diminutive body size. Small ancestral body size suggests that the extreme rarity of early ornithodirans in the fossil record owes more to taphonomic artifact than true reflection of the group’s evolutionary history.
Dinosauria includes the largest terrestrial animals in Earth’s history (1–4), and the median body mass of Mesozoic dinosaurs far surpasses that of extant mammals, the largest modern animals (5–8). Following their appearance in the Late Triassic record, dinosaur lineages across multiple clades attained sizes previously unknown among terrestrial vertebrates (7–9). Intriguingly, however, dinosaurs exhibited no comparable cross-clade tendencies toward smaller sizes. A lower bound on tetrapod body size is determined by physiological constraints (10, 11), but this limit was rarely approached by Mesozoic dinosaurs, which failed to occupy size ranges successfully inhabited by thousands of species of mammals, lepidosaurian reptiles, and lissamphibians (12). Only in the lineage leading to birds (avian dinosaurs) did dinosaurs exhibit a sustained trend toward miniaturization (13), eventually approaching the lower extremes of tetrapod body size (14). The cause of this apparent canalization in dinosaurian body size remains poorly understood.
Although body size evolution has been studied extensively within Dinosauria (2, 3, 7, 8, 13, 15), the lineage leading to dinosaurs has received less attention. This largely reflects the paucity of fossils of early members of the dinosaur stem lineage, Avemetatarsalia, which includes dinosaurs (including birds), pterosaurs, and all taxa more closely related to them than to crocodiles. Although avemetatarsalians must have diverged no later than the Early Triassic (∼251 million years ago) (16), avemetatarsalian body fossils are rare prior to the radiation of saurischian dinosaurs at the end of the Carnian (Late Triassic), roughly 20 million years later (17, 18). Historically, early-diverging members of the dinosaurian stem lineage were known only from the Upper Triassic Los Chañares Formation of Argentina and consisted solely of members of Dinosauromorpha (avemetatarsalians more closely related to dinosaurs than to pterosaurs) (19, 20). Although the enigmatic Scleromochlus taylori from the Upper Triassic Lossiemouth Sandstone of Scotland has been interpreted as an early-diverging member of the pterosaur line (Pterosauromorpha) (21), the relationships of this taxon are uncertain, and some analyses have recovered it as a dinosauromorph (22).
Recent discoveries have substantially expanded the temporal and geographic range of early avemetatarsalians (19, 22–25). Nesbitt and coworkers (22, 26) described a new taxon, Teleocrater rhadinus, from the ?Middle Triassic Manda Beds of Tanzania, the first known avemetatarsalian outside of Ornithodira (the clade containing the common ancestor of dinosaurs and pterosaurs and all of its descendants). Furthermore, the well-preserved remains of Teleocrater permit reinterpretation of several other poorly known Triassic reptiles (Russian Dongusuchus efremovi, Brazilian Spondylosoma absconditum, and Indian Yarasuchus deccanensis), showing that these taxa form a clade (Aphanosauria) at the base of Avemetatarsalia (22). A number of new or newly recognized basal (i.e., nondinosaur, nonpterosaur) ornithodirans have also been recently described. Most of this newly recognized material belongs to the Silesauridae, a specialized clade of herbivorous or omnivorous, beaked quadrupeds typified by Silesaurus from the Upper Triassic of Poland (23). Silesaurids are identified as the nearest relatives of Dinosauria in the most recent comprehensive analyses of archosaur relationships (19, 22), although alternative hypotheses for their relationships exist (25, 27).
Lagerpetidae, a second, less species-rich clade traditionally considered to be nondinosaurian dinosauromorphs, is known primarily from hindlimb material from Upper Triassic deposits in North and South America (27). Five lagerpetid species are currently known: Lagerpeton chanarense from the Upper Triassic of Argentina (20) (species name emended under International Code of Zoological Nomenclature [ICZN] Art. 31.2 because -erpeton is neuter), Ixalerpeton polesinense from the Upper Triassic of Brazil (28) (also emended as above), and three species of Dromomeron from the Upper Triassic of Argentina and the southwestern United States (24, 29, 30). Two additional, unnamed lagerpetid morphotypes are known from fragmentary material in the Upper Triassic of Argentina (31) and Brazil (32).
Here, we describe a lagerpetid taxon representing the smallest known member of the clade, which extends the distribution of this group outside of the Americas. The type material of the lagerpetid was recovered from the Mid-to-Upper Triassic “basal Isalo II beds” in the southern Morondava Basin of southwestern Madagascar. These beds have produced a diverse tetrapod fauna, including a variety of synapsids and nonarchosaurian archosauromorphs (33), but crown archosaurs are rare components of the fauna. We also reexamine body size evolution in Triassic archosaurs in light of the wealth of new data available for early avemetatarsalians. Many nondinosaurian dinosauromorphs are similar in size to the earliest dinosaurs (e.g., ref. 23), and previous analyses have estimated that the ancestral body sizes for Archosauria, Ornithodira, and Dinosauromorpha were comparable (34). Here, we present evidence instead for a profound miniaturization event near the base of Ornithodira, with implications for the physiology and evolutionary history of dinosaurs and pterosaurs.
Systematic Paleontology
Archosauria Cope, 1869 (35)
Avemetatarsalia Benton, 1999 (21)
Lagerpetidae Arcucci, 1986 (36); sensu Nesbitt et al., 2009 (29)
Kongonaphon kely gen. et sp. nov.
Etymology.
Name meaning “tiny bug slayer,” derived from kongona (Malagasy, “bug”) and φον (variant of ancient Greek φονεύς, “slayer”), referring to the probable diet of this animal; kely (Malagasy, “small”), referring to the diminutive size of this specimen.
Holotype.
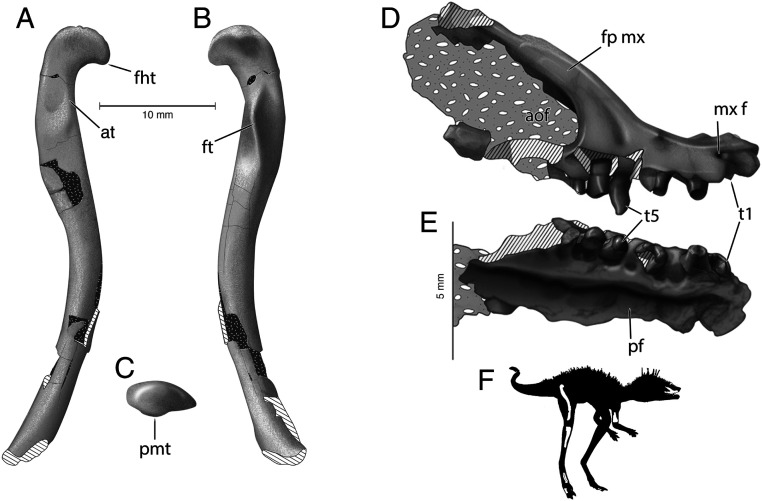
UA 10618 is a partial skeleton composed of a right maxilla (Fig. 1 A–C), distal portion of the humerus, right femur (missing the distal tip) (Fig. 1 D and E), proximal portions of the right and left tibia, proximal portion of the fibula, problematic elongate elements that may represent two metatarsals, metatarsal II articulated to a pedal phalanx, an additional phalanx, an isolated caudal vertebra, and indeterminate skeletal fragments (refer to SI Appendix for further details).
Fig. 1.
Anatomy of the femur and maxilla of Kongonaphon kely gen. et sp. nov. (UA 10618). (A) Right femur in anterolateral, (B) posteromedial, and (C) proximal views. (D) Right maxilla in right lateral and (E) palatal views. (F) Preserved elements in the holotype, UA 10618, presented in a silhouette of Kongonaphon. aof, antorbital fenestra; at, anterior trochanter; fht, tip of femoral head; fp mx, facial process of maxilla; ft, fourth trochanter; mx f, maxillary foramen; pf, palatine fossa; pmt, posterior medial tubercle; t, maxillary tooth. Illustrations credit: American Museum of Natural History/Frank Ippolito.
Locality and Horizon.
UA 10618 was collected in coarse-grained gray sandstone in the southern Morondava Basin of southwestern Madagascar. The type locality also has produced several cynodont specimens, including the holotypes of the traversodontids Menadon besairiei and Dadadon isaloi, and specimens of the rhynchosaur Isalorhynchus genovefae (33, 37). The age of the basal Isalo II deposits is not well constrained, as no radiometric dates are yet available for this unit. The shared presence of the distinctive cynodont Menadon in the basal Isalo II and the Santacruzodon Assemblage Zone (Santa Maria Supersequence) of Brazil suggests that the former is early Carnian, based on biostratigraphic and radioisotopic analyses of South American tetrapod-bearing beds (38, 39). However, additional research is needed to confirm this age, considering the sometimes lengthy stratigraphic ranges of Triassic tetrapod genera and the paucity of robust dates for other Triassic cynodont-bearing beds worldwide. Here, we retain Flynn et al.’s (37) circumspect treatment of the basal Isalo II deposits as Ladinian–Carnian (Mid-to-Upper Triassic) pending further study.
Diagnosis.
A basal ornithodiran archosaur distinguishable from all members of the group other than lagerpetids by the hook-like femoral head with a concave ventral margin: distinguished from Dromomeron by the presence of a sharp, blade-like fourth trochanter; distinguished from Lagerpeton by the fourth trochanter angled medially, without a lateral depression, and the presence of a ridge in the fourth trochanter depression; and distinguished from both Dromomeron and Lagerpeton by a more gracile, elongate, and curved femoral shaft. Femoral proportions also distinguish Kongonaphon from the recently described Ixalerpeton. The holotype femur of Ixalerpeton polesinense is 64 mm long and 6 mm in diameter at midshaft, compared with 38-mm length and 2.9-mm midshaft diameter in UA 10618, indicating a more gracile femur in Kongonaphon. Furthermore, the femur of Ixalerpeton exhibits substantial distal expansion (28), whereas in Kongonaphon, the diameter of the preserved portion of the femur changes little from the midshaft distally. The distance between the proximal tip of the femoral head and fourth trochanter also are proportionally greater in Kongonaphon than in Ixalerpeton.
Remarks.
The Isalo II lagerpetid, K. kely, is represented by a partial skeleton, the holotype specimen UA 10618. Although mostly disarticulated, close physical association of the skeletal elements when collected, avemetatarsalian synapomorphies of individual bones, and lack of overlapping elements indicate that the holotype represents a single individual. The right maxilla of UA 10618 (Fig. 1 D and E) expands our knowledge of the lagerpetid cranium (part of the skull roof was previously known for Ixalerpeton, but no dentition). This element bears unserrated, simple, conical teeth, suggestive of an insectivorous diet. The right femur of UA 10618 is extremely elongate and gracile, even compared with juvenile specimens of other lagerpetids (29), suggesting a light build. In all other regards, the morphology of Kongonaphon agrees closely with that of other lagerpetids, which are generally considered small, predatory bipeds (20, 24).
The most remarkable aspect of Kongonaphon is its extraordinarily small size, with a preserved femoral length of only 38 mm (estimated total length, ∼40 mm). Although the femora of some early pterosaurs are similarly small, the limb proportions of pterosaurs (elongate forelimbs and proportionally short hindlimbs; ref. 40) are the reverse of those in other ornithodirans, indicating that Kongonaphon would have been smaller than a pterosaur with a femur of equivalent size. Triassic dinosaurs comparable in mature body size to Kongonaphon are unknown, as all specimens within this size range have proven to be perinates (41). We can confidently demonstrate that the holotype of K. kely does not represent a perinate. Histological sectioning of the right tibia of UA 10618 revealed the presence of two lines of arrested growth as well as parallel-fibered bone in the outer cortex indicative of a reduced growth rate in this individual. Based on osteohistological and gross skeletal growth indicators (SI Appendix), we hypothesize that the preserved elements of UA 10618 accurately reflect a diminutive mature body size (although we cannot exclude the possibility that other individuals of this taxon could achieve larger maximum size). Scleromochlus, with an estimated femoral length of 32 mm (16), is the only Triassic avemetatarsalian smaller than Kongonaphon. Histological assessment of the Scleromochlus material is not possible, as it consists entirely of sandstone molds. However, unlike Kongonaphon, Scleromochlus is known from multiple specimens (seven individuals in total), all of comparable size, only two of which were found in association, so there is no evidence they represent a clutch or age-associated sibling group. The Lossiemouth Sandstone in which Scleromochlus occurs also yields abundant fossils of much larger reptiles (e.g., aetosaurs, rhynchosaurs), so there is no apparent taphonomic bias that would favor the preservation of only small individuals of Scleromochlus. We consider it most likely that Scleromochlus was also small-bodied at maturity and that it and Kongonaphon represent a historically undersampled size class of early avemetatarsalians.
Discussion
To test the phylogenetic position of Kongonaphon, we included this taxon in the comprehensive matrix of a recent analysis of Triassic archosaur relationships (22) (modified following ref. 27 and based on our examination of silicone peels of the known Scleromochlus specimens; see SI Appendix for details). In our expanded phylogenetic analysis, Kongonaphon was consistently recovered as a member of Lagerpetidae, albeit in an unresolved polytomy with the other lagerpetid genera. However, the position of Lagerpetidae as a whole was found to be unstable. In analyses where Scleromochlus was not included, Lagerpetidae was recovered in its traditional position (e.g., ref. 24), as the earliest-diverging clade of dinosauromorphs. When Scleromochlus was included, this genus and Lagerpetidae were both recovered as early-diverging pterosauromorphs (with Scleromochlus as the sister-taxon of Pterosauria). A possible pterosauromorph placement for Lagerpetidae has been previously proposed (42), but this position is based on very limited evidence. Although an intriguing possibility worthy of future investigation, the support for lagerpetids-as-pterosauromorphs generally is weak and rendered problematic by extensive missing data for the majority of lagerpetid taxa. More complete and better-preserved specimens are needed to further test the position of Lagerpetidae in avemetatarsalian phylogeny. At present, we consider their placement equivocal and prefer to depict the base of Ornithodira as an unresolved polytomy between Lagerpetidae, Scleromochlus, Pterosauria, and Dinosauriformes (Fig. 2).
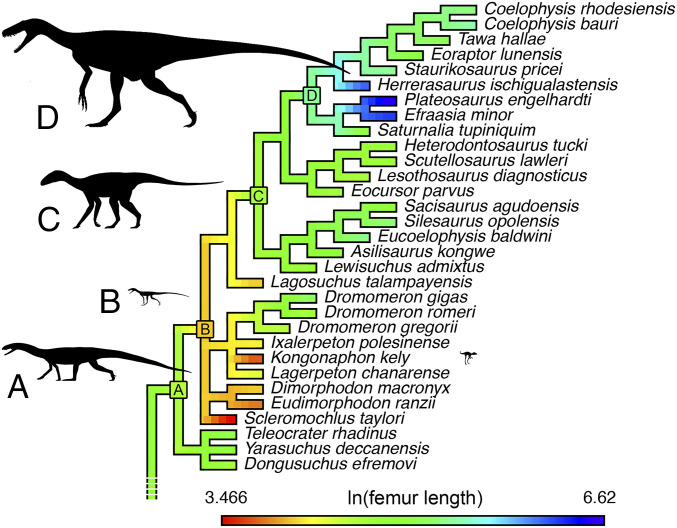
Fig. 2.
Body size of early avemetatarsalian (bird line) archosaurs mapped onto a consensus supertree, based on the current phylogenetic analysis (SI Appendix) and recent analyses (22). Silhouettes are scaled to estimated femoral lengths for the labeled nodes (SI Appendix, Table S1): A, base of Avemetatarsalia (represented by Teleocrater); B, base of Ornithodira (represented by Ixalerpeton); C, base of Dracohors (Silesauridae + Dinosauria) (represented by Silesaurus); and D, base of Saurischia (represented by Herrerasaurus). Silhouettes credit: Phylopic/Scott Hartman/Mathew Wedel, which is licensed under CC BY 3.0. Silhouette of Kongonaphon to the right of the taxon label is to scale (adapted from Fig. 1).
Previous analyses of body-size evolution in Triassic archosaurs (e.g., refs. 7, 34) included only a limited sample of basal avemetatarsalians (as few were known at the time), which does not reflect current knowledge of the size range of this grade. The discovery of nonornithodiran avemetatarsalians such as Teleocrater and other aphanosaurs has provided evidence for larger-bodied taxa at the very base of Avemetatarsalia, and at the other end of the size range, taxa such as Kongonaphon and Scleromochlus are among the smallest nonavian avemetatarsalians known (7, 9). Integrating data for Kongonaphon, Scleromochlus, and Aphanosauria (22) into a body size dataset for archosaurs (34) indicates that an abrupt and pronounced miniaturization event occurred near the base of Ornithodira (Fig. 2). The exact scale and scope of this miniaturization event are somewhat variable, depending on the underlying tree topology and divergence dates used, but a sharp reduction in body size between Archosauria and Ornithodira or its immediate subtaxa is recovered in all analyses (SI Appendix, Tables S1 and S2). Treating lagerpetids as dinosauromorphs, Scleromochlus as a pterosauromorph, and enforcing ornithodiran divergence in the Early Triassic—which has been argued based on footprint records (43), although substantial uncertainty exists as to the trackmaker identification of these prints—yields the most extreme miniaturization pattern, with the ancestral ornithodiran (∼63-mm femur length) estimated to be less than half the size of the ancestral archosaur (∼133 mm). In this analysis, the earliest-diverging dinosauromorphs are also miniaturized (∼65 mm) compared with the ancestral archosaur, and the earliest-diverging pterosauromorphs occupy the lower reaches of nonavian archosaur size (∼34 mm). When both Scleromochlus and lagerpetids are treated as pterosauromorphs, there is still some reduction in size between the ancestral archosaur (∼170 mm) and the ancestral ornithodiran (∼140 mm) and dinosauromorph (∼138 mm), but evidence for miniaturization is restricted to the pterosauromorph branch (∼65 mm). As discussed above, we currently consider either of these topologies to be possible but would note that the more extreme results of the first analysis more closely accord with observed sizes in Dinosauriformes, in which the earliest-diverging taxon (Lagosuchus) is also the smallest Triassic member of the clade (57-mm femur length). Other variations in topology, removal of Kongonaphon or Scleromochlus from the analysis, or increasing femoral length for these genera (to simulate potential larger maximum body size) yield results within the bounds of the aforementioned two analyses (see SI Appendix for full details).
The selective advantages underlying observed miniaturization within Ornithodira are obscure. Archosaurs diversified in the wake of the end-Permian mass extinction, an event correlated with postextinction size reduction (Lilliput effect) in several animal clades (44). The Lilliput effect is unlikely to account for ornithodiran size diminution, however, given that coeval archosauriform clades (proterosuchids, erythrosuchids, etc.) diversified at comparatively large sizes, and the earliest-diverging clade within Avemetatarsalia (Aphanosauria) consists of relatively large animals (22). It is notable, though, that small-bodied ornithodirans only appeared following the extinction of insectivorous synapsids following the end-Permian mass extinction. The marginal dentition of Kongonaphon is characterized by close-packed, unserrated, conical teeth. This dental morphology is atypical for Triassic archosauromorphs and otherwise unknown in early avemetatarsalians. Scleromochlus also is characterized by a close-packed, isodont dentition, but preservation of known specimens is too poor to indicate fine details of its teeth, such as whether serrations were present. In general, the dental morphology of Kongonaphon most closely resembles that of small-bodied extant squamates specializing in arthropod prey, suggesting insectivory in Kongonaphon. Potential insectivory in Kongonaphon also is supported by microwear on the labial surface of the fifth preserved maxillary tooth, which exhibits fine pitting (SI Appendix, Fig. S5) characteristic of resistant foodstuffs such as insect cuticle (45). Among avemetatarsalians, discovery of taxa occupying this ecological niche demonstrates a growing pattern that the closest relatives of dinosaurs were more trophically diverse than previously suspected (22, 23). Together, diversification of dental morphology and reduction in body size may have enabled avemetatarsalians to invade resource zones not previously occupied by archosaurs.
Whatever the evolutionary impetus of ornithodiran miniaturization, this marked shift in body size has several important implications for understanding the evolution and fossil record of the group. The small size estimated for the ancestral ornithodiran (or at least pterosauromorph), and the persistence of small body sizes among early ornithodirans (e.g., Kongonaphon and the basal dinosauriform Lagosuchus), may account for the perplexing absence of ornithodirans in well-known Early and Middle Triassic faunas (46, 47) worldwide. Although footprint evidence has been argued to support the presence of ornithodirans in the Early–Middle Triassic of Europe (43), no definitive skeletal fossils from the clade are known from this interval. Our results suggest that this absence can be explained as a taphonomic consequence of the extremely small body size of early members of the group (a known correlate of poor preservation potential; ref. 48), rather than a true indication of their scarcity or geographic restriction. It is telling that for decades, most known basal ornithodirans were from the Los Chañares Formation, a Lagerstätte that preferentially preserves small-bodied animals (49). Although rare in collections, basal ornithodirans may have been substantially more abundant in their ecosystems than the known record indicates, highlighting the need for greater sampling of Triassic sites preserving small tetrapod fossils.
In addition to opening a niche (small-bodied insectivore) previously unavailable to archosaurs, reduced body size in early ornithodirans may bear on key aspects of the later evolutionary success of dinosaurs and pterosaurs. Since miniaturization appears to be a necessary precursor to the development of flight in vertebrates (13), the origin of pterosaurs, the first vertebrates capable of powered flight, is likely related to their ancestry among already-small-bodied early ornithodirans (50). Although the origin of an erect, bipedal gait (which characterizes dinosaurs ancestrally) is not restricted to amniotes of small body size (see, e.g., shuvosaurids among crocodile-line archosaurs; ref. 51), initial cursorial or possibly saltatorial habits in small-bodied early ornithodirans would also explain the bipedal ancestry of Triassic dinosaurs (an important aspect of their radiation, regardless of whether it conferred any competitive advantage over coeval crocodile-line archosaurs). Finally, small body size makes heat retention difficult in vertebrates, a serious problem given the climatic extremes characteristic of the Triassic (52). If the filamentous body covering now known to be present in pterosaurs and various dinosaur groups is homologous, as has been argued recently (53), it likely originated as insulation in small-bodied ancestral ornithodirans, as has been invoked to explain the origin of fur at the same time in the ancestors of mammals (54).
Methods
Phylogenetic Analysis.
The phylogenetic data matrix consists of codings for 422 characters and 90 archosauromorph taxa (predominantly Triassic archosaurs). Data were analyzed in PAUP* Version 4.0a (build 165) (55) using heuristic searching. Two primary analyses were run, the first excluding S. taylori and the second including it. Bootstrap values were produced using “fast” stepwise addition on 10,000 replicates. Refer to Dataset S1 for the matrix and the SI Appendix for full details of the analysis, including discussion of modifications made to previous versions of the data matrix.
Ancestral Body Size Estimation.
Data for ancestral body size estimation were taken from a previous analysis (34) with the addition of a variety of recently recognized avemetatarsalian taxa (see SI Appendix for details). Size data consist of maximum known femoral lengths, log-transformed for inclusion in the analysis. Ancestral body-size estimation was performed using the “fastAnc” function in the R package “phytools” (56).
Nomenclatural Acts.
This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank Life Science Identifiers (LSIDs) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix zoobank.org/. The LSIDs for this publication are as follows: 5D376FFA-D2FC-4433-A6F9-39C5F94DE403; BE48486B-E8F2-48BA-B49C-57775A9C43F4; FA34A83C-46C8-4F76-A1F0-C7B47A76E7D3.
Supplementary Material
Acknowledgments
We are grateful for long-term collaborations with the Département de Paléontologie et d’Anthropologie Biologique, Université d’Antananarivo, Madagascar. We thank Frank Ippolito for his drawings and reconstruction. We thank Christopher Griffin for discussions about growth in early avemetatarsalians and Sarah Werning for detailed photographs of the osteohistological sections. This research was supported by a Gerstner Scholars Fellowship from the Gerstner Family Foundation and the Richard Gilder Graduate School (to C.F.K.); the Division of Paleontology of the American Museum of Natural History; National Geographic Society Grant 6271-98, for expeditionary support (to J.J.F. and A.R.W.); and the World Wide Fund for Nature, Madagascar, for expedition logistical support. S.J.N.’s research at the Field Museum of Natural History was supported by a Meeker Family Fellowship.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the International Code of Zoological Nomenclature. The ZooBank Life Science Identifiers (LSIDs) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://www.zoobank.org/. The LSIDs for this publication are as follows: 5D376FFA-D2FC-4433-A6F9-39C5F94DE403; BE48486B-E8F2-48BA-B49C-57775A9C43F4; FA34A83C-46C8-4F76-A1F0-C7B47A76E7D3.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1916631117/-/DCSupplemental.
Data Availability.
The data supporting the findings of this study are available in Datasets 1–4. The specimen UA 10618 belongs to the collections of the Université d’Antananarivo, Antananarivo, Madagascar.
References
- 1.Klein N. et al., Biology of the Sauropod Dinosaurs: Understanding the Life of Giants, (University of Indian Press, Bloomington, IN, 2011). [Google Scholar]
- 2.Sander P. M. et al., Biology of the sauropod dinosaurs: The evolution of gigantism. Biol. Rev. Camb. Philos. Soc. 86, 117–155 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lacovara K. J. et al., A gigantic, exceptionally complete titanosaurian sauropod dinosaur from southern Patagonia, Argentina. Sci. Rep. 4, 6196 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carballido J. L. et al., A new giant titanosaur sheds light on body mass evolution among sauropod dinosaurs. Proc. Biol. Sci. 284, 20171219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janis C. M., Carrano M., Scaling of reproductive turnover in archosaurs and mammals: Why are large terrestrial mammals so rare? Acta Zool. Fenn. 28, 201–216 (1992). [Google Scholar]
- 6.Smith F. A. et al., Similarity of mammalian body size across the taxonomic hierarchy and across space and time. Am. Nat. 163, 672–691 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Sookias R. B., Butler R. J., Benson R. B. J., Rise of dinosaurs reveals major body-size transitions are driven by passive processes of trait evolution. Proc. Biol. Sci. 279, 2180–2187 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benson R. B. J. et al., Rates of dinosaur body mass evolution indicate 170 million years of sustained ecological innovation on the avian stem lineage. PLoS Biol. 12, e1001853 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irmis R. B., Evaluating hypotheses for the early diversification of dinosaurs. Earth Environ. Sci. Trans. R. Soc. Edinb. 101, 397–426 (2011). [Google Scholar]
- 10.Pearson O. P., Metabolism of small mammals, with remarks on the lower limit of mammalian size. Science 108, 44 (1948). [DOI] [PubMed] [Google Scholar]
- 11.Alexander R. M., Optima for Animals, (Princeton University Press, Princeton, NJ, 1996). [Google Scholar]
- 12.Orme C. D. L. et al., Body size does not predict species richness among the metazoan phyla. J. Evol. Biol. 15, 235–247 (2002). [Google Scholar]
- 13.Lee M. S. Y., Cau A., Naish D., Dyke G. J., Sustained miniaturization and anatomical innovation in the dinosaurian ancestors of birds. Science 345, 562–566 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Dunning J. B., Jr., CRC Handbook of Avian Body Masses, (CRC Press, Boca Raton, FL, 2007). [Google Scholar]
- 15.Zanno L. E., Makovicky P. J., No evidence for directional evolution of body mass in herbivorous theropod dinosaurs. Proc. Biol. Sci. 280, 20122526 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nesbitt S. J., Liu J., Li C., A sail-backed suchian from the Heshanggou formation (Early Triassic: Olenekian) of China. Earth Environ. Sci. Trans. R. Soc. Edinb. 101, 271–284 (2011). [Google Scholar]
- 17.Brusatte S. L., Benton M. J., Ruta M., Lloyd G. T., Superiority, competition, and opportunism in the evolutionary radiation of dinosaurs. Science 321, 1485–1488 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Nesbitt S. J. et al., Ecologically distinct dinosaurian sister group shows early diversification of Ornithodira. Nature 464, 95–98 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Sereno P. C., Arcucci A. B., Dinosaurian precursors from the Middle Triassic of Argentina: Lagerpeton chanarensis. J. Vertebr. Paleontol. 13, 385–399 (1993). [Google Scholar]
- 20.Sereno P. C., Arcucci A. B., Dinosaurian precursors from the Middle Triassic of Argentina: Marasuchus lilloensis, gen. nov. J. Vertebr. Paleontol. 14, 53–73 (1994). [Google Scholar]
- 21.Benton M. J., Scleromochlus taylori and the origin of dinosaurs and pterosaurs. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354, 1423–1446 (1999). [Google Scholar]
- 22.Nesbitt S. J. et al., The earliest bird-line archosaurs and the assembly of the dinosaur body plan. Nature 544, 484–487 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Dzik J., A beaked herbivorous archosaur with dinosaur affinities from the early Late Triassic of Poland. J. Vertebr. Paleontol. 23, 556–574 (2003). [Google Scholar]
- 24.Irmis R. B. et al., A Late Triassic dinosauromorph assemblage from New Mexico and the rise of dinosaurs. Science 317, 358–361 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Langer M. C., Ezcurra M. D., Bittencourt J. S., Novas F. E., The origin and early evolution of dinosaurs. Biol. Rev. Camb. Philos. Soc. 85, 55–110 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Nesbitt S. J., et al. , The anatomy of Teleocrater rhadinus, an early avemetatarsalian from the lower portion of the Lifua member of the Manda beds (Middle Triassic). J. Vertebr. Paleontol. 37, 142–177 (2018). [Google Scholar]
- 27.Müller R. T., Langer M. C., Dias-da-Silva S., Ingroup relationships of Lagerpetidae (Avemetatarsalia: Dinosauromorpha): A further phylogenetic investigation on the understanding of dinosaur relatives. Zootaxa 4392, 149–158 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Cabreira S. F. et al., A unique Late Triassic dinosauromorph assemblage reveals dinosaur ancestral anatomy and diet. Curr. Biol. 26, 3090–3095 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Nesbitt S. J. et al., Hindlimb osteology and distribution of basal dinosauromorphs from the Late Triassic of North America. J. Vertebr. Paleontol. 29, 498–516 (2009). [Google Scholar]
- 30.Martínez R. N. et al., A Norian lagerpetid dinosauromorph from the Quebrada del Barro Formation, northwestern Argentina. Ameghiniana 53, 1–13 (2016). [Google Scholar]
- 31.Martínez R. N. et al., Vertebrate succession in the Ischigualasto formation. J. Vertebr. Paleontol. 12, 10–30 (2013). [Google Scholar]
- 32.Garcia M. S. et al., The oldest known co-occurrence of dinosaurs and their closest relatives: A new lagerpetid from a Carnian (Upper Triassic) bed of Brazil with implications for dinosauromorph biostratigraphy, early diversification and biogeography. J. S. Am. Earth Sci. 91, 302–319 (2019). [Google Scholar]
- 33.Flynn J. J. et al., A Triassic fauna from Madagascar, including early dinosaurs. Science 286, 763–765 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Turner A. H., Nesbitt S. J., Body size evolution during the Triassic archosauriform radiation. Geol. Soc. Lond. Spec. Publ. 379, 573–597 (2013). [Google Scholar]
- 35.Cope E. D., Synopsis of the extinct Batrachia, Reptilia and Aves of North America. Trans. Am. Philos. Soc. 14, 1–252 (1869). [Google Scholar]
- 36.Arcucci A. B., Nuevos materiales y reinterpretación de Lagerpeton chanarensis Romer (Thecodontia, Lagerpetonidae nov.) del Triásico medi de La Rioja, Argentina. Ameghiniana 23, 233–242 (1986). [Google Scholar]
- 37.Flynn J. J. et al., New traversodontids (Synapsida: Eucynodontia) from the Triassic of Madagascar. J. Vertebr. Paleontol. 20, 422–427 (2000). [Google Scholar]
- 38.Schmitt M. R., et al. , On the occurrence of the traversodontid Massetognathus ochagaviae (Synapsida, Cynodontia) in the early late Triassic Santacruzodon Assemblage Zone (Santa Maria Supersequence, southern Brazil): Taxonomic and biostratigraphic implications. J. S. Am. Earth Sci. 93, 36–50 (2019). [Google Scholar]
- 39.Ezcurra M. D. et al., Deep faunistic turnovers preceded the rise of dinosaurs in southwestern Pangaea. Nat. Ecol. Evol. 1, 1477–1483 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Dyke G. J., Nudds R. L., Rayner J. M., Limb disparity and wing shape in pterosaurs. J. Evol. Biol. 19, 1339–1342 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Cerda I. A., Pol D., Chinsamy A., Osteohistological insight into the early stages of growth in Mussaurus patagonicus (Dinosauria, Sauropodomorpha). Hist. Biol. 26, 110–121 (2014). [Google Scholar]
- 42.Nesbitt S. J., The early evolution of archosaurs: Relationships and the origin of major clades. Bull. Am. Mus. Nat. Hist. 352, 1–292 (2011). [Google Scholar]
- 43.Brusatte S. L., Niedźwiedzki G., Butler R. J., Footprints pull origin and diversification of dinosaur stem lineage deep into Early Triassic. Proc. Biol. Sci. 278, 1107–1113 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Twitchett R. J., The Lilliput effect in the aftermath of the end-Permian extinction event. Palaeogeogr. Palaeoclimatol. Palaeoecol. 252, 132–144 (2007). [Google Scholar]
- 45.Taylor M. E., Hannam A. G., Tooth microwear and diet in the African Viverridae. Can. J. Zool. 65, 1696–1702 (1987). [Google Scholar]
- 46.Benton M. J. et al., The Age of Dinosaurs in Russia and Mongolia, (Cambridge University Press, Cambridge, UK, 2000). [Google Scholar]
- 47.Damiani R., et al. , Barendskraal, a diverse amniote locality from the Lystrosaurus Assemblage Zone, Early Triassic of South Africa. Palaeontol. Afr. 39, 53–62 (2003). [Google Scholar]
- 48.Kidwell S. M., Flessa K. W., The quality of the fossil record: Populations, species, and communities. Annu. Rev. Ecol. Syst. 26, 269–299 (1995). [Google Scholar]
- 49.Rogers R. R., et al. , Paleoenvironment and taphonomy of the Chañares Formation tetrapod assemblage (Middle Triassic), northwestern Argentina: Spectacular preservation in volcanogenic concretions. Palaios 16, 461–481 (2001). [Google Scholar]
- 50.Dalla Vecchia F. M., Triassic pterosaurs. Geol. Soc. Lond. Spec. Publ. 379, 119–155 (2013). [Google Scholar]
- 51.Nesbitt S. J., Norell M. A., Extreme convergence in the body plans of an early suchian (Archosauria) and ornithomimid dinosaurs (Theropoda). Proc. Biol. Sci. 273, 1045–1048 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winguth A. M. E., Shields C. A., Winguth C., Transition into a hothouse world at the Permian-Triassic boundary—A model study. Palaeogeogr. Palaeoclimatol. Palaeoecol. 440, 316–327 (2015). [Google Scholar]
- 53.Yang Z. et al., Pterosaur integumentary structures with complex feather-like branching. Nat. Ecol. Evol. 3, 24–30 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Hopson J. A., Endothermy, small size, and the origin of mammalian reproduction. Am. Nat. 107, 446–452 (1973). [Google Scholar]
- 55.Swofford D. L., PAUP*, Phylogenetic Analysis Using Parsimony (*and Other Methods) (Version 4, Sinauer Associates, Sunderland, MA, 2002).
- 56.Revell G., phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available in Datasets 1–4. The specimen UA 10618 belongs to the collections of the Université d’Antananarivo, Antananarivo, Madagascar.