Significance
Red blood cells perform the unique function of capturing certain pathogens in blood and presenting them to the immune cells in the spleen. We developed a strategy based on this innate immune function of red blood cells to deliver vaccine nanoparticles to the spleen. This biomimetic strategy induced a strong vaccination response without the need for foreign adjuvants.
Keywords: biomimetic, spleen targeting, immunization, vaccination, erythrocyte hitchhiking
Abstract
Erythrocytes naturally capture certain bacterial pathogens in circulation, kill them through oxidative stress, and present them to the antigen-presenting cells (APCs) in the spleen. By leveraging this innate immune function of erythrocytes, we developed erythrocyte-driven immune targeting (EDIT), which presents nanoparticles from the surface of erythrocytes to the APCs in the spleen. Antigenic nanoparticles were adsorbed on the erythrocyte surface. By engineering the number density of adsorbed nanoparticles, (i.e., the number of nanoparticles loaded per erythrocyte), they were predominantly delivered to the spleen rather than lungs, which is conventionally the target of erythrocyte-mediated delivery systems. Presentation of erythrocyte-delivered nanoparticles to the spleen led to improved antibody response against the antigen, higher central memory T cell response, and lower regulatory T cell response, compared with controls. Enhanced immune response slowed down tumor progression in a prophylaxis model. These findings suggest that EDIT is an effective strategy to enhance systemic immunity.
Erythrocytes, accounting for over 80% of cells in the human body, serve the primary function of oxygen delivery to tissues. In addition to oxygen transport, erythrocytes also perform several additional functions that are of high immunological relevance. For example, upon reaching the end of their natural lifespan, senescent erythrocytes are phagocytosed in the spleen in a noninflammatory pathway (1). This unique mechanism has been elegantly exploited to develop tolerance to antigens for applications in autoimmune disorders and reducing anti-drug antibody production (2–4). Specifically, antigenic peptides, attached to erythrocyte membranes, are captured in the spleen along with senescent erythrocytes, thus generating a tolerogenic response to antigens due to the noninflammatory pathway of capture unique to erythrocytes.
Recently, erythrocytes have been implicated in another interesting and contrasting innate immune function (5, 6). Specifically, they capture immune complexes and bacteria in circulation on their surface and hand them to Kupffer cells in the liver and professional antigen-presenting cells (APCs) in the spleen without the capture of the carrier erythrocyte (7–11). Bacterial species in the blood such as Staphylococcus and Propionibacterium attach to erythrocyte membrane due to electrostatic attraction and are killed by oxycytosis by the carrier erythrocyte. Thereafter, erythrocytes hand them over to the cells in the liver and spleen, without themselves being sequestered (9, 12). While the exact mechanism of selective cargo uptake by APCs is unclear, transient membrane alteration induced by the bacterial cargo is implicated in the increased cross talk between the erythrocytes and APCs (13, 14). Here, we leverage this innate and unique ability of erythrocytes to present antigens in the spleen to develop a biomimetic strategy for generating cellular and humoral immune responses to antigens (Fig. 1).
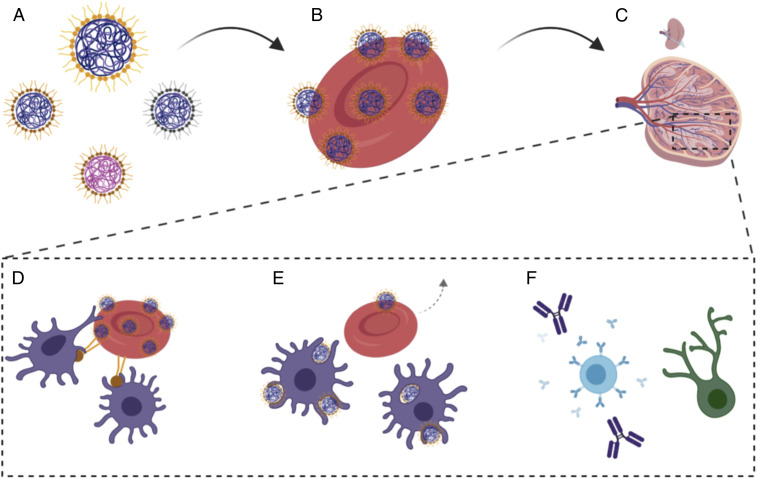
Fig. 1.
Schematic for engineering a handoff of nanoparticles at the spleen via erythrocyte hitchhiking. (A) Protein-capped polymeric nanoparticles used for the study (different sizes, materials, or coated with different proteins). (B) The number of nanoparticles loaded on erythrocytes was tuned for protein loading and to induce temporary up-regulation of phosphatidylcholine. (C) Intravenous injection of hitchhiked nanoparticles leads to high discharge in the spleen. (D) Up-regulated phosphatidylcholine and masking CD47 improves interactions with antigen-presenting cells in the spleen. (E) Improved erythrocyte interactions facilitate nanoparticle uptake by the APCs while the erythrocytes return back to the circulation. (F) Handoff of nanoparticles at the spleen improves both humoral and cellular immune responses.
Attachment of molecules to erythrocytes has been leveraged for several biomedical applications (15). A range of payloads including proteins (2–4), therapeutics (16), and nanoparticles (17–19) have been attached to the erythrocyte surface or encapsulated within erythrocytes (20) for various therapeutic applications. The attachment of cargo to the erythrocyte surface has been brought about by chemical conjugation (16), binding to specific receptors like glycophorin A (4), sortagging (2), or passive adsorption (19), without compromising their physiological function of oxygen transport. All previous approaches of hitchhiking on erythrocytes are based on induction of minimal perturbation to the carrier erythrocytes, which has led to either their extended circulation or capture in the capillary endothelia after injection (17, 19, 21). Here, we engineered a hitchhiking system that induces the delivery of the attached nanoparticles predominantly to the spleen instead of lungs to achieve cellular and humoral immunity, a process that we refer to as erythrocyte-driven immune targeting (EDIT).
Results
Synthesis and Characterization of Antigenic Cargo.
Ovalbumin (OVA) was selected as a model antigen and was capped on the surface of 200-nm polystyrene carboxylate (PS-COOH) to generate protein-capped nanoparticles (NPs) that were attached to erythrocytes (Fig. 2A). OVA was attached to 200-nm NPs using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) chemistry, as previously reported (22). Loading of OVA on NPs could be controlled over a wide range (SI Appendix, Fig. S1); however, an intermediate loading of ∼43 μg/mg of particles was used for the remainder of the studies (Fig. 2B). OVA attachment to nanoparticles was confirmed by size and zeta-potential measurements. OVA attachment increased the hydrodynamic size of NPs from 191 to 234 nm (Fig. 2C). Further, conjugation of the carboxylate groups on the NPs was also evident from the decrease in zeta potential from −40.4 to −21.4 mV (Fig. 2D). OVA conjugation did not affect NP polydispersity (Fig. 2E). This was further confirmed by performing scanning electron microscopy (SEM). SEM images of plain and conjugated nanoparticles show monodisperse nanoparticles (Fig. 2F) in both cases, indicating that OVA conjugation had a negligible effect on polydispersity.
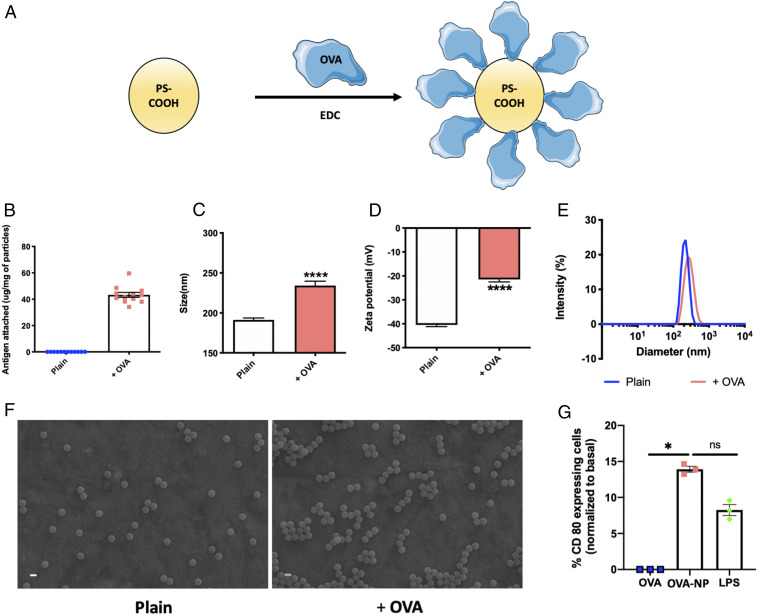
Fig. 2.
Characterization of protein-capped nanoparticles. (A) Scheme of protein attachment to polystyrene carboxylate nanoparticles using EDC chemistry. (B) Amount of antigen attached to PS-COOH (n = 12). (C) Particle size in nanometers of plain and protein-capped nanoparticles (n = 6). Significantly different (Student’s t test): ****P < 0.0001. (D) Zeta potential in millivolts of plain and protein-capped nanoparticles (n = 6). Significantly different (Student’s t test): ****P < 0.0001. (E) Particle size distribution of plain and protein-capped nanoparticles. (F) Scanning electron micrographs of plain and protein-capped nanoparticles. (Scale bars, 200 nm.) (G) Dendritic cell maturation evaluated in terms of % CD80 expression (normalized to basal expression) (n = 3 for all groups). Significantly different (one-way ANOVA followed by Tukey’s HSD test): *P < 0.05; ns, not significantly different. Data in B–D and G are expressed as mean ± SEM.
Apart from characterization of physicochemical properties, we also characterized the OVA-NPs for internalization by and activation of dendritic cells. Both OVA and OVA-NPs were taken up by dendritic cells (SI Appendix, Fig. S2A). However, OVA-NPs were taken up in significantly higher quantities compared with free OVA, which was also confirmed by confocal scanning laser microscopy images (SI Appendix, Fig. S2B). Costimulatory effects on dendritic cells, evaluated in terms of CD80 up-regulation, revealed that OVA-NPs significantly up-regulated CD80 expression compared with their soluble counterpart and were comparable to the positive-control lipopolysaccharide (LPS) (Fig. 2G). We also capped 500-nm PS with OVA, 200-nm poly(lactic-co-glycolic acid) (PLGA) with OVA (PLGA-OVA-200), and 200-nm PS-COOH with subunit 1 of keyhole limpet hemocyanin (KLH) (PS-KLH-200) to confirm the generality of this approach. Respective proteins were attached to different particle types using the same EDC chemistry. Physicochemical properties of these combination particles were evaluated (SI Appendix, Table S1) and these particles were also characterized for their ability to get internalized by the dendritic cells and consequently activate them (SI Appendix, Fig. S3). All particles were monodispersed and showed excellent internalization by and activation of dendritic cells. Though bare nanoparticles are themselves capable of maturating the cells (23), they are not of specific consequence in assessing the benefits of hitchhiking OVA-NPs and hence were not included in the study.
Engineering Nanoparticle–Erythrocyte Hitchhiking to Achieve a Handoff in the Spleen.
Hitchhiking of nanoparticles occurs through two steps that are physical in nature (17, 19, 24): adsorption of nanoparticles on the erythrocyte surface to initiate contact, and spreading of the membrane around the nanoparticles to enhance adhesion strength. Either one of them is not sufficient. If nanoparticles do not make contact with the erythrocyte, the adhesion is not initiated, and if the membrane spreading is inhibited, the adhesion is weak and the nanoparticles fall off during washing. Introduction of competitor proteins (serum) during attachment essentially inhibits the hitchhiking. This inhibitory effect is seen even at a 25% addition of serum. At the same time, by using glutaraldehyde-fixed red blood cells (RBCs), our data demonstrate that rigidifying the membrane nearly eliminates hitchhiking (SI Appendix, Fig. S4). Detailed mechanistic studies of the hitchhiking process, such as varying the method of membrane rigidification through different degrees of cross-linking or heat inactivation, will need to be carried out in future studies to investigate the role of active adsorption. As the NP:erythrocyte ratio during incubation increased from 75:1 to 300:1, the number of nanoparticles that attached to erythrocytes increased from 12 to 24 per cell. However, further increasing the ratio to 600:1 surprisingly decreased nanoparticle loading to about 18 per erythrocyte, possibly due to the presence of excessive nanoparticles in the hitchhiking suspension, thereby hampering the necessary erythrocyte–nanoparticle interactions (Fig. 3A). The presence of nanoparticles on the erythrocytes was confirmed by SEM (SI Appendix, Fig. S5) and flow cytometry analyses of hitchhiked erythrocytes. Particularly, the percentage of erythrocytes carrying nanoparticles increased from 68% at a ratio of 75:1 to >95% at a ratio of 600:1 (Fig. 3B).
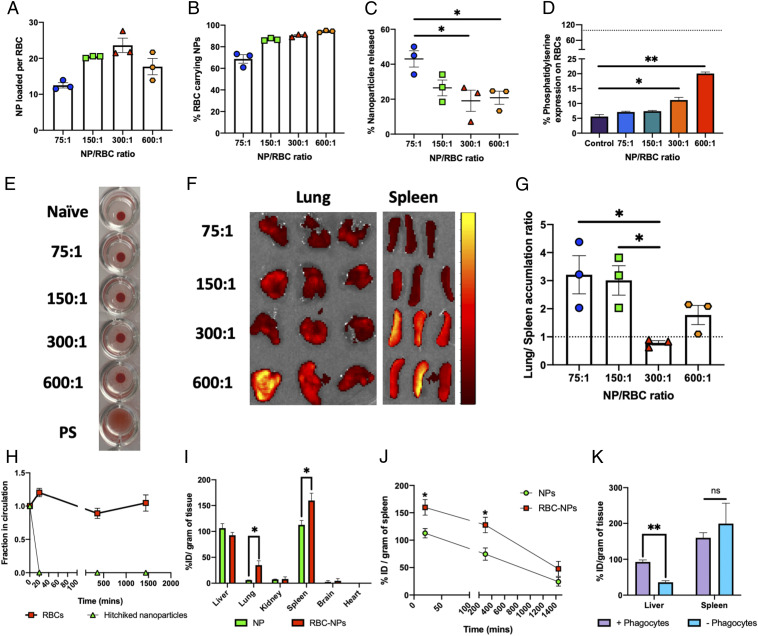
Fig. 3.
Engineering nanoparticle–erythrocyte–hitchhiking parameters to achieve spleen targeting. (A) Nanoparticles loaded per erythrocyte for different feed ratios of nanoparticles to erythrocytes (n = 3 for all groups). (B) Percentage of erythrocytes carrying nanoparticles (determined by flow cytometry) for different feed ratios of nanoparticles to erythrocytes (n = 3 for all groups). (C) Percentage of nanoparticles released from erythrocytes following in vitro shear studies at the lung corresponding to shear stress (6 Pa). Significantly different (one-way ANOVA followed by Tukey’s HSD test): *P < 0.05. (D) Erythrocyte damage caused by nanoparticles, evaluated by changes in percentage of phosphatidylserine expression, for different feed ratios of nanoparticles to erythrocytes (n = 3 for all groups). The dotted line indicates positive-control (polystyrene beads) mean value. Significantly different (one-way ANOVA followed by Tukey’s HSD test): *P < 0.05, **P < 0.01. (E) Optical agglutination assay demonstrating minimal aggregates induced by nanoparticles to erythrocytes. All of the tested nanoparticle-to-erythrocyte ratios were similar to naïve control as opposed to polystyrene beads which induced matrix-shaped aggregates. (F) IVIS images of lungs and spleen harvested from mice 20 min after being injected with erythrocytes incubated at different nanoparticle-to-erythrocyte ratios. The scale indicates low (maroon) to high (bright yellow) radiant efficiency. (G) Lung-to-spleen accumulation ratios computed by using radiant efficiencies of these organs from IVIS imaging (n = 3 for all groups). The dotted line indicates equal lung and spleen accumulation. Significantly different (one-way ANOVA followed by Tukey’s HSD test): *P < 0.05. (H) Fraction of particles and erythrocytes remaining in circulation, evaluated by their parallel tracking using flow cytometry (n = 5). (I) Biodistribution of free nanoparticles and hitchhiked nanoparticles in different organs, expressed in terms of % injected dose per gram of tissue, harvested 20 min after injection (n = 3 for all groups). Significantly different (Student’s t test): *P < 0.05. (J) Kinetics of spleen accumulation of free and hitchhiked nanoparticles monitored over 24 h after injection (n = 3 for all groups). Significantly different (Student’s t test): *P < 0.05. (K) Effect of phagocyte depletion on hitchhiked nanoparticle biodistribution in the two most important organs of the mononuclear phagocytic system, 20 min after injection (n = 3 for all groups). Significantly different (Student’s t test): **P < 0.01. Data in A–D and G–J are expressed as mean ± SEM.
Erythrocyte hitchhiking has been previously explored for lung targeting since the nanoparticles on the erythrocyte surface are sheared off in the lungs owing to high shear stress and squeezing of erythrocytes due to close contact with the endothelium in lung capillaries (17, 18). Reducing lung uptake is essential to enable nanoparticle-carrying erythrocytes to escape lungs and deliver their cargo to other organs, in this case, spleen. To that effect, we tested the in vitro shear resistance of hitchhiked nanoparticles as a function of NP:erythrocyte ratio at a shear stress of 6 Pa, which corresponds to lung capillaries. Release of NPs from erythrocytes decreased with increasing loading from 75:1 to 600:1 (Fig. 3C), likely due to the stiffening of erythrocytes at high particle loadings (24). Thus, sufficient fluidity/shear resistance at higher nanoparticle loadings is needed to escape the mechanical dislodgement of particles in the lungs.
Spleen targeting was mediated by maintaining sufficient loading, shear resistance to escape mechanical dislodgement in the lungs, and induction of erythrocyte membrane alterations to prompt capture in the spleen. The extent of alterations in the erythrocyte in the membrane was controlled by NP dose. Erythrocyte membrane alteration was quantified in terms of expression of phosphatidylserine on the erythrocyte membrane. Incubation of erythrocytes at NP:erythrocyte ratios of 300:1 and 600:1 caused a moderate increase in the expression of phosphatidylserine compared with unloaded naïve erythrocytes (Fig. 3D). The hitchhiking process also decreased CD47 expression, possibly due to physical masking by the nanoparticles (SI Appendix, Fig. S6A). Further, an optical agglutination assay indicated that there is no visual aggregation/rouleau formation of erythrocytes incubated with nanoparticles compared with positive-control polystyrene beads which formed matrix-shaped aggregates (Fig. 3E). The lack of aggregation indicates that NP-hitchhiking erythrocytes can be injected in vivo (25).
The effect of nanoparticle loading on in vivo nanoparticle distribution was evaluated by performing biodistribution 20 min after intravenous (i.v.) injection of all different loading ratios but injecting the same volume of erythrocytes. Fluorescence intensities of harvested organs, particularly lungs and spleen, were evaluated (Fig. 3F). Low NP:erythrocyte ratios (75:1 and 150:1) led to a high lung:spleen accumulation ratio (∼3) whereas high loading (NP:erythrocyte ratio of 300:1) showed higher spleen accumulation than lung accumulation (lung:spleen ratio ∼0.8). Increasing the ratio further to 600:1 again favored lung accumulation, possibly due to lower nanoparticle attachment than that of 300:1 (Fig. 3A). The phosphatidylserine expression data (Fig. 3D) indicated that the erythrocyte membrane is most impacted at an incubation ratio of 600:1. Collectively, these findings suggest that 300:1 is the optimal loading ratio for spleen targeting (Fig. 3G). Hence, an NP:erythrocyte ratio of 300:1 was selected for the remainder of the studies. The lung:spleen accumulation ratio for our optimal system is less than 1 (<40% injected dose [ID] per gram in lungs). This ratio in a typical study on erythrocyte-hitchhiking nanoparticles targeting the lungs is ∼10 (to >100% ID per gram in the lungs) (17). We also evaluated the pharmacokinetics of the injected hitchhiked nanoparticles (NP:erythrocyte ratio of 300:1) by separately tracking erythrocytes and nanoparticles by flow cytometry. The fraction of injected erythrocytes did not change with time (≤24 h) after injection, while the hitchhiked nanoparticles rapidly disappeared out of the bloodstream with less than 1% remaining in the circulation as early as 20 min after the injection, suggesting rapid clearance from the bloodstream (Fig. 3H). This clearly indicated that erythrocytes were able to quickly deliver their payloads to specific organs while themselves resisting clearance, possibly due to a decrease in the phosphatidylserine expression on hitchhiked erythrocytes after nanoparticle handoff (SI Appendix, Fig. S6B).
Next, we performed a time-course biodistribution of hitchhiked nanoparticles at 20 min, 6 h, and 24 h after i.v. injection and compared it with the biodistribution of equivalent free nanoparticles (Fig. 3I and SI Appendix, Fig. S7). Free nanoparticles accumulated in the liver and spleen. Erythrocyte-hitchhiked NPs exhibited higher spleen accumulation (Fig. 3J). A splenic dose of ∼150% ID per gram was achieved using erythrocyte hitchhiking. The higher accumulation (∼1.5-fold improvement over control) in spleen was significant even after 6 h compared with free nanoparticles and was maintained for up to 24 h after injection (Fig. 3J). Further studies revealed that other particle combinations studied were also able to induce transient damage and this strategy was capable of carrying out handoffs for a variety of particles (SI Appendix, Fig. S8).
To assess whether the nanoparticles delivered by erythrocytes to the spleen are picked up by phagocytes or by professional APCs, we carried out phagocyte depletion in mice using clodronate (26) and performed biodistribution at 20 min post injection of the hitchhiked nanoparticles, and two immunologically active organs, liver and spleen, were evaluated for changes in delivery efficiency. Clodronate liposomes transiently incapacitate the macrophages in the reticuloendothelial system in hepatic sinuses and spleen (26). This intervention leads to delegation of the functions of recognition, phagocytosis, and presentation of foreign compounds to other cells including dendritic cells taking over antigen-presenting functions in the host defense. Phagocyte depletion significantly reduced liver uptake (∼2.5-fold) but caused no significant change in splenic uptake, indicating that nanoparticles in the spleen are viable and internalized by APCs and not phagocytosed (Fig. 3K).
Immunological Consequences of Nanoparticle Handoff in the Spleen.
We characterized both the humoral and cellular responses of hitchhiked nanoparticles delivered to the spleen from the erythrocyte surface. For humoral immunity, we used a vaccination schedule comprising one injection per week for 3 wk followed by two alum-based humoral challenges (Fig. 4A). Anti-OVA antibody (immunoglobulin G; IgG) titer, 1 d before the challenge (day −1), indicated no significant differences between hitchhiked OVA-NPs, free OVA-NPs, or soluble OVA. Antibody titers evaluated 13 d after the last challenge were highest for hitchhiked OVA-NPs (EDIT), and significantly higher than those for free nanoparticles (∼3-fold) and soluble protein (∼4-fold). No difference was found between OVA-NPs and free OVA (Fig. 4B). This demonstrated the ability of EDIT to induce higher OVA-specific humoral responses compared with the other groups.
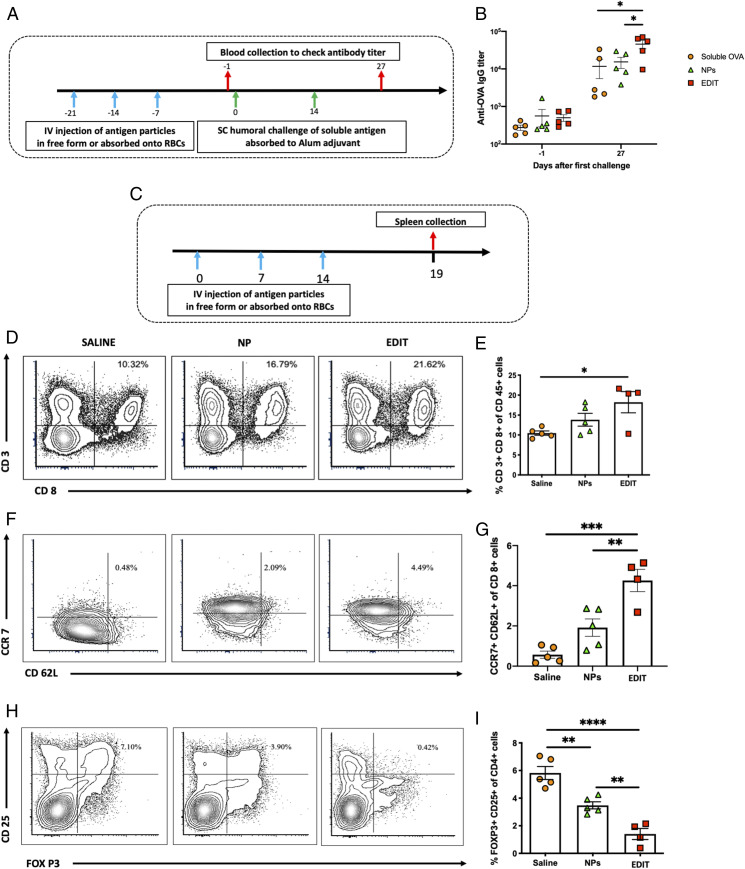
Fig. 4.
Immunological consequences of nanoparticle spleen handoff. (A) Schedule for evaluating systemic antibody (humoral) responses of hitchhiked nanoparticles. (B) Anti-OVA IgG titer evaluated 1 d before the first immune challenge (day −1) and 13 d after the second immune challenge (day 27) (n = 5 for all groups). Significantly different (one-way ANOVA followed by Tukey’s HSD test): *P < 0.05. (C) Schedule for evaluating systemic cellular immune responses of hitchhiked nanoparticles. (D) Representative flow cytometry analysis images of CD3+ CD8+ cells in the spleen. (E) Quantitative analysis of the percentage of CD3+ CD8+ cells in the spleen (n = 4 for the EDIT group; n = 5 for all other groups). Significantly different (one-way ANOVA followed by Tukey’s HSD test): *P < 0.05. (F) Representative flow cytometry analysis images of CCR7+ CD62L+ cells in the spleen. (G) Quantitative analysis of the percentage of CCR7+ CD62L+ cells in the spleen (n = 4 for the EDIT group; n = 5 for all other groups). Significantly different (one-way ANOVA followed by Tukey’s HSD test): **P < 0.01, ***P < 0.001. (H) Representative flow cytometry analysis images of CD25+ FOXP3+ cells in the spleen. (I) Quantitative analysis of the percentage of CD25+ FOXP3+ cells in the spleen (n = 4 for the EDIT group; n = 5 for all other groups). Significantly different (one-way ANOVA followed by Tukey’s HSD test): *P < 0.05, **P < 0.01, ****P < 0.0001. Data in B, E, G, and I are expressed as mean ± SEM.
Cellular immunity generated by EDIT was also assessed. Mice were immunized by EDIT or OVA-NPs once a week for 3 wk, and comprehensive immune profiling from the harvested splenocytes was performed 5 d after the last vaccination (Fig. 4C). Flow cytometry analysis indicated that EDIT showed significant enhancement in CD3+ CD8+ cells in the spleen compared with the control group (∼1.7-fold). Interestingly, free NPs alone did not show the same effect (Fig. 4 D and E). Carrying out a deeper analysis of CD8 subtypes, we found that CCR7+ CD62L+ T cells, which correspond to a group of antigen-experienced T cells (27, 28), were remarkably increased in EDIT compared with both free NPs and the control group. Specifically, EDIT had 8-fold and 2.2-fold more antigen-experienced cells than untreated and OVA-NP groups, respectively (Fig. 4 F and G). Furthermore, our analysis also revealed that the increase in antigen-experienced central memory T cells is also associated with a corresponding decrease in the CD25+ FOXP3+ regulatory T cell phenotype, with EDIT having 4-fold and 2.5-fold fewer Treg cells than untreated or the OVA-NP group, respectively (Fig. 4 H and I). No significant cellular immune effects were seen locally in the lung tissue (SI Appendix, Fig. S9), suggesting that spleen delivery and consequent systemic effects are more dominant. Additional studies such as tetramer analysis should be performed in future studies to further characterize the antigen specificity of the EDIT platform.
Enhanced Immune Response Improves the Interventional Window in a Prophylactic Tumor Model.
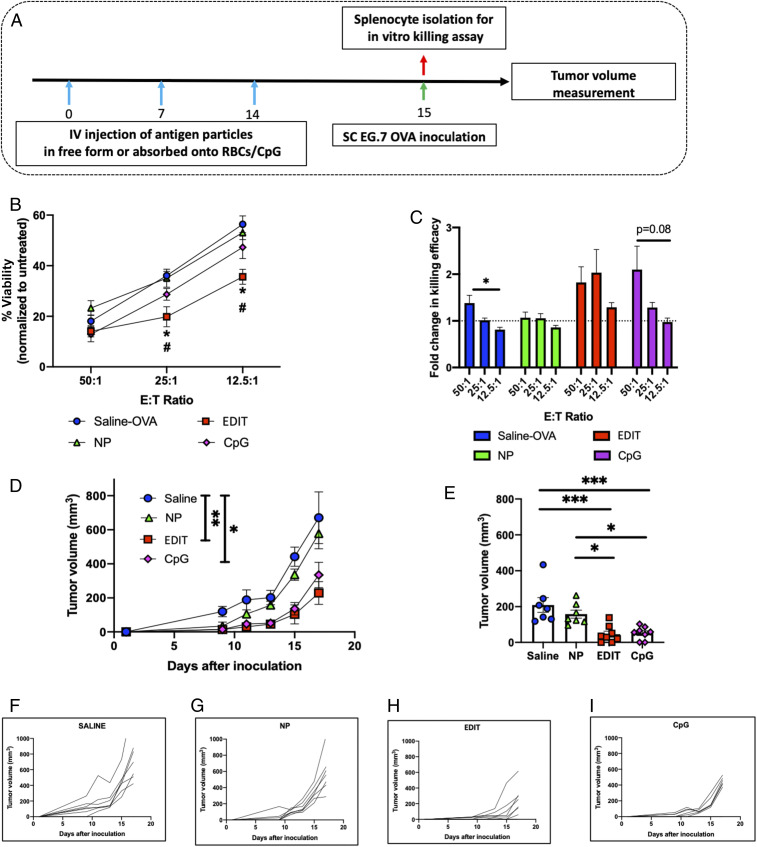
To test the ability of EDIT to induce a cellular therapeutic response, we designed a prophylactic vaccination study where the mice were immunized once a week for 3 wk with OVA, EDIT, or free OVA-NPs. CpG was used as a positive control. One day after the last vaccination, mice were challenged by subcutaneous (s.c.) inoculation of EG-7 OVA cells and tumor growth was monitored (Fig. 5A). None of the treatment groups induced obvious toxicities during vaccination as indicated by body weight (SI Appendix, Fig. S10). Also, after the last vaccination, splenocytes were isolated from mice injected with different treatment groups to evaluate their in vitro specific target cell-killing ability. Splenocytes from mice immunized with EDIT demonstrated significant specific killing even at low effector-to-target (E:T) ratios (Fig. 5B). Only the EDIT group maintained the fold change of killing efficiency above 1 for all of the ratios tested (Fig. 5C). Both these studies indicated that EDIT induced higher OVA-specific responses compared with any other vaccination. Tumor growth kinetics clearly demonstrated EDIT immunization was effective. Specifically, 17 d after tumor inoculation, EDIT immunization resulted in ∼2.9-fold slower growth as compared with the control group but the free NP group exhibited no significant difference compared with the control group (Fig. 5D), while on day 13, EDIT resulted in ∼4.6-fold and ∼3.5-fold compared with the control and NP groups (Fig. 5E). In other words, central memory induced by EDIT immunization successfully manifests in effector immune responses against EG-7 OVA when stimulated with the antigen and is able to significantly slow down the tumor growth rate as effectively as the positive control, CpG, without the need for a foreign adjuvant. Free NPs on their own show no such memory effects. Individual tumor growth curves from all different treatment groups indicate that in control and NP groups, growth curves exponentiate far more quickly as compared with the EDIT and CpG groups (Fig. 5 F–I). Remarkably, one mouse from the EDIT group remained tumor-free throughout the course of the study. EDIT significantly prolonged the tumor exponentiation, thereby increasing the window for therapeutic interventions with alternate strategies.
Fig. 5.
Therapeutic extension of immune modulation of hitchhiked nanoparticles for vaccination. (A) Schedule for prophylactic vaccination studies. (B) In vitro cell-killing data post immunization by various treatment groups evaluated as percent viability normalized to the untreated control at different effector-to-target ratios (n = 3 mice for all groups). Significantly different: saline OVA vs. EDIT and NPs vs. EDIT (one-way ANOVA followed by Tukey’s HSD test): *P < 0.05, #P < 0.05. (C) Fold change in in vitro cell-killing assay, comparison of fold change within each treatment group as a function of effector-to-target ratio (n = 3 mice for all groups). Significantly different (one-way ANOVA followed by Tukey’s HSD): *P < 0.05. (D) Tumor growth curves for mice inoculated after prophylactic vaccinations by different treatment groups. Statistical analysis within this figure was carried out on day 17. (E) Evaluation of tumor volumes for different groups on day 13. For D and E (n = 8 for the EDIT and CpG groups; n = 7 for the saline and NP groups), significant different (one-way ANOVA followed by Tukey’s HSD): *P < 0.05, **P < 0.01, ***P < 0.001. (F–I) Tumor growth kinetics for individual mice in (F) saline, (G) NP, (H) EDIT, and (I) CpG treatment groups. Data in B–E are expressed as mean ± SEM.
Discussion
Erythrocytes play an important role in maintaining physiological homeostasis by carrying out the process of oxygenation. However, erythrocytes are also an active member of the innate immune system. It has been reported that certain pathogens can attach to the erythrocyte cell membrane, get neutralized by oxidative species from within the erythrocytes, and ultimately are physically handed off to the immune cells in the spleen (8, 9). This offers a genuine opportunity to develop a biomimetic strategy to target spleen, erythrocyte-driven immune targeting (EDIT), which leverages antigen presentation to the spleen from the surface of the erythrocyte.
Conventionally, erythrocyte hitchhiking has been explored for lung-targeting applications since the shear stresses experienced by stretched erythrocytes in lung capillaries are able to dislodge particles in the lungs (17, 18). This makes it challenging to deliver the cargo to the spleen. The dominant factor in skewing the distribution of nanoparticles from the lung to the spleen was the initial feed ratio of nanoparticle to erythrocytes. Modulation of this parameter helped in improving shear resistance in the lungs, thus allowing a smaller fraction of nanoparticles to detach in the lungs and making a larger fraction available to target elsewhere. At the same time, the slight alteration induced to the erythrocyte membrane enabled the spleen as a natural target. In vitro shear studies indicated that increasing NP:erythrocyte feed ratios significantly reduced shear-induced detachment. Higher nanoparticle density on hitchhiked erythrocytes for higher NP:erythrocyte feed ratios is the likely cause for this improved shear resistance. Highly loaded erythrocytes are more rigid, thus resisting the biomechanical stretching in the lung capillaries (24) and thereby reducing lung accumulation. The natural pathway of pathogen transfer from the surface of erythrocytes to the APCs in the spleen has been unclear; however, membrane alteration caused by adherent pathogens has been strongly implicated. This attribute was engineered in our system by controlling the NP:erythrocyte ratio in the feed and assessing temporary damage in terms of phosphatidylserine up-regulation. Phosphatidylserine up-regulation is known to promote interactions of dendritic cells with erythrocytes (13, 14). This, combined with the masking of CD47 receptors at higher nanoparticle-to-erythrocyte ratios, likely makes the nanoparticles on the “missing self” erythrocyte more prone to uptake by these cells (29).
Based on the effect of NP:erythrocyte ratio on in vitro shear resistance and transient phosphatidylserine expression, an optimal NP:erythrocyte ratio of 300:1 was selected. This ratio also led to efficient delivery and sustained presence of nanoparticles in the spleen. In contrast to past studies involving erythrocytes or their membranes, where their senescence was exploited for targeting the spleen, in our case, particles are only delivered to the spleen, while the erythrocytes continue to remain in circulation, indicating that the damage to erythrocyte membrane is temporary, sufficient for spleen handoff, but does not cause the erythrocytes themselves to be sequestered. Thus, EDIT offers a different pathway for targeting the spleen, particularly the antigen-presenting cells in the spleen. Phagocyte depletion studies illustrated that particles in the spleen are not located within the phagocytes, suggesting their presence within APCs which could be exploited for immunomodulation.
Overall, for therapeutic evaluation of the humoral and cellular immune responses, respective OVA challenges were received after the treatments were given and therapeutic outcomes were monitored. Thus, by the design of these experiments, we were able to track the memory responses to our prophylactic vaccinations. Humoral and cellular immune responses showed a strong vaccination potential, with EDIT exhibiting 3-fold higher antibody titer, 2.2-fold higher antigen-experienced central memory T cells, and 2.5-fold lower regulatory T cells, compared with free nanoparticles. Moreover, the outcomes were assessed by enzyme-linked immunosorbent assay (ELISA) (for anti-OVA IgG antibody) and specific cell-killing assay (for splenocyte cytotoxicity), indicating that these responses are highly specific. However, additional studies such as tetramer analysis should be performed in future studies to further characterize the antigen specificity and memory responses of the EDIT platform. This adjuvant effect could be effectively used for vaccinations against blood-borne infections, such as malaria, and the overall concept could be extrapolated to develop systemic or tissue-specific memory responses following i.v. vaccinations (17, 30). As a proof of concept, the immune response generated by EDIT was successfully utilized to drive therapeutic responses in a prophylaxis model. EDIT-mediated immunization was able to significantly slow down tumor growth by increasing the equilibrium phase of the cancer immunity cycle (31), performing equally as well as a foreign adjuvant CpG, thereby increasing the window of therapeutic interventions. Several differences can be noted between the strategies of CpG and EDIT. Unlike CpG, which is a nonnative molecule, the RBC here is a native cell type in the body. Further, CpG is generally admixed with the vaccine, thus allowing it to diffuse away from the injection site. In contrast, EDIT is active only when the nanoparticle is attached to the perturbed erythrocyte. Finally, our data confirm that EDIT can incorporate nanoparticles beyond 200-nm PS including those of different sizes, synthetic materials, or biological materials.
Thus, EDIT offers a different perspective for vaccination strategies. Several adjuvants have been reported in the literature and used in the clinic (32). Often, the adjuvants are of nonhuman origin and that is the principal reason why an immune response gets triggered. Such adjuvant-based strategies are based on mixing the antigen with some kind of foreign chemical/material that stimulates the immune system, as the first Gaston Ramon’s alum adjuvant. In contrast, we report erythrocyte-mediated delivery of the antigen that stimulates the immune response. Adjuvant-free therapies based on the “self” cell of the body represent a unique way of propelling development of vaccines. While future studies can focus on understanding the similarities and differences between EDIT and other adjuvants, availability of additional adjuvants, especially self-based ones like perturbed erythrocytes, may significantly benefit the scientific community engaged in adjuvant and vaccine research.
In summary, we have developed a biomimetic strategy that exploits the innate immune function of erythrocytes to engineer an efficient nanoparticle handoff to the spleen. Fundamentally, it represents a different pathway to deliver nanoparticles to the spleen that does not involve extensive modifications to the nanoparticles themselves. Nanoparticle handoff by EDIT led to a strong immunological memory that can drive therapeutic responses. With further research and performing more specific immunological studies, this platform can be used as a versatile strategy to target several off-the-shelf nanoparticles to the spleen without specific modifications.
Materials and Methods
Materials.
Carboxylic acid polystyrene nanoparticles were purchased from Polysciences. PLGA nanoparticles and hexamethyldisilazane (HMDS) were purchased from Sigma-Aldrich. Granulocyte–macrophage colony-stimulating factor (GM-CSF) was obtained from PeproTech. The Nunc Lab-Tek II Chamber Slide System, cell-staining buffer, Alexa Fluor 647 ovalbumin, Alexa Fluor 647 N-hydroxysuccinimide reagent, phosphate-buffered saline (PBS) (1×), EDC, and 2-(N-morpholino)ethanesulfonic acid (MES) were obtained from Thermo Fisher Scientific. Lithium heparin-coated BD Microtainer tubes were obtained from BD Medical Technology. Tissue dissociation tubes and a lung dissociation kit were obtained from Miltenyi Biotec. Saline solution (0.9%) was obtained from Teknova. Paraformaldehyde was obtained from Electron Microscopy Sciences. Clodrosome was obtained from Encapsula NanoSciences. All fluorescent probe-conjugated antibodies for immune cell staining were purchased from BioLegend.
Preparation and Characterization of Antigen-Coated Polystyrene Nanoparticles.
Antigen-coated polystyrene nanoparticles were prepared using an EDC-based method. Briefly, 2 mg of polystyrene nanoparticles with carboxylic acid surface groups was suspended in MES buffer (pH 5.5) for 15 min to activate the carboxylic group. Antigen protein (1 mg) was subsequently added and allowed to react for 4 h under gentle shaking at room temperature. Unconjugated protein was eliminated by centrifugation of the nanoparticles at 12,000 × g for 15 min. Protein conjugation efficiency was measured by quantifying the unconjugated protein in the supernatant using a fluorescence-based method. Protein-coated nanoparticles were washed twice using deionized (DI) water. The particles were resuspended in DI water and assessed for their size, zeta potential, and polydispersity index using dynamic light scattering (Malvern Zen3600) and scanning electron microscopy (Zeiss FESEM Supra 55VP, Zeiss FESEM Ultra 55). Nanoparticles were resuspended in 1× PBS immediately before their use. Antigen-coated PLGA nanoparticles were prepared using the same method.
Internalization of Nanoparticles by Dendritic Cells and Activation of Dendritic Cells by Nanoparticles.
JAWII dendritic cells (DCs) were obtained from the ATCC (CRL-11904). They were cultured in Alpha minimum essential medium with ribonucleosides, deoxyribonucleosides, 4 mM l-glutamine, 1 mM sodium pyruvate, and 80% 5 ng/mL murine GM-CSF, 20% fetal bovine serum (FBS). Internalization of antigen-coated nanoparticles was evaluated by flow cytometry and confocal microscopy. For flow cytometry analysis, 2 × 106 JAWII DCs were seeded in a 12-well plate and allowed to adhere overnight. Media were replaced before adding nanoparticles. Alexa Fluor 647-labeled antigen-coated nanoparticles (30 μg) were added to each well and allowed to incubate for 24 h at 37 °C. Media were then removed and cells were washed three times using PBS. The cells were gently scraped using a cell scraper. These cells were analyzed by flow cytometry (BD LSR Analyzer II). For confocal microscopy, 2 × 105 JAWII DCs were seeded in a two-well chamber and treated similarly as for the flow cytometry analysis. After washing cells with PBS, cells were fixed with 4% paraformaldehyde for 10 min. Cells were then permeabilized with 0.01% Triton X-100 and cell nuclei were stained with DAPI. The processed cells were imaged by confocal microscopy (Upright Zeiss LSM 710 NLO ready).
To evaluate the activation of DCs by antigen-coated nanoparticles, 2 × 106 JAWII DCs were seeded in a 12-well plate and allowed to adhere overnight. Cells were incubated with antigen-coated nanoparticles using the same protocol as for flow cytometry analysis of nanoparticle uptake. After treatment, cells were washed three times with PBS and detached from the wells using 0.25% trypsin/ethylenediaminetetraacetate solution. The cells were washed twice using flow staining buffer and stained for CD80 using a PE-CD80 antibody (BioLegend). The stained cells were analyzed by flow cytometry (BD LSR Analyzer II).
Hitchhiking of Antigen-Coated Nanoparticles to Red Blood Cells.
Mouse whole blood was collected via terminal cardiac puncture using a heparin-coated syringe and stored in a Microtainer blood collection tube. After sitting for >30 min on ice, the collected whole blood was centrifuged at 1,000 × g for 10 min at 4 °C to remove the serum and buffy-coat layer. The RBC layer was washed three times using cold PBS and centrifuged at 650 × g for 15 min at 4 °C. The washed RBCs were resuspended in PBS at a hematocrit of 10% (RBC stock solution) and stored at 4 °C for later use.
The hitchhiking of antigen-coated nanoparticles to RBCs was conducted using a previously reported method (18). In brief, equal volumes of antigen-coated nanoparticles and a 10% RBC stock solution were mixed by inversion and pipetting. The mixture was then rotated on a revolver at 12 rpm for 40 min. The hitchhiked RBCs were separated from unbound nanoparticles by centrifugation at 100 × g for 5 min at 4 °C. The hitchhiked samples were then washed twice using PBS and finally resuspended in PBS at a 10% (volume [vol]/vol) concentration for further characterization and later use. The number of hitchhiked nanoparticles on RBCs was quantified using a fluorescence-based method. Hitchhiked RBC samples (25 μL; with a known number of RBCs) were lysed using DI water, and the nanoparticle concentration was quantified by measuring the fluorescence of nanoparticles on a plate reader. The percentage of RBCs carrying nanoparticles for different nanoparticle-to-RBC ratios was determined using flow cytometry (BD LSR Analyzer II) using Alexa Fluor 647 fluorescence and confirmed by confocal microscopy (Upright Zeiss LSM 710 NLO ready). Scanning electron microscopy (Zeiss FESEM Supra 55VP, Zeiss FESEM Ultra 55) was used to confirm the hitchhiking of antigen-coated nanoparticles to RBCs. Briefly, hitchhiked samples were fixed for 1 h using 4% glutaraldehyde. They were washed twice with PBS to remove unreacted glutaraldehyde. Next, fixed hitchhiked cells were subjected to successive washes with increasing ethanol concentration (50 to 100%; vol/vol) before finally resuspending them in HMDS followed by imaging. In vitro shear studies were performed as described before (18). Briefly, hitchhiked RBCs were resuspended in 10 mL FBS and a rotary shear of 6 Pa was applied for 20 min using a couette viscometer (AR-G2, TA Instruments). The nanoparticles remaining attached were quantified using fluorescence as described before.
The impact of nanoparticle hitchhiking on the carrier RBCs was evaluated by agglutination assay (25) and phosphatidylserine assay (24) as reported before. In brief, for the agglutination assay, naïve or hitchhiked RBCs of 1% hematocrit were dispensed into a 96-well U-bottom plate. The plate was allowed to sit at 37 °C for 1 h and the agglutination was then assessed. Carboxylic acid polystyrene nanoparticle (200-nm)-hitchhiked RBCs were used as a positive control considering its reported damage to the carrier erythrocytes. For the phosphatidylserine assay, naïve and hitchhiked RBCs of 0.01% hematocrit were incubated with fluorescent Annexin V-Alexa Fluor 488 (binding to phosphatidylserine) for 15 min in buffer containing 2 mM CaCl2. After staining, samples were analyzed using flow cytometry (BD LSR Analyzer II).
Animals.
Female BALB/c and C57BL/6 mice (7 to 9 wk of age) were purchased from Charles River Laboratories. All animal experiments were performed according to approved protocols by the Institutional Animal Care and Use Committee of the Faculty of Arts and Sciences, Harvard University.
Biodistribution Study.
All biodistribution studies were performed in healthy female BALB/c mice. Alexa Fluor 647-labeled antigen was used to prepare antigen-coated nanoparticles for the biodistribution studies. In brief, female BALB/c mice (7 to 9 wk of age) were i.v. administered with free or hitchhiked antigen nanoparticles at a dose containing 7 μg antigen. For studies involving phagocyte depletion, phagocytes were depleted by i.v. administration of 200 μL clodrosome containing 5 mg/mL clodronate 48 h before i.v. injection of formulations. Twenty minutes, 6 h, or 24 h after formulation administration, mice were euthanized and major organs including blood, liver, spleen, kidney, heart, lung, and brain were extracted. The extracted organs were imaged using in vivo imaging (PerkinElmer IVIS Spectrum). Fluorescence in organs was quantified using IVIS software by analyzing the region of interest of organs. Percent injected dose of nanoparticles accumulated in organs was estimated by dividing the fluorescence in the organ of interest by the total fluorescence in all of the tested organs.
For the in vivo tracking of the hitchhiked system, RBCs were labeled by CellTrace carboxyfluorescein succinimidyl ester and antigen-coated nanoparticles were labeled by Alexa Fluor 647. The double-labeled hitchhiked system was i.v. administered to female BALB/c mice (7 to 9 wk of age). Blood was collected at predetermined time points (0 min, 20 min, 6 h, and 24 h after administration). The collected blood was diluted in flow staining buffer at a 1:100 dilution and analyzed by flow cytometry (BD LSR Analyzer II).
Characterization of Immune Responses Induced by EDIT.
The humoral and cellular immune responses induced by EDIT were assessed in healthy BALB/c mice. To evaluate the humoral response, female BALB/c mice (7 wk of age) were i.v. administered free ovalbumin, OVA-coated nanoparticles, and hitchhiked OVA-coated nanoparticles at a dose containing 7 μg of OVA on days 0, 7, and 14. Subsequently, 7 and 14 d after the three doses of immunization, mice were s.c. challenged with two doses of OVA adjuvanted with alum (7 μg OVA and 70 μg alum). Blood was collected 1 d before the first dose of challenge and 13 d after the second dose of challenge. Anti-OVA IgG antibody titer in the collected blood was measured by ELISA using a previously reported method (33).
To evaluate the cellular response, female BALB/c mice (7 wk of age) were i.v. administered saline, OVA-coated nanoparticles, and hitchhiked OVA-coated nanoparticles at a dose of 7 μg of OVA every week for three doses (on days 0, 7, and 14). Five days after the last dose (on day 19), spleens and lungs of mice were collected. A single-cell suspension of organ cells was formed using corresponding organ dissociation kits (Miltenyi Biotec) according to the manufacturer’s instructions. The cells were stained with antibodies and analyzed by flow cytometry (BD LSR Analyzer II). Different panels of antibody mixtures were made from CD45 (BioLegend, catalog no. 103116, clone 30-F11), CD3 (BioLegend, no. 100218, clone 17A2), CD4 (BioLegend, no. 100421, clone GK1.5), CD8a (BioLegend, no. 100711, clone 53-6.7), NKp46 (BioLegend, no. 137606, clone 29A1.4), CD11c (BioLegend, no. 1c7307, clone N418), granzyme B (BioLegend, no. 372208, clone QA16A02), IFNγ (BioLegend, no. 505849, clone XMG1.2), IFNγ (BioLegend, no. 505806, clone XMG1.2), CD86 (BioLegend, no. 105011, clone GL-1), and an AmCyan Live/Dead Cell Staining Kit (Thermo Fisher Scientific). All antibodies were diluted at optimized dilutions prior to their use.
Tumor Studies.
EG-7 OVA was obtained from the ATCC (CRL-2113). Cells were cultured in RPMI medium 1640 with 2 mM l-glutamine adjusted to contain 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mM Hepes, and 1.0 mM sodium pyruvate and supplemented with 0.05 mM 2-mercaptoethanol and 90% 0.4 mg/mL G418, 10% FBS. Cells of low passage number were passaged two or three times before their in vivo use.
The efficacy of EDIT in controlling the growth of EG-7 OVA tumors was studied in a prophylactic model. Female C57BL/6 mice (7 wk of age) were immunized with free OVA (in saline), OVA nanoparticles, hitchhiked OVA nanoparticles, and free OVA + CpG ODN 1826 (10 μg) at a dose containing 7 μg OVA on days 0, 7, and 14. One day after the last immunization (on day 15), 5 × 105 EG-7 OVA cells were s.c. inoculated into the right mammary fat pad. The tumor size and body weight of mice were monitored after tumor inoculation.
Statistical Analysis.
All of the experiments were conducted with at least three replicates. All statistical analyses were carried out using GraphPad Prism 8 software. Normality tests were used to determine data normality. Student’s t test and one-way ANOVA with Tukey’s HSD (honestly significant difference) test were used to determine significance: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. All of the flow cytometry analyses were carried out using FCS Express 7.0 software.
Supplementary Material
Acknowledgments
We thank Prof. Joerg Lahann (University of Michigan, Ann Arbor) for his valuable insights. We acknowledge the use of https://www.biorender.com in creating schematics. This work was financially supported by the Wyss Institute at Harvard University. We acknowledge funding from the NIH (1R01HL143806-01).
Footnotes
Competing interest statement: A.U., Z.Z., and S.M. are inventors on a patent application that covers aspects of the technology described in this manuscript. The patent application is assigned to and managed by Harvard University.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2002880117/-/DCSupplemental.
Data Availability.
All relevant data and methods are reported in the manuscript. Refer to SI Appendix for additional information on material characterization, mechanistic analysis, and biodistribution.
References
- 1.Klei T. R., Meinderts S. M., van den Berg T. K., van Bruggen R., From the cradle to the grave: The role of macrophages in erythropoiesis and erythrophagocytosis. Front. Immunol. 8, 73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pishesha N. et al., Engineered erythrocytes covalently linked to antigenic peptides can protect against autoimmune disease. Proc. Natl. Acad. Sci. U.S.A. 114, 3157–3162 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorentz K. M., Kontos S., Diaceri G., Henry H., Hubbell J. A., Engineered binding to erythrocytes induces immunological tolerance to E. coli asparaginase. Sci. Adv. 1, e1500112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kontos S., Kourtis I. C., Dane K. Y., Hubbell J. A., Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proc. Natl. Acad. Sci. U.S.A. 110, E60–E68 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson H. L., Brodsky I. E., Mangalmurti N. S., The evolving erythrocyte: Red blood cells as modulators of innate immunity. J. Immunol. 201, 1343–1351 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pretini V. et al., Red blood cells: Chasing interactions. Front. Physiol. 10, 945 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baum J., Ward R. H., Conway D. J., Natural selection on the erythrocyte surface. Mol. Biol. Evol. 19, 223–229 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Minasyan H., Mechanisms and pathways for the clearance of bacteria from blood circulation in health and disease. Pathophysiology 23, 61–66 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Minasyan H., Phagocytosis and oxycytosis: Two arms of human innate immunity. Immunol. Res. 66, 271–280 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Halma C. et al., Elimination of soluble 123I-labeled aggregates of IgG in patients with systemic lupus erythematosus. Effect of serum IgG and numbers of erythrocyte complement receptor type 1. Arthritis Rheum. 34, 442–452 (1991). [DOI] [PubMed] [Google Scholar]
- 11.Edberg J. C., Wright E., Taylor R. P., Quantitative analyses of the binding of soluble complement-fixing antibody/dsDNA immune complexes to CR1 on human red blood cells. J. Immunol. 139, 3739–3747 (1987). [PubMed] [Google Scholar]
- 12.Damgaard C. et al., Viable bacteria associated with red blood cells and plasma in freshly drawn blood donations. PLoS One 10, e0120826 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen D. B. et al., Regulation of phosphatidylserine exposure in red blood cells. Cell. Physiol. Biochem. 28, 847–856 (2011). [DOI] [PubMed] [Google Scholar]
- 14.de Back D. Z., Kostova E. B., van Kraaij M., van den Berg T. K., van Bruggen R., Of macrophages and red blood cells; a complex love story. Front. Physiol. 5, 9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villa C. H., Anselmo A. C., Mitragotri S., Muzykantov V., Red blood cells: Supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv. Drug Deliv. Rev. 106, 88–103 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danielyan K. et al., Cerebrovascular thromboprophylaxis in mice by erythrocyte-coupled tissue-type plasminogen activator. Circulation 118, 1442–1449 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenner J. S. et al., Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat. Commun. 9, 2684 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Z., Ukidve A., Gao Y., Kim J., Mitragotri S., Erythrocyte leveraged chemotherapy (ELeCt): Nanoparticle assembly on erythrocyte surface to combat lung metastasis. Sci. Adv. 5, eaax9250 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anselmo A. C. et al., Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano 7, 11129–11137 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourgeaux V., Lanao J. M., Bax B. E., Godfrin Y., Drug-loaded erythrocytes: On the road toward marketing approval. Drug Des. Devel. Ther. 10, 665–676 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Z., Ukidve A., Krishnan V., Mitragotri S., Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv. Drug Deliv. Rev. 143, 3–21 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Kumar S., Anselmo A. C., Banerjee A., Zakrewsky M., Mitragotri S., Shape and size-dependent immune response to antigen-carrying nanoparticles. J. Control. Release 220, 141–148 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Frick S. U. et al., Functionalized polystyrene nanoparticles trigger human dendritic cell maturation resulting in enhanced CD4+ T cell activation. Macromol. Biosci. 12, 1637–1647 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Pan D. C. et al., Nanoparticle properties modulate their attachment and effect on carrier red blood cells. Sci. Rep. 8, 1615 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan D. et al., The effect of polymeric nanoparticles on biocompatibility of carrier red blood cells. PLoS One 11, e0152074 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreno S. G., Depleting macrophages in vivo with clodronate-liposomes. Methods Mol. Biol. 1784, 259–262 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Martin M. D., Badovinac V. P., Defining memory CD8 T cell. Front. Immunol. 9, 2692 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unsoeld H., Pircher H., Complex memory T-cell phenotypes revealed by coexpression of CD62L and CCR7. J. Virol. 79, 4510–4513 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi T. et al., Splenic dendritic cells survey red blood cells for missing self-CD47 to trigger adaptive immune responses. Immunity 43, 764–775 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darrah P. A. et al., Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 577, 95–102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen D. S., Mellman I., Oncology meets immunology: The cancer-immunity cycle. Immunity 39, 1–10 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Di Pasquale A., Preiss S., Tavares Da Silva F., Garçon N., Vaccine adjuvants: From 1920 to 2015 and beyond. Vaccines (Basel) 3, 320–343 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Z. et al., Engineering of a hybrid nanoparticle-based nicotine nanovaccine as a next-generation immunotherapeutic strategy against nicotine addiction: A focus on hapten density. Biomaterials 123, 107–117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data and methods are reported in the manuscript. Refer to SI Appendix for additional information on material characterization, mechanistic analysis, and biodistribution.