Abstract
Much evidence implicates the serotonergic regulation of the amygdala in anxiety. Thus the present study was undertaken to characterize the influence of serotonin (5-HT) on principal neurons (PNs) of the rat lateral amygdala (LA), using whole cell recordings in vitro. Because inhibition is a major determinant of PN activity, we focused on the control of GABAergic transmission by 5-HT. IPSCs were elicited by local electrical stimulation of LA in the presence of glutamate receptor antagonists. We found that 5-HT reduces GABAA inhibitory postsynaptic currents (IPSCs) via presynaptic 5-HT1B receptors. While the presynaptic inhibition of GABA release also attenuated GABAB currents, this effect was less pronounced than for GABAA currents because 5-HT also induced a competing postsynaptic enhancement of GABAB currents. That is, GABAB currents elicited by pressure application of GABA or baclofen were enhanced by 5-HT. In addition, we obtained evidence suggesting that 5-HT differentially regulates distinct subsets of GABAergic synapses. Indeed, GABAA IPSCs were comprised of two components: a relatively 5-HT-insensitive IPSC that had a fast time course and a 5-HT-sensitive component that had a slower time course. Because the relative contribution of these two components varied depending on whether neurons were recorded at proximity versus at a distance from the stimulating electrodes, we speculate that distinct subtypes of local-circuit cells contribute the two contingents of GABAergic synapses. Overall, our results indicate that 5-HT is a potent regulator of synaptic inhibition in LA.
NEW & NOTEWORTHY We report that 5-HT, acting via presynaptic 5-HT1B receptors, attenuates GABAA IPSCs by reducing GABA release in the lateral amygdala (LA). In parallel, 5-HT enhances GABAB currents postsynaptically, such that GABAB inhibitory postsynaptic currents (IPSCs) are relatively preserved from the presynaptic inhibition of GABA release. We also found that the time course of 5-HT-sensitive and -insensitive GABAA IPSCs differ. Together, these results indicate that 5-HT is a potent regulator of synaptic inhibition in LA.
Keywords: 5-HT1B receptor, GABAA receptor, GABAB receptor, lateral amygdala, serotonin
INTRODUCTION
Converging lines of evidence implicate a dysregulation of the amygdala, possibly mediated by serotonin (5-HT), in pathological anxiety. First, the amygdala plays a critical role in the genesis of defensive behaviors (LeDoux 2000) and it is hyperactive in anxiety disorders (Bremner 2005; Shin et al. 2006). Second, serotonin (5-HT) uptake inhibitors figure among the recommended pharmacological treatments for anxiety disorders (reviewed in Bandelow et al. 2017) and the amygdala receives serotonergic inputs from the dorsal raphe (Ma et al. 1991; Sadikot and Parent 1990). Third, dorsal raphe neurons fire at higher rates during emotional arousal (Cohen et al. 2015; Grahn et al. 1999; Schweimer and Ungless 2010), causing a rise in amygdala levels of 5-HT (Kawahara et al. 1993; Kirby et al. 1995; Yokoyama et al. 2005).
To shed light on how 5-HT regulates emotional behaviors, several in vitro studies have examined the effects of 5-HT in the basolateral complex of the amygdala (BLA). Unexpectedly, contrasting results were obtained in the lateral (LA) and basolateral (BL) nuclei. In principal neurons (PNs) of LA but not BL, 5-HT was found to exert various excitatory effects, including a slow depolarization, the enhancement of depolarization-induced slow afterdepolarizations, and the inhibition of Ca2+-dependent K+ currents (Faber and Sah 2002; Yamamoto et al. 2012, 2014). In BL by contrast, most studies reported that 5-HT increases the frequency of spontaneous IPSCs in PNs, an effect dependent on the depolarization of interneurons via 5-HT2A receptors (Bocchio et al. 2015; Jiang et al. 2009; Rainnie 1999).
So far, the regulation of inhibition by 5-HT has not been examined in LA. Yet, this question is of particular significance because LA is the main input station of the amygdala for sensory inputs and inhibition is a major determinant of PN activity in LA (reviewed in Paré et al. 2003). Indeed, in vivo intracellular recordings of PNs have revealed that their activity is dominated by inhibitory synaptic potentials of high amplitude and rapid onset (Lang and Paré 1997). As a result, PNs rarely spike, generally at rates <0.1 Hz (Paré and Gaudreau 1996). Consistent with these results, synaptic boutons immunoreactive for glutamic acid decarboxylase (GAD) or GABA are concentrated in the perisomatic region of PNs, although they are also present in their dendritic tree (Carlsen 1988; McDonald et al. 2002). Since deafferenting the BLA only causes slight decreases in local GAD levels (Le Gal LaSalle et al. 1978), it is likely that these inhibitory inputs mostly arise from GABAergic cells intrinsic to LA. In support of this, LA contains a similar variety of GABAergic interneurons subtypes (Mascagni and McDonald 2003; reviewed in Spampanato et al. 2011) as in cortex (Freund and Buzsáki 1996; Markram et al. 2004). For example, these include a numerically dominant parvalbumin group and somatostatin-expressing neurons, which, respectively, target the somatic region and dendrites of PNs (Muller et al. 2006, 2007a; Smith et al. 1998).
Thus, given the importance of inhibition in the functioning of LA, the present study aimed to characterize how 5-HT regulates GABAA and GABAB inhibition, identify the receptor subtype mediating these effects, and examine the possibility that these receptors are differentially expressed by different subsets of inhibitory inputs.
MATERIALS AND METHODS
Experiments were performed in accordance with the guiding principles of the Physiological Society of Japan and were approved by the Animal Care Committee of Kanazawa Medical University.
Slice preparations.
Wistar rats (21–30 days old, 29 males and 11 females) were decapitated under deep isoflurane anesthesia. The brains were dissected out and then sectioned using a vibrating microtome (Pro7; Dosaka, Kyoto, Japan) at a thickness of 300 µm in an ice-cold cutting solution composed (in mM) of 103 N-methyl-d-glucamine, 2.5 KCl, 10 MgSO4, 30 NaHCO3, 1.2 NaH2PO4, 0.5 CaCl2, 25 glucose, 20 HEPES, 2 thiourea, 3 Na-pyruvate, and 12 N-acetyl-l-cysteine. Slices were kept at 30°C for 5 min in the above solution and then incubated at room temperature for more than 1 h in a normal artificial cerebrospinal fluid (ACSF) composed (in mM) of 124 NaCl, 2.5 KCl, 2.0 CaCl2, 1.2 MgSO4, 1.3 NaH2PO4, 26 NaHCO3, and 20 glucose (~310 mOsm; ~7.2 pH). All solutions were oxygenated with a mixture of 95% O2-5% CO2. Recordings were made from slices submerged in normal ACSF at 30°C.
Electrophysiological recordings.
Slices were placed in a recording chamber on the stage of an upright microscope (BX51WI; Olympus, Tokyo, Japan) with a ×60 water-immersion objective (LUMPlanFI/IR, Olympus). Whole cell recordings were made from the soma of visually identified pyramidal-like neurons located in LA using glass pipettes (resistance 5–8 MΩ) pulled from borosilicate glass capillaries with a Narishige pipette puller (PP-830, Tokyo, Japan). Glass pipette electrodes were filled with a solution containing (in mM) 130 K-gluconate, 10 KCl, 2 MgCl2, 2 Na2-ATP, 0.4 Na2-GTP, 0.2 EGTA, 10 HEPES, and 5 K2-phosphocreatine with pH adjusted to 7.2–7.3 with KOH.
For miniature IPSC (mIPSC) recordings, pipettes were filled with a cesium-based solution containing (in mM) 50 Cs-methanesulfonate, 85 CsCl, 2 MgCl2, 2 Na2-ATP, 0.4 Na2-GTP, 0.2 EGTA, 10 HEPES, and 5 QX-314 with pH adjusted to 7.2–7.3 with CsOH. Osmolarity was adjusted to 295–300 mOsm. The liquid junction potential for the potassium-based solution was ~10 mV and for the cesium-based solution was ~1 mV. However, the membrane potential values reported below were not corrected for the junction potential. We only considered neurons with a stable resting membrane potential negative to −55 mV. Series resistance was monitored. If it changed by >20%, the recording was discarded. We recorded only one neuron per slice and used two to five slices per animal.
To evoke synaptic responses, a bipolar tungsten stimulation electrode was placed in the center of LA. During the recordings, electrical stimuli (100 µs, 0.06 mA) were applied through this electrode every 15 s. Whole cell recordings were obtained 50–350 µm from the tips of the stimulating electrodes. To isolate inhibitory responses, AMPA/kainate and NMDA receptor antagonists were added to the perfusate [10 µM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 50 µM dl-2-amino-5-phosphonopentanoic acid (dl-APV)]. To isolate GABAA IPSCs, the GABAB antagonist CGP55845 (1 µM) was also added to the perfusate. Voltage-clamp recordings of electrically evoked inhibitory postsynaptic currents (IPSCs) were performed at a holding potential of −50 mV. GABAA miniature IPSCs (mIPSCs) were recorded in the presence of tetrodotoxin (1 µM) at a holding potential of −80 mV for 1 min. A Picospritzer II (Parker Hannifin, Hollis, NH) was used for pressure application of drugs in the vicinity of recorded neurons. Recordings were obtained with a Multiclamp 700A amplifier (Molecular Devices, Sunnyvale, CA) and digitized at 20 kHz with a Digidata-1550 interface (Molecular Devices) controlled by pClamp-10.3 (Molecular Devices). When mIPSCs were recorded, the data were filtered at 1 kHz.
Drugs.
Depending on the purpose of the experiments, one or more of the following drugs were added to the perfusate: baclofen (20 µM; Tocris, Bristol, UK), CGP55845 (1 µM; Sigma, St. Louis, MO), CNQX (10 µM; Wako, Osaka, Japan), CP93129 (5 µM; Tocris), dl-APV (50 µM; Sigma), DOI (10 µM; Tocris), GABA (50 µM; Wako), picrotoxin (100 µM; Wako), QX-314 (5 mM; Tocris), serotonin (5-HT; 5 µM; Wako), sumatriptan (5 µM; Tocris), tetrodotoxin (TTX; 1 µM; Latoxan, Portes lès Valence, France), and WAY100635 (10 µM; Tocris).
Data analysis.
Data are expressed as means ± SE. Paired or unpaired t tests as well as one-way ANOVA with post hoc Dunnett tests were used for assessing statistical significance using a threshold P < 0.05. For statistical testing and computing linear regressions, we used SPSS (IBM, Chicago, IL) or MATLAB (Mathworks, Natick, MA). mIPSCs were automatically detected offline using the software Mini Analysis (Synaptosoft, Decatur, GA) followed by manual corrections.
RESULTS
5-HT suppresses inhibitory inputs to PNs via 5-HT1B receptors.
Whole cell recordings of PNs (n = 141) were obtained under visual guidance with infrared and differential interference contrast microscopy. Their electrophysiological characteristics were consistent with previous reports (Faber et al. 2001; Washburn and Moises 1992). Synaptic responses were elicited by electrical stimuli (100 µs, 0.06 mA), delivered every 15 s through bipolar electrodes positioned in the center of LA. To isolate the inhibitory components of these responses, CNQX (10 µM) and dl-APV (50 µM) were added to the perfusate. In five cells, we confirmed that the residual response was abolished by a combination of picrotoxin (100 µM) and CGP55845 (1 µM).
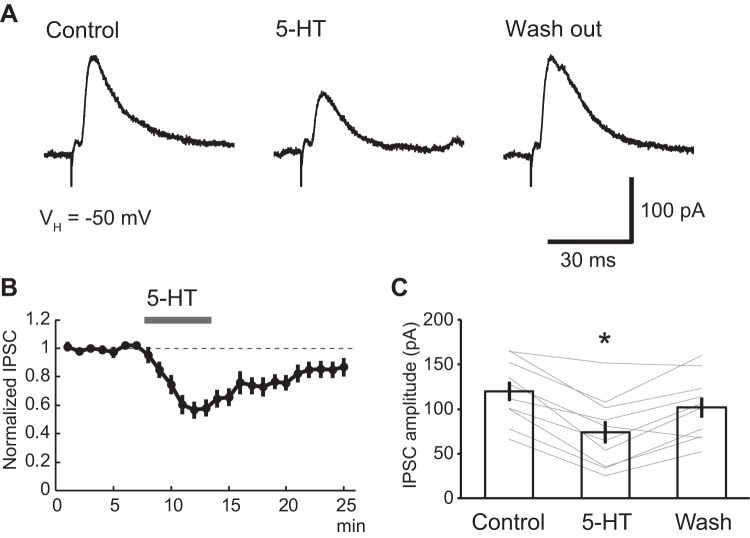
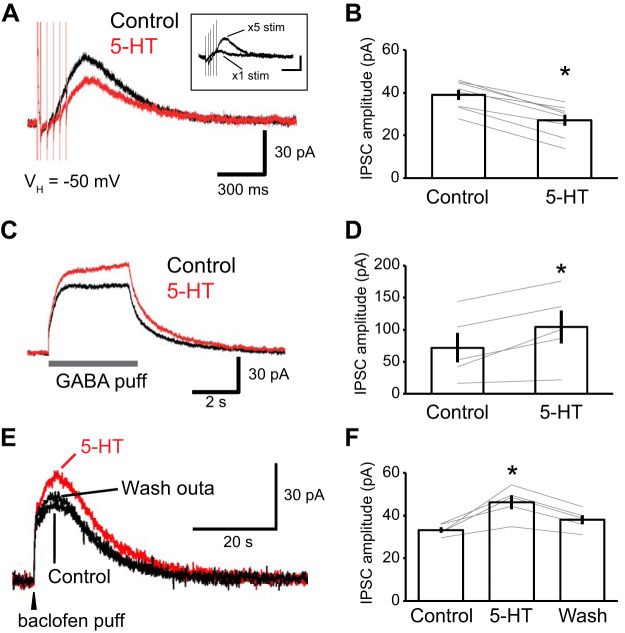
Pharmacologically isolated inhibitory postsynaptic currents (IPSCs) were studied in voltage-clamp mode, from a holding potential of −50 mV (Fig. 1). Addition of 5-HT (5 µM) to the perfusate reduced IPSC amplitudes by 41.6 ± 6.0% (Fig. 1, A and C; n = 10). Although this effect of 5-HT outlasted the period of drug application by several minutes (Fig. 1B), IPSC amplitudes gradually recovered (control, 119.8 ± 11.0 pA; 5-HT, 73.6 ± 12.5 pA; washout, 101.6 ± 11.4 pA; one-way repeated ANOVA; df = 2; F = 3.93; P = 0.03; post hoc Dunnett test, control vs. 5-HT, P = 0.017; control vs. washout, P = 0.445; n = 10 from 5 rats). Consistent with our previous report (Yamamoto et al. 2014), 5-HT also induced an inward current (15.7 ± 6.4 pA; n = 10 from 5 rats).
Fig. 1.
5-HT-induced suppression of inhibitory postsynaptic currents (IPSCs) in principal lateral amygdala (LA) neurons. A: representative IPSC traces induced by local electrical stimulation of LA in the presence of CNQX (10 µM) and dl-APV (50 µM). Bath application of 5-HT (5 µM) reduced IPSC amplitudes. B: averaged time course of normalized IPSC amplitudes before and after 5-HT application from 10 neurons. C: modulation of IPSC amplitudes by 5-HT in 10 neurons. 5-HT significantly reduced IPSC amplitudes (control vs. 5-HT: from 119.8 ± 11.0 to 73.6 ± 12.5 pA; one-way repeated ANOVA; df = 2; F = 3.93; P = 0.03; post hoc Dunnett test, control vs. 5-HT, P = 0.017; control vs. washed out: from 119.8 ± 11.0 to 101.6 ± 11.4 pA; post hoc Dunnett test, control vs. washout, P = 0.445; n = 10). *P < 0.05.
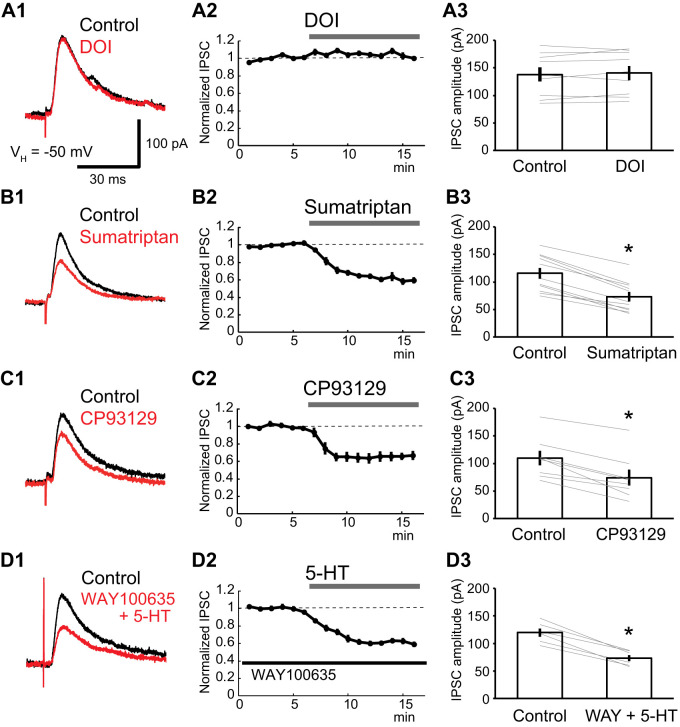
To identify the 5-HT receptor subtype responsible for the reduction of GABAA IPSCs, we tested the effects of 5-HT agonists that differ in their receptor selectivity, in the presence of the GABAB antagonist CGP55845 (1 µM). Addition of DOI (10 µM) to the perfusate, a broad 5-HT2 receptor agonist, had no effect on GABAA IPSCs (Fig. 2A; paired t test; df = 8; t = −0.88; P = 0.405; n = 9 from 4 rats), while sumatriptan (5 µM), a 5-HT1 receptor agonist, attenuated GABAA IPSCs by 37.2 ± 3.6% (Fig. 2B; paired t test; df = 10; t = 11.03; P < 0.001; n = 11 from 5 rats). CP93129 (5 µM), a selective 5-HT1B receptor agonist, reduced GABAA IPSCs by 35.5 ± 5.6%, (Fig. 2C; paired t test; df = 7; t = 6.80; P < 0.001; n = 8 from 3 rats), nearly as much as sumatriptan. To determine whether 5-HT1A receptors are involved in the 5-HT induced suppression of IPSCs, we also tested the effect of WAY100635 (10 µM), a 5-HT1A receptor antagonist. Even in the presence of WAY100365, 5-HT reduced the amplitude of IPSCs by 39.2 ± 2.7% (Fig. 2D; paired t test; df = 5; t = 9.15; P < 0.001; n = 6 from 3 rats). Together, these results indicate that 5-HT1B receptors mediate the inhibition of GABAA IPSCs by 5-HT.
Fig. 2.
5-HT1B receptor subtype mediates the GABAA inhibitory postsynaptic current (IPSC) reduction by 5-HT. The GABAA IPSCs were isolated with CNQX (10 µM), dl-APV (50 µM), and CGP55845 (1 µM). A–D: experiments testing the effects of the broad 5-HT2 receptor antagonist DOI (10 µM; A), the 5-HT1 receptor agonist sumatriptan (5 µM; B), the 5-HT1B receptor agonist CP93129 (5 µM; C), and 5-HT in the presence of the 5-HT1A receptor antagonist WAY100635 (D; 10 µM). For each type of experiment, the left shows representative IPSC traces (black: control; red: drug), the middle shows averaged time course of normalized IPSC amplitudes, and the right shows the result obtained in all tested cells (A, n = 9; B, n = 11; C, n = 8; D, n = 6). The broad 5-HT2 receptor agonist DOI (10 µM) failed to alter IPSC amplitudes (A; from 138.0 ± 13.1 to 140.7 ± 12.9 pA; paired t test; df = 8; t = −0.88; P = 0.405, n = 9). The 5-HT1 receptor agonist sumatriptan reduced the IPSC amplitudes (B; from 115.6 ± 9.9 to 73.1 ± 8.4 pA; paired t test; df = 10; t = 11.03; P < 0.001; n = 11). The 5-HT1B receptor agonist CP93129 reduced the IPSC amplitudes to the same extent as sumatriptan (C; from 109.8 ± 13.1 to 74.0 ± 14.3 pA; paired t test; df = 7; t = 6.80; P < 0.001; n = 8). 5-HT reduced the IPSC amplitude in the presence of Way100635 (D; from 119.6 ± 7.6 to 73.2 ± 5.7 pA; paired t test; df = 5; t = 9.15; P < 0.001; n = 6). *P < 0.05.
The reduction of GABAA IPSCs by 5-HT depends on a presynaptic mechanism.
To test whether the reduction of GABAA IPSCs by 5-HT depends on pre- or postsynaptic mechanisms, we used two approaches. In the first, we examined the effects of 5-HT on the paired-pulse ratio. In the second, we tested whether 5-HT also reduced IPSCs elicited by exogenous application of GABA. Both tests were carried out in the presence of the GABAB antagonist CGP55845 (1 µM).
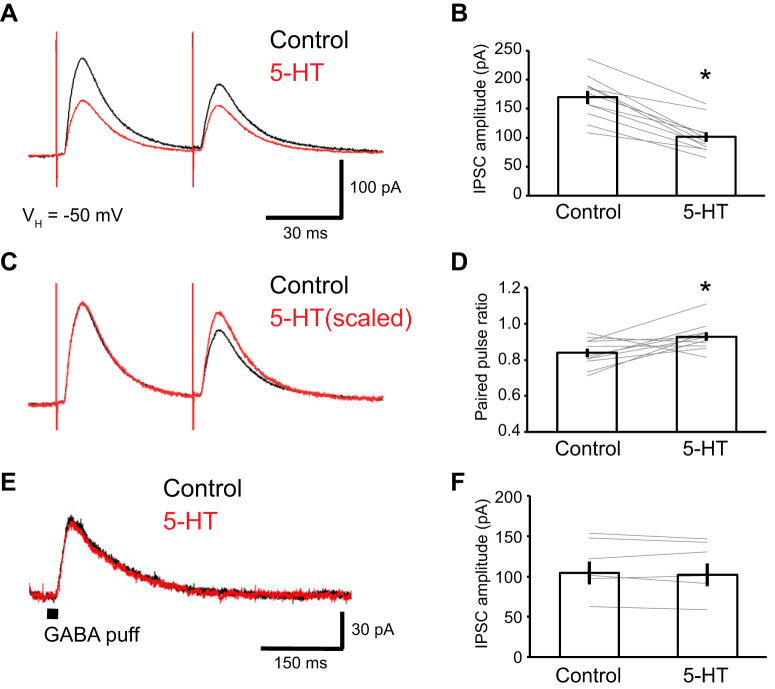
For the first test, we elicited IPSCs by applying two identical electrical stimuli in rapid succession (50-ms interstimulus interval). Addition of 5-HT (5 µM) to the perfusate attenuated the GABAA IPSCs evoked by the first stimulus by 40.2 ± 3.4% (paired t test; df = 10; t = 8.69; P < 0.001; n = 11 from 4 rats; Fig. 3, A and B), similar to the effect observed in our first experiment. In contrast, IPSCs elicited by the second stimulus were more resistant to 5-HT (reduction of 33.9 ± 3.5%; paired t test; df = 10; t = −2.49; P = 0.032; n = 11 from 4 rats) such that the amount of paired-pulse depression was reduced (Fig. 3, C and D; IPSC2/IPSC1; from 0.84 ± 0.02 to 0.93 ± 0.02; paired t test; df = 10; t = −2.58; P = 0.027; n = 11 from 4 rats).
Fig. 3.
Presynaptic origin of the suppression of GABAA inhibitory postsynaptic currents (IPSCs) by 5-HT. The GABAA IPSCs were isolated with CNQX (10 µM), dl-APV (50 µM), and CGP55845 (1 µM). A: representative traces of paired-pulse IPSCs in the absence (black) and presence (red) of 5-HT. B: amplitude of the first IPSC induced by paired-pulse stimulation in control conditions and in the presence of 5-HT (from 170.0 ± 11.3 to 101.0 ± 8.7 pA; paired t test; df = 10; t = 8.69; P < 0.001; n = 11). The extent of suppression was much the same as for the single pulse IPSCs. C: scaled traces for comparing the paired-pulse ratio. D: paired-pulse-ratio in 11 tested neurons, in the absence (black) or presence (red) of 5-HT. The paired-pulse ratio was significantly increased by 5-HT (IPSC2/IPSC1; from 0.84 ± 0.02 to 0.93 ± 0.02; paired t test; df = 10; t = −2.58; P = 0.027; n = 11). E: representative traces of GABAA current induced by a direct GABA (50 µM) puff to the recorded neuron. F: amplitude of GABAA current induced by direct GABA puff applications in 6 tested neurons (from 114.2 ± 14.0 to 111.7 ± 14.03 pA, paired t test; df = 5; t = 0.85; P = 0.431, n = 6). *P < 0.05.
In prior experiments with such paired-stimulus paradigms, it was observed that depending on the synapse type, the second stimulus elicited PSCs of higher or lower amplitude (paired-pulse facilitation or depression, respectively) and that manipulations that increased release probability decreased the amount of facilitation (or enhanced the depression) and conversely (Manabe et al. 1993). As a result, transmitter release probability is thought to be inversely proportional to the amount of facilitation (or proportional to the amount of depression).
Thus the above results suggest that the reduction of IPSCs by 5-HT depends on a presynaptic mechanism, namely a reduction of GABA release. If this interpretation is correct, one would expect GABAA IPSCs evoked by exogenous GABA to be insensitive to 5-HT. To test this prediction, we performed local pressure application of GABA in the vicinity of recorded cells (see materials and methods). As predicted, addition of 5-HT (5 µM) to the perfusate did not alter the IPSCs elicited by exogenous GABA (Fig. 3, E and F; from 114.2 ± 14.0 to 111.7 ± 14.03 pA; paired t test; df = 5; t = 0.85; P = 0.431; n = 6 from 4 rats), again supporting the conclusion that 5-HT presynaptically inhibits IPSCs by reducing GABA release.
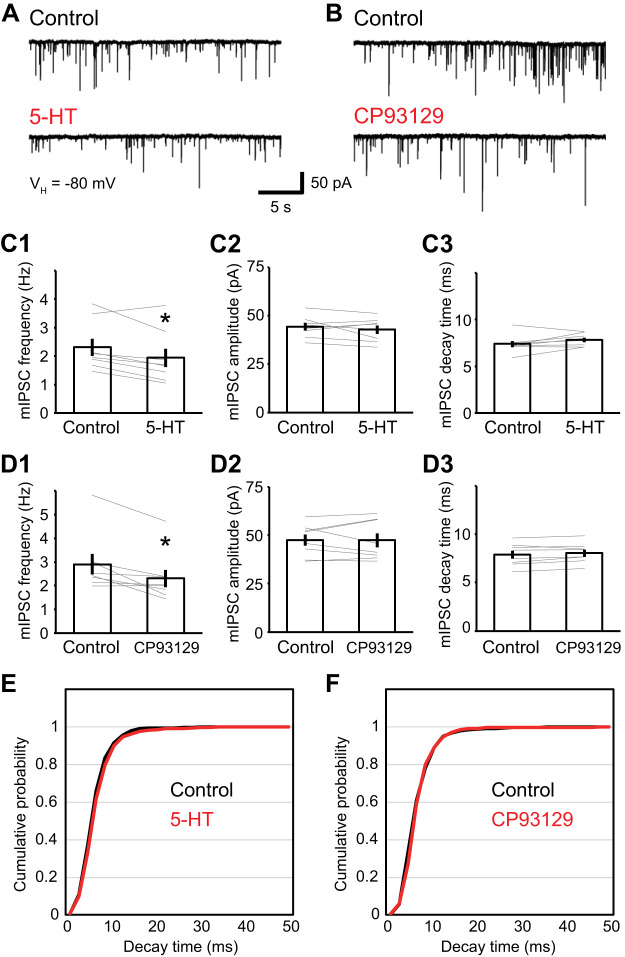
To test this idea further, we examined the effects of 5-HT on pharmacologically isolated miniature GABAA IPSCs (mIPSCs) recorded from a holding potential of −80 mV and in the presence of tetrodotoxin (1 µM) and CGP55845 (1 µM). In this experiment, we used a cesium-based internal solution to improve space clamp. Application of 5-HT (5 µM) decreased the frequency of mIPSCs by 18.1 ± 4.2% (Fig. 4, A and C1; paired t test; df = 7; t = 3.01; P = 0.020; n = 8 from 5 rats), leaving their amplitude and decay time unchanged (Fig. 4, C2, C3, and E; amplitude, 3.1 ± 3.0%; paired t test; df = 7; t = 0.98; P = 0.362; decay, −7.0 ± 3.1%; paired t test; df = 7; t = −1.89; P = 0.100; n = 8 from 5 rats). Moreover, the 5-HT1B receptor agonist CP93129 (5 µM) yielded qualitatively identical results. That is, CP93129 decreased mIPSC frequency by 19.7 ± 5.5% (Fig. 4, B and D1; paired t test; df = 7; t = 3.32; P = 0.013; n = 8 from 4 rats) but did not alter their amplitude or decay time (Fig. 4, D2, D3, and F; amplitude, 0.3 ± 3.3%; paired t test; df = 7; t = 0.02; P = 0.982; decay, −2.3 ± 1.3%; paired t test; df = 7; t = −1.53; P = 0.169; n = 8 from 4 rats). We also examined the effect of 5-HT and CP93129 on the distribution of mIPSC decay time, but no change was noted (Fig. 4, E and F).
Fig. 4.
Effect of 5-HT on GABAA miniature inhibitory postsynaptic currents (mIPSCs). Recordings were performed in the presence of TTX (1 µM), CNQX (10 µM), dl-APV (50 µM), and CGP55845 (1 µM). A and B: representative traces of mIPSCs before (top) and after (bottom) 5-HT (5 µM; A) or CP93129 (5 µM; B) application. VH, holding potential. C1–3: 5-HT reduced the frequency of mIPSC (C1; from 2.3 ± 0.3 Hz to 1.9 ± 0.3 Hz; paired t test; df = 7; t = 3.01; P = 0.020; n = 8) but did not alter their amplitude (C2; from 44.2 ± 2.0 pA to 42.7 ± 2.1 pA; paired t test; df = 7; t = 0.98; P = 0.362; n = 8) and decay time of mIPSC (C3; from 7.4 ± 0.3 ms to 7.8 ± 0.2 ms; paired t test; df = 7; t = −1.89; P = 0.100; n = 8). D1–3: CP93129 reduced the frequency of mIPSC (D1; from 2.9 ± 0.4 Hz to 2.3 ± 0.4 Hz; paired t test; df = 7; t = 3.32; P = 0.013; n = 8) but did not alter their amplitude (D2; from 47.4 ± 2.9 to 47.4 ± 3.6 pA; paired t test; df = 7; t = 0.02; P = 0.982; n = 8) and decay time of mIPSC (D3; from 7.9 ± 0.4 to 8.0 ± 0.4 ms; paired t test; df = 7; t = −1.53; P = 0.169; n = 8). E and F: cumulative probability distribution of the decay time of mIPSCs in control conditions (black) and after application (red) of 5-HT (E) or CP93129 (F). *P < 0.05.
These results further support the conclusion that 5-HT decreases GABA release probability by acting on presynaptic 5-HT1B receptors.
GABAB IPSCs are also regulated by a postsynaptic action of 5-HT.
Collectively, the above experiments indicate that 5-HT causes a reduction in the amplitude of GABAA IPSCs by activating presynaptic 5-HT1B receptors that reduce GABA release. Thus, in principle, 5-HT should also reduce GABAB IPSCs and to a similar extent as for GABAA IPSCs. To test this prediction, we examined the effect of 5-HT on pharmacologically isolated GABAB IPSCs; that is, IPSCs we recorded in the presence of CNQX (10 µM), dl-APV (50 µM), and picrotoxin (100 µM). However, when elicited by single electrical stimuli, the residual (GABAB) IPSCs were unstable and of low amplitude (Fig. 5A, inset). Repetitive electrical stimuli (5 stimuli at 25 Hz) were required to elicit robust GABAB currents. As expected, 5-HT attenuated these synaptically evoked GABAB IPSCs but by only 31.7 ± 1.1% (Fig. 5, A and B; paired t test; df = 7; t = 10.61; P < 0.001; n = 8 from 3 rats), a smaller reduction than seen with GABAA IPSCs (41.6 ± 6.0%) elicited by single electrical stimuli. When we compared the 5-HT-induced reduction of GABAA IPSCs elicited by repetitive stimuli, as in the GABAB experiments, the difference was even larger: the GABAA IPSC was reduced by 45.9 ± 0.1% whereas the GABAB IPSC was reduced by 31.7 ± 1.1% (t test; df = 10; t = 2.47; P = 0.033).
Fig. 5.
The enhancement of the GABAB current by 5-HT is mediated postsynaptically. Recordings in A–D were performed in the presence of picrotoxin (100 µM). A–C: experiments testing the effects of 5-HT (red, 5 µM; control, black) on electrically evoked (5 stimuli at 25 Hz) GABAB inhibitory postsynaptic currents (IPSCs; A) and on IPSCs evoked by pressure application of GABA (50 µM; B) or baclofen (20 µM; C). GABAA and glutamate receptor antagonists were present throughout (see materials and methods). For each type of experiments, the left shows traces from a representative cell whereas the right shows the results obtained in all tested cells (A, n = 8; B, n = 5; C, n = 5). While 5-HT significantly reduced the amplitude of electrically evoked GABAB IPSCs (A: from 39.1 ± 6.7 to 27.1 ± 7.5 pA; paired t test; df = 7; t = 10.61; P < 0.001, n = 8), it enhanced GABAB responses to exogenous GABA (B; from 71.9 ± 23.0 pA to 104.0 ± 25.8 pA; paired t test; df = 4; t = −3.82; P = 0.019; n = 5) and baclofen (C; control, 33.3 ± 1.3 pA; baclofen, 46.3 ± 3.3 pA; washout, 38.0 ± 2.2 pA; one-way repeated ANOVA; df = 2; F = 7.703; P = 0.03; post hoc Dunnett test, control vs. baclofen, P = 0.004; control vs. washout, P = 0.302; n = 5). A, inset: representative examples of GABAB currents evoked by single versus repetitive electrical stimuli. *P < 0.05.
This discrepancy suggested that the regulation of GABAB IPSCs by 5-HT might be more complex than for GABAA currents. To examine this possibility, in the presence of picrotoxin (100 µM), we performed local pressure applications of GABA in the vicinity of recorded cells, allowing us to examine the effects of 5-HT on GABAB currents, independently of any presynaptic effects. Addition of 5-HT (5 µM) to the perfusate enhanced postsynaptic GABAB currents by 57.1 ± 21.5% (Fig. 5, C and D; paired t test; df = 4; t = −3.82; P = 0.019; n = 5 from 3 rats).
To obtain an independent corroboration of this finding, we also examined the effect of 5-HT on GABAB currents elicited by pressure application of baclofen. In control conditions, a brief pulse of baclofen (0.5 s) induced a long-lasting (>30 s) GABAB current (Fig. 5E; control). Addition of 5-HT to the perfusate enhanced the GABAB current by 39.4 ± 9.5%, and the current returned to control values after washout (Fig. 5, E and F; control, 33.3 ± 1.3 pA; baclofen, 46.3 ± 3.3 pA; washout, 38.0 ± 2.2 pA; one-way repeated ANOVA; df = 2; F = 7.703; P = 0.03; post hoc Dunnett test, control vs. baclofen, P = 0.004; control vs. washout, P = 0.302; n = 5 from 2 rats). Together, these results indicate that 5-HT enhances GABAB currents postsynaptically, thereby partially counteracting the reduction caused by the presynaptic inhibition of GABA release.
Negative correlation between GABAA IPSC amplitudes and their attenuation by 5-HT.
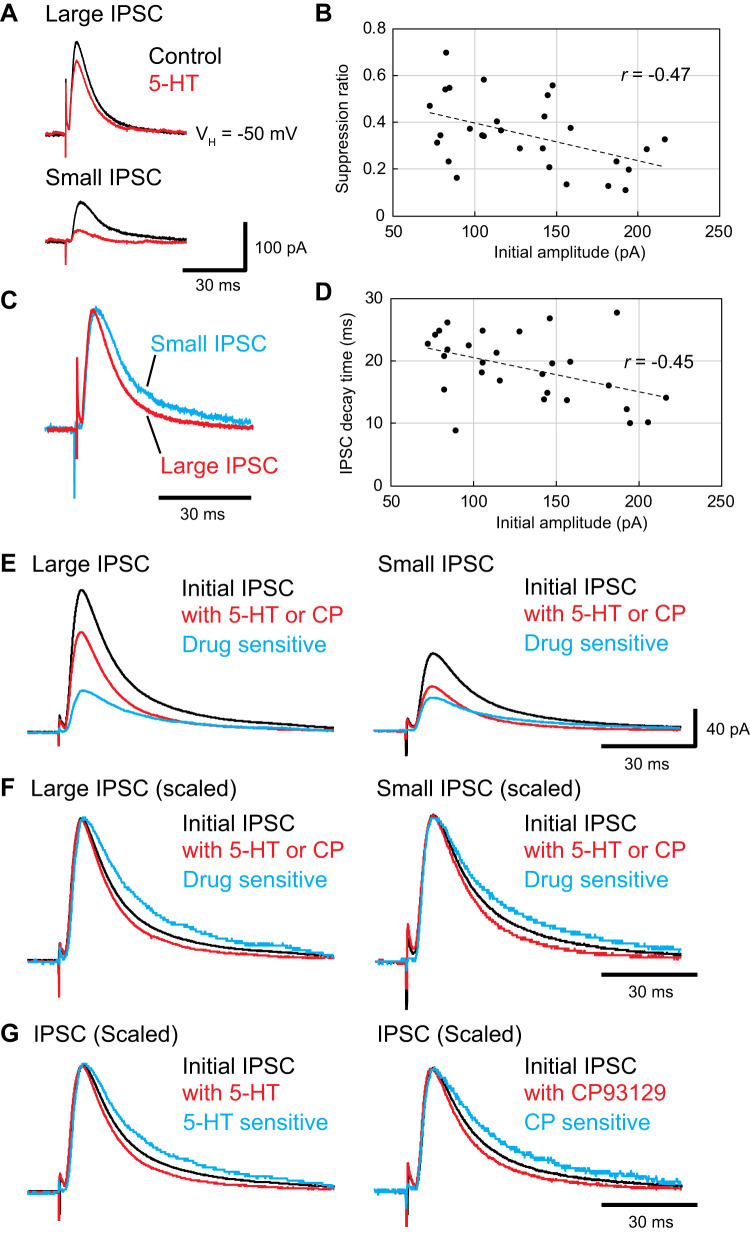
The above experiments indicate that GABAB currents are more resistant to the attenuating effects of 5-HT than GABAA IPSCs. However, in contrast with GABAA IPSCs, repetitive stimuli were required to elicit robust GABAB currents (Fig. 6A, inset), raising the possibility that 5-HT effects on GABAA currents might differ depending on IPSC amplitudes. These considerations led us to revisit our data set on the attenuation of electrically evoked GABAA IPSCs by 5-HT (n = 20 from 9 rats) and CP93129 (n = 8; recorded in Fig. 2). Specifically, we examined the relationship between the initial amplitude of GABAA IPSCs and the magnitude of their attenuation by 5-HT and CP93129 in the presence of the GABAB antagonist CGP55845 (1 µM). Indeed, even though we used a fixed stimulation intensity, IPSC amplitudes varied widely between cells (72.5 to 216.2 pA; n = 28 from 13 rats). This effect was strongly related to the distance between the stimulating electrodes and recorded neurons: the lower the distance, the higher the IPSC amplitude (Pearson r = –0.60; P = 0.004; n = 20 from 4 rats).
Fig. 6.
High-amplitude inhibitory postsynaptic currents (IPSCs) are less sensitive to 5-HT than low-amplitude IPSCs. Recordings were performed in the presence of CNQX (10 µM), dl-APV (50 µM), and CGP55845 (1 µM). A: representative traces showing IPSCs in control conditions (black) and in the presence of 5-HT agonists (5- HT; 5 µM, n = 20; CP93129; 5 µM; n = 8; red) in cells with high- (top) and low-amplitude (bottom) IPSCs. The low-amplitude IPSC was suppressed to a greater extent by the 5-HT agonists than the high-amplitude IPSC. B: IPSC suppression ratio (y-axis) as a function of control IPSC amplitude (x-axis). C: differing time course of low (blue)- and high-amplitude (red) IPSCs. Low amplitude IPSC was scaled up to match the peak amplitude of a high-amplitude IPSC, highlighting the slower decay time of low-amplitude IPSCs. D: IPSC half-width (y-axis) as a function of IPSC amplitude (x-axis). E: for high (left)- and low-amplitude (right) IPSCs, 3 superimposed traces are shown: control IPSC (black), IPSC in the presence of 5-HT agonist (red), and 5-HT-sensitive component (blue). The 5-HT-sensitive IPSC was estimated by subtracting the 5-HT-depressed IPSC from the initial IPSC. F: for high (left)- and low-amplitude (right) IPSCs, we show 3 superimposed traces scaled so that their peak amplitude would match: control IPSC (black), IPSC in the presence of the 5-HT agonist (red) and 5-HT-sensitive component (blue). G: averaged IPSCs recorded in the experiments testing the effects of 5-HT (left) or CP93129 (right), irrespective of control IPSC amplitudes. In both cases, we show 3 superimposed traces scaled so that their peak amplitude would match: control IPSC (black), IPSC in the presence of 5-HT agonist (red), and the difference between these 2 traces.
Unexpectedly, the impact of 5-HT and CP93129 varied depending on control IPSC amplitudes. As shown in the representative examples of Fig. 6A, control IPSCs of high amplitude were less sensitive to 5-HT than IPSCs of low amplitude. In fact, there was a significant negative correlation between initial IPSC amplitudes and their sensitivity to 5-HT and CP93129 (Fig. 6B; Pearson r = –0.47; P = 0.012; n = 28 from 13 rats).
The contrasting sensitivity of high versus low-amplitude GABAA IPSCs to 5-HT and CP93129 suggests that they are mediated by different complements of GABAergic synapses, with a larger proportion of the synapses generating low-amplitude IPSCs expressing 5-HT1B receptors than in high-amplitude IPSCs and a greater fraction of those mediating high-amplitude IPSCs devoid of 5-HT1B receptors than in low-amplitude IPSCs. To further characterize the two sets of inhibitory synapses, we first examined the time course of the pharmacologically isolated GABAA IPSCs and determined if it correlated with their amplitude. As shown in Fig. 6D, IPSC durations (decay time) correlated negatively with their amplitude (Pearson r = –0.45; P = 0.017; n = 28 from 13 rats), whereas their rise time (20–80%) did not (Pearson r = 0.12; P = 0.528; n = 28 from 13 rats).
Furthermore, 5-HT and CP93129 accelerated the decaying phase of the IPSCs (from 18.9 ± 1.5 to 14.6 ± 1.4 ms; paired t test; df = 27; t = 6.51; P < 0.001; n = 28 from 13 rats). This is evident in Fig. 6, E and F, which illustrates, for the 14 highest (left) and 14 lowest (right) amplitude IPSCs, the control IPSC, the IPSC in the presence of 5-HT or CP93129, and the difference between the two (i.e., the 5-HT-sensitive component). As shown in Fig. 6, in both subsets of cells, the 5-HT-sensitive component had a markedly slower time course than the 5-HT-resistant component (the decay time was ~12 ms longer; 26.5 ± 2.1 vs. 14.6 ± 0.9 ms; paired t test; df = 27; t = −5.82; P < 0.001; n = 28 from 13 rats). Importantly, while the amplitude of the 5-HT-sensitive current (Table 1) did not differ between the two subsets of cells (131.8%; high, 51.5 ± 5.2; low, 39.1 ± 3.7 pA; t test, df = 26; t = 1.96; P = 0.06), that of the 5-HT-resistant component was more than twice higher (219.2%) in cells with high (120.0 ± 9.3 pA) than low (54.7 ± 4.4 pA) IPSC amplitudes (t test; df = 26; t = 6.34; P < 0.001). We also analyzed the effect of 5-HT (5 µM) and CP93129 (5 µM) on the decay time of IPSCs recorded separately. With 5-HT, the decay time of GABAA IPSCs became faster (Fig. 6G, left; from 18.4 ± 1.4 to 14.2 ± 1.3 ms; paired t test; df = 19; t = 4.86; P < 0.001; n = 20 from 9 rats). With CP93129, also the decay time of GABAA IPSCs became faster (Fig. 6G, right; from 20.2 ± 1.0 to 15.7 ± 0.7 ms; paired t test; df = 7; t = 5.17; P < 0.002; n = 8 from 4 rats). This result indicates the serotonergic modulation of the IPSCs time course is also dependent on 5-HT1B receptors.
Table 1.
Properties of low- and high-amplitude IPSCs in control conditions in the presence of drugs as well as the difference between them
| Amplitude, pA | Rise Time, ms | Decay Time, ms | Half Width, ms | |
|---|---|---|---|---|
| Control IPSCs | ||||
| High | 167.2 ± 7.5* | 1.91 ± 0.15 | 17.3 ± 1.5 | 14.3 ± 1.0 |
| Low | 92.3 ± 3.8* | 2.21 ± 0.13 | 20.6 ± 1.2 | 16.2 ± 0.9 |
| Drug-resistant component | ||||
| High | 120.0 ± 9.3* | 1.76 ± 0.16 | 13.7 ± 1.5 | 12.5 ± 0.9 |
| Low | 54.7 ± 4.4* | 2.05 ± 0.18 | 15.5 ± 1.2 | 14.0 ± 0.8 |
| Drug-sensitive component | ||||
| High | 51.5 ± 5.2 | 2.64 ± 0.35 | 24.9 ± 3.7 | 19.7 ± 2.9 |
| Low | 39.1 ± 3.6 | 2.32 ± 0.22 | 28.1 ± 1.9 | 20.1 ± 1.6 |
Values are means ± SE for control conditions in the presence of drugs (5-HT or CP93129; drug-resistant component) and the difference between them (drug-sensitive component); n = 14. In cells with low- and high-amplitude inhibitory postsynaptic currents (IPSCs), the 5-HT-sensitive component had a slower decay time than the 5-HT-resistant component (P < 0.001; see detailed statistics in main text). Whereas the amplitude of the 5-HT-sensitive current did not differ between the 2 subsets of cells, that of the 5-HT-resistant component was significantly higher in cells with high than low IPSC amplitudes (P < 0.001). CP93129 also shortened the decay time of GABAA IPSCs (P < 0.002).
P < 0.05, statistically significant differences between high vs. low.
Together, these findings suggest that high- and low-amplitude control IPSCs both contain a fast, relatively 5-HT-insensitive component and a slower, 5-HT-sensitive component. The fast, 5-HT-resistant component accounts for a larger portion of the overall IPSC in cells with high- compared with low-amplitude IPSCs. The slow, 5-HT-sensitive component accounts for a larger portion of the overall IPSC in cells with low- compared with high-amplitude IPSCs. The absolute size of the slow component is about the same for both the large and small control IPSCs. However, because the fast component is much larger in high-amplitude IPSCs, they appear relatively resistant to 5-HT.
DISCUSSION
This study was undertaken to shed light on the regulation of GABAergic inhibition by 5-HT in LA. The interest of this question stems from prior observations indicating that inhibition is a major determinant of PN activity in LA (reviewed in Paré et al. 2003) and converging lines of evidence implicating a dysregulation of the amygdala, in part mediated by 5-HT, in pathological anxiety (Bandelow et al. 2017; Bremner 2005; LeDoux 2000; Lowry et al. 2005; Shin et al. 2006).
Our results indicate that by binding to presynaptic 5-HT1B receptors and inhibiting GABA release, 5-HT attenuates GABAergic IPSCs. However, this effect was more evident when monitoring GABAA than GABAB IPSCs because 5-HT also caused a postsynaptic enhancement of GABAB currents, which partially counteracted for the presynaptic inhibition of GABA release. Unexpectedly, we also observed that 5-HT differentially regulates distinct subsets of GABAergic synapses. That is, 5-HT did not seem to have much influence on a set of inhibitory inputs that mediates fast GABAA IPSCs but strongly inhibited another one that generates slower GABAA responses.
Prior work on the regulation of neuronal excitability by 5-HT in the basolateral complex of the amygdala.
The regulation of neuronal excitability by 5-HT is extremely complex because the density of 5-HT inputs varies between structures (Steinbusch 1981) and the expression of the many 5-HT receptor subtypes is heterogeneous, regionally as well as between cell types (for example, see Andrade 1998; Chameau and van Hooft 2006; Palacios 2016). The basolateral complex of the amygdala is no exception. For instance, a higher density of 5-HT axons is found in the BL than LA (Muller et al. 2007b; Sengupta et al. 2017) and the concentration of 5-HT receptors varies between these two nuclei. For instance, 5-HT2C receptors are more concentrated in LA than BL (Greenwood et al. 2012), whereas the opposite is true for 5-HT1A and 5-HT1B receptors (Saha et al. 2010). There is also heterogeneity at the level of single cells. For instance, in BL at least, 5-HT2A receptors are expressed by PNs and parvalbumin-positive interneurons but not by cholecystokinin interneurons (McDonald and Mascagni 2007). In contrast, 5-HT3A receptors are only found in a specific subtype of local-circuit cell that does not express calcium-binding proteins (Mascagni and McDonald 2007; see Bocchio et al. 2016 for an extensive description of the cell-type specific expression of 5-HT receptors).
Expectedly given these variations, electrophysiological studies on the effect of 5-HT in LA and BL have yielded different results. In LA PNs, 5-HT was found to exert a variety of excitatory effects including a depolarization, the inhibition of Ca2+-dependent K+ currents, and the enhancement of depolarization-induced slow afterdepolarizations (Faber and Sah 2002; Yamamoto et al. 2012, 2014). In BL by contrast, optogenetic excitation of 5-HT terminals inhibited most PNs, an effect mediated by the activation of 5-HT1A receptors combined with a direct excitation of local-circuit cells (Sengupta et al. 2017). Indeed, the optogenetic activation of 5-HT terminals excited a large proportion of interneurons via 5-HT2A receptors; only a minority of interneurons were inhibited and via 5-HT1A receptors (Sengupta et al. 2017). Consistent with this, the pharmacological activation of 5-HT2A receptors depolarized interneurons in the BLA (Bocchio et al. 2015; Jiang et al. 2009).
In addition to the postsynaptic actions reviewed above, 5-HT also acts presynaptically to regulate transmitter release in the basolateral complex of the amygdala. Indeed, an electron microscopic study found numerous axon terminals immunopositive for 5-HT in close apposition with nonserotonergic axon terminals (Muller et al. 2007b). Consistent with this, several studies reported that 5-HT presynaptically inhibits (Cheng et al. 1998; Rainnie 1999; Yamamoto et al. 2012) transmitter release in the basolateral complex of the amygdala.
5-HT inhibits GABA release via presynaptic 5-HT1B receptors.
In LA and BL, the influence of 5-HT over glutamate release appears to be always inhibitory (Cheng et al. 1998; Guo et al. 2017; Yamamoto et al. 2012). While several candidate 5-HT receptors were identified for this effect (Cheng et al. 1998; Guo et al. 2017; Yamamoto et al. 2012), the receptors responsible for the modulation of GABA release remained unknown. By contrasting the impact of a variety of 5-HT receptor agonists and antagonists, our study established that 5-HT1B receptors mediate the inhibitory influence of 5-HT on GABA release in LA.
The 5-HT1B receptor is a Gi/o-coupled receptor that reduces neural activity, is predominantly found on axon terminals (Boschert et al. 1994) and is similar in structure to the 5-HT1A receptor, another Gi/o-coupled receptor (Hoyer et al. 2002). In many other brain regions, activation of 5-HT1B receptors causes a suppression of both excitatory and inhibitory synaptic transmission. These include the suprachiasmatic nucleus (Pickard et al. 1999), basal forebrain (Nishijo and Momiyama 2016), cerebellar nuclei (Saitow et al. 2009), bed nucleus of stria terminalis (Guo and Rainnie 2010), and various cortical regions (Kjaerby et al. 2016; Liao and Lee 2014; Mlinar et al. 2003). In all these cases, the suppression depended on a presynaptic mechanism.
Our findings in LA agree with these earlier observations. Indeed, we also found that the 5-HT1B receptor-dependent suppression of GABA transmission is mediated presynaptically. That is, even though 5-HT reduced electrically evoked GABAA IPSCs, it did not alter the inhibitory responses elicited by pressure application of GABA. Moreover, we found that 5-HT reduced the amount of paired-pulse depression normally exhibited by electrically evoked IPSCs and the frequency (but not amplitude) of GABAA mIPSCs.
Postsynaptic enhancement of GABAB IPSCs by 5-HT.
We noticed that GABAA and GABAB IPSCs were not reduced to the same extent by 5-HT, which seemed inconsistent with the notion that 5-HT acts presynaptically, by inhibiting GABA release. However, control experiments revealed that GABAB IPSCS were also potentiated by 5-HT through a postsynaptic mechanism. Indeed, when GABAB currents were induced by directly puffing GABA or baclofen, 5-HT actually enhanced GABAB currents. Together, these results suggest that GABAB currents are partially spared from the 5-HT-induced suppression of GABA release by a competing postsynaptic enhancement of GABAB currents.
While the 5-HT receptors responsible for this effect remain unknown, it should be noted that there are precedents in the literature for this effect (Jacobson et al. 2017; Strahlendorf et al. 1991). Also, it seems unlikely that the direct convergence of 5-HT and GABA acting via GABAB receptors on the same K+ conductance (Andrade et al. 1986) is responsible for the postsynaptic enhancement of GABAB currents since this mechanism would be expected to reduce GABAB currents through occlusion.
A clue pointing to the possible significance of this dual regulation of GABAB currents by 5-HT comes from the contrasting intensity of the afferent drive required to elicit GABAA compared with GABAB IPSCs. Whereas a single low-intensity stimulus reliably elicits GABAA IPSCs, activation of robust GABAB currents requires repetitive stimulation. Thus the dual regulation of GABAB IPSCs might preserve the dynamics of inhibitory tone in LA.
5-HT differentially regulates distinct subsets of GABAergic synapses.
Several observations suggest that the electrical stimuli we used to elicit IPSCs recruited different contingent of GABAergic synapses and that 5-HT regulates them differentially. First, depending on the distance between the stimulating electrodes and recorded cells, IPSCs varied in amplitude, with cells closer to the stimulating electrodes exhibiting higher amplitude IPSCs than more distant neurons. Second, 5-HT caused a greater attenuation of low- than high-amplitude IPSCs. Third, for both low- and high amplitude IPSCs, the time course of the 5-HT-sensitive and -resistant IPSCs differed markedly: the 5-HT-insensitive component rose (~2 ms) and decayed faster (~15 ms) than the 5-HT-sensitive component (~3 and 30 ms, respectively).
The differing time course of the 5-HT-resistant and -sensitive components is reminiscent of the GABAA,fast and GABAA,slow first described in the hippocampus (Banks et al. 1998; Pearce 1993) and later in a variety of other brain regions (reviewed in Capogna and Pearce 2011). In these various structures, mounting evidence suggests that GABAA,slow is generated by dendrite-targeting interneurons such as the NPY-expressing neurogliaform (NGF) cells (Price et al. 2005; Szabadics et al. 2007). These cells are unusual because they have an extensive axonal field that often crosses regional boundaries. Various mechanisms have been invoked to explain the prolonged time course of GABAA,slow (reviewed in Armstrong et al. 2012), including dendritic filtering, asynchronous vesicular release, GABA spillover, and differences in GABA receptor subunit composition. While these factors may contribute to explain the slow time course of GABAA,slow, the main one appears to be the spatiotemporal profile of GABA concentration at the synapse (Karayannis et al. 2010; Krook-Magnuson and Huntsman 2007; Szabadics et al. 2007). Indeed, many of the synapses formed by NGF cells are appositions rather than conventional synapses with narrow clefts, such that the GABA transient is prolonged (Oláh et al. 2009; Szabadics et al. 2007).
Since LA also contains NGF neurons (McDonald 1984), could they be responsible for the 5-HT-sensitive GABAA,slow we observed? In support of this possibility, an electron microscopic study in the basolateral complex of the amygdala reported that 5-HT-positive axonal boutons contact NPY-positive somata (Bonn et al. 2013). Moreover, NGF cells have an extensive axonal arbor, contribute frequent en passant axonal varicosities, and form many appositions, as opposed to conventional synaptic contacts, which should prolong GABA transients (Mańko et al. 2012). Consistent with this, it was reported that the inhibitory responses elicited by the activation of NGF cells last longer than those evoked by other interneuronal classes of the basolateral amygdala (Mańko et al. 2012; reviewed in Capogna 2014).
While LA also contains NGF neurons (McDonald 1984), lacking experiments directly testing the impact of 5-HT on the IPSCs generated by NGF cells, we can only speculate as to their involvement in the genesis of the slow 5-HT-sensitive IPSC component. Furthermore, it is currently unclear why the slow IPSC component accounted for a greater proportion of the IPSCs seen in PNs distant from our stimulating electrodes. We presume that it reflects differences in the axonal arbors of the various interneuronal subtypes, with those devoid of 5-HT1B receptors having narrower axonal fields.
An important challenge for future investigations will be to ascertain whether NGF cells play a critical role in the regulation of LA by 5-HT. This could be achieved by examining whether NPY+ axon terminals express 5-HT1B receptors and by studying the impact of 5-HT on the IPSCs generated by optogenetically excited NGF cells.
GRANTS
This work was supported partly by KAKENHI Grants 18K07572 and 18KK0468 (to R. Yamamoto), 19K16192 (to T. Furuyama), 19K09918 (to M. Ono), and 17H02223 (to N. Kato), Kanazawa Medical University Grant C2018-1 (to N. Kato), a grant from the Nakatomi Foundation (to R. Yamamoto), and National Institute of Mental Health Grant MH-119854 (to D. Pare).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.Y. conceived and designed research; R.Y. and T.F. performed experiments; R.Y., T.F., T.S., D.P., and N.K. analyzed data; R.Y., T.S., M.O., D.P., and N.K. interpreted results of experiments; R.Y. prepared figures; R.Y., D.P., and N.K. drafted manuscript; R.Y. and D.P. edited and revised manuscript; R.Y., T.F., T.S., M.O., D.P., and N.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank S. Muramoto, K. Yamada, and S. Tsukurimichi for assistance.
REFERENCES
- Andrade R. Regulation of membrane excitability in the central nervous system by serotonin receptor subtypes. Ann N Y Acad Sci 861: 190–203, 1998. doi: 10.1111/j.1749-6632.1998.tb10191.x. [DOI] [PubMed] [Google Scholar]
- Andrade R, Malenka RC, Nicoll RA. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science 234: 1261–1265, 1986. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Armstrong C, Krook-Magnuson E, Soltesz I. Neurogliaform and ivy cells: a major family of nNOS expressing GABAergic neurons. Front Neural Circuits 6: 23, 2012. doi: 10.3389/fncir.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B, Michaelis S, Wedekind D. Treatment of anxiety disorders. Dialogues Clin Neurosci 19: 93–107, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MI, Li TB, Pearce RA. The synaptic basis of GABAA, slow. J Neurosci 18: 1305–1317, 1998. doi: 10.1523/JNEUROSCI.18-04-01305.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchio M, Fucsina G, Oikonomidis L, McHugh SB, Bannerman DM, Sharp T, Capogna M. Increased serotonin transporter expression reduces fear and recruitment of parvalbumin interneurons of the amygdala. Neuropsychopharmacology 40: 3015–3026, 2015. doi: 10.1038/npp.2015.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchio M, McHugh SB, Bannerman DM, Sharp T, Capogna M. Serotonin, amygdala and fear: assembling the puzzle. Front Neural Circuits 10: 24, 2016. doi: 10.3389/fncir.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn M, Schmitt A, Lesch KP, Van Bockstaele EJ, Asan E. Serotonergic innervation and serotonin receptor expression of NPY-producing neurons in the rat lateral and basolateral amygdaloid nuclei. Brain Struct Funct 218: 421–435, 2013. doi: 10.1007/s00429-012-0406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschert U, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience 58: 167–182, 1994. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Effects of traumatic stress on brain structure and function: relevance to early responses to trauma. J Trauma Dissociation 6: 51–68, 2005. doi: 10.1300/J229v06n02_06. [DOI] [PubMed] [Google Scholar]
- Capogna M. GABAergic cell type diversity in the basolateral amygdala. Curr Opin Neurobiol 26: 110–116, 2014. doi: 10.1016/j.conb.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Capogna M, Pearce RA. GABA A,slow: causes and consequences. Trends Neurosci 34: 101–112, 2011. doi: 10.1016/j.tins.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Carlsen J. Immunocytochemical localization of glutamate decarboxylase in the rat basolateral amygdaloid nucleus, with special reference to GABAergic innervation of amygdalostriatal projection neurons. J Comp Neurol 273: 513–526, 1988. doi: 10.1002/cne.902730407. [DOI] [PubMed] [Google Scholar]
- Chameau P, van Hooft JA. Serotonin 5-HT(3) receptors in the central nervous system. Cell Tissue Res 326: 573–581, 2006. doi: 10.1007/s00441-006-0255-8. [DOI] [PubMed] [Google Scholar]
- Cheng LL, Wang SJ, Gean PW. Serotonin depresses excitatory synaptic transmission and depolarization-evoked Ca2+ influx in rat basolateral amygdala via 5-HT1A receptors. Eur J Neurosci 10: 2163–2172, 1998. doi: 10.1046/j.1460-9568.1998.00229.x. [DOI] [PubMed] [Google Scholar]
- Cohen JY, Amoroso MW, Uchida N. Serotonergic neurons signal reward and punishment on multiple timescales. eLife 4: e06346, 2015. doi: 10.7554/eLife.06346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Callister RJ, Sah P. Morphological and electrophysiological properties of principal neurons in the rat lateral amygdala in vitro. J Neurophysiol 85: 714–723, 2001. doi: 10.1152/jn.2001.85.2.714. [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci 22: 1618–1628, 2002. doi: 10.1523/JNEUROSCI.22-05-01618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus 6: 347–470, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res 826: 35–43, 1999. doi: 10.1016/S0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Loughridge AB, Day HE, Clark PJ, Mika A, Hellwinkel JE, Spence KG, Fleshner M. 5-HT2C receptors in the basolateral amygdala and dorsal striatum are a novel target for the anxiolytic and antidepressant effects of exercise. PLoS One 7: e46118, 2012. doi: 10.1371/journal.pone.0046118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JD, O’Flaherty BM, Rainnie DG. Serotonin gating of cortical and thalamic glutamate inputs onto principal neurons of the basolateral amygdala. Neuropharmacology 126: 224–232, 2017. doi: 10.1016/j.neuropharm.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JD, Rainnie DG. Presynaptic 5-HT(1B) receptor-mediated serotonergic inhibition of glutamate transmission in the bed nucleus of the stria terminalis. Neuroscience 165: 1390–1401, 2010. doi: 10.1016/j.neuroscience.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71: 533–554, 2002. doi: 10.1016/S0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Hoyer D, Fehlmann D, Bettler B, Kaupmann K, Cryan JF. Blunted 5-HT1A receptor-mediated responses and antidepressant-like behavior in mice lacking the GABAB1a but not GABAB1b subunit isoforms. Psychopharmacology (Berl) 234: 1511–1523, 2017. doi: 10.1007/s00213-016-4521-5. [DOI] [PubMed] [Google Scholar]
- Jiang X, Xing G, Yang C, Verma A, Zhang L, Li H. Stress impairs 5-HT2A receptor-mediated serotonergic facilitation of GABA release in juvenile rat basolateral amygdala. Neuropsychopharmacology 34: 410–423, 2009. doi: 10.1038/npp.2008.71. [DOI] [PubMed] [Google Scholar]
- Karayannis T, Elfant D, Huerta-Ocampo I, Teki S, Scott RS, Rusakov DA, Jones MV, Capogna M. Slow GABA transient and receptor desensitization shape synaptic responses evoked by hippocampal neurogliaform cells. J Neurosci 30: 9898–9909, 2010. doi: 10.1523/JNEUROSCI.5883-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara H, Yoshida M, Yokoo H, Nishi M, Tanaka M. Psychological stress increases serotonin release in the rat amygdala and prefrontal cortex assessed by in vivo microdialysis. Neurosci Lett 162: 81–84, 1993. doi: 10.1016/0304-3940(93)90565-3. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Allen AR, Lucki I. Regional differences in the effects of forced swimming on extracellular levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res 682: 189–196, 1995. doi: 10.1016/0006-8993(95)00349-U. [DOI] [PubMed] [Google Scholar]
- Kjaerby C, Athilingam J, Robinson SE, Iafrati J, Sohal VS. Serotonin 1B receptors regulate prefrontal function by gating callosal and hippocampal inputs. Cell Rep 17: 2882–2890, 2016. doi: 10.1016/j.celrep.2016.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Huntsman MM. The transience of interneuron circuit diversity just “sped” up. Proc Natl Acad Sci USA 104: 16723–16724, 2007. doi: 10.1073/pnas.0708149104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang EJ, Paré D. Similar inhibitory processes dominate the responses of cat lateral amygdaloid projection neurons to their various afferents. J Neurophysiol 77: 341–352, 1997. doi: 10.1152/jn.1997.77.1.341. [DOI] [PubMed] [Google Scholar]
- Le Gal LaSalle G, Paxinos G, Emson P, Ben-Ari Y. Neurochemical mapping of GABAergic systems in the amygdaloid complex and bed nucleus of the stria terminalis. Brain Res 155: 397–403, 1978. doi: 10.1016/0006-8993(78)91037-5. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184, 2000. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Liao CC, Lee LJ. Presynaptic 5-HT1B receptor-mediated synaptic suppression to the subplate neurons in the somatosensory cortex of neonatal rats. Neuropharmacology 77: 81–89, 2014. doi: 10.1016/j.neuropharm.2013.08.040. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress 8: 233–246, 2005. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Ma QP, Yin GF, Ai MK, Han JS. Serotonergic projections from the nucleus raphe dorsalis to the amygdala in the rat. Neurosci Lett 134: 21–24, 1991. doi: 10.1016/0304-3940(91)90499-J. [DOI] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol 70: 1451–1459, 1993. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- Mańko M, Bienvenu TC, Dalezios Y, Capogna M. Neurogliaform cells of amygdala: a source of slow phasic inhibition in the basolateral complex. J Physiol 590: 5611–5627, 2012. doi: 10.1113/jphysiol.2012.236745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5: 793–807, 2004. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Res 976: 171–184, 2003. doi: 10.1016/S0006-8993(03)02625-8. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. A novel subpopulation of 5-HT type 3A receptor subunit immunoreactive interneurons in the rat basolateral amygdala. Neuroscience 144: 1015–1024, 2007. doi: 10.1016/j.neuroscience.2006.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Neuronal organization of the lateral and basolateral amygdaloid nuclei in the rat. J Comp Neurol 222: 589–606, 1984. doi: 10.1002/cne.902220410. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Neuronal localization of 5-HT type 2A receptor immunoreactivity in the rat basolateral amygdala. Neuroscience 146: 306–320, 2007. doi: 10.1016/j.neuroscience.2007.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Muller JF, Mascagni F. GABAergic innervation of alpha type II calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. J Comp Neurol 446: 199–218, 2002. doi: 10.1002/cne.10204. [DOI] [PubMed] [Google Scholar]
- Mlinar B, Falsini C, Corradetti R. Pharmacological characterization of 5-HT(1B) receptor-mediated inhibition of local excitatory synaptic transmission in the CA1 region of rat hippocampus. Br J Pharmacol 138: 71–80, 2003. doi: 10.1038/sj.bjp.0705026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol 494: 635–650, 2006. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J Comp Neurol 500: 513–529, 2007a. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Serotonin-immunoreactive axon terminals innervate pyramidal cells and interneurons in the rat basolateral amygdala. J Comp Neurol 505: 314–335, 2007b. doi: 10.1002/cne.21486. [DOI] [PubMed] [Google Scholar]
- Nishijo T, Momiyama T. Serotonin 5-HT1B receptor-mediated calcium influx-independent presynaptic inhibition of GABA release onto rat basal forebrain cholinergic neurons. Eur J Neurosci 44: 1747–1760, 2016. doi: 10.1111/ejn.13273. [DOI] [PubMed] [Google Scholar]
- Oláh S, Füle M, Komlósi G, Varga C, Báldi R, Barzó P, Tamás G. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature 461: 1278–1281, 2009. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios JM. Serotonin receptors in brain revisited. Brain Res 1645: 46–49, 2016. doi: 10.1016/j.brainres.2015.12.042. [DOI] [PubMed] [Google Scholar]
- Paré D, Gaudreau H. Projection cells and interneurons of the lateral and basolateral amygdala: distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. J Neurosci 16: 3334–3350, 1996. doi: 10.1523/JNEUROSCI.16-10-03334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Royer S, Smith Y, Lang EJ. Contextual inhibitory gating of impulse traffic in the intra-amygdaloid network. Ann N Y Acad Sci 985: 78–91, 2003. doi: 10.1111/j.1749-6632.2003.tb07073.x. [DOI] [PubMed] [Google Scholar]
- Pearce RA. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron 10: 189–200, 1993. doi: 10.1016/0896-6273(93)90310-N. [DOI] [PubMed] [Google Scholar]
- Pickard GE, Smith BN, Belenky M, Rea MA, Dudek FE, Sollars PJ. 5-HT1B receptor-mediated presynaptic inhibition of retinal input to the suprachiasmatic nucleus. J Neurosci 19: 4034–4045, 1999. doi: 10.1523/JNEUROSCI.19-10-04034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Cauli B, Kovacs ER, Kulik A, Lambolez B, Shigemoto R, Capogna M. Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J Neurosci 25: 6775–6786, 2005. doi: 10.1523/JNEUROSCI.1135-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol 82: 69–85, 1999. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Parent A. The monoaminergic innervation of the amygdala in the squirrel monkey: an immunohistochemical study. Neuroscience 36: 431–447, 1990. doi: 10.1016/0306-4522(90)90439-B. [DOI] [PubMed] [Google Scholar]
- Saha S, Gamboa-Esteves FO, Batten TF. Differential distribution of 5-HT 1A and 5-HT 1B-like immunoreactivities in rat central nucleus of the amygdala neurones projecting to the caudal dorsomedial medulla oblongata. Brain Res 1330: 20–30, 2010. doi: 10.1016/j.brainres.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Saitow F, Murano M, Suzuki H. Modulatory effects of serotonin on GABAergic synaptic transmission and membrane properties in the deep cerebellar nuclei. J Neurophysiol 101: 1361–1374, 2009. doi: 10.1152/jn.90750.2008. [DOI] [PubMed] [Google Scholar]
- Schweimer JV, Ungless MA. Phasic responses in dorsal raphe serotonin neurons to noxious stimuli. Neuroscience 171: 1209–1215, 2010. doi: 10.1016/j.neuroscience.2010.09.058. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Bocchio M, Bannerman DM, Sharp T, Capogna M. Control of amygdala circuits by 5-HT neurons via 5-HT and glutamate cotransmission. J Neurosci 37: 1785–1796, 2017. doi: 10.1523/JNEUROSCI.2238-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci 1071: 67–79, 2006. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Smith Y, Paré JF, Paré D. Cat intraamygdaloid inhibitory network: ultrastructural organization of parvalbumin-immunoreactive elements. J Comp Neurol 391: 164–179, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- Spampanato J, Polepalli J, Sah P. Interneurons in the basolateral amygdala. Neuropharmacology 60: 765–773, 2011. doi: 10.1016/j.neuropharm.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience 6: 557–618, 1981. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Strahlendorf JC, Lee MH, Strahlendorf HK. Serotonin modulates muscimol- and baclofen-elicited inhibition of cerebellar Purkinje cells. Eur J Pharmacol 201: 239–242, 1991. doi: 10.1016/0014-2999(91)90352-Q. [DOI] [PubMed] [Google Scholar]
- Szabadics J, Tamás G, Soltesz I. Different transmitter transients underlie presynaptic cell type specificity of GABAA,slow and GABAA,fast. Proc Natl Acad Sci USA 104: 14831–14836, 2007. doi: 10.1073/pnas.0707204104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci 12: 4066–4079, 1992. doi: 10.1523/JNEUROSCI.12-10-04066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Hatano N, Sugai T, Kato N. Serotonin induces depolarization in lateral amygdala neurons by activation of TRPC-like current and inhibition of GIRK current depending on 5-HT(2C) receptor. Neuropharmacology 82: 49–58, 2014. doi: 10.1016/j.neuropharm.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Yamamoto R, Ueta Y, Sugai T, Kato N. A serotonergic discrimination favoring synaptic inputs that accompany robust spike firing in lateral amygdala neurons. Neuroscience 220: 119–130, 2012. doi: 10.1016/j.neuroscience.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Suzuki E, Sato T, Maruta S, Watanabe S, Miyaoka H. Amygdalic levels of dopamine and serotonin rise upon exposure to conditioned fear stress without elevation of glutamate. Neurosci Lett 379: 37–41, 2005. doi: 10.1016/j.neulet.2004.12.047. [DOI] [PubMed] [Google Scholar]