Abstract
Acute kidney injury (AKI) remains a major global healthcare problem, and there is a need to develop human-based models to study AKI in vitro. Toward this goal, we have characterized induced pluripotent stem cell-derived human kidney organoids and their response to cisplatin, a chemotherapeutic drug that induces AKI and preferentially damages the proximal tubule. We found that a single treatment with 50 µM cisplatin induces hepatitis A virus cellular receptor 1 (HAVCR1) and C-X-C motif chemokine ligand 8 (CXCL8) expression, DNA damage (γH2AX), and cell death in the organoids but greatly impairs organoid viability. DNA damage was not specific to the proximal tubule but also affected the distal tubule and interstitial cell populations. This lack of specificity correlated with low expression of proximal tubule-specific SLC22A2/organic cation transporter 2 (OCT2) for cisplatin. To improve viability, we developed a repeated low-dose regimen of 4 × 5 µM cisplatin over 7 days and found this caused less toxicity while still inducing a robust injury response that included secretion of known AKI biomarkers and inflammatory cytokines. This work validates the use of human kidney organoids to model aspects of cisplatin-induced injury, with the potential to identify new AKI biomarkers and develop better therapies.
Keywords: acute kidney injury, acute kidney injury biomarker, cisplatin, cytokine, inflammation, kidney organoids, nephrotoxicity, proximal tubule, repeated low-dose regimen
INTRODUCTION
Acute kidney injury (AKI) is defined by the rapid decline of kidney function and can be caused by nephrotoxic side effects of clinical drugs. AKI is a serious condition that predisposes patients to chronic kidney disease (CKD) and mortality. However, there are no targeted therapies for AKI. This is partly due to a lack of clinically relevant models that are required to gain a better understanding of the pathological mechanisms involved in AKI and for the identification of early reliable biomarkers to improve diagnostic accuracy of AKI (24, 31).
Cisplatin is a chemotherapeutic DNA cross-linking agent that is used to treat solid tumors. The antitumor efficacy of cisplatin is limited by severe nephrotoxicity, particularly affecting the S3 segment of the proximal tubule due to the high expression of drug transporters such as SLC22A2/organic cation transporter 2 (OCT2) (2, 28). The renal pathology of cisplatin-induced AKI is multifactorial, consisting of inflammation, vascular injury, oxidative stress, and direct toxicity via generation of reactive oxygen species (17).
In recent years, kidney organoids differentiated from human pluripotent stem cells (hPSCs) have been established to study various types of kidney disorders (10, 14). Kidney organoids were reported to be susceptible to proximal tubule injury caused by nephrotoxins such as cisplatin (5, 15, 25, 27). However, a full characterization of the effects of cisplatin in this system has been lacking.
We have developed a novel kidney organoid protocol that uses a simple, cost-effective method to generate large numbers of organoids, making it ideally suited for injury modeling and drug development (19). Here, we test the hypothesis that cisplatin treatment can model specific proximal tubule nephrotoxicity in these kidney organoids. We found that a single high dose of cisplatin induces upregulation of the proximal tubule injury marker hepatitis A virus cellular receptor 1 (HAVCR1) and the inflammatory cytokine C-X-C motif chemokine ligand 8 (CXCL8), DNA damage, and cell death that compromises organoid viability. Cisplatin-induced DNA damage was not specific to the proximal tubule but found in the distal tubules and interstitial cells as well. We further developed a repeated low-dose regimen of cisplatin that not only improved organoid viability but also induced substantial AKI biomarker expression and cytokine secretion, reminiscent of rodent cisplatin-AKI models.
MATERIALS AND METHODS
Induced pluripotent stem cell maintenance and organoid generation.
All work was carried out with the approval of the University of Auckland Human Participants Ethics and Health and Disability Ethics Committees (UAHPEC 8712 and HDEC 17/NTA/204, respectively) and the University of Auckland Biological Safety Committee. Induced pluripotent stem cell (iPSC) maintenance and kidney organoid generation were performed as previously described (19). All experiments were performed using organoids generated from the MANZ-2 iPSC line. All quantitative PCR analyses were performed using MANZ-2 cells plus a second independent iPSC line (RiPS or MANZ-4; see Ref. 19).
Cisplatin treatment.
For the single high-dose regimen, a whole organoid assay of ∼500–800 organoids was evenly split into five wells of a six-well ultra-low attachment plate (Corning). Cisplatin was added at 0, 5, 10, 25, or 50 µM to “stage II” medium (19) on day 12. Samples were collected 24 and 48 h posttreatment and then analyzed using quantitative PCR and immunohistochemistry. For the repeated low-dose regimen, 5 µM cisplatin was added on day 12 and subsequently every other day with the medium change for a total of 4 treatments over 7 days. Samples were collected on day 19.
RNA extraction, cDNA synthesis, and quantitative PCR.
Organoids were washed in PBS and homogenized in TRIzol. Total RNA was extracted using the GENEzol TriRNA Pure kit (Geneaid). RNA from fetal and adult kidney tissue was purchased from Takara. cDNA was synthesized using qScript cDNA SuperMix (Quanta). Quantitative PCR was performed using the PerfeCTa SYBR Green FastMix reagents (Quanta) on a QuantStudio 6 Flex machine. Gene expression was calculated using the ΔCT method (where CT is threshold cycle) using hypoxanthine-guanine phosphoribosyltransferase (HPRT1) for normalization. Error bars represent SDs of triplicate measurements.
Histology and immunohistochemistry.
Organoids were fixed in 4% paraformaldehyde-PBS. Paraffin embedding and sectioning were performed as previously described (19). Immunohistochemistry (IHC) was performed using standard procedures, including heat-induced antigen retrieval. Hoechst 33342 was used for nuclear staining. The antibodies and dyes used were kidney injury molecule-1 (KIM-1; AF1750, R&D Systems), γH2AX (CTE2577S and no. 14-9865-80, ThermoFisher), MAF BZIP transcription factor B (MAFB; NBP1-81342, Novus), E-cadherin (CDH1; no. 610181, BD Biosciences), MEIS1/2/3 (no. 39796, Active Motif), and Lotus tetragonolobus lectin (LTL; no. 1321, Vectorshield). Cell death was measured by TUNEL assay (ApopTag Plus Fluorescein In Situ Detection kit, Millipore). Fluorescently stained sections were imaged on a Zeiss LSM710 confocal microscope.
Quantification and statistics.
Quantification of IHC was performed on ≥10 organoids/sample using ImageJ. For quantification of marker colocalization, double-positive cells for each marker and yH2AX were counted from sections through the middle of ≥10 individual organoids. For each section, the number of double-positive cells was normalized to the area of the stained tissue using ImageJ, and the average number of cells per area was plotted. Statistical significance was determined using one-way ANOVA or unpaired t test in Prism (GraphPad). P values of ≤0.05 were considered to be statistically significant.
Cytokine array.
At least 20 organoids per condition were cultured in a single well of a 24-well ultra-low attachment plate in 500 µL of stage II medium with or without cisplatin for either 48 h (single-dose cisplatin regimen) or for the last 48 h of the repeated low-dose regimen. Conditioned culture media were analyzed using the Proteome Profiler Human XL Cytokine Array (R&D Systems). Signals were visualized with enhanced chemiluminescence on a Bio-Rad ChemiDoc MP Imaging system. Intensities of the duplicate signals were quantified using ImageJ.
RNA sequencing.
Total RNA from quadruplicate samples of control and 4 × 5 µM cisplatin-treated organoids (>100 organoids/sample) was prepared as described above. Library preparation (Collibri Stranded RNA Library Kit, ThermoFisher) and 1 × 75 bp sequencing on an Illumina NextSeq 500 were performed by Auckland Genomics. For analysis, reads were quality (Phred score of 20) and adapter trimmed using Trim Galore (version 0.6.4) and then aligned to GRCh38.p13 using STAR (4). Read counts were obtained using featureCounts (8). The edgeR (22) and limma (21) packages were used for differential expression analysis, with a threshold of log fold change = 1 and adjusted P value of <0.05. Gene set enrichment analysis (GSEA) was done for the hallmark gene sets from the MSigDB collections using the clusterProlifer package (9, 30).
Data availability.
The RNA sequencing (RNA-Seq) data have been deposited in the National Center for Biotechnology Information’s GEO database (Accession No. GSE145085).
RESULTS
Cisplatin induces AKI marker expression, DNA damage, and cell death in kidney organoids.
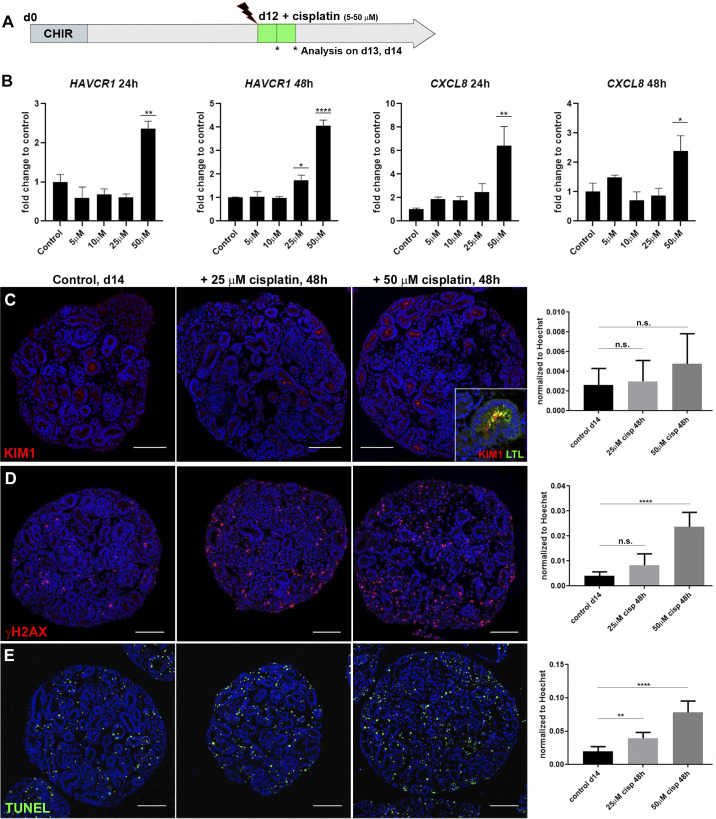
Previous studies have reported cisplatin treatment of kidney organoids with doses ranging from 5 to 100 μM leading to the induction of injury markers, apoptosis, and DNA damage (5, 15, 27). To investigate whether these findings were reproducible on kidney organoids generated with our protocol (19), we added single doses of 5, 10, 25, or 50 μM cisplatin to the culture medium of day 12 organoids and collected samples 24 and 48 h posttreatment (Fig. 1A). Following the 24-h treatment, expression of AKI marker HAVCR1 (encoding KIM-1) remained unchanged after exposure to 5–25 μM cisplatin but increased 2.4-fold with 50 μM cisplatin. After 48 h, both 25 μM and 50 μM cisplatin induced HAVCR1 expression significantly (1.7- and 4.1-fold). A similar dose response was observed for expression of the inflammatory cytokine CXCL8. No significant change in expression was measured with 5–25 μM cisplatin, whereas 50 μM cisplatin resulted in a 6.5- and 2.4-fold induction of CXCL8 24 h and 48 h posttreatment, respectively (Fig. 1B). Using IHC on organoid sections, we detected an increase in KIM-1 protein upon cisplatin treatment (Fig. 1C, inset showing colocalization of KIM-1 to a LTL+ proximal tubule). To determine the extent of DNA damage and cell death as well as the spatial distribution of cells affected by cisplatin, we next performed γH2AX antibody and TUNEL staining. Image analysis revealed that both markers were rare in the nuclei of control organoids but increased with 25 and 50 μM cisplatin (Fig. 1, D and E). γH2AX+ and TUNEL+ cells were scattered throughout the organoid sections with no obvious accumulation to tubules. These findings suggest that our kidney organoids respond to cisplatin treatment with induction of AKI markers as well as dose-dependent DNA damage and cell death, consistent with previous data (15, 27).
Fig. 1.
Cisplatin induces tubular injury, DNA damage, and cell death in kidney organoids. A: schematic of cisplatin treatment on kidney organoids. CHIR, chemical compound CHIR99021 used as in Ref. 19. B: quantitative PCR showing elevated expression of hepatitis A virus cellular receptor 1 (HAVCR1) and C-X-C motif chemokine ligand 8 (CXCL8) with increasing doses of cisplatin. C–E: immunohistochemistry staining on paraffin sections of day 14 organoids and quantification showing levels of tubular injury marker kidney injury molecule-1 [KIM1; colocalized to Lotus tetragonolobus lectin (LTL)+ proximal tubule; C, inset], DNA damage marker γH2AX, and cell death marker TUNEL increased with 25 and 50 µM cisplatin. d, day; ns, not significant. *P ≤ 0.05; **P ≤ 0.01; ****P ≤ 0.0001. Scale bars = 100 μm.
Cisplatin targets interstitial cells.
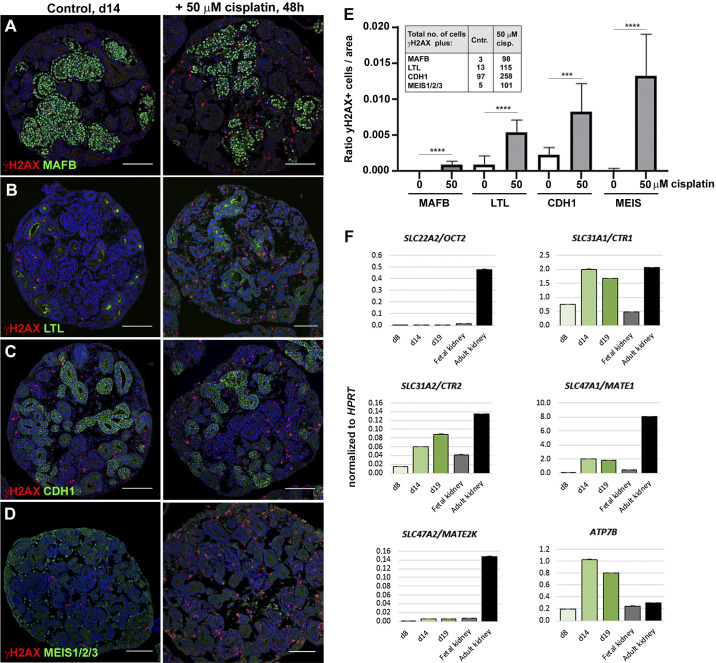
We next investigated whether our injury model could recapitulate the proximal tubule-specific cell damage found in cisplatin-treated patients and animal models. Upon coimmunostaining of γH2AX with antibodies for podocytes, proximal tubule cells, distal tubule cells, and interstitial cells, we observed that MAFB+ podocytes were the least damaged by cisplatin (Fig. 2A). In contrast, γH2AX colabeled a subset of LTL+ proximal tubule cells, CDH1+ distal tubule cells, and MEIS1/2/3+ interstitial cells in cisplatin-treated organoids (Fig. 2, B–D). Quantification revealed that 50 µM cisplatin induced a 5.5-fold increase in γH2AX+ proximal tubule cells and a 3.7-fold increase in distal tubule cells compared with untreated controls (of n ≥ 10 organoids). Strikingly, the largest increase in γH2AX was found in interstitial cells (67-fold; Fig. 2E). This result demonstrates that cisplatin predominantly targets the stromal compartment in kidney organoids.
Fig. 2.
Cisplatin predominantly targets interstitial cells in kidney organoids. A–D: immunohistochemistry staining for colocalization of DNA damage marker γH2AX with MAF BZIP transcription factor B (MAFB)+ podocytes, Lotus tetragonolobus lectin (LTL)+ proximal tubules, E-cadherin (CDH1)+ distal tubules, and MEIS1/2/3+ interstitial cells in control (Cntr) and 50 µM cisplatin-treated organoids. E: ratio of γH2AX+ cells per marker. Inset shows the total numbers of double-positive cells counted. F: quantitative PCR showing expression of cisplatin transporters in day 8 (d8), day 14 (d14), and day 19 (d19) kidney organoids and fetal and adult human kidneys. OCT2, organic cation transporter 2; CTR1 and CTR2, copper transporter 1 and 2, respectively; MATE1 and MATE2K, multidrug and toxin extrusion proteins 1 and 2, respectively; ATP7B, ATPase copper transporting-β; HPRT, hypoxanthine-guanine phosphoribosyltransferase 1. ***P ≤ 0.001; ****P ≤ 0.0001. Scale bars = 100 μm.
Low expression of cisplatin transporters in kidney organoids.
To elucidate why tubular cells were less susceptible to cisplatin-induced injury than interstitial cells, we measured expression levels of cisplatin influx transporters encoded by SLC22A2/OCT2, a major cisplatin transporter that is specifically expressed by proximal tubule cells, and SLC31A1/copper transporter 1 (CTR1) and SLC31A2/copper transporter 2 (CTR2) (16). For cisplatin efflux, we measured expression of ATPase copper transporting-β (ATP7B) and SLC47A1/multidrug and toxin extrusion protein 1 (MATE1) and SLC47A2/multidrug and toxin extrusion protein 2 (MATE2K) transporter genes, of which SLC47A2/MATE2K is uniquely expressed in the proximal tubule (7, 13). Three stages of organoid development (days 8, 14, and 19) were analyzed alongside commercially available RNA of fetal and adult human kidneys using quantitative PCR. We found that expression of SLC32A1/CTR1, SLC32A2/CTR2, ATP7B, and SLC47A1/MATE1 increased with organoid maturation and was comparable with (or exceeded) the levels of these markers in fetal or adult kidney tissue. In contrast, expression of proximal tubule-specific SLC22A2/OCT2 and SLC47A2/MATE2K transporters was equally low in organoids and the fetal kidney (Fig. 2F). This low expression of SLC22A2/OCT2 may be responsible for the lack of proximal tubule-specific damage induced by cisplatin.
Repeated low-dose cisplatin treatment reduces cytotoxicity.
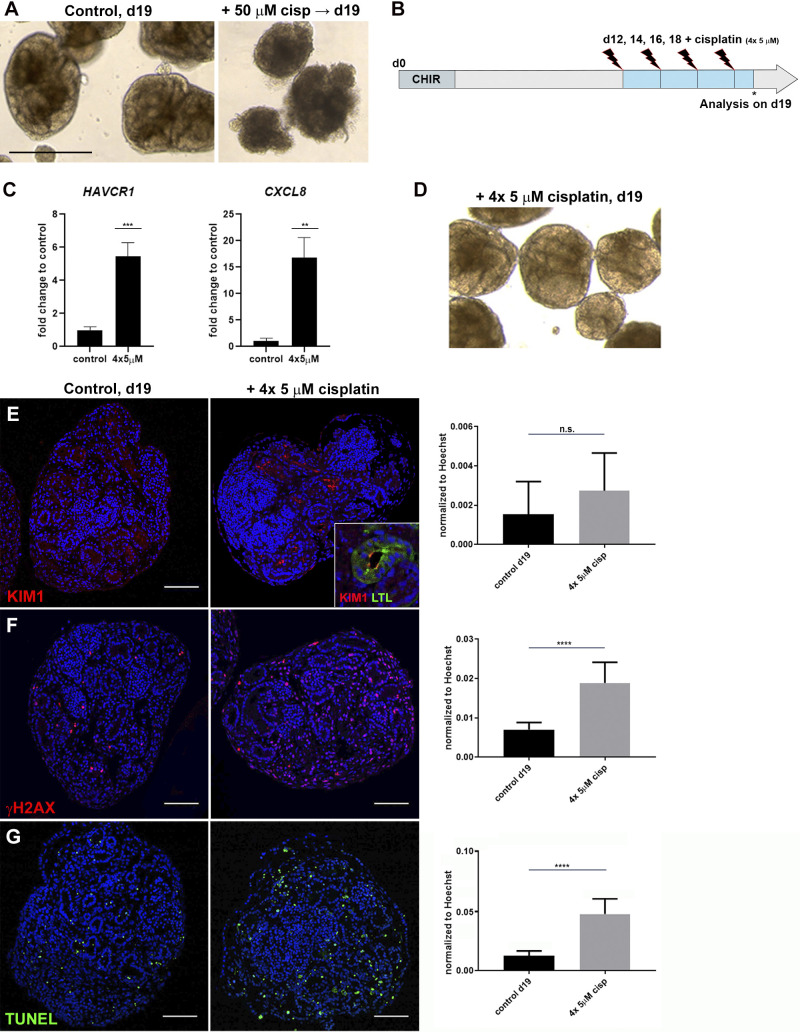
As shown above, exposure of organoids to 25 and 50 µM cisplatin for 48 h leads to acute DNA damage and cell death (Fig. 1, D and E). At collection on day 14, cisplatin-treated organoids appeared healthy and similar in morphology to untreated controls (not shown). However, treated organoids deteriorated at later stages of culture, whereas untreated controls retained a healthy appearance (Fig. 3A). To increase organoid viability and to more closely mimic the repeated dosing regimen of chemotherapy, we tested a repeated low-dose cisplatin regimen on the organoids. To do this, 5 µM cisplatin was added on days 12, 14, 16, and 18 (4 × 5 µM) before collection on day 19 (Fig. 3B). Quantitative PCR revealed a 5.5-fold induction of HAVCR1 and 19-fold induction of CXCL8 in 4 × 5 µM cisplatin-treated organoids compared with controls (Fig. 3C). Importantly, no tissue disintegration was observed at later stages, indicative of improved organoid viability (Fig. 3D).
Fig. 3.
Repeated exposure to low-dose cisplatin reduces cell death and structural deterioration. A: bright-field imaging showing healthy organoids on day 19 (d19) and organoid deterioration after 50 µM cisplatin. B: schematic of repeated low-dose cisplatin treatment. C: quantitative PCR analysis of day 19 organoids showing hepatitis A virus cellular receptor 1 (HAVCR1) and C-X-C motif chemokine ligand 8 (CXCL8) expression increased upon 4 × 5 µM cisplatin. D: organoids treated with 4 × 5 µM cisplatin maintained tubular structures. E–G: immunohistochemistry showing levels of kidney injury molecule-1 [KIM1; colocalized to Lotus tetragonolobus lectin (LTL)+ proximal tubule; E, inset], γH2AX, and TUNEL increased with 4 × 5 µM cisplatin. ns, not significant. **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. Scale bars = 400 μm in A and 100 μm in E–G.
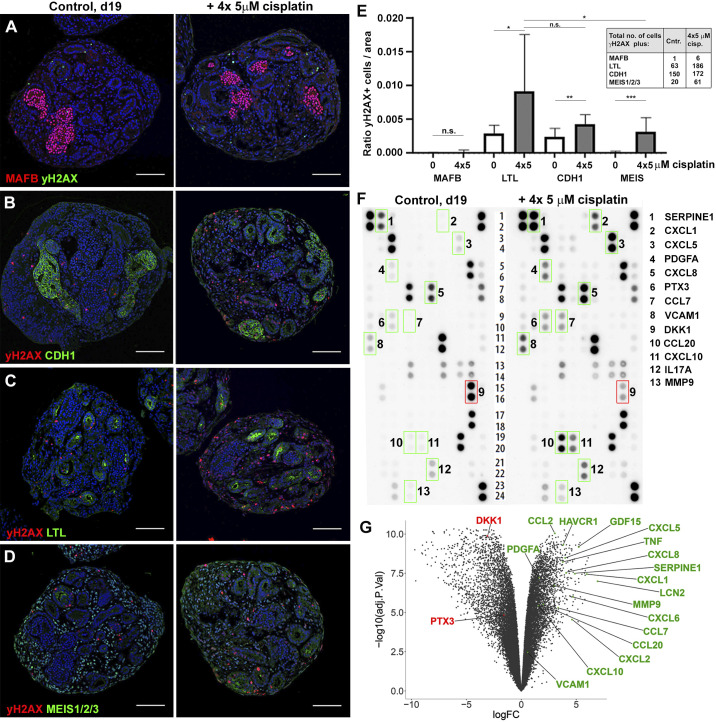
An analysis of 4 × 5 µM-treated organoids by IHC showed that KIM-1, γH2AX, and TUNEL were increased to a similar extent as with the single treatment (Fig. 3, E–G). Colabeling of γH2AX+ nuclei with the tissue markers MAFB, LTL, CDH1, and MEIS1/2/3 (Fig. 4, A–D) showed that cisplatin had the greatest effect on interstitial cells (20-fold), whereas for the tubular compartment there was a trend for more proximal tubule injury (3.1-fold) relative to distal (1.7-fold; Fig. 4E). Taken together, the repeated low-dose cisplatin treatment recapitulated the induction of kidney injury marker expression observed upon exposure to single high-dose cisplatin yet exhibited less DNA damage in interstitial cells and improved organoid viability.
Fig. 4.
Organoids secrete acute kidney injury biomarkers and cytokines in response to cisplatin. A–D: immunohistochemistry on day 19 (d19) control (Cntr) and 4 × 5 µM cisplatin-treated organoids for colocalization of DNA damage (γH2AX) with kidney tissues [MAF BZIP transcription factor B (MAFB) + podocytes, Lotus tetragonolobus lectin (LTL)+ proximal tubules, E-cadherin (CDH1)+ distal tubules, and MEIS1/2/3+ interstitial cells]. E: quantification of γH2AX colocalization with kidney tissues. Inset shows total numbers of double-positive cells counted. F: cytokine array analysis using culture media collected from control and 4 × 5 µM cisplatin-treated organoids. Factors with higher (green boxes) or lower (red box) secretion in cisplatin-treated versus control organoids are shown. G: volcano plot showing the result of RNA sequencing profiling of control and 4 × 5 µM cisplatin-treated organoids. The genes encoding the differentially secreted cytokines and a selection of acute kidney injury biomarkers that are differentially expressed upon cisplatin treatment are highlighted. ns, not significant. See text for abbreviations. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. Scale bars = 100 μm.
Cisplatin-treated organoids (4 × 5 µM) express AKI biomarkers and secrete inflammatory cytokines.
Having improved the injury regimen, we next surveyed the cytokines secreted into the medium in response to cisplatin using the Proteome Profiler Human XL Cytokine Array, a membrane-based sandwich immunoassay that can detect 102 cytokines. We found 12 secreted factors with at least a 2-fold increase in signal intensity upon cisplatin treatment, including cytokines implicated in acute or chronic kidney injury [C-X-C motif chemokine ligands 1 and 5 (CXCL1 and CXCL5), C-C motif chemokine ligands 7 and 20 (CCL7 and CCL20), and IL17A] and renal fibrosis [serpin family E member 1 (SERPINE1), platelet-derived growth factor-A (PDGFA), and matrix metalloproteinase-9 (MMP9)] (Fig. 4F, green boxes) (3, 11, 26, 29) and decreased secretion of the Wnt inhibitor DKK1 (Fig. 4F, red box). To validate this finding at the transcriptional level, we performed RNA-Seq on control and 4 × 5 µM-cisplatin treated organoids. This analysis revealed that genes encoding the proteins identified from the cytokine array were differentially expressed as expected [with the exception of pentraxin 3 (PTX3) and IL17A]. Furthermore, we identified a large set of classic AKI biomarkers among the highest induced transcripts, including TNFα, CXCL2, CCL2, HAVCR1, lipocalin 2 (LCN2), and growth/differentiation factor 15 (GDF15) (Fig. 4G) (11, 12, 17, 26). “Hallmark pathways” analysis showed that “TNF-α signaling” was one of the top enriched gene categories in cisplatin-treated organoids, which is in line with TNF-α being a major mediator of cisplatin-induced injury (20).
DISCUSSION
The main finding of this work is that cisplatin, both the single high-dose and repeated low-dose regimen, damages all cellular compartments in the organoids but has the most profound effect on interstitial cells. This is in contrast to previous reports, where a single dose of 5 µM cisplatin was suggested to cause DNA damage and apoptosis specifically to proximal tubules (15, 27). Given the low expression of SLC22A2/OCT2 in our kidney organoids, we speculate that cisplatin uptake occurs via widely expressed CTRs and thus accounts for the lack of proximal tubule-specific damage (18). Because SLC22A2/OCT2 is not normally expressed in the fetal human kidney, and because kidney organoids represent a fetal stage of differentiation (19, 27), the lack of SLC22A2/OCT2 expression is most likely due to immaturity of our organoids. This lack of specificity may not apply to kidney organoids generated by other protocols that can be cultured for longer periods. The high rate of stromal cell proliferation, compared with the tubular cells (19), may be responsible for the enriched susceptibility to DNA damage we observe in these cells, given that cisplatin targets dividing cells (28). Together, our findings suggest that cisplatin exerts a general cytotoxic effect on kidney organoids reminiscent of the impact of the drug on tumor cells, rather than acting as a specific proximal tubule nephrotoxin.
We found that there was a trend for more DNA damage in the proximal tubule over the distal tubule when cisplatin was administered with the repeated low-dose regimen. Whether this trend is the result of subtle differences in injury susceptibility between proximal and distal tubules or due to differences in proliferation between these cell types remains to be determined. We speculate that the 4 × 5 µM regimen avoids the acute cell death response of the 50 µM dose and instead leads to a milder yet accumulative injury phenotype that manifests in robust activation of known AKI markers. Such a scenario is more representative of chemotherapeutic low-dose cisplatin administered over an extended period of time, whereby sustained inflammation is a contributing factor to the transition from AKI to renal fibrosis and CKD (23). Our 4 × 5 µM approach is also consistent with recent work in mice, where repeated low-dose cisplatin regimens were shown to induce inflammatory cytokines and fibrosis markers in contrast to the acute toxicity, high mortality, and lack of fibrosis seen with a single high-dose treatment (1, 6, 24).
Finally, we show that the proteome array allows for detection of proteins secreted in response to a nephrotoxic insult, analogous to measuring serum and urine markers in AKI patients. This assay provides a proof of principle for more sensitive detection tools, such as ELISA, to be used to identify new biomarkers of injury. Alternatively, we show that bulk RNA-Seq can also reveal transcriptional changes in AKI biomarkers and can now be mined for new candidates. In summary, our organoid model of cisplatin-induced kidney injury shows limited proximal tubule specificity but a strong representation of the inflammatory response to nephrotoxic insults. Thus, organoids provide a valuable system for drug and biomarker discovery to improve the treatment of patients with cisplatin-induced AKI and potentially other causes of nephrotoxicity.
GRANTS
This work was supported by the Health Research Council of New Zealand (17/425), the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-069403), and the United States Army Medical Research and Development Command (W81XWH-17-1-0610).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.S. conceived and designed research; J.L.M.D., A.P., and V.S. performed experiments; J.L.M.D., T.V., A.P., and V.S. analyzed data; J.L.M.D., A.J.D. and V.S. interpreted results of experiments; J.L.M.D. and V.S. prepared figures; V.S. drafted manuscript; A.J.D. edited and revised manuscript; J.L.M.D., T.V., A.P., A.J.D., and V.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank G. Chang for critical reading of the manuscript.
Present address of A. Przepiorski: Department of Developmental Biology, University of Pittsburgh, Pittsburgh, PA.
REFERENCES
- 1.Black LM, Lever JM, Traylor AM, Chen B, Yang Z, Esman SK, Jiang Y, Cutter GR, Boddu R, George JF, Agarwal A. Divergent effects of AKI to CKD models on inflammation and fibrosis. Am J Physiol Renal Physiol 315: F1107–F1118, 2018. doi: 10.1152/ajprenal.00179.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciarimboli G, Ludwig T, Lang D, Pavenstädt H, Koepsell H, Piechota H-J, Haier J, Jaehde U, Zisowsky J, Schlatter E. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol 167: 1477–1484, 2005. doi: 10.1016/S0002-9440(10)61234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortvrindt C, Speeckaert R, Moerman A, Delanghe JR, Speeckaert MM. The role of interleukin-17A in the pathogenesis of kidney diseases. Pathology 49: 247–258, 2017. doi: 10.1016/j.pathol.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, Saad AF, Li MK, Hughes MR, Werff RV, Peters DT, Lu J, Baccei A, Siedlecki AM, Valerius MT, Musunuru K, McNagny KM, Steinman TI, Zhou J, Lerou PH, Bonventre JV. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun 6: 8715, 2015. doi: 10.1038/ncomms9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu Y, Cai J, Li F, Liu Z, Shu S, Wang Y, Liu Y, Tang C, Dong Z. Chronic effects of repeated low-dose cisplatin treatment in mouse kidneys and renal tubular cells. Am J Physiol Renal Physiol 317: F1582–F1592, 2019. doi: 10.1152/ajprenal.00385.2019. [DOI] [PubMed] [Google Scholar]
- 7.Harrach S, Ciarimboli G. Role of transporters in the distribution of platinum-based drugs. Front Pharmacol 6: 85, 2015. doi: 10.3389/fphar.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930, 2014. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 9.Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 1: 417–425, 2015. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Little MH, Combes AN. Kidney organoids: accurate models or fortunate accidents. Genes Dev 33: 1319–1345, 2019. doi: 10.1101/gad.329573.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Kumar S, Dolzhenko E, Alvarado GF, Guo J, Lu C, Chen Y, Li M, Dessing MC, Parvez RK, Cippà PE, Krautzberger AM, Saribekyan G, Smith AD, McMahon AP. Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion. JCI Insight 2: e94716, 2017. doi: 10.1172/jci.insight.94716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Kumar S, Heinzel A, Gao M, Guo J, Alvarado GF, Reindl-Schwaighofer R, Krautzberger AM, Cippà PE, McMahon J, Oberbauer R, McMahon AP. Renoprotective and immunomodulatory effects of GDF15 following AKI invoked by ischemia-reperfusion injury. J Am Soc Nephrol. In press. doi: 10.1681/ASN.2019090876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masuda S, Terada T, Yonezawa A, Tanihara Y, Kishimoto K, Katsura T, Ogawa O, Inui K. Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J Am Soc Nephrol 17: 2127–2135, 2006. doi: 10.1681/ASN.2006030205. [DOI] [PubMed] [Google Scholar]
- 14.Miyoshi T, Hiratsuka K, Saiz E, Morizane R. Kidney organoids in translational medicine: Disease modeling and regenerative medicine. Dev Dyn 249: 34–45, 2020. doi: 10.1002/dvdy.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33: 1193–1200, 2015. doi: 10.1038/nbt.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otsuka M, Matsumoto T, Morimoto R, Arioka S, Omote H, Moriyama Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc Natl Acad Sci USA 102: 17923–17928, 2005. doi: 10.1073/pnas.0506483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res Int 2014: 1–17, 2014. doi: 10.1155/2014/967826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pabla N, Murphy RF, Liu K, Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol 296: F505–F511, 2009. doi: 10.1152/ajprenal.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Przepiorski A, Sander V, Tran T, Hollywood JA, Sorrenson B, Shih J-H, Wolvetang EJ, McMahon AP, Holm TM, Davidson AJ. A simple bioreactor-based method to generate kidney organoids from pluripotent stem cells. Stem Cell Reports 11: 470–484, 2018. doi: 10.1016/j.stemcr.2018.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramesh G, Reeves WB. TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110: 835–842, 2002. doi: 10.1172/JCI200215606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43: e47, 2015. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140, 2010. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato Y, Yanagita M. Immune cells and inflammation in AKI to CKD progression. Am J Physiol Renal Physiol 315: F1501–F1512, 2018. doi: 10.1152/ajprenal.00195.2018. [DOI] [PubMed] [Google Scholar]
- 24.Sharp CN, Siskind LJ. Developing better mouse models to study cisplatin-induced kidney injury. Am J Physiol Renal Physiol 313: F835–F841, 2017. doi: 10.1152/ajprenal.00285.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soo JY, Jansen J, Masereeuw R, Little MH. Advances in predictive in vitro models of drug-induced nephrotoxicity. Nat Rev Nephrol 14: 378–393, 2018. doi: 10.1038/s41581-018-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Späth MR, Bartram MP, Palacio-Escat N, Hoyer KJR, Debes C, Demir F, Schroeter CB, Mandel AM, Grundmann F, Ciarimboli G, Beyer A, Kizhakkedathu JN, Brodesser S, Göbel H, Becker JU, Benzing T, Schermer B, Höhne M, Burst V, Saez-Rodriguez J, Huesgen PF, Müller RU, Rinschen MM. The proteome microenvironment determines the protective effect of preconditioning in cisplatin-induced acute kidney injury. Kidney Int 95: 333–349, 2019. doi: 10.1016/j.kint.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 27.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526: 564–568, 2015. [Erratum in Nature 536: 238, 2016.] doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]
- 28.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci 334: 115–124, 2007. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 29.Yimit A, Adebali O, Sancar A, Jiang Y. Differential damage and repair of DNA-adducts induced by anti-cancer drug cisplatin across mouse organs. Nat Commun 10: 309, 2019. doi: 10.1038/s41467-019-08290-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16: 284–287, 2012. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuk A, Bonventre JV. Acute kidney injury. Annu Rev Med 67: 293–307, 2016. doi: 10.1146/annurev-med-050214-013407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The RNA sequencing (RNA-Seq) data have been deposited in the National Center for Biotechnology Information’s GEO database (Accession No. GSE145085).