Abstract
Consumption of a Western diet (WD) induces central aortic stiffening that contributes to the transmittance of pulsatile blood flow to end organs, including the kidney. Our recent work supports that endothelial epithelial Na+ channel (EnNaC) expression and activation enhances aortic endothelial cell stiffening through reductions in endothelial nitric oxide (NO) synthase (eNOS) and bioavailable NO that result in inflammatory and oxidant responses and perivascular fibrosis. However, the role that EnNaC activation has on endothelial responses in the renal circulation remains unknown. We hypothesized that cell-specific deletion of the α-subunit of EnNaC would prevent WD-induced central aortic stiffness and protect the kidney from endothelial dysfunction and vascular stiffening. Twenty-eight-week-old female αEnNaC knockout and wild-type mice were fed either mouse chow or WD containing excess fat (46%), sucrose, and fructose (17.5% each). WD feeding increased fat mass, indexes of vascular stiffening in the aorta and renal artery (in vivo pulse wave velocity and ultrasound), and renal endothelial cell stiffening (ex vivo atomic force microscopy). WD further impaired aortic endothelium-dependent relaxation and renal artery compliance (pressure myography) without changes in blood pressure. WD-induced renal arterial stiffening occurred in parallel to attenuated eNOS activation, increased oxidative stress, and aortic and renal perivascular fibrosis. αEnNaC deletion prevented these abnormalities and support a novel mechanism by which WD contributes to renal arterial stiffening that is endothelium and Na+ channel dependent. These results demonstrate that cell-specific EnNaC is important in propagating pulsatility into the renal circulation, generating oxidant stress, reduced bioavailable NO, and renal vessel wall fibrosis and stiffening.
Keywords: endothelial epithelial Na+ channel, endothelial nitric oxide synthase, oxidative stress, renal
INTRODUCTION
Consumption of a Western diet (WD) high in saturated fat and refined carbohydrates is associated with vascular stiffening and the development of cardiovascular and kidney disease (3, 16, 20). In this regard, obesity and kidney disease have been associated with vascular stiffness regardless of age and race, particularly in women (22, 27, 32, 35, 37). Central aortic stiffening contributes to the propagation of pulsatile waves into the kidney, where there is high flow but low precapillary resistance (14, 34). Ultimately, this excess kinetic energy to the kidney contributes to alterations in myogenic tone, wall strain and remodeling, and perivascular fibrosis that eventually leads to end-organ injury (14, 22, 36). Recent studies have supported a role for endothelial stiffness and impaired endothelial nitric oxide (NO) production in endothelium-mediated vascular and renal inflammatory responses as well as the extracellular matrix remodeling that contribute to vascular and renal fibrosis (19, 25, 37). Our recent work supports that stiffening initially occurs at the level of the endothelium and that these changes precede the development of perivascular fibrosis and associated myocardial and kidney tissue fibrosis (3, 15, 20, 29). The process of vascular stiffening is enhanced by consumption of a WD and occurs more rapidly in women compared with their male counterparts (20).
Our recent work in a female rodent model of overnutrition and insulin resistance further supports the hypothesis that renal vascular endothelial stiffening and vascular fibrosis are associated with enhanced oxidative stress and attenuated endothelial NO synthase (eNOS) activation by enhanced endothelial cell mineralocorticoid receptor (MR) signaling (3). These data revealed that the endothelium epithelial Na+ channel (ENaC) in endothelial cells (EnNaC) is a downstream mediator of endothelial cell MRs as MR activation promotes translocation of EnNaC to the plasma membrane of the arterial endothelium and activation of this Na+ channel (16). In this context, a number of studies have supported that EnNaC contributes to shear sensing in conduit and resistance arteries and regulates vascular stiffness and cardiac function (1, 4, 11, 17, 31). ENaC is a member of the ENaC/degenerin superfamily of cation-selective ion channels and consists of three subunits (αENaC, βENaC, and γENaC). All three subunits are core subunits important in the activation of the channel (16, 31), and their deletion has been shown to attenuate aortic endothelial cell stiffness (29). However, the role of the different subunits is unknown, and, specifically, the role of the α-subunit of EnNaC in the pathogenesis of renal artery stiffening and fibrosis is not understood. Therefore, we hypothesized that deletion of the α-subunit of EnNaC in the endothelium would attenuate both aorta and renal artery stiffening, early renal arterial fibrosis, and remodeling in WD feeding.
MATERIALS AND METHODS
Animals and treatments.
All animal procedures were performed in accordance with guidelines from the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Use and Care Committee of the University of Missouri-Columbia and the Subcommittee for Animal Safety at the Harry S. Truman Veterans’ Memorial Hospital. The EnNaC α-subunit knockout (αEnNaC−/−) model was obtained by crossing αENaCf/f floxed mice with transgenic mice expressing Cre recombinase under the control of the Tie2 promoter on the C57BL/6 genetic background (The Jackson Laboratory, Bar Harbor, ME), as previously described (16). αENaCf/f littermates lacking the Tie2-Cre transgene were used as controls. Groups of 28-wk-old female mice were fed either a control diet or WD (Test Diet 58Y1,5APC), consisting of high fat (46%) and a high-carbohydrate component constituted of sucrose (17.5%) and high-fructose corn syrup (17.5%) and water for 12 wk.
Body composition, blood pressure, and proteinuria.
After 12 wk of feeding, mice underwent body composition analysis for whole body/lean fat mass using an EchoMRI-500 for quantitative magnetic resonance analysis (Echo Medical Systems, Houston, TX) by a method previously established in our laboratory (8, 20). Mean arterial pressure was determined by tail-cuff blood pressure measurements, the protocols of which involved collection of several dozen measurements, with the software establishing the validity of each measurement. Data were tabulated in Excel files, and valid readings were used to calculate mean values for each mouse (Kent Scientific, Torrington, CT), as previously described (8, 9, 15). Blood samples were collected at euthanasia, and plasma was separated and stored at −80°C for further analysis. Urine samples were collected into chilled 4°C tubes from mice placed in metabolic cages for 24 h within 1 wk before euthanasia and were subsequently stored at −80°C for further analysis of total protein and creatinine, as previously described (3).
Aortic and renal artery stiffening.
In vivo aortic stiffness was measured via in vivo pulse wave velocity (PWV) using a Doppler ultrasound (Indus Mouse Doppler System, Webster, TX) according to a previously established protocol (3, 16). Briefly, PWV is based on the difference in time of a Doppler pulse wave between two locations along the aorta at a known distance apart. PWV is then calculated as the ratio of the distance traveled by the pulse wave between the two locations and is expressed in meters per second (33). The calculated incremental PWV is the value of the in vivo PWV of the renal arteries, as obtained using the Moens-Korteweg equation, where the incremental modulus of elasticity (Einc), internal diameter (D), and wall thickness (τ) are measured for a given intraluminal pressure and ρ is the density of the intraluminal buffer (ρ ≈ 1,005 kg/m3) used in the experiments (2). The ex vivo stiffness of aortic and renal artery explants were evaluated by an atomic force microscope (AFM), as previously described (3, 29). Briefly, 2-mm segments of the thoracic aorta and 0.5- to 1-mm segments of the renal artery were isolated from mice, separately opened longitudinally, securely fixed on glass coverslips using Cell Tak, and kept at room temperature (~25°C). After exposure of the vascular endothelial surface for access by AFM, the stiffness of vascular endothelial cells was measured using a cell nano-indentation protocol, as previously described (2, 3, 16, 29).
Vascular activity in the aorta and renal arteries.
Ex vivo aortic activity was investigated as previously described (2, 3, 16). Briefly, aortic contractile capacity was ascertained by exposure to KCl (80 mM). Subsequently, aortas were preconstricted with the thromboxane A2 receptor antagonist U46619 (30 nM). Arterial relaxation to ACh (1 nM−10 μM) and sodium nitroprusside (1 nM−10 μM) were assessed by cumulative addition of agonist to the vessel bath (2, 3, 16). For ex vivo renal arterial activity, samples of renal arteries were isolated, cannulated onto glass micropipettes, pressurized at 70 mmHg without flow, and warmed to 37°C in commercial myograph chambers (Living Systems Instrumentation, Burlington, VT), as previously described (3, 8). Only arteries that constricted more than 20% upon exposure to 80 mM K+ solution were used in the analyses. After the exposure to high K+, the arteries were washed three times with fresh physiological saline solution. Vessels were then placed in Ca2+-free physiological saline solution and exposed to consecutive changes in intraluminal pressure from 5 to 120 mmHg while under passive conditions to determine the elastic properties of the arteries, as previously reported (3, 8). Throughout the experiment, chambers were mounted on inverted microscopes with charge-coupled device cameras. Luminal diameter and wall thicknesses were recorded using video calipers (Living Systems Instrumentation) and PowerLab data-acquisition systems (ADInstruments).
Conventional and real-time PCR.
Conventional PCR was used as previously described (26). The prrimer sequences used were as follows: αENaC, forward: 5′-CAGACTGCTTCTACCAGACATAC-3′ and reverse: 5′-CCAGGGCTTCCTTTCTCATAC-3′; βENaC, forward: 5′-ACTGGGCCTATTGCTATCTAAAC-3′ and reverse: 5′-CTTCCTGCTCAGGGTGATATTC-3′; and γENaC, forward: 5′-CGTCTCTGTCTCCATCAAAGTC-3′ and reverse: 5′-GATTCCTGAGCTGAAGGTGTAG-3′. For quantitative PCR, first-strand cDNA synthesis was performed using 1 μg total RNA with oligo dT (1μg), 5× reaction buffer, MgCl2, dNTP mix, RNase inhibitor, and Improm II reverse transcriptase using an Improm II reverse transcription kit (Promega, Madison, WI). Real-time PCR was performed using 8 μL cDNA, 10 μL SYBR Green PCR master mix (Bio-Rad Laboratories, Hercules, CA), forward and reverse primers (10 pM/μL) (Integrated DNA Technologies, San Diego, CA), and a real-time PCR system (CFX96, Bio-Rad Laboratories). The primer sequences used were as follows: podocin, forward: 5′-GTGAGGAGGGCACGGAAG-3′ and reverse: 5′-AGGGAGGCGAGGACAAGA-3′; nephrin, forward: 5′-CCCAGGTACACAGAGCACAA-3′ and reverse: 5′-CTCACGCTCACAACCTTCAG-3′; and GAPDH, forward: 5′-GGAGAAACCTGCCAAGTATGA-3′ and reverse: 5′- TCCTCAGTGTAGCCCAAGA-3′. PCR protocols consisted of 5 min at 95°C for initial denaturation and 40 cycles of 30 s at 95°C, 30 s at 58°C, and 30 s at 72°C. Results were normalized against the housekeeping gene GAPDH.
Immunohistochemistry.
Segments of tissues were fixed in 3% paraformaldehyde, dehydrated in an ethanol series, paraffin embedded, and transversely sectioned (5-μm slices). Sections were incubated with either antibodies to either 3-nitrotyrosine (3-NT; Millipore, Billerica, MA), which is a product of tyrosine nitration generated by reactive nitrogen species such as peroxynitrite anion and nitrogen dioxide, or picrosirius red to detect collagen type I/III and tissue fibers. 4-Hydroxynonenal (4-HNE) antibody (Millipore, Billerica, MA) was further used to investigate the lipid peroxidation chain reaction and tissue oxidative stress. The intensities of staining were quantified using MetaVue. Isolated and cannulated renal arteries were fixed in 4% paraformaldehyde while pressurized intraluminally to 70 mmHg. Subsequently, the arteries were incubated with 0.5 μg/mL DAPI to stain nuclei, 0.2 μM Alexa Fluor 633 hydrazide (Molecular Probes) to stain elastin, and 0.02 μM Alexa Fluor 546 phalloidin (Molecular Probes) to stain F-actin, as previously described (3, 23). Images detecting all fluorescent probes and collagen with second harmonic image generation within the renal vessels were acquired using a Leica SP5 confocal/multiphoton microscope with a 63×/1.2 numerical aperture water objective (3, 5). After staining, colors on the fluorescent images were blocked by computer, and only the color indicative of the expression of the protein of interest was left, which was then quantified by MetaVue (5). For transmitted light images, colors were deleted except for dark brown, specific for 3-NT and 4-HNE, or pink for fibrosis. Intensities and areas of the colors were then quantified as “grayscale intensities” and normalized to the size of the arteries. The values were averaged, and statistical analysis was performed (5).
Statistical analysis.
Results are reported as means ± SD. Differences in measured parameters were determined using two-way ANOVA with repeated measures and the subsequent application of a Tukey highly significant difference post hoc test. Data presented as bars were analyzed by one-way ANOVA using the same post hoc test. Differences were considered statistically significant when P < 0.05. All statistical analyses were performed using Prism (version 8, GraphPad Software, San Diego, CA).
RESULTS
Effect of 12 wk of WD on characteristics of αEnNaC mice.
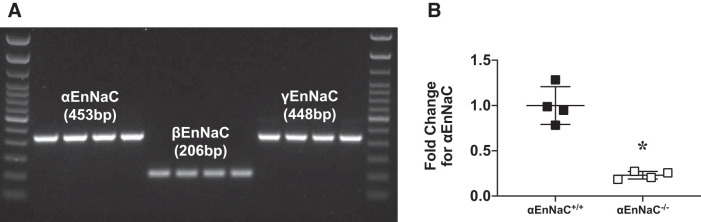
To obtain primary renal endothelial cells from αEnNaC−/− mice, mouse kidneys were removed, digested with collagenase type IV, and then passed across anti-CD45 antibody- and anti-CD31 antibody-conjugated microbeads (Miltenyi Biotec, Auburn, CA), as previously described (24). αENaC, βENaC, and γENaC were found to be present in isolated renal microvascular endothelial cells by conventional PCR (Fig. 1A), and an ~80% reduction in αEnNaC deletion was observed in endothelial cells from αEnNaC+/+ mice by real-time PCR (Fig. 1B). Consistent with our previous work (15, 20, 29), 12 wk of WD resulted in increased fat mass without changes in lean body mass (Table 1). There were no changes in body weight, mean arterial pressure, or proteinuria between groups. To corroborate the lack of between-group differences in proteinuria measures, we also assessed nephrin and podocin in renal cortical tissue by real-time PCR (Table 1). Similarly, there were no between-group differences in either nephrin or podocin expression. Furthermore, there were no apparent difference in urine protein/creatinine and mRNA expression of renal podocin and nephrin between control αEnNaC+/+ and αEnNaC−/− groups (see Supplemental Fig S1, available online at https://doi.org/10.6084/m9.figshare.12009279.v2).
Fig. 1.
Expression of α- β-, and γ-subunits of the endothelial epithelial Na+ channel (EnNaC) in in vitro isolated renal vascular endothelial cells (A) and confirmation of EnNaC deletion in αEnNaC−/− mice (B). Data are expressed as means ± SD; n = 4. *P < 0.01 vs. control diet-fed αEnNaC+/+ mice.
Table 1.
Characteristics of CD-fed αEnNaC+/+, WD-fed αEnNaC+/+, and WD-fed αEnNaC−/− mice
| Measure | CD-Fed αEnNaC+/+ | WD-Fed αEnNaC+/+ | WD-Fed αEnNaC−/− |
|---|---|---|---|
| Fat mass, g | 3.12 ± 0.33 (6) | 4.22 ± 0.32 (7)* | 4.41 ± 0.34 (5)* |
| Lean mass, g | 15.84 ± 0.39 (6) | 15.08 ± 0.34 (7) | 15.60 ± 0.80 (5) |
| Body weight, g | 20.30 ± 0.53 (6) | 20.62 ± 0.43 (7) | 21.25 ± 0.73 (5) |
| Mean arterial pressure, mmHg | 69.48 ± 2.29 (4) | 78.69 ± 4.58 (5) | 75.23 ± 2.69 (5) |
| Fasting glucose, mg/dL | 152.3 ± 2.2 (4) | 180.2 ± 18.3 (6) | 173.4 ± 10.3 (5) |
| Urine protein/creatinine | 1.49 ± 0.22 (8) | 2.80 ± 1.40 (7) | 1.97 ± 0.24 (5) |
| Nephrin, fold change of mRNA expression | 1.00 ± 0.143 (6) | 0.83 ± 0.08 (7) | 1.16 ± 0.11 (6) |
| Podocin, fold change of mRNA expression | 1.00 ± 0.14 (6) | 0.96 ± 0.14 (7) | 1.06 ± 0.09 (6) |
Values are presented as means ± SE; values in parentheses are numbers of animals/group. CD-fed αEnNaC+/+, control diet-fed α-endothelial epithelial Na+ channel (αEnNaC; wild-type control) mice; WD-fed αEnNaC+/+, Western diet-fed αEnNaC (wild-type control) mice; WD-fed αEnNaC−/−, Western diet-fed αEnNaC knockout mice.
P < 0.05 vs. CD-fed αEnNaC+/+ mice.
αEnNaC−/− prevented WD-induced central aortic and renal arterial stiffening as well as impaired endothelium-dependent relaxation but not wall remodeling.
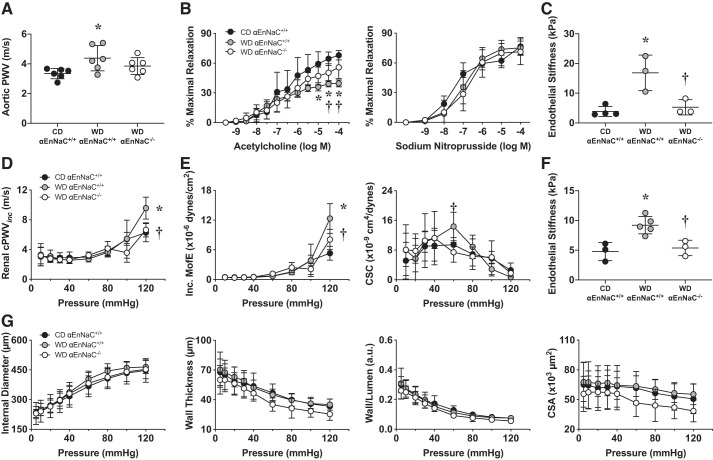
There is a link between aortic vascular stiffness and microcirculatory impairments in end organs such as the kidney (3, 14). In this context, 12 wk of WD induced an increase in central aortic stiffness, as determined by PWV (Fig. 2A), that was associated with impaired aortic endothelium (NO)-dependent vasodilatory responses on wire myography (Fig. 2B) and increased ex vivo endothelial cell stiffening, as observed with AFM (Fig. 2C). Renal arteries also displayed increased stiffening with WD feeding, as evidenced with increases in calculated incremental PWV measures (Fig. 2D) and reductions in vessel cross-sectional compliance (Fig. 2E) on pressure-dependent curves. These findings that occurred in parallel with endothelial cell stiffening were assessed by AFM (Fig. 2F). However, there were no changes in indexes of wall remodeling between groups (Fig. 2G). Importantly, these abnormalities were prevented in αEnNaC−/− mice, suggesting that αEnNaC−/− prevented both WD-induced aortic and renal arterial endothelial cell stiffness and impaired endothelium-dependent relaxation.
Fig. 2.
α-Endothelial epithelial Na+ channel (αEnNaC) mediates Western diet (WD)-induced aortic and renal arterial stiffening and impaired endothelium-dependent vascular relaxation. A: aortic pulse wave velocity (PWV) determined on Doppler ultrasound. B: aortic vasorelaxation response to the endothelium-dependent dilator ACh and the vascular smooth muscle-dependent dilator sodium nitroprusside. C: aortic endothelial cell stiffness as evaluated by atomic force microscopy. D: renal artery PWV using pressure-diameter analysis. cPWVinc, calculated incremental PWV. E: renal microvessel mechanical and functional experiments. Microvessel stiffness was evaluated with incremental moduli of elasticity (Inc. MofE) and cross-sectional compliance (CSC) using pressure myography in renal artery vessels. F: renal endothelial cell stiffness as evaluated by atomic force microscopy. G: renal artery wall measurements on pressure-diameter curves for internal diameter, wall thickness, wall-to-lumen ratio, and cross-sectional area (CSA). Data are expressed as means ± SD; n = 4–7. *P < 0.05 vs. control diet (CD)-fed αEnNaC+/+ mice; †P < 0.05 vs. WD-fed αEnNaC+/+ mice.
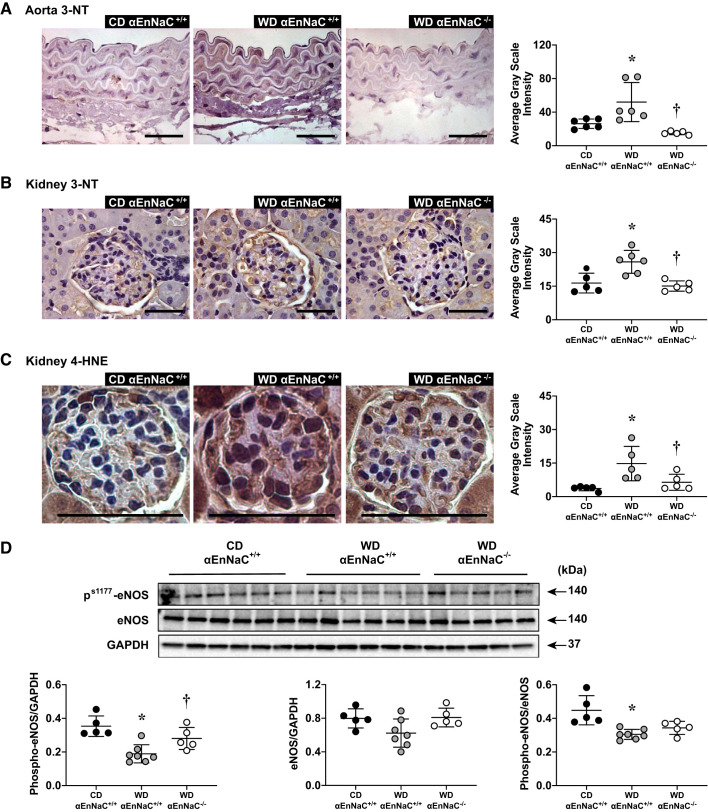
αEnNaC−/− prevented WD-induced aortic and renal arterial oxidative stress and related reduced eNOS activity.
Our previous studies have shown that enhanced αEnNaC expression or activity induced by aldosterone infusion (16) or WD feeding (29) contribute to increased aortic oxidative stress and suppression of eNOS activation (phosphorylation). This study further showed that 12 wk of WD also induced an increase of aortic 3-NT, renal arterial 3-NT, and 4-HNE, which are markers of peroxynitrite formation, lipid peroxidation chain reaction, and excessive tissue oxidative stress (Fig. 3, A–C). Meanwhile, 12 wk of WD and related oxidative stress also inhibited phosphorylation of eNOS (Fig. 3D). However, there were no observed differences in aortic and renal arterial 3-NT and phosphorylated eNOS/eNOS expression between control αEnNaC+/+ and αEnNaC−/− groups (see Supplemental Fig. S1). These data suggest that αEnNaC deletion prevented WD-induced aortic and renal arterial oxidative stress and related reduced eNOS activity (Fig. 3).
Fig. 3.
α-Endothelial epithelial Na+ channel (αEnNaC) mediates Western diet (WD)-induced and aortic and renal oxidant stress and reduction of endothelial nitric oxide synthase (eNOS) activation. A−C: representative images of immunostaining for aorta 3-nitrotyrosine (3-NT; A), kidney 3-NT (B), and kidney 4-hydroxynonenal (4-HNE) (C) with corresponding quantitative analysis. Scale bars = 50 µm. D: Western blots of Ser1177 phosphorylated (p-)eNOS or total eNOS normalized to GAPDH and ratio of p-eNOS to eNOS with representative quantitative analyses. Data are expressed as means ± SD; n = 5 or 6. *P < 0.05 vs. control diet (CD)-fed αEnNaC+/+ mice; †P < 0.05 vs. WD-fed αEnNaC+/+ mice.
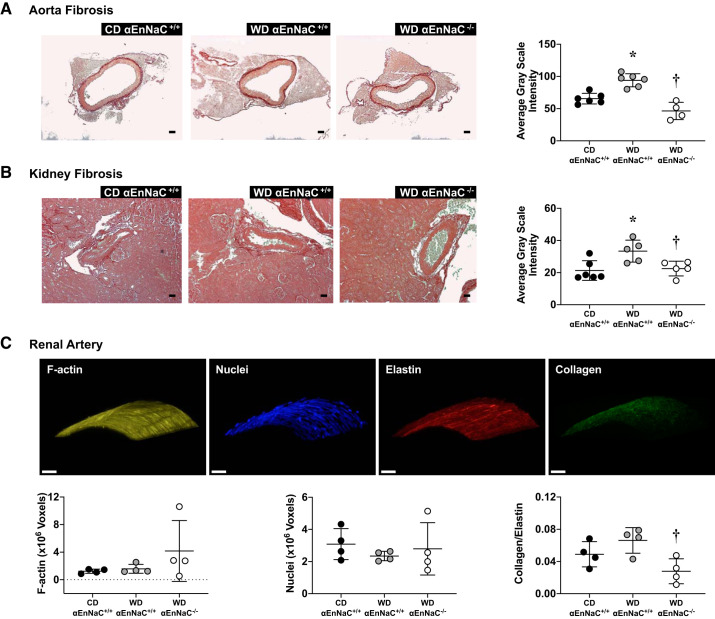
αEnNaC−/− prevented WD-induced aortic and renal arterial fibrosis and improved elasticity but had no impact on inward remodeling or hypertrophy.
Increased oxidative stress contributes to tissue fibrosis and vessel wall remodeling. Consistent with this notion, our present data support that 12 wk of WD induced both aortic and renal arterial fibrosis and that these abnormalities were prevented in αEnNaC−/− mice, suggesting that αEnNaC is involved in diet-induced tissue fibrosis (Fig. 4, A and B). However, there were no difference in aortic and renal arterial fibrosis and wall remodeling between control αEnNaC+/+ and αEnNaC−/− groups (see Supplemental Fig. S1). To further determine the role that WD has on the structural characteristics of renal vessels, we used three-dimensional reconstructive confocal imaging to visualize and quantify F-actin fibers along with collagen and elastin content in isolated renal arteries. There were no between-group differences in F-actin cytoskeletal content (Fig. 4C), consistent with wall remodeling indexes observed on pressure-diameter curves (Fig. 1), suggesting no inward remodeling or wall hypertrophy occurring with the observed fibrosis (Fig. 4B). Also consistent with the increased stiffness and fibrosis determined in the functional and immunohistochemical studies, we observed a nonsignificant increase of the collagen-to-elastin ratio in WD-fed mice that is significantly prevented with αEnNaC deletion (Fig. 4C). These data further support that WD-dependent engagement of αEnNaC had a specific effect on fibrosis generation that influenced the elasticity and compliance of the renal microcirculation.
Fig. 4.
α-Endothelial epithelial Na+ channel (αEnNaC) mediates Western diet (WD)-induced both aortic and renal arterial fibrosis as well as vessel wall elasticity. A and B: representative images of vessel fibrosis in aorta (A) and kidney (B) with corresponding quantitative analyses. Scale bar = 50 µm. C: three-dimensional reconstructive confocal imaging of F-actin, collagen, and elastin stained with nuclei. CD, Control diet. Data are expressed as means ± SD; n = 5 or 6. *P < 0.05 vs. CD. †P < 0.05 vs. WD EnNaC+/+.
DISCUSSION
Central aortic stiffening contributes to end-organ injury, as occurs in the kidney. Cumulative evidence indicates that periaortic fibrosis contributes to aortic vessel wall stiffening, which, in turn, facilitates pulsatility. However, there is little information on stiffening of the renal circulation. Furthermore, the role of the endothelium in the development of renal vessel wall stiffening and fibrosis is unknown. This present investigation demonstrates a novel mechanism by which WD feeding contributes to renal artery stiffening, which is endothelium and ENaC dependent. We have previously shown that WD-induced aortic endothelial cell and vascular stiffening is mitigated in αEnNaC−/− mice (16, 29). Here, we demonstrate through measures of in vivo PWV that endothelial cell-specific deletion of the α-subunit of EnNaC attenuates WD-induced central aortic and renal artery stiffening. This is associated with improvement in ex vivo measures of endothelial cell stiffness, impaired aortic endothelium-dependent relaxation, and renal endothelial stiffening. Multiple observations show that deletion of the α-subunit of EnNaC improved vessel wall stiffness, endothelium-dependent responses, and elasticity, which occurred contemporaneous with improvement in phosphorylated eNOS and decreased oxidant stress (e.g., 3-NT) as well as perivascular fibrosis in the renal circulation, extends previous work from conduit vessel aortic studies into the renal circulation (15, 16, 29). Because we observed no differences in growth and remodeling markers Akt and mammalian target of rapamycin, suggesting that stiffening primarily occurs through changes in cellular stiffness and extracellular matrix modifications, including the collagen-to-elastin ratio and tissue fibrosis. Of note, we recently reported that 20 wk of WD feeding induces proteinuria, a hallmark of kidney injury (3). In the present study, we observed neither elevated proteinuria nor expression of renal injury markers nephrin and podocin, suggesting that the process of renal arterial stiffening and perivascular fibrosis precedes renal injury in WD-fed mice.
Consumption of a WD plays a key role in macrovessel dysfunction, such as aorta stiffness, fibrosis, and atherosclerosis (3, 16). Consistent with this notion, we observed significant increases in central aortic stiffness by in vivo PWV, impaired aortic endothelium-dependent vasodilation, and aortic endothelial cell stiffening by ex vivo AFM with our WD high in fat and refined carbohydrates compared with control diet-fed mice. These findings occurred contemporaneously with increased oxidant stress (e.g., 3-NT content) and periaortic fibrosis. In this context, recent work has focused on how central aortic stiffening contributes to alterations in microcirculatory hemodynamics in target organs, such as the kidney (10, 37). Pulsatile (kinetic) energy created by central aortic stiffening alters autoregulatory mechanisms through chronic increases in myogenic tone and consequent wall strain and remodeling that over time may lead to renal vascular stiffening. Current work from our laboratories and others support the concept that obesity increases central aortic stiffness, which extends to renal artery stiffening and microvessel impairment changes, which are dependent on endothelial cell MRs (3). Consistent with our previous studies and those of others (29, 31), there were no apparent differences in physiological parameters (including blood pressure and aortic relaxation) between control αEnNaC+/+ and αEnNaC−/− animals. Although EnNaC is likely a key component in the maintenance of normal endothelium function, we do not think that its deletion necessarily leads to a significant arterial phenotype under control conditions (i.e., when fed a normal chow diet). Our present work extends this to support that renal artery stiffening occurs in response to a WD-induced central aortic stiffening, as determined by renal artery duplex measures of in vivo PWV are endothelium dependent. This finding is supported by our observation that WD contributed to increased endothelial stiffening and impairments in endothelial ACh-dependent vasodilation but not sodium nitroprusside-dependent vascular smooth muscle vasodilation. These novel data support that stiffening in the renal arteries is driven by the endothelium.
Our observation that genetic deletion of the α-subunit, as one of the functional core subunits of EnNaC, attenuated stiffening in the renal artery advances our understanding of the unique contributions of EnNaC to microcirculation function beyond what is known in the aortic macrocirculation. Studies have indicated that ENaC is regulated by shear stress in collecting duct cells (28) and that ENaC subunits are molecular components of the arterial baroreceptor complex (13). Recent data also suggest that ENaC is present in vascular smooth muscle cells and can function as a mechanotransducer to regulate blood flow in the kidney, brain, and mesentery (7, 12, 18). The endothelium-specific α-subunit of ENaC then plays a critical role in endothelial function (31) as well as shear stress sensing and flow-mediated vascular dilation, and our present data suggest that EnNaC is involved in vasoregulation and mechanotransduction in the renal microcirculation. Our previous work in this area supports the fact that a WD increases EnNaC promoter activity and EnNaC expression in the aortic endothelium as well as αEnNaC subunit-mediated Na+ channel activity, inward Na+ currents, and endothelial stiffness, which contribute to central aortic stiffening (16, 29). In our present study, genetic deletion of the α-subunit of EnNaC extends this work and support its role on improved renal artery stiffening through pressure-dependent measures of cross-sectional compliance, the incremental modulus of elasticity, and stiffness measures on endothelial cells from microvessels using AFM. Our data also extend recent work that αEnNaC deletion is protective of tubular injury in renal ischemia-reperfusion injury through an eNOS-dependent mechanism (30).
In concert with our present findings, pharmacological inhibition of ENaC in aortic and mesenteric arteries has been shown to improve phosphorylation of eNOS and bioavailable NO that directly relate to vessel stiffening and relaxation (15, 21, 29). Genetic deletion of αEnNaC improved Ser1177 phosphorylation of eNOS in relation to improved renal endothelial cell stiffening on AFM and compliance. This complements the findings from recent work on αEnNaC as a protective mechanism in proximal tubular injury that was NO dependent (30, 31). Our work defines, for the first time, a role for αEnNaC in the regulation of NO and vessel tone in the renal artery. Additionally, αEnNaC deletion rescued phosphorylation of eNOS and attenuated renal cortical tissue oxidative stress, supporting a role for endothelial regulation of renal tissue oxidant stress. Although there has been significant work to explore the relationship between NO and oxidant and inflammatory pathways that regulate stiffening and vessel wall relaxation, there is increasing interest in these pathways as they relate to fibrosis. In this regard, WD induced not only renal artery stiffening that was dependent on NO but also the development of vessel wall fibrosis. In cultured aortic endothelial cells, eNOS activation and NO production have been shown to directly regulate nitrosylation, translocation, and extracellular release of matrix-producing enzymes that contribute to cell cortical stiffness, increased cell permeability, and synthesis of collagens (3, 6). In this study, we observed that αEnNaC deletion attenuated aortic and renal arterial perivascular fibrosis and improved elasticity, supporting, for the first time, that αEnNaC regulation of NO dictates renal vessel wall fibrosis.
The rationale for choosing female mice is based on our previous work that female C57BL6J mice fed a WD develop insulin resistance earlier and a more rapid onset of aortic (21) and cardiac stiffness (20) compared with male mice. In addition, WD-induced plasma aldosterone levels are higher in female subjects than in male subjects (20). Thus, it is acknowledged that the impact of sexual dimorphism is an important topic for future studies in this area. In summary, we demonstrate a novel mechanism by which WD feeding contributes to renal arterial stiffening that is endothelium and ENaC dependent. Furthermore, we demonstrated that endothelial cell-specific EnNaC activation is important in increasing pulsatility into the renal microcirculation and regulation of oxidant stress, bioavailable NO, renal vessel wall stiffening, compliance, and fibrosis. The findings in this investigation address an important role of αEnNaC on WD-induced renal arterial compliance, stiffening, and dysfunction, thereby providing a potential therapeutic strategy in the prevention of kidney disease.
GRANTS
This work was supported by Veterans Affairs Merit System Grants BX003391 (to A. Whaley-Connell) and BX001981 (to J. R. Sowers) and by National Institutes of Health Grants R01-HL-73101-01A and HL-107910-01 (to J. R. Sowers), R01-HL-088105 (to L. A. Martinez-Lemus), and RO1-HL-085119 (to M. A. Hill). G. Jia is supported by American Diabetes Association Grant 1-17-IBS-201. Y. Xiong is supported by the State Scholarship Fund, Natural Science Foundation of China Grants 81760734 and 31760313, Natural Science Foundation of Yunnan Province Grant 2017FA 048, Diabetic Innovation Team Grant 2019HC002, and Clinical Endocrinology of Yunnan Province, the fund for medical leaders in Yunnan Province, Grant L-201709.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.M.-A., G.L., L.A.M.-L., J.R.S., and A.W.-C. conceived and designed research; Y.X., A.R.A., F.I.R.-P., G.J., J.H., D.C., and V.G.D. performed experiments; A.R.A., F.I.R.-P., G.J., J.H., D.C., V.G.D., and A.W.-C. analyzed data; A.R.A., F.I.R.-P., G.J., J.H., C.M.-A., G.L., F.J., and A.W.-C. interpreted results of experiments; Y.X., A.R.A., F.I.R.-P., G.J., J.H., V.G.D., and A.W.-C. prepared figures; Y.X., A.R.A., G.J., and A.W.-C. drafted manuscript; Y.X., A.R.A., G.J., L.A.M.-L., F.J., J.R.S., and A.W.-C. edited and revised manuscript; A.W.-C. approved final version of manuscript.
ACKNOWLEDGMENTS
The data sets generated during and analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
REFERENCES
- 1.Alvarez de la Rosa D, Canessa CM, Fyfe GK, Zhang P. Structure and regulation of amiloride-sensitive sodium channels. Annu Rev Physiol 62: 573–594, 2000. doi: 10.1146/annurev.physiol.62.1.573. [DOI] [PubMed] [Google Scholar]
- 2.Aroor AR, Das NA, Carpenter AJ, Habibi J, Jia G, Ramirez-Perez FI, Martinez-Lemus L, Manrique-Acevedo CM, Hayden MR, Duta C, Nistala R, Mayoux E, Padilla J, Chandrasekar B, DeMarco VG. Glycemic control by the SGLT2 inhibitor empagliflozin decreases aortic stiffness, renal resistivity index and kidney injury. Cardiovasc Diabetol 17: 108, 2018. doi: 10.1186/s12933-018-0750-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aroor AR, Habibi J, Nistala R, Ramirez-Perez FI, Martinez-Lemus LA, Jaffe IZ, Sowers JR, Jia G, Whaley-Connell A. Diet-induced obesity promotes kidney endothelial stiffening and fibrosis dependent on the endothelial mineralocorticoid receptor. Hypertension 73: 849–858, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashley Z, Mugloo S, McDonald FJ, Fronius M. Epithelial Na+ channel differentially contributes to shear stress-mediated vascular responsiveness in carotid and mesenteric arteries from mice. Am J Physiol Heart Circ Physiol 314: H1022–H1032, 2018. doi: 10.1152/ajpheart.00506.2017. [DOI] [PubMed] [Google Scholar]
- 5.Bender SB, Castorena-Gonzalez JA, Garro M, Reyes-Aldasoro CC, Sowers JR, DeMarco VG, Martinez-Lemus LA. Regional variation in arterial stiffening and dysfunction in Western diet-induced obesity. Am J Physiol Heart Circ Physiol 309: H574–H582, 2015. doi: 10.1152/ajpheart.00155.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castorena-Gonzalez JA, Staiculescu MC, Foote CA, Polo-Parada L, Martinez-Lemus LA. The obligatory role of the actin cytoskeleton on inward remodeling induced by dithiothreitol activation of endogenous transglutaminase in isolated arterioles. Am J Physiol Heart Circ Physiol 306: H485–H495, 2014. doi: 10.1152/ajpheart.00557.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi SK, Yeon SI, Kwon Y, Byeon S, Lee YH. Involvement of epithelial Na+ channel in the elevated myogenic response in posterior cerebral arteries from spontaneously hypertensive rats. Sci Rep 7: 45996, 2017. doi: 10.1038/srep45996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley-Connell A, Sowers JR. Low-dose mineralocorticoid receptor blockade prevents Western diet-induced arterial stiffening in female mice. Hypertension 66: 99–107, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeMarco VG, Johnson MS, Habibi J, Pulakat L, Gul R, Hayden MR, Tilmon RD, Dellsperger KC, Winer N, Whaley-Connell AT, Sowers JR. Comparative analysis of telmisartan and olmesartan on cardiac function in the transgenic (mRen2)27 rat. Am J Physiol Heart Circ Physiol 300: H181–H190, 2011. doi: 10.1152/ajpheart.00883.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Djudjaj S, Boor P. Cellular and molecular mechanisms of kidney fibrosis. Mol Aspects Med 65: 16–36, 2019. doi: 10.1016/j.mam.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Downs CA, Johnson NM, Coca C, Helms MN. Angiotensin II regulates δ-ENaC in human umbilical vein endothelial cells. Microvasc Res 116: 26–33, 2018. doi: 10.1016/j.mvr.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Drummond HA, Stec DE. βENaC acts as a mechanosensor in renal vascular smooth muscle cells that contributes to renal myogenic blood flow regulation, protection from renal injury and hypertension. J Nephrol Res 1: 1–9, 2015. doi: 10.17554/j.issn.2410-0579.2015.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drummond HA, Welsh MJ, Abboud FM. ENaC subunits are molecular components of the arterial baroreceptor complex. Ann N Y Acad Sci 940: 42–47, 2001. doi: 10.1111/j.1749-6632.2001.tb03665.x. [DOI] [PubMed] [Google Scholar]
- 14.Jia G, Aroor AR, Sowers JR. Arterial stiffness: a nexus between cardiac and renal disease. Cardiorenal Med 4: 60–71, 2014. doi: 10.1159/000360867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia G, Habibi J, Aroor AR, Hill MA, DeMarco VG, Lee LE, Ma L, Barron BJ, Whaley-Connell A, Sowers JR. Enhanced endothelium epithelial sodium channel signaling prompts left ventricular diastolic dysfunction in obese female mice. Metabolism 78: 69–79, 2018. doi: 10.1016/j.metabol.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Jia G, Habibi J, Aroor AR, Hill MA, Yang Y, Whaley-Connell A, Jaisser F, Sowers JR. Epithelial sodium channel in aldosterone-induced endothelium stiffness and aortic dysfunction. Hypertension 72: 731–738, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosari F, Sheng S, Li J, Mak DO, Foskett JK, Kleyman TR. Subunit stoichiometry of the epithelial sodium channel. J Biol Chem 273: 13469–13474, 1998. doi: 10.1074/jbc.273.22.13469. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Fung E. Multifaceted functions of epithelial Na+ channel in modulating blood pressure. Hypertension 73: 273–281, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12330. [DOI] [PubMed] [Google Scholar]
- 19.Lipphardt M, Song JW, Matsumoto K, Dadafarin S, Dihazi H, Müller G, Goligorsky MS. The third path of tubulointerstitial fibrosis: aberrant endothelial secretome. Kidney Int 92: 558–568, 2017. doi: 10.1016/j.kint.2017.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manrique C, DeMarco VG, Aroor AR, Mugerfeld I, Garro M, Habibi J, Hayden MR, Sowers JR. Obesity and insulin resistance induce early development of diastolic dysfunction in young female mice fed a Western diet. Endocrinology 154: 3632–3642, 2013. doi: 10.1210/en.2013-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Lemus LA, Aroor AR, Ramirez-Perez FI, Jia G, Habibi J, DeMarco VG, Barron B, Whaley-Connell A, Nistala R, Sowers JR. Amiloride improves endothelial function and reduces vascular stiffness in female mice fed a Western diet. Front Physiol 8: 456, 2017. doi: 10.3389/fphys.2017.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985) 105: 1652–1660, 2008. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Padilla J, Ramirez-Perez FI, Habibi J, Bostick B, Aroor AR, Hayden MR, Jia G, Garro M, DeMarco VG, Manrique C, Booth FW, Martinez-Lemus LA, Sowers JR. Regular exercise reduces endothelial cortical stiffness in Western diet-fed female mice. Hypertension 68: 1236–1244, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padilla J, Woodford ML, Lastra-Gonzalez G, Martinez-Diaz V, Fujie S, Yang Y, Lising AMC, Ramirez-Perez FI, Aroor AR, Morales-Quinones M, Ghiarone T, Whaley-Connell A, Martinez-Lemus LA, Hill MA, Manrique-Acevedo C. Sexual dimorphism in obesity-associated endothelial ENaC activity and stiffening in mice. Endocrinology 160: 2918–2928, 2019. doi: 10.1210/en.2019-00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perry HM, Okusa MD. Endothelial dysfunction in renal interstitial fibrosis. Nephron 134: 167–171, 2016. doi: 10.1159/000447607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez-Lázaro D, Hernández M. Confirmation of isolates of Listeria by conventional and real-time PCR. Methods Mol Biol 1157: 31–38, 2014. doi: 10.1007/978-1-4939-0703-8_3. [DOI] [PubMed] [Google Scholar]
- 27.Sakurai M, Kobayashi J, Takeda Y, Nagasawa SY, Yamakawa J, Moriya J, Mabuchi H, Nakagawa H. Sex differences in associations among obesity, metabolic abnormalities, and chronic kidney disease in Japanese men and women. J Epidemiol 26: 440–446, 2016. doi: 10.2188/jea.JE20150208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na+ channels are regulated by flow. Am J Physiol Renal Physiol 280: F1010–F1018, 2001. doi: 10.1152/ajprenal.2001.280.6.F1010. [DOI] [PubMed] [Google Scholar]
- 29.Sowers JR, Habibi J, Aroor AR, Yang Y, Lastra G, Hill MA, Whaley-Connell A, Jaisser F, Jia G. Epithelial sodium channels in endothelial cells mediate diet-induced endothelium stiffness and impaired vascular relaxation in obese female mice. Metabolism 99: 57–66, 2019. doi: 10.1016/j.metabol.2019.153946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarjus A, González-Rivas C, Amador-Martínez I, Bonnard B, López-Marure R, Jaisser F, Barrera-Chimal J. The absence of endothelial sodium channel α (αENaC) reduces renal ischemia/reperfusion injury. Int J Mol Sci 20: 3132, 2019. doi: 10.3390/ijms20133132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarjus A, Maase M, Jeggle P, Martinez-Martinez E, Fassot C, Loufrani L, Henrion D, Hansen PBL, Kusche-Vihrog K, Jaisser F. The endothelial αENaC contributes to vascular endothelial function in vivo. PLoS One 12: e0185319, 2017. doi: 10.1371/journal.pone.0185319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Townsend RR, Anderson AH, Chirinos JA, Feldman HI, Grunwald JE, Nessel L, Roy J, Weir MR, Wright JT Jr, Bansal N, Hsu CY; CRIC Study Investigators . Association of pulse wave velocity with chronic kidney disease progression and mortality: findings from the CRIC Study (Chronic Renal Insufficiency Cohort). Hypertension 71: 1101–1107, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension . Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 66: 698–722, 2015. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whaley-Connell A, Sowers JR. Obesity and kidney disease: from population to basic science and the search for new therapeutic targets. Kidney Int 92: 313–323, 2017. doi: 10.1016/j.kint.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 35.Wildman RP, Mackey RH, Bostom A, Thompson T, Sutton-Tyrrell K. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension 42: 468–473, 2003. doi: 10.1161/01.HYP.0000090360.78539.CD. [DOI] [PubMed] [Google Scholar]
- 36.Woodard T, Sigurdsson S, Gotal JD, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Gudnason V, Harris TB, Launer LJ, Levey AS, Mitchell GF. Mediation analysis of aortic stiffness and renal microvascular function. J Am Soc Nephrol 26: 1181–1187, 2015. doi: 10.1681/ASN.2014050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang SH, Kim YC, An JN, Kim JH, Lee J, Lee HY, Cho JY, Paik JH, Oh YK, Lim CS, Kim YS, Lee JP. Active maintenance of endothelial cells prevents kidney fibrosis. Kidney Res Clin Pract 36: 329–341, 2017. doi: 10.23876/j.krcp.2017.36.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]