Abstract
Carvedilol is an FDA-approved β-blocker commonly used for treatment of high blood pressure, congestive heart failure, and cardiac tachyarrhythmias, including atrial fibrillation. We investigated at the cellular level the mechanisms through which carvedilol interferes with sarcoplasmic reticulum (SR) Ca2+ release during excitation-contraction coupling (ECC) in single rabbit atrial myocytes. Carvedilol caused concentration-dependent (1–10 µM) failure of SR Ca2+ release. Failure of ECC and Ca2+ release was the result of dose-dependent inhibition of voltage-gated Na+ (INa) and L-type Ca2+ (ICa) currents that are responsible for the rapid depolarization phase of the cardiac action potential (AP) and the initiation of Ca2+-induced Ca2+ release from the SR, respectively. Carvedilol (1 µM) led to AP duration shortening, AP failures, and peak INa inhibition by ~80%, whereas ICa was not markedly affected. Carvedilol (10 µM) blocked INa almost completely and reduced ICa by ~40%. No effect on Ca2+-transient amplitude, ICa, and INa was observed in control experiments with the β-blocker metoprolol, suggesting that the carvedilol effect on ECC is unlikely the result of its β-blocking property. The effects of carvedilol (1 µM) on subcellular SR Ca2+ release was spatially inhomogeneous, where a selective inhibition of peripheral subsarcolemmal Ca2+ release from the junctional SR accounted for the cell-averaged reduction in Ca2+-transient amplitude. Furthermore, carvedilol significantly reduced the probability of spontaneous arrhythmogenic Ca2+ waves without changes of SR Ca2+ load. The data suggest a profound antiarrhythmic action of carvedilol in atrial myocytes resulting from an inhibitory effect on the SR Ca2+ release channel.
NEW & NOTEWORTHY Here we show that the clinically widely used β-blocker carvedilol has profound effects on Ca2+ signaling and ion currents, but also antiarrhythmic effects in adult atrial myocytes. Carvedilol inhibits sodium and calcium currents and leads to failure of ECC but also prevents spontaneous Ca2+ release from cellular sarcoplasmic reticulum (SR) Ca2+ stores in form of arrhythmogenic Ca2+ waves. The antiarrhythmic effect occurs by carvedilol acting directly on the SR ryanodine receptor Ca2+ release channel.
Keywords: arrhythmogenic Ca2+ waves, atrial myocyte, calcium and sodium currents, excitation-contraction coupling, intracellular Ca2+ release
INTRODUCTION
The cellular mechanism of excitation-contraction coupling (ECC) reveals marked differences between atrial and ventricular cells (6, 7). Atrial myocytes, in contrast to ventricular myocytes, lack or only have a rudimentary and irregular transverse tubule (TT) system. The absence of TTs causes characteristic spatial and temporal inhomogeneities during action potential (AP)-induced Ca2+ release (6–9, 36). Ca2+ release initiates in the periphery with the activation of L-type Ca2+ channels (LTCCs), where Ca2+ influx triggers Ca2+-induced Ca2+ release (CICR) through ryanodine receptor (RyR) Ca2+ release channels from junctional sarcoplasmic reticulum (j-SR). Subsequently, CICR from central nonjunction SR (nj-SR) Ca2+ release sites propagates actively in centripetal direction, ultimately leading to global, whole cell elevation of intracellular Ca2+ concentration ([Ca2+]i) and atrial contraction (18, 34).
The physiological mechanism of Ca2+ release during ECC in atrial cells is reminiscent of the mechanism that sustains pathological arrhythmogenic Ca2+ waves. The similarity lies in the fact that both rely on propagating CICR. However, in contrast to the physiological mechanism of atrial ECC, pathological Ca2+ waves typically occur spontaneously (i.e., in the absence of an initial electrical trigger in form of an AP and ICa), require an increased SR Ca2+ load, and RyR and Ca2+ release activation by luminal Ca2+. Ca2+ waves have pathological ramifications and have been linked to cardiac arrhythmias, including atrial fibrillation (10, 13, 20, 24, 25, 35).
β-Adrenergic receptor antagonists (β-blockers), including carvedilol, are clinically used for heart failure treatment to reduce arrhythmia risk, including atrial fibrillation (16), and to prevent sudden cardiac death (15, 40). In vitro, carvedilol has also been described to significantly reduce spontaneous Ca2+ waves, presumably by modulating the RyR (43, 45) through reducing mean open time and curtailing Ca2+ flux through the channel. Ca2+ waves and the physiological mechanism of atrial ECC have in common that both mechanisms involve propagating CICR. Since carvedilol has profound effects on spontaneous Ca2+ waves and affects RyR gating, we set out to characterize the effects of carvedilol on atrial ECC and Ca2+ release. We tested the effect of the drug on the key elements of ECC, from activation of voltage-gated Na+ (INa) and Ca2+ (ICa) currents, AP morphology to activation of RyR-mediated CICR. With the combination of voltage- and current-clamp recordings and spatially resolved Ca2+ imaging in atrial myocytes, we demonstrate that carvedilol has inhibitory effects on INa and ICa, shortens or abolishes the AP, and disrupts physiological Ca2+ release in atrial myocytes but protects from spontaneous arrhythmogenic Ca2+ waves.
METHODS
Myocyte isolation.
Atrial myocytes were isolated from male New Zealand White rabbits (2.5 kg; 41 rabbits; Charles River, Wilmington, MA). Rabbits were anesthetized with an intravenous injection of pentobarbital sodium (Euthasol; 50 mg/kg) together with heparin (1,000 IU/kg). Hearts were excised and mounted on a Langendorff apparatus and retrogradely perfused with nominally Ca2+-free Tyrode (see composition below) solution containing heparin (1,000 IU/kg) for 5 min, followed by perfusion with minimal essential medium Eagle (MEM) solution containing 20 μM Ca2+ and 22.5 μg/ml Liberase TH (Roche Applied Science, Indianapolis, IN) for ∼20 min at 37°C. The left atrium was removed and digested for an additional 5 min in the enzyme solution at 37°C. Digested tissue was then minced, filtered, and washed in a MEM solution containing 50 μM Ca2+ and 10 mg/ml bovine serum albumin. Isolated cells were kept in MEM solution with 50 μM Ca2+ at room temperature (22–24°C) until experimentation. All aspects of animal husbandry, animal handling, anesthesia, surgery, and euthanasia were fully approved by the Institutional Animal Care and Use Committee (IACUC) of Rush University Chicago and comply with United States and United Kingdom regulations on animal experimentation.
Patch-clamp experiments.
Electrophysiological signals were recorded from single atrial myocytes in the whole cell ruptured patch-clamp configuration using an Axopatch 200B patch‐clamp amplifier, the Axon Digidata 1550B interface and pCLAMP 11 software (Molecular Devices, Sunnyvale, CA). Current and voltage recordings were low‐pass filtered at 5 kHz and digitized at 10 kHz. Patch pipettes (1.5–3 MΩ filled with internal solution) were pulled from borosilicate glass capillaries (AM-Systems, Squim, WA) with a horizontal puller (model P‐97; Sutter Instruments, Novato, CA). For Na+ (INa) and Ca2+ (ICa) current measurements, myocytes were voltage clamped and held at a membrane potential (Vm) of −80 mV. In experiments testing drug effects on membrane currents, a two-step voltage-clamp protocol was applied to measure INa and ICa during the same experiment. INa was elicited with a 50-ms voltage step from −80 to −50 mV, immediately followed by a voltage step (350 ms) to 0 mV to elicit ICa (the voltage-clamp protocol is shown in Fig. 3A, inset). Upon completion of each step command, the command potential was returned to −80 mV for 1.4 s. During extended INa and ICa recordings, a substantial current rundown was observed, especially of INa. To correct for such rundown, the same protocol was used to record INa and ICa in the absence of the drug. INa and ICa were normalized for Imax, and the averaged normalized current data from the carvedilol experiments were divided by the averaged normalized data recorded in the absence of carvedilol. To establish the current-voltage (I–V) relationship of ICa, the holding potential (VH) was −80 mV and cells were depolarized by a prepulse to −50 mV (50 ms) immediately followed by depolarization steps of 350-ms duration in increments of 10 mV to a maximum depolarization of +60 mV. For the measurement of the I–V relationship for INa, single myocytes were held at −80 mV, and 30-ms voltage steps were applied from −80 to +30 mV in 5-mV increments. For current-clamp measurements, the whole cell fast current-clamp mode of the Axopatch 200B was used, and APs were evoked by 5-ms stimulation pulses of a magnitude ∼1.5 times higher than AP activation threshold. Vm measurements were corrected for a junction potential error of −10 mV.
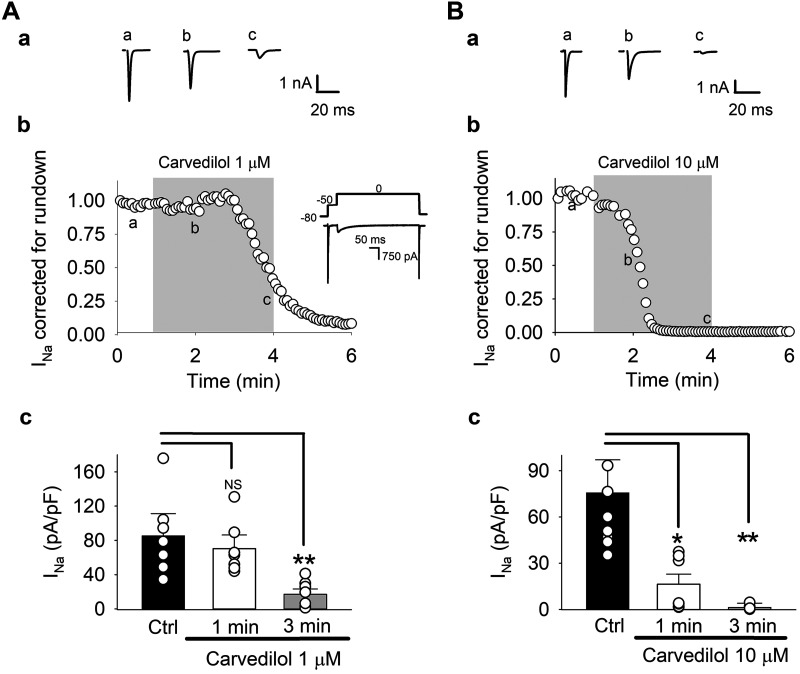
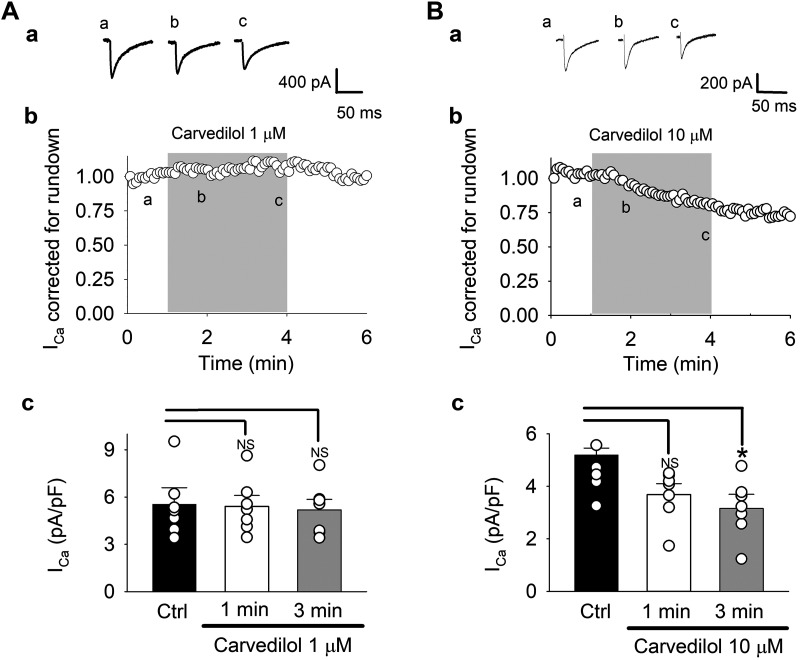
Fig. 3.
Carvedilol effect on voltage-gated Na+ current (INa). A and B: effect of 1 µM (A) and 10 µM (B) on INa: representative INa traces (a) and time course (b) of carvedilol effect on average peak INa corrected and normalized for rundown. A,b, insert: voltage-clamp protocol. Myocytes were held at membrane potential = −80 mV. A 2-step voltage-clamp protocol was applied to measure INa and L-type Ca2+ current (ICa) (Fig. 4) during the same experiment. INa was elicited with a 50-ms voltage step from −80 to −50 mV, immediately followed by a voltage step (350 ms) to 0 mV to elicit ICa. Upon completion of each step command, the command potential was returned to −80 mV for 1.4 s. A,c and B,c: summary data for uncorrected currents in control (Ctrl) and after 1- and 3-min carvedilol exposure. Means ± SE and individual cell data are shown (n = 8). *P < 0.02; **P < 0.003 (ANOVA). NS, not significant.
For INa and ICa measurements, the internal solution consisted of (in mM) 130 Cs-glutamate, 20 CsCl, 0.33 MgCl2, 4 MgATP, 5 EGTA, and 10 HEPES with pH adjusted to 7.2 with CsOH. External Tyrode solution was composed of (in mM) 135 NaCl, 4 CsCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 d-glucose (pH 7.4 with NaOH). For I–V curve of INa, the internal solution consisted of (in mM) 5 NaCl, 20 CsCl, 5 EGTA, 4 MgATP, and 10 HEPES (pH 7.2 with CsOH), and the low-sodium external solution consisted of (in mM) 20 NaCl, 120 CsCl, 1 MgCl2, 10 HEPES, and 10 d-glucose (pH 7.4 with CsOH). Current-clamp and [Ca2+]i experiments were conducted using external Tyrode solution containing (in mM) 135 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 10 d-glucose, and 10 HEPES (pH 7.4 with NaOH). For AP measurements, the internal solution contained (in mM) 130 K-glutamate, 5 NaCl, 15 KCl, 0.33 MgCl2, 4 MgATP, and 10 HEPES (pH 7.2). Experiments were conducted at room temperature (22–24°C).
[Ca2+]i measurements.
To monitor cytosolic Ca2+ transients and Ca2+ waves, atrial myocytes were loaded with the membrane-permeable Ca2+ indicator Fluo-4 AM (10 μM) and 0.2% of pluronic acid (Invitrogen) for 20 min and then washed twice for 10 min with Tyrode solution. Confocal microscopy (Bio-Rad Radiance 2000 and Nikon A1R) were used to image [Ca2+]i. Fluo-4 was excited at 488 nm and emission collected at wavelengths > 515 nm. Fluo-4 [Ca2+]i signals are presented as F/F0 ratios, where F0 represents diastolic [Ca2+]i during electrical pacing. Ca2+-transient (CaT) amplitudes are quantified as ∆F/F0, where ∆F/F0 = (F − F0)/F0. [Ca2+]i measurements were conducted in the line scan mode (512 lines/s) using a ×60 water-immersion (Bio-Rad Radiance 2000) and a ×60 oil-immersion (Nikon A1R) objective lens. The scan line was placed along the transversal axis of the cell avoiding the nucleus with a pixel size of 0.08–0.15 µm. Cells were superfused with external Tyrode solution. CaTs were elicited by electrical field stimulation of intact atrial myocytes using a pair of platinum electrodes (voltage set at ∼50% above the threshold for contraction, 3-ms duration). Ca2+ waves were recorded during a 20-s period of rest after electrical stimulation at 1 Hz. To increase the probability of spontaneous Ca2+ waves in these experiments, extracellular Ca2+ concentration ([Ca2+]o) was increased to 7 mM. Experiments were conducted at room temperature (22–24°C).
Chemicals.
Chemicals were from Sigma‐Aldrich (St. Louis, MO), unless otherwise stated. Fluo‐4 AM was obtained from Thermo Fisher Scientific (Waltham MA). Carvedilol and metoprolol were dissolved in DMSO at a concentration of 40 mM. The final DMSO concentration in extracellular solutions was 0.025%.
Data analysis and presentation.
Results are presented as individual observations or as means ± SE, and n represents the number of individual cells. Statistical significance was evaluated using unpaired and paired Student's t-test for single comparisons, and two-way ANOVA, followed by Tukey's post hoc test for multiple comparisons. Statistical analysis was conducted with SigmaPlot (v. 11; Systat Software). Differences were considered significant at P < 0.05. NS indicates statitically not significant.
RESULTS
Carvedilol effect on atrial ECC and SR Ca2+ release.
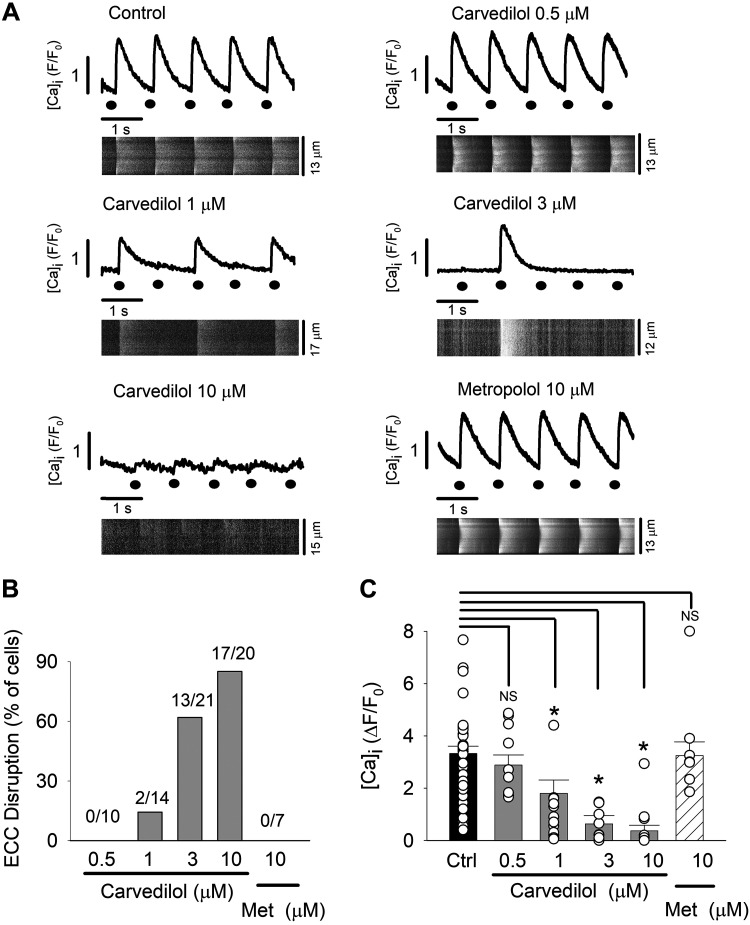
We tested the effect of carvedilol on AP-induced Ca2+ release in isolated rabbit atrial myocytes. Cytosolic CaTs were elicited by electrical field stimulation (1 Hz). We found that carvedilol had profound, dose-dependent effects on electrically evoked CaTs. As shown in Fig. 1A, carvedilol diminished the CaT amplitude in a dose-dependent manner and led to disruption of ECC that became more severe with increasing carvedilol concentration. ECC disruption was defined here as the failure of electrical stimulation to elicit CaTs. Figure 1B summarizes the degree of ECC disruption. A single failure of Ca2+ release during a test period of 10 s counted as a disruption of ECC. At the lowest concentration tested (0.5 µM), carvedilol did not disrupt ECC (n = 0/10). At higher concentrations, failure of electrical stimulation to elicit CaTs was observed in 14% (1 µM; n = 2/14), 61% (3 µM; n = 13/21), and 85% (10 µM; n = 17/20) of cells, respectively. Figure 1C summarizes the carvedilol concentration-dependent decrease in whole cell CaT amplitude (∆F/F0). CaT amplitudes were determined by averaging the amplitudes of 10 consecutive CaTs (including disruptions). In control, mean CaT amplitude was ∆F/F0 = 3.32 ± 0.27, compared with 2.88 ± 0.38 (carvedilol 0.5 μM; n = 10, P = 0.14), 1.79 ± 0.51 (1 μM, n = 14, P < 0.005), 0.63 ± 0.31 (3 μM, n = 21, P < 0.005), and 0.36 ± 0.21 (10 μM, n = 20, P < 0.005). Since carvedilol is a clinically used β-blocker, we tested whether the carvedilol effects on CaTs and ECC resulted from its antiadrenergic action. In contrast to carvedilol, however, metoprolol (FDA-approved β-blocker) did not significantly affect CaT amplitude (3.24 ± 0.52; n = 7, P = 0.21; Fig. 1C), arguing against an antiadrenergic effect.
Fig. 1.
Excitation-contraction coupling (ECC) failure by carvedilol. Carvedilol inhibits action potential-induced Ca2+ release during ECC. ECC disruption was defined as the inability of electrical filed stimulation (1 Hz) to elicit Ca2+ transient (CaTs) in isolated rabbit atrial myocytes. A: representative whole cell intracellular Ca2+ concentration ([Ca2+]i) profiles and corresponding line scan images in control (n = 65) and during exposure to 0.5 µM (n = 10 cells), 1 µM (n = 14), 3 µM (n = 21), and 10 µM (n = 20) carvedilol and the negative control with metoprolol (Met; 10 µM, n = 7). Black circles indicate time of electrical stimulus. B: fraction of cells (%) showing ECC disruption. A single failure of Ca2+ release during the test period of 10 s counted as a disruption of ECC. C: average CaT amplitudes from control and carvedilol-treated atrial myocytes. The [Ca2+]i responses to 10 consecutive electrical stimulations were averaged, including failures. The Fluo-4 Ca2+ signal was averaged over the entire width of the cell. For presentation purposes, control (Ctrl) CaT amplitudes were pooled; however, for statistical analysis (t-test for paired measurements), each carvedilol concentration and the metoprolol group had its own control, since experiments were conducted as a paired measurement with recording control CaTs followed by drug application to the same cell. Means ± SE and individual cell data (white circles) are shown. *P < 0.05. NS, not significant.
In the following we systematically tested the key steps of ECC that might be critically affected by carvedilol, from AP generation, membrane depolarization-induced activation of voltage-gated Na+ and Ca2+ currents, to release of Ca2+ from the SR via the RyR Ca2+ release channel.
Carvedilol effects on AP.
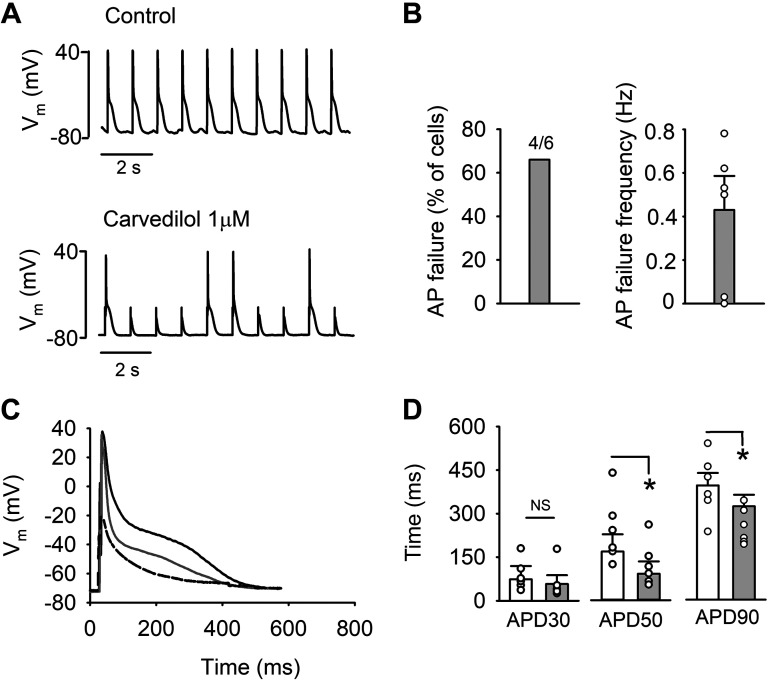
First, we tested the effect of carvedilol (1 μM) on AP morphology using the whole cell current-clamp technique. APs were elicited by applying 5-ms stimulation pulses of a magnitude ∼1.5 times higher than AP activation threshold at 1 Hz. Representative traces of APs recorded before and during application of carvedilol are shown in Fig. 2A. APs failed after carvedilol treatment in 67% of cells (n = 4/6; Fig. 2B). AP failures occurred at an average rate of 0.43 ± 0.15 Hz during a 10-s test period, whereas in control no AP failures were recorded (Fig. 2A). Resting membrane potential (Ctrl, −73.7 ± 1.7; carvedilol, −76.4 ± 3.2 mV, n = 6, P = 0.11) was similar in control and after drug exposure. Typical APs in control (black), during carvedilol treatment (gray), as well as an AP failure (dashed), are shown in Fig. 2C. After reaching steady state, AP duration (APD) was determined at 30, 50, and 90% repolarization (APD30, APD50, and APD90, respectively) using the average of 10 consecutive APs (no failures included). Carvedilol treatment resulted in APD shortening [APD30 decreased by 21 ± 19% (P = 0.19, n = 6); APD50 by 46 ± 18% (P = 0.04), and APD90 by 24 ± 6% (P = 0.04)]. Summary of the AP shortening is shown in Fig. 2D.
Fig. 2.
Action potential (AP) failure by carvedilol. A: representative AP traces in control (top) and after carvedilol (1 µM) exposure (bottom). APs were recorded in current-clamp mode and evoked by 5-ms stimulation pulses of a magnitude ∼1.5 times higher than AP activation threshold. B: summary of AP failure in percentage of cells (left) and AP failure frequency (right) after carvedilol treatment. C: representative traces of AP waveform in control (black), after carvedilol exposure (gray), and AP failure (dashed). D: average AP duration (APD) at 30, 50, and 90% repolarization (APD30, APD50, and APD90, respectively; n = 6) in control (white bars) and in presence of carvedilol (gray bars). AP failures are not included in APD calculations. *P < 0.05 (paired t-test). Vm, membrane potential; NS, not significant.
Carvedilol effects on INa and ICa.
To investigate the cause of ECC and AP failures and AP shortening, we investigated the effect of carvedilol on INa and ICa using the whole cell voltage-clamp technique. In a first set of experiments, INa and ICa were recorded during the same voltage protocol. INa and ICa were elicited by applying a two-step depolarization protocol (Fig. 3A, inset). From a holding potential of −80 mV, cells were depolarized to −50 mV for 50 ms to elicit INa, immediately followed by further depolarization to 0 mV for 350 ms to elicit ICa. The advantage of this protocol is that both currents are recorded from the same cell at essentially the same time; however, at a test potential of −50 mV, only ~60% of peak INa is activated (see below Fig. 5). The effect of carvedilol on INa and ICa was tested for two drug concentrations (1 and 10 µM).
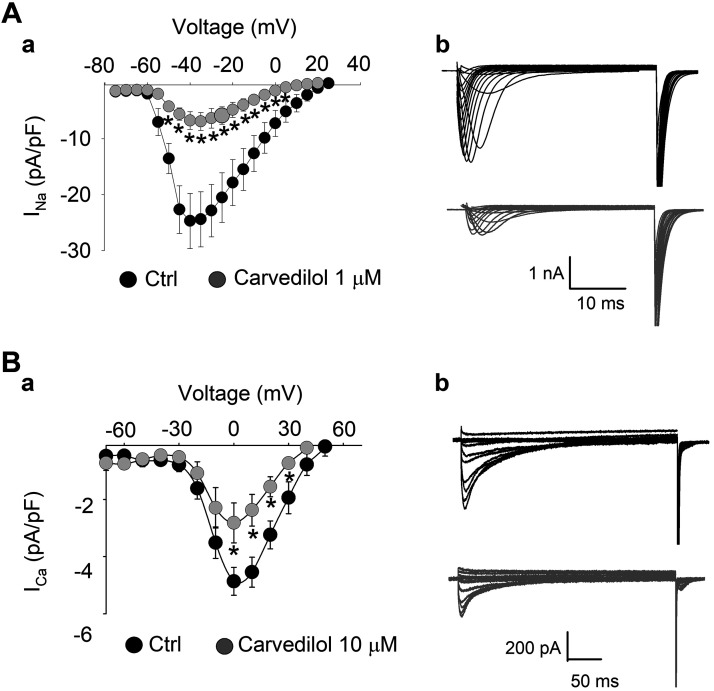
Fig. 5.
Current density-voltage (I–V) curves of voltage-gated Na+ current (INa; A) and L-type Ca2+ current (ICa; B). A,a and B,a: I–V relationships in control (black) and during carvedilol treatment (gray). Means ± SE. A,b and B,b: family of current traces at all test voltages in control (Ctrl; black) and during carvedilol treatment (gray) recorded from same cell. The following voltage-clamp protocols were used. ICa, holding potential (VH) = −80 mV; depolarization to −50 mV, followed by 350-ms depolarization steps in increments of 10 mV to a maximum depolarization of +60 mV. INa, VH = −80 mV; 30-ms voltage steps −80 to +30 mV in 5-mV increments. INa, n = 8 (carvedilol, 1 µM), *P < 0.03; ICa, n = 5 (carvedilol, 10 µM), *P < 0.04 (ANOVA).
Figure 3 shows the effect of carvedilol on INa. Representative INa traces showing the effect of 1 µM (Fig. 3A,a) and 10 µM (Fig. 3B,a) carvedilol are presented for control (a) and 1-min (b) and 3-min (c) carvedilol treatment. Carvedilol had a profound inhibitory effect on INa. Figure 3, A,b and B,b, shows the time course of the averaged, run-down corrected INa. During these extended INa and ICa recordings, we observed a substantial current rundown, especially of INa. To correct for such rundown, the same protocol was used to record INa and ICa in the absence of the drug. INa and ICa were normalized for Imax, and the averaged normalized current data from the carvedilol experiments were divided by the averaged normalized data recorded in the absence of carvedilol. Figure 3, A,c and B,c, shows average INa amplitudes in control and at 1- and 3-min exposure to carvedilol before run-down correction. With 1 µM carvedilol, INa density in control was 85.5 ± 25.8 compared with 70.3 ± 15.9 (1-min carvedilol) and 17.0 ± 6.3 pA/pF (3 min). At 10 µM, carvedilol mean control INa density was 75.8 ± 21.3 and decreased to 16.3 ± 6.4 after 1 min and 1.2 ± 0.6 pA/pF after 3 min of carvedilol exposure. While both carvedilol concentrations eventually inhibited INa almost completely, the time course of inhibition was accelerated in 10 µM carvedilol (t1/2 of maximal inhibition, 1 µM, 163 s; 10 µM, 77 s).
Figure 4 presents the effect of 1 and 10 µM carvedilol on ICa. In contrast to INa, the inhibitory effect of carvedilol on ICa was much less pronounced. At 1 µM, the average run-down corrected current was only minimally affected (Fig. 4A,b), whereas at 10 µM, an ~21% inhibition of ICa was observed after 3 min of drug exposure (Fig. 4B,b). With 1 µM carvedilol, ICa density before run-down correction (Fig. 4A,c) was 5.5 ± 1.1 pA/pF in control, compared with 5.4 ± 0.7 (1 min) and 5.2 ± 0.7 (3 min) pA/pF in the presence of the drug. At 10 µM (Fig. 4B,c), mean control ICa density was 5.1 ± 0.7 pA/pF and decreased to 3.7 ± 0.4 pA/pF after 1 min and to 3.2 ± 0.4 pA/pF after 3 min of carvedilol treatment (n = 8, P < 0.05).
Fig. 4.
Carvedilol effect on L-type Ca2+ current (ICa). A and B: effect of 1 µM (A) and 10 µM (B) on ICa. For voltage-clamp protocol, see Fig. 3. Representative ICa traces (a) and time course (b) of carvedilol effect on average peak ICa corrected and normalized for rundown. A,c and B,c: summary data for uncorrected currents in control and after 1- and 3-min carvedilol exposure. Means ± SE and individual cell data are shown (n = 8). *P < 0.02 (ANOVA). NS, not significant.
In a second set of experiments, we evaluated the I–V relationship for INa and ICa (Fig. 5). For INa, depolarization steps (30 ms) were applied from a holding potential of −80 mV in 5-mV increments to a maximum depolarization of +30 mV in the presence and absence of carvedilol (1 µM). An inhibition of INa was observed across the entire voltage range tested. Peak INa was measured at −40 mV where carvedilol decreased the current from −24.7 ± 4.9 in control to −6.7 ± 2.1 pA/pF after drug exposure (72% inhibition; Fig. 5A,a). At −50 mV, INa was −13.7 ± 2.7 pA/pF in control (56% of peak INa) and decreased by 70% to −4.2 ± 1.9 pA/pF in carvedilol. Representative traces of the INa family in control (black) and after carvedilol treatment (gray) are depicted in Fig. 5A,b. In the voltage range from −50 to +10 mV, INa inhibition was statistically significant (P < 0.05, n = 8).
The I–V relationship of ICa (Fig. 5B) was evaluated by applying a 50-ms prepulse from a holding potential of −80 to −50 mV to inactivate INa, immediately followed by depolarization steps of 350-ms duration in increments of 10 mV to a maximum depolarization of +60 mV. The I–V curves of ICa measured at peak inward current are shown in Fig. 5B,a. Carvedilol reduced the ICa amplitude without affecting the voltage dependence of ICa. On average, carvedilol (10 µM) decreased maximum ICa measured at 0 mV by 42 ± 6%. ICa was significantly reduced at 0, +10, and +20 mV (n = 5, P < 0.04). Figure 5B,b shows representative traces of the ICa family over the voltage range tested in control (black) and during carvedilol treatment (gray).
For both currents, the washout of the drug was slow, incomplete, and variable from cell to cell (data not shown). Therefore, reversibility of current inhibition by carvedilol was not further investigated.
Metoprolol effect on INa and ICa.
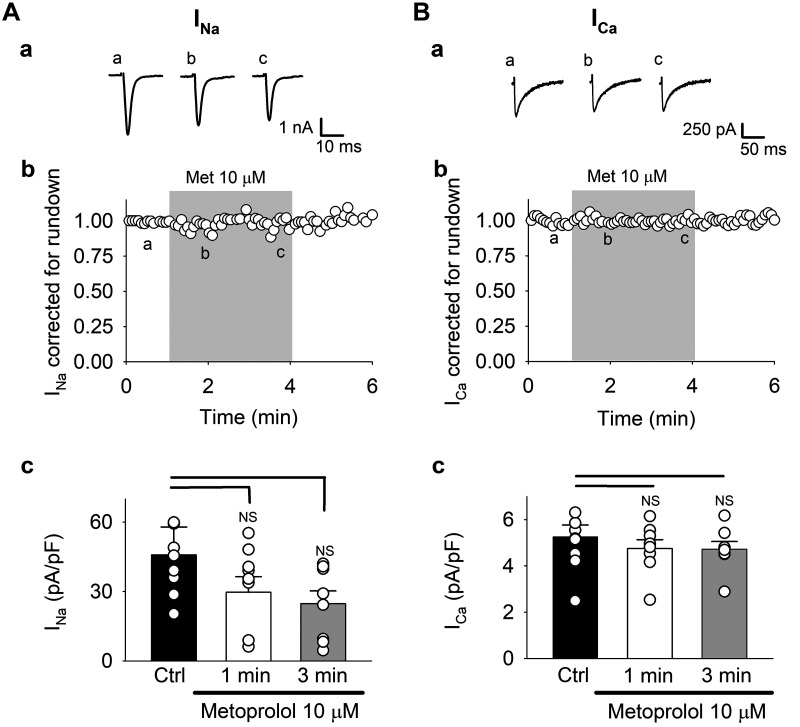
Again, we tested whether the observed carvedilol effect on ion currents were due to its β-blocking action. We applied the same two-step protocol, but using the β-blocker metoprolol (Fig. 6) instead of carvedilol. Figure 6A shows the effect on INa. Figure 6A,a shows exemplary INa traces before and after 1- and 3-min metoprolol (10 µM) exposure. The time course of run-down corrected average peak INa (Fig. 6A,b) revealed that metoprolol had no effect on INa. Figure 6A,c shows uncorrected average INa data. In this set of experiments, INa in control was 46.8 ± 12.0 and decreased to 29.7 ± 6.7 pA/pF after 1 min of metoprolol exposure and further to 24.8 ± 5.5 pA/pF after 3 min.
Fig. 6.
Metoprolol effect on voltage-gated Na+ current (INa; A) and L-type Ca2+ current (ICa; B). A,a and B,a: representative INa and ICa traces. For voltage-clamp protocol, see Fig. 3. A,b and B,b: time course of metoprolol (Met) effect on average run-down corrected currents. A,c and B,c: summary data for uncorrected currents in control (Ctrl) and after 1- and 3-min metoprolol exposure. Means ± SE and individual cell data are shown (n = 8). *P < 0.05 (ANOVA). NS, not significant.
Figure 6B shows metoprolol effects on ICa. Representative ICa traces of the metoprolol effect are shown in Fig. 6B,a. Like INa, ICa was completely unaffected by metoprolol when data were corrected for rundown (Fig. 6B,b). Uncorrected average data showed that ICa in control was 5.3 ± 0.5 and 4.7 ± 0.4 (1 min) and 4.7 ± 0.3 (3 min) pA/pF in the presence of metoprolol (Fig. 6B,c). From these experiments we concluded that the observed effects of carvedilol, in line with metoprolol effects on CaTs (Fig. 1), were not mediated by the β-blocking potency of this compound.
Effect of carvedilol on RyR function and SR Ca2+ release.
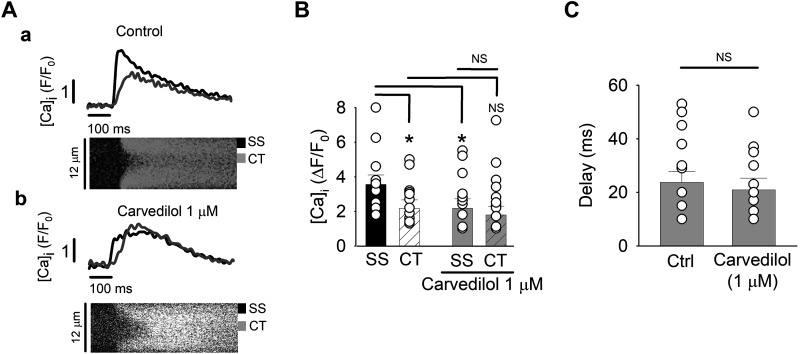
In the next set of experiments, we turned our attention to Ca2+ release from the SR. Due to the lack or only rudimentary developed TT system in atrial cells, Ca2+ release during physiological ECC is spatially and temporally inhomogeneous. Triggered by the AP, ICa induces CICR from subsarcolemmal (SS) SR Ca2+ release sites (j-SR) from where the elevated subsarcolemmal Ca2+ initiates a propagating CICR wave from nj-SR toward the center (CT) of the cell. This spatial inhomogeneity caused by the propagating Ca2+ release is visualized in transverse line scan images and has previously referred to as a U-shaped CaT (18) (Fig. 7A). Previous studies have demonstrated that carvedilol suppressed spontaneous Ca2+ waves (43, 45). Generally speaking, Ca2+ waves propagate by an analogous mechanism to the one that is in operation during physiological atrial ECC. Therefore, we explored the effect of carvedilol (1 µM) on the spatiotemporal attributes of AP-induced Ca2+ release in isolated atrial cells. Figure 7A shows SS and CT [Ca2+]i profiles (F/F0 spatially averaged over 2 µm) in the presence and absence of carvedilol. Under control conditions (Fig. 7B), SS CaT amplitude was 67% larger than CT CaTs (P = 0.04). Carvedilol (1 µM) reduced the SS CaT amplitude to the level of the CT CaT (∆F/F0, Ctrl 3.5 ± 0.6 vs. treated 2.3 ± 0.4; n = 14; P = 0.03) but did not significantly affect CT Ca2+ release (∆F/F0, Ctrl 2.1 ± 0.8 vs. treated 1.7 ± 0.5; n = 14; P = 0.15). Thus, even though 1 µM carvedilol inhibited SS Ca2+ release from the j-SR, resulting in a decreased amplitude of the averaged whole cell CaT (Fig. 1C), the local SS elevation of [Ca2+]i was sufficient to initiate robust centripetal propagation of CICR that remained largely unaffected by the drug, although there was a tendency toward at reduced CT CaT amplitude. Furthermore, carvedilol did not affect the delay between SS and CT Ca2+ release (Δt between SS half-maximum and CT half-maximum CaT amplitude, control 23.7 ± 3.1 ms; carvedilol 20.9 ± 3.1 ms; Fig. 7C), indicating that propagation velocity of activation was not affected by carvedilol (n = 14; P = 0.12).
Fig. 7.
Carvedilol effect on subcellular intracellular Ca2+ concentration ([Ca2+]i) during excitation-contraction coupling. Carvedilol (1 µM) reduces subsarcolemmal (SS) junctional sarcoplasmic reticulum (SR), but not central (CT) nonjunctional-SR Ca2+ transient amplitude. A: line scan images and corresponding [Ca2+]i profiles from peripheral (SS, black trace) and central (CT, gray trace) Ca2+ release sites in control (a) and after carvedilol treatment (b). Subcellular [Ca2+]i profiles were spatially averaged over 2 µm. B: average SS and CT Ca2+-transient amplitudes from control and carvedilol-treated atrial myocytes. *P < 0.04 (ANOVA). C: average delay between peripheral and central Ca2+ release in control (Ctrl) and carvedilol (1 µM)-treated atrial myocytes (paired t-test). Means ± SE and individual cell data are shown (n = 14). NS, not significant.
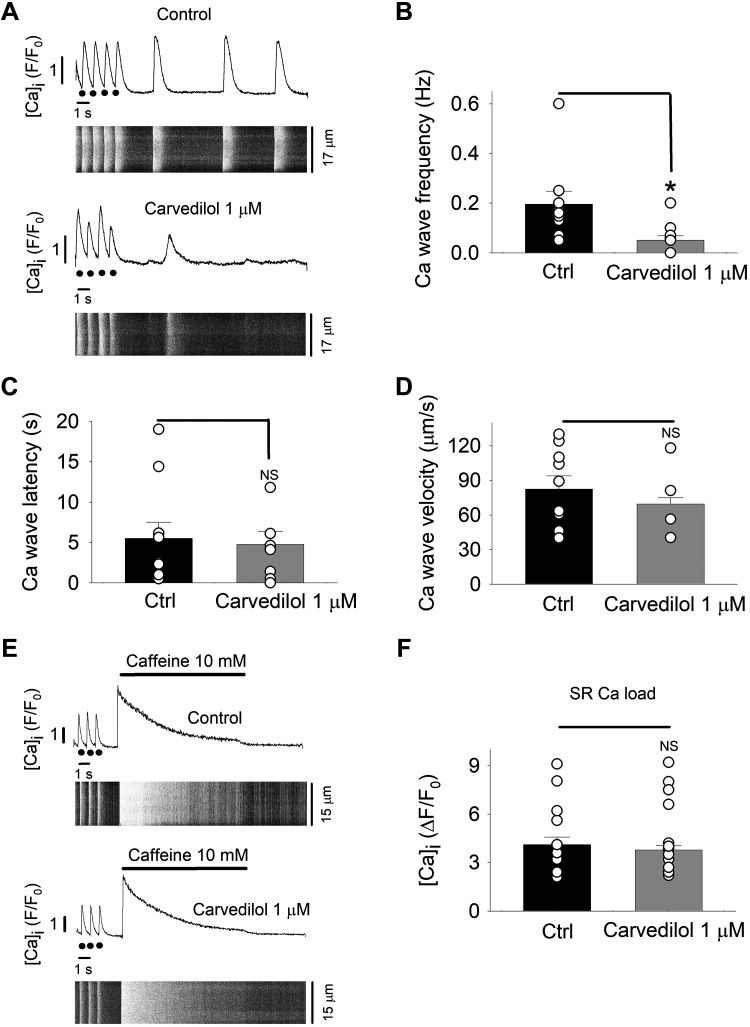
Since carvedilol is known to reduce the mean open time of the RyR Ca2+ release channel (43, 45), we explored the effect of the drug on spontaneous Ca2+ release events in the form of spontaneous CaTs and Ca2+ waves. To increase the propensity of such events, atrial myocytes were electrically paced in elevated extracellular Ca2+ (7 mM). Cells were stimulated at 1 Hz for 30 s, pacing was then stopped, and the occurrence of spontaneous activity was recorded during a 20-s interval of rest, using line-scanning confocal imaging. Representative confocal images in control and after carvedilol treatment together with their respective whole cell [Ca2+]i profiles are depicted in Fig. 8A. Carvedilol (1 µM) significantly decreased the frequency of spontaneous Ca2+ waves from 0.19 ± 0.05 in control to 0.05 ± 0.01 Hz in the presence of carvedilol (n = 10; P = 0.002; Fig. 8B). However, Ca2+ wave latency (time between the last electrically evoked CaT and the first Ca2+ wave; Fig. 8C) was not statistically different (Ctrl, 5.5 ± 2.0 s; carvedilol, 4.8 ± 1.6 s; n = 10; P = 0.8). There was a tendency toward slowing of Ca2+ wave propagation velocity in the presence of carvedilol (Fig. 8D); however, the difference was not statistically significant (Ctrl, 82.4 ± 11.5 µm/s; carvedilol 69.6 ± 5.3 µm/s; n = 10; P = 0.26).
Fig. 8.
Carvedilol (1 µM) suppresses spontaneous Ca2+ waves. Ca2+ wave frequency was quantified during a period of rest (20 s) after pacing (1 Hz) in elevated extracellular Ca2+ concentration ([Ca2+]o; 7 mM). A; whole cell [Ca2+]i profiles and confocal line scan images in control (top) and carvedilol-treated (bottom) atrial myocytes (n = 10). Black circles indicate stimulus during electrical pacing. B: average carvedilol effect on Ca2+ wave frequency. *P < 0.004 (paired t-test). C: average wave latency [Δt between last electrically elicited Ca2+-transient (CaT) and first Ca2+ wave] in control (Ctrl) and carvedilol-treated atrial myocytes (unpaired t-test). D: average Ca2+ wave velocity in control and carvedilol-treated atrial myocytes (unpaired t-test). E: carvedilol effect on sarcoplasmic reticulum (SR) Ca2+ load. SR load was quantified as the amplitude of a CaT elicited by rapid superfusion with caffeine (10 mM) in control (top) and in the presence of carvedilol (1 µM; bottom). Whole cell intracellular Ca2+ concentration ([Ca2+]i) profiles and corresponding line scan images are shown. F: summary of CaT amplitudes in response to 10 mM caffeine (n = 11; paired t-test). NS, not significant.
Furthermore, we tested for the possibility that the decreased Ca2+ wave frequency resulted from a reduced SR Ca2+ load. For this purpose, atrial cells were rapidly exposed to caffeine (10 mM; Fig. 8E), and the amplitude of the caffeine-induced CaT served as a measure of SR Ca2+ load (Fig. 8F). Carvedilol did not significantly affect releasable SR Ca2+ content (∆F/F0, Ctrl 4.1 ± 0.6; caffeine 3.8 ± 0.7; n = 11; P = 0.15). The significant reduction of the propensity of spontaneous Ca2+ waves by carvedilol in the absence of a difference in SR Ca2+ load suggests a direct action of carvedilol on the RyR Ca2+ release channel and demonstrates an antiarrhythmic effect of carvedilol in atrial myocytes.
DISCUSSION
In this study we investigated the effect of the clinically widely used β-blocker carvedilol on SR Ca2+ release and ECC in rabbit atrial myocytes. The main findings are as follows: 1) carvedilol causes failure of SR Ca2+ release and ECC in a dose-dependent manner; 2) the primary cause of ECC failure by carvedilol is a dose-dependent inhibition of voltage-gated INa and ICa; 3) at low carvedilol concentration (1 µM), the decrease of the whole cell CaT amplitude by over 55% results from a specific inhibition of subsarcolemmal CICR from j-SR presumably due to INa and ICa inhibition and AP shortening, while propagating CICR from nj-SR remains largely unaffected; and 4) the frequency of spontaneous arrhythmogenic Ca2+ waves is significantly reduced despite identical SR Ca2+ load, suggesting a direct effect of carvedilol on RyR function.
Carvedilol is a nonspecific β-blocker clinically used for the treatment of cardiovascular diseases, including ventricular tachyarrhythmia and atrial fibrillation (16, 19, 26, 32), has vasodilatory effects, and has proven to reduce hospitalization and mortality of patients with congestive heart failure (29). Most clinical benefits stem from the β-blocker drug action (15); however, carvedilol is not entirely specific to β-adrenoceptors. It also has α-adrenoreceptor blocking effects (31), can act as an antioxidant (14, 42), and has antiproliferative properties in vascular smooth muscle (28).
At the cellular level we observed a dose-dependent failure of AP-induced SR Ca2+ release in atrial myocytes (Fig. 1), reminiscent of a previously reported reversible loss of responsiveness to field stimulation in ventricular myocytes (40). We demonstrate that carvedilol-induced ECC failure is the result of inhibition of INa and ICa (Figs. 3, 4 and 5), resulting in AP failure (Fig. 2, A and B) and shortening (Fig. 2C). An inhibitory effect of carvedilol on several membrane currents in cardiac cells (ventricle and pacemaker, but not in the atrial myocardium) has been described before. Inhibition of voltage-gated ICa (11, 12, 27, 40, 41), INa (1, 2), K+ currents (IK) (11, 22), pacemaker current If (41), and NCX1 current (INCX1) (37) have been reported. For example, carvedilol was found to inhibit LTCCs in vascular smooth muscle (27) and ventricular cells (11) by 64 and 43%, respectively, compared with our observation that 10 µM carvedilol inhibited ICa by 21%. Carvedilol (1 μM) disrupted ECC in 14% of cells (Fig. 1B) and reduced the whole cell CaT amplitude by 55% (Fig. 1C) but apparently failed to significantly inhibit ICa (Fig. 5F) in voltage-clamp experiments when the cell membrane was depolarized with rectangular depolarization pulses. However, AP recordings revealed that at 1 µM carvedilol, APD was already shortened in the voltage range where ICa is maximally activated. In other words, under physiological conditions when ion channel activation is dictated by the AP, the data are consistent with an ICa inhibition already at 1 µM. In other studies, 1 µM carvedilol blocked ICa in ventricular myocytes by ∼10%, but by ~50% with 10 µM carvedilol (40), whereas in pacemaker cells (sinoatrial and atrioventricular node cells), the degree of inhibition of ICa was higher (45–50%) and the spontaneous firing frequency was reduced (41). Carvedilol (10 μM) had no effect on ICa voltage dependence in atrial myocytes (Fig. 5), similar to the results obtained in vascular smooth cells (27), but different from pacemaker cells, where carvedilol shifted the voltage dependence by 10 mV toward more positive values (41). A dose-dependent INa inhibition by carvedilol has been reported using a heterologous expression system (HEK-293 cells) for NaV1.5 and NaV1.6 channels (1, 2) and in cultured bovine adrenal cells (21). It has been reported that half maximal inhibitory concentration for INa and ICa inhibition by carvedilol is rather similar [3.59 and 3.83 µM, respectively (1, 11, 27)]. In our hands, however, inhibition of INa for any given concentration tested was much more pronounced than the effect on ICa. Carvedilol plasma levels for therapeutic doses of the drug have been reported to be in the range of 0.1 to 0.6 µM (23, 38, 41), somewhat lower than the concentration (1 µM) where we started to see an inhibitory action on ECC. Specifically, at 0.5 µM we did not see an acute effect after 3 min of drug exposure; however, we found ECC disruption after 15 min of treatment (data not shown). The reason for this discrepancy between in vitro and in vivo data is unclear. The profound current inhibition in vitro, especially of INa at the 1 µM concentration, might put doubts on the clinical usefulness of this drug and appears at odds with the fact that it is widely used clinically and is considered rather safe. Carvedilol is highly lipophilic, and the drug concentration at the target might differ from plasma levels. Furthermore, the INa inhibitory action could be advantageous for the use of carvedilol as an antiarrhythmic drug since Na+ channel blockers are clinically used for this purpose.
Since carvedilol has well-documented diverse effects, we tested whether the observed inhibition of INa and ICa was due to the β-blocking effect of the drug. For that purpose, we tested the specific β-blocker metoprolol. In contrast to carvedilol, metoprolol (10 μM) had no effect on CaT amplitude (Fig. 1) and on INa and ICa (Fig. 6). This result implies that carvedilol inhibition of INa and ICa is not due to the β-blocker action, consistent with previous studies on INa (3, 5, 39) and ICa where significantly higher (30 µM) drug concentrations were required to elicit an inhibitory effect (3, 33). These data suggest that carvedilol acts directly on the ion channels involved in ECC, either the relevant voltage-gated surface membrane channels or the SR Ca2+ release channel (RyR).
Previous studies have tested the effect of β-blockers on RyR function. Zhou et al. (45) tested over a dozen β-blockers for effects on RyR-mediated Ca2+ release. With the exception of carvedilol, none of the blockers tested had any effect on spontaneous Ca2+ release events (Ca2+ waves). Carvedilol, however, was capable of significantly suppressing spontaneous SR Ca2+ release. The suppression of Ca2+ release could be pinpointed to a significant reduction of the mean open time of the RyR Ca2+ release channel. The reduction in mean open time as the key mechanism underlying the action of carvedilol was further confirmed by the observation that the non-β-blocking R-enantiomer of carvedilol (43) and the compound VK-II-86 (45), which also reduces RyR mean open time, both suppressed spontaneous Ca2+ waves. Presumably through its ability to curtail RyR activity, carvedilol can facilitate Ca2+ alternans in hearts harboring a suppression-of-function RyR mutation (44). Furthermore, when compared with other β-blockers (e.g., metoprolol), carvedilol had a higher antiarrhythmic effect due to the combination of β-blocking and RyR-modulating actions (45). In addition, the INa inhibition observed here adds another facet of antiarrhythmic action of carvedilol since Na+ channel blockers are therapeutically used for treatment of supraventricular arrhythmias, including atrial fibrillation.
A key finding in our study was the observation that in adult atrial myocytes, carvedilol also suppressed spontaneous Ca2+ waves (Fig. 8) by decreasing their frequency by ~75%. SR Ca2+ overload (17) accompanies certain pathological conditions and ultimately generates Ca2+ waves (30). Such spontaneous Ca2+ waves can become arrhythmogenic because they induce a depolarizing membrane current (via Na+/Ca2+ exchange) that can result in arrhythmia and contractile dysfunction (4, 10). As shown here, carvedilol has an antiarrhythmic effect in atrial cells by significantly reducing the probability of Ca2+ waves. Remarkably, the reduction in Ca2+ wave frequency could not be explained by a decreased SR Ca2+ load, since SR Ca2+ content was the same under both conditions (Fig. 8F), suggesting again that the suppression of spontaneous SR Ca2+ release is a consequence of a direct action of carvedilol on the RyR channel.
As detailed in introduction, the mechanism of atrial Ca2+ release and ECC differs significantly from ventricular cells due to the absence or paucity of the TT system. Once activated by an AP, CICR propagates through the nj-SR network independent of ICa (18, 34), only sustained by the Ca2+ gradients established by intracellular SR Ca2+ release. Thus, the activation of the majority of Ca2+ release sites in atrial cells reside in the membrane of the nj-SR and are activated in a propagating Ca2+ wave fashion. We observed that carvedilol reduced the whole cell CaT amplitude in a dose-dependent manner (Fig. 1). Confocal Ca2+ imaging studies revealed (Fig. 7) that carvedilol primarily reduced the peripheral CaT, i.e., release from j-SR. This is consistent with the notion that the trigger for CICR from j-SR release site is Ca2+ influx through LTCCs, which is reduced by carvedilol. We propose, however, that the elevation of [Ca2+]i in the subsarcolemmal space in the presence of 1 µM carvedilol is still high enough to induce CICR from a nearby nj-SR release site [note that the diffusional distance between j-SR and the most peripheral array of nj-SR release site is only 1 to 2 µm (6)] from where CICR continues to propagate in centripetal direction. Overall, we found a slight reduction in central CaT amplitude (Fig. 7B), but no impediment of CICR propagation velocity (Fig. 7C). In an earlier study we showed that in atrial myocytes, ICa and j-SR CaT amplitudes have the well-known, bell-shaped membrane potential dependence (34). In contrast, the relationship between membrane potential and CT nj-SR CaT amplitude is relatively flat, i.e., the atrial ECC machinery is highly reliable in that there is a substantial degree of safety inherent to the system which allows a wide range of variability of peripheral j-SR Ca2+ release without jeopardizing nj-SR Ca2+ release and CICR propagation. The observation that Ca2+ wave propagation velocity is not affected by carvedilol once waves have been triggered is consistent with this hypothesis. The inhibitory carvedilol effect on the RyR, however, renders luminal RyR activation by luminal Ca2+ less effective and explains the reduction of spontaneous Ca2+ wave frequency.
In summary, as shown here for the first time in atrial cells, carvedilol inhibited INa and ICa in a concentration-dependent manner, resulting in CaTs of reduced amplitude and ultimately failure of APs and SR Ca2+ release during ECC. The effect of carvedilol on electrically evoked Ca2+ signals was subcellularly heterogenous where the drug preferentially affected peripheral Ca2+ realese from the j-SR, whereas propagating SR Ca2+ release from nj-SR, once initiated, was not further affected. Moreover, carvedilol had a prominent inhibitory effect on arrhythmogenic Ca2+ waves, an effect that was not associated with differences in SR Ca2+ load and is consistent with the known effect of reducing the mean open time of the RyR. These cellular findings are in line with the clinical finding of carvedilol's efficiency of suppressing cardiac arrhythmias, including atrial fibrillation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-057832, HL-132871, and HL-134781.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.M.-H. and L.A.B. conceived and designed research; E.M.-H. performed experiments; E.M.-H. and L.A.B. analyzed data; E.M.-H. and L.A.B. interpreted results of experiments; E.M.-H. and L.A.B. prepared figures; E.M.-H. and L.A.B. drafted manuscript; E.M.-H. and L.A.B. edited and revised manuscript; E.M.-H. and L.A.B. approved final version of manuscript.
REFERENCES
- 1.Atkin TA, Maher CM, Gerlach AC, Gay BC, Antonio BM, Santos SC, Padilla KM, Rader J, Krafte DS, Fox MA, Stewart GR, Petrovski S, Devinsky O, Might M, Petrou S, Goldstein DB. A comprehensive approach to identifying repurposed drugs to treat SCN8A epilepsy. Epilepsia 59: 802–813, 2018. doi: 10.1111/epi.14037. [DOI] [PubMed] [Google Scholar]
- 2.Bankston JR, Kass RS. Molecular determinants of local anesthetic action of beta-blocking drugs: implications for therapeutic management of long QT syndrome variant 3. J Mol Cell Cardiol 48: 246–253, 2010. doi: 10.1016/j.yjmcc.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrington PL, Ten Eick RE. Characterization of the electrophysiological effects of metoprolol on isolated feline ventricular myocytes. J Pharmacol Exp Ther 252: 1043–1052, 1990. [PubMed] [Google Scholar]
- 4.Berlin JR, Cannell MB, Lederer WJ. Cellular origins of the transient inward current in cardiac myocytes. Role of fluctuations and waves of elevated intracellular calcium. Circ Res 65: 115–126, 1989. doi: 10.1161/01.RES.65.1.115. [DOI] [PubMed] [Google Scholar]
- 5.Besana A, Wang DW, George AL Jr, Schwartz PJ. Nadolol block of Nav1.5 does not explain its efficacy in the long QT syndrome. J Cardiovasc Pharmacol 59: 249–253, 2012. doi: 10.1097/FJC.0b013e31823d2fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blatter LA. The intricacies of atrial calcium cycling during excitation-contraction coupling. J Gen Physiol 149: 857–865, 2017. doi: 10.1085/jgp.201711809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blatter LA, Kockskämper J, Sheehan KA, Zima AV, Hüser J, Lipsius SL. Local calcium gradients during excitation-contraction coupling and alternans in atrial myocytes. J Physiol 546: 19–31, 2003. doi: 10.1113/jphysiol.2002.025239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodi I, Mikala G, Koch SE, Akhter SA, Schwartz A. The L-type calcium channel in the heart: the beat goes on. J Clin Invest 115: 3306–3317, 2005. doi: 10.1172/JCI27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandenburg S, Kohl T, Williams GS, Gusev K, Wagner E, Rog-Zielinska EA, Hebisch E, Dura M, Didié M, Gotthardt M, Nikolaev VO, Hasenfuss G, Kohl P, Ward CW, Lederer WJ, Lehnart SE. Axial tubule junctions control rapid calcium signaling in atria. J Clin Invest 126: 3999–4015, 2016. doi: 10.1172/JCI88241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng H, Lederer MR, Lederer WJ, Cannell MB. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol 270: C148–C159, 1996. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- 11.Cheng J, Niwa R, Kamiya K, Toyama J, Kodama I. Carvedilol blocks the repolarizing K+ currents and the L-type Ca2+ current in rabbit ventricular myocytes. Eur J Pharmacol 376: 189–201, 1999. doi: 10.1016/S0014-2999(99)00368-4. [DOI] [PubMed] [Google Scholar]
- 12.Deng C, Rao F, Wu S, Kuang S, Liu X, Zhou Z, Shan Z, Lin Q, Qian W, Yang M, Geng Q, Zhang Y, Yu X, Lin S. Pharmacological effects of carvedilol on T-type calcium current in murine HL-1 cells. Eur J Pharmacol 621: 19–25, 2009. doi: 10.1016/j.ejphar.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 13.Denham NC, Pearman CM, Caldwell JL, Madders GW, Eisner DA, Trafford AW, Dibb KM. Calcium in the pathophysiology of atrial fibrillation and heart failure. Front Physiol 9: 1380, 2018. doi: 10.3389/fphys.2018.01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diogo CV, Deus CM, Lebiedzinska-Arciszewska M, Wojtala A, Wieckowski MR, Oliveira PJ. Carvedilol and antioxidant proteins in a type I diabetes animal model. Eur J Clin Invest 47: 19–29, 2017. doi: 10.1111/eci.12696. [DOI] [PubMed] [Google Scholar]
- 15.Foody JM, Farrell MH, Krumholz HM. β-Blocker therapy in heart failure: scientific review. JAMA 287: 883–889, 2002. doi: 10.1001/jama.287.7.883. [DOI] [PubMed] [Google Scholar]
- 16.Gheorghiade M, Robbins JD, Lukas MA. Role of carvedilol in atrial fibrillation: insights from clinical trials. Am J Cardiol 93, Suppl 1: 53–57, 2004. doi: 10.1016/j.amjcard.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 17.Hove-Madsen L, Llach A, Bayes-Genís A, Roura S, Rodriguez Font E, Arís A, Cinca J. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation 110: 1358–1363, 2004. doi: 10.1161/01.CIR.0000141296.59876.87. [DOI] [PubMed] [Google Scholar]
- 18.Hüser J, Lipsius SL, Blatter LA. Calcium gradients during excitation-contraction coupling in cat atrial myocytes. J Physiol 494: 641–651, 1996. doi: 10.1113/jphysiol.1996.sp021521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue H, Atarashi H, Okumura K, Yamashita T, Fukuzawa M, Shiosakai K, Kimura T. Heart rate control by carvedilol in Japanese patients with chronic atrial fibrillation: The AF Carvedilol study. J Cardiol 69: 293–301, 2017. doi: 10.1016/j.jjcc.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi P, Zhang L, Chen SR. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ Res 97: 1173–1181, 2005. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- 21.Kajiwara K, Yanagita T, Nakashima Y, Wada A, Izumi F, Yanagihara N. Differential effects of short and prolonged exposure to carvedilol on voltage-dependent Na(+) channels in cultured bovine adrenal medullary cells. J Pharmacol Exp Ther 302: 212–218, 2002. doi: 10.1124/jpet.302.1.212. [DOI] [PubMed] [Google Scholar]
- 22.Karle CA, Kreye VA, Thomas D, Röckl K, Kathöfer S, Zhang W, Kiehn J. Antiarrhythmic drug carvedilol inhibits HERG potassium channels. Cardiovasc Res 49: 361–370, 2001. doi: 10.1016/S0008-6363(00)00265-0. [DOI] [PubMed] [Google Scholar]
- 23.Kim YH, Choi HY, Noh YH, Lee SH, Lim HS, Kim C, Bae KS. Dose proportionality and pharmacokinetics of carvedilol sustained-release formulation: a single dose-ascending 10-sequence incomplete block study. Drug Des Devel Ther 9: 2911–2918, 2015. doi: 10.2147/DDDT.S86168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lou Q, Belevych AE, Radwański PB, Liu B, Kalyanasundaram A, Knollmann BC, Fedorov VV, Györke S. Alternating membrane potential/calcium interplay underlies repetitive focal activity in a genetic model of calcium-dependent atrial arrhythmias. J Physiol 593: 1443–1458, 2015. doi: 10.1113/jphysiol.2014.280784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maxwell JT, Blatter LA. Facilitation of cytosolic calcium wave propagation by local calcium uptake into the sarcoplasmic reticulum in cardiac myocytes. J Physiol 590: 6037–6045, 2012. doi: 10.1113/jphysiol.2012.239434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naccarelli GV. Does carvedilol have antiarrhythmic properties? Nat Clin Pract Cardiovasc Med 2: 338–339, 2005. doi: 10.1038/ncpcardio0245. [DOI] [PubMed] [Google Scholar]
- 27.Nakajima T, Ma J, Iida H, Iwasawa K, Jo T, Omata M, Nagai R. Inhibitory effects of carvedilol on calcium channels in vascular smooth muscle cells. Jpn Heart J 44: 963–978, 2003. doi: 10.1536/jhj.44.963. [DOI] [PubMed] [Google Scholar]
- 28.Ohlstein EH, Douglas SA, Sung CP, Yue TL, Louden C, Arleth A, Poste G, Ruffolo RR Jr, Feuerstein GZ. Carvedilol, a cardiovascular drug, prevents vascular smooth muscle cell proliferation, migration, and neointimal formation following vascular injury. Proc Natl Acad Sci USA 90: 6189–6193, 1993. doi: 10.1073/pnas.90.13.6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH; U.S. Carvedilol Heart Failure Study Group . The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 334: 1349–1355, 1996. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 30.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res 108: 871–883, 2011. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruffolo RR., Jr Fundamentals of receptor theory: basics for shock research. Circ Shock 37: 176–184, 1992. [PubMed] [Google Scholar]
- 32.Ruwald AC, Gislason GH, Vinther M, Johansen JB, Nielsen JC, Philbert BT, Torp-Pedersen C, Riahi S, Jøns C. Importance of beta-blocker dose in prevention of ventricular tachyarrhythmias, heart failure hospitalizations, and death in primary prevention implantable cardioverter-defibrillator recipients: a Danish nationwide cohort study. Europace 20: f217–f224, 2018. doi: 10.1093/europace/euy077. [DOI] [PubMed] [Google Scholar]
- 33.Sánchez-Chapula J. Effects of metoprolol on action potential and membrane currents in guinea-pig ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol 345: 342–348, 1992. doi: 10.1007/BF00168696. [DOI] [PubMed] [Google Scholar]
- 34.Sheehan KA, Blatter LA. Regulation of junctional and non-junctional sarcoplasmic reticulum calcium release in excitation-contraction coupling in cat atrial myocytes. J Physiol 546: 119–135, 2003. doi: 10.1113/jphysiol.2002.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stuyvers BD, Boyden PA, ter Keurs HE. Calcium waves: physiological relevance in cardiac function. Circ Res 86: 1016–1018, 2000. doi: 10.1161/01.RES.86.10.1016. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka H, Kawanishi T, Shigenobu K. Optical bioimaging: from living tissue to a single molecule, atrio-ventricular difference in myocardial excitation-contraction coupling--sequential versus simultaneous activation of SR Ca2+ release units. J Pharmacol Sci 93: 248–252, 2003. doi: 10.1254/jphs.93.248. [DOI] [PubMed] [Google Scholar]
- 37.Tashiro M, Watanabe Y, Yamakawa T, Yamashita K, Kita S, Iwamoto T, Kimura J. Suppressive effect of carvedilol on Na+/Ca2+ exchange current in isolated guinea-pig cardiac ventricular myocytes. Pharmacology 99: 40–47, 2017. doi: 10.1159/000450753. [DOI] [PubMed] [Google Scholar]
- 38.Tenero D, Boike S, Boyle D, Ilson B, Fesniak HF, Brozena S, Jorkasky D. Steady-state pharmacokinetics of carvedilol and its enantiomers in patients with congestive heart failure. J Clin Pharmacol 40: 844–853, 2000. doi: 10.1177/00912700022009576. [DOI] [PubMed] [Google Scholar]
- 39.Wang DW, Mistry AM, Kahlig KM, Kearney JA, Xiang J, George AL Jr. Propranolol blocks cardiac and neuronal voltage-gated sodium channels. Front Pharmacol 1: 144, 2010. doi: 10.3389/fphar.2010.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao A, Kohmoto O, Oyama T, Sugishita Y, Shimizu T, Harada K, Matsui H, Komuro I, Nagai R, Matsuo H, Serizawa T, Maruyama T, Takahashi T. Characteristic effects of alpha1-beta1,2-adrenergic blocking agent, carvedilol, on [Ca2+]i in ventricular myocytes compared with those of timolol and atenolol. Circ J 67: 83–90, 2003. doi: 10.1253/circj.67.83. [DOI] [PubMed] [Google Scholar]
- 41.Yokoyama A, Sato N, Kawamura Y, Hasebe N, Kikuchi K. Electrophysiological effects of carvedilol on rabbit heart pacemaker cells. Int Heart J 48: 347–358, 2007. doi: 10.1536/ihj.48.347. [DOI] [PubMed] [Google Scholar]
- 42.Yue TL, Cheng HY, Lysko PG, McKenna PJ, Feuerstein R, Gu JL, Lysko KA, Davis LL, Feuerstein G. Carvedilol, a new vasodilator and beta adrenoceptor antagonist, is an antioxidant and free radical scavenger. J Pharmacol Exp Ther 263: 92–98, 1992. [PubMed] [Google Scholar]
- 43.Zhang J, Zhou Q, Smith CD, Chen H, Tan Z, Chen B, Nani A, Wu G, Song LS, Fill M, Back TG, Chen SR. Non-β-blocking R-carvedilol enantiomer suppresses Ca2+ waves and stress-induced ventricular tachyarrhythmia without lowering heart rate or blood pressure. Biochem J 470: 233–242, 2015. doi: 10.1042/BJ20150548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhong X, Sun B, Vallmitjana A, Mi T, Guo W, Ni M, Wang R, Guo A, Duff HJ, Gillis AM, Song LS, Hove-Madsen L, Benitez R, Chen SR. Suppression of ryanodine receptor function prolongs Ca2+ release refractoriness and promotes cardiac alternans in intact hearts. Biochem J 473: 3951–3964, 2016. doi: 10.1042/BCJ20160606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Q, Xiao J, Jiang D, Wang R, Vembaiyan K, Wang A, Smith CD, Xie C, Chen W, Zhang J, Tian X, Jones PP, Zhong X, Guo A, Chen H, Zhang L, Zhu W, Yang D, Li X, Chen J, Gillis AM, Duff HJ, Cheng H, Feldman AM, Song LS, Fill M, Back TG, Chen SR. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca2+ release. Nat Med 17: 1003–1009, 2011. doi: 10.1038/nm.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]