Abstract
Purpose:
Recent trends in payer and patient preferences increasingly incentivize time-efficient (≤2-week treatment time) prostate cancer treatments.
Methods and Materials:
National Medicare claims from January 1, 2011, through December 31, 2014, were analyzed to identify newly diagnosed prostate cancers. Three “radical treatment” cohorts were identified (prostatectomy, brachytherapy, and stereotactic body radiation therapy [SBRT]) and matched to an active surveillance (AS) cohort by using inverse probability treatment weighting via propensity score. Total costs at 1 year after biopsy were calculated for each cohort, and treatment-specific costs were estimated by subtracting total 1-year costs in each radical treatment group from those in the AS group.
Results:
Mean 1-year adjusted costs were highest among patients receiving SBRT ($26,895), lower for prostatectomy ($23,632), and lowest for brachytherapy ($19,980), whereas those for AS were $9687. Costs of radical modalities varied significantly by region, with the Mid-Atlantic and New England regions having the highest cost ranges (>$10,000) and the West South Central and Mountain regions the lowest range in costs (<$2000). Quantification of toxic effects showed that prostatectomy was associated with higher genitourinary incontinence (hazard ratio [HR] = 10.8 compared with AS) and sexual dysfunction (HR = 3.5), whereas the radiation modalities were associated with higher genitourinary irritation/bleeding (brachytherapy HR = 1.7; SBRT HR = 1.5) and gastrointestinal ulcer/stricture/fistula (brachytherapy HR = 2.7; SBRT HR = 3.0). Overall mean toxicity costs were highest among patients treated with prostatectomy ($3500) followed by brachytherapy ($1847), SBRT ($1327), and AS ($1303).
Conclusions:
Time-efficient treatment techniques exhibit substantial variability in toxicity and costs. Furthermore, geographic location substantially influenced treatment costs.
Introduction
Prostate cancer is the most common malignancy and second leading cause of cancer-related death among men in the United States.1 With an aging population and improved treatments for cardiac disease and stroke, the mortality, morbidity, and associated costs of prostate cancer are expected to increase.2 Recent advances in treatment delivery, surgical techniques, and use of ancillary devices (eg, SpaceOAR) have contributed to increased acceptance of new time-efficient radiation techniques, specifically stereotactic body radiation therapy (SBRT) and high-dose-rate (HDR) brachytherapy, and improvements in the more established low-dose-rate (LDR) brachytherapy and prostatectomy.3-5 These time-efficient radiation modalities, which facilitate treatment completion in ≤2 weeks, provide a menu of expedient, effective, and cost-effective treatment options. In contrast, timeintensive treatments (eg, intensity modulated radiation therapy [IMRT] and proton therapy) are known to be more costly than time-efficient techniques; however, few data have compared time-efficient techniques.6-10
Understanding the landscape of nationwide costs for these time-efficient treatment strategies in the current fee-for-service environment is crucial for informing the transition to value-based payments, as exemplified by the recently announced Radiation Oncology—Alternative Payment Model (RO-APM).11 Furthermore, the unique technical expertise, equipment, and infrastructure requirements for each time-efficient technique mean that individual institutions often preferentially develop proficiency in only a subset of these techniques. The divergent evolution of technical expertise has often created strong institutional preferences that make randomized comparisons difficult. We therefore sought to analyze costs and adverse events after an initial diagnosis of prostate cancer, with a focus on time-efficient treatments. The goal of this study was to leverage the scope of national Medicare data to comprehensively compare these time-efficient techniques.
Methods and Materials
Analysis population
We analyzed national Medicare claims data from January 1, 2011, through December 31, 2014, with the inclusion criterion of an associated prostate biopsy from 2012 to 2013, to allow ≥12 months of claims before and after diagnosis. During the 12 months before the initial prostate biopsy, patients must not have had any cancerassociated claim. The date of this first biopsy was considered to be the date of initial diagnosis. This algorithm to identify the initial date of prostate cancer diagnosis has been validated with the Surveillance, Epidemiology, and End Results (SEER) database and found to exhibit 99.8% specificity.12,13
Additional exclusion criteria were age <66 years at the time of first biopsy, death within 1 year after the initial diagnosis, and continuous enrollment in Medicare parts A and B (Table E1). This study was reviewed and found to be exempt by the appropriate institutional review board.
Defining treatment groups
Patients were grouped according to primary treatment: (1) active surveillance (AS), (2) prostatectomy, (3) brachytherapy monotherapy, and (4) SBRT. Prostatectomy, brachytherapy, and SBRT were considered “radical treatment” groups.
Patients were included in the AS group if they had no prior androgen deprivation therapy or definitive cancer treatment in the year after initial diagnosis. This rubric for identifying AS was found to have the highest sensitivity among a number of algorithms in a Medicare cohort (sensitivity of 88.2% and specificity of 93.5%).12 Patients were included in the prostatectomy group if they had received definitive prostatectomy, regardless of approach. Patients were included in the brachytherapy cohort if they had received LDR or HDR brachytherapy. Patients were included in the SBRT cohort if they had received ≥3 fractions of SBRT within 30 days of starting radiation. The diagnostic and treatment codes used to define treatment cohorts are presented in Table E2.
Defining patient variables and outcomes
Adverse events were defined a priori by using a set of established International Classification of Diseases, Ninth Revision, Clinical Modification diagnostic and procedure codes based on expert opinion and the published literature (Table E3).7-9,14-16 Total costs were calculated from all Medicare claims regardless of association and adjusted to 2015 US dollars.17 Medicare claims analyzed include inpatient, outpatient, and carrier claims charges and did not include Part D drug costs. Prostatectomy costs included all prostatectomy patients in aggregate and were not separately analyzed based on different types of prostatectomy (eg, robotic, laparoscopic, open). Claims filed within 1 year before initial diagnosis were used to calculate a baseline Charlson comorbidity score.18 Toxicity-associated costs were defined as all costs incurred on days toxic effects were coded.
Statistical analysis
The inverse probability treatment weighted method was used to adjust for potential selection bias. Three separate logistic regression models were used to adjust the probability of selecting each radical treatment group against the AS group.19 By using the propensity score (PS), patients in the radical treatment groups were assigned weights of PS / (1 — PS), whereas patients in the AS group were assigned a weight of 1.19 The following covariates were used to calculate the PS: year of biopsy, age, race, Charlson score, region, state buy-in, county radiation oncologist density, county median income, and baseline toxic effects.
For all cost analyses, a generalized linear model was used to estimate costs, comparing each radical treatment group against the AS group. To assess toxicity burden, multivariate Cox proportional hazards models were implemented to evaluate associations between treatment group and time-to-toxicity, with the AS group used as the reference group. Proportional hazards assumptions were confirmed by inspection of the log(−log[survival]) curves. To assess early toxicity, administrative censoring was implemented at 12 months. To assess late toxicity, only those events that occurred after 12 months were considered. For each radical treatment group, hazard ratios (HR) for toxicity incidence were referenced to those for the AS group. Cell sizes of fewer than 11 patients were suppressed as stipulated by the Centers for Medicare and Medicaid Services privacy policy. Statistical analyses were done with SAS version 9.3 (SAS Institute, Cary, NC), and R version 3.5.1.
Results
Patient characteristics
A total of 24,843 men met the study criteria, of whom 11,877 (48%) were in the AS group, 9509 (38%) in the prostatectomy group, 2679 (11%) in the brachytherapy group, and 778 (3%) in the SBRT group. Within the brachytherapy group, 2479 (93%) patients received LDR and 153 (7%) received HDR with a median of 2 fractions (interquartile range, 2-3). The median number of SBRT fractions was 5 (interquartile range, 5-5). The median follow-up time from initial diagnosis was 25 months (range, 12-36 months; Table 1). All unadjusted comparisons were significant (all P < .001); however, after adjustment, all variables achieved balance, with standardized difference ≤10%.
Table 1.
Baseline patient characteristics with adjusted and unadjusted proportions
| Characteristics | Active surveillance N = 11,877 |
Prostatectomy N = 9509 |
Brachytherapy N = 2679 |
SBRT N = 778 |
||||
|---|---|---|---|---|---|---|---|---|
| n | %* | n | %* | n | %* | n | %* | |
| Median age (y) | 73 | 70 | 71 | 72 | ||||
| Age, y | ||||||||
| 66-69 | 3005 | 25 (25) | 4591 | 48 (26) | 847 | 32 (26) | 193 | 25 (24) |
| 70-74 | 4442 | 37 (37) | 4075 | 43 (38) | 1171 | 44 (38) | 321 | 41 (38) |
| 75-79 | 2782 | 23 (23) | 784 | 8 (23) | 545 | 20 (23) | 203 | 26 (24) |
| ≥80 | 1648 | 14 (14) | 59 | 1 (14) | 116 | 4 (14) | 61 | 8 (14) |
| Year of first biopsy | ||||||||
| 2012 | 6215 | 52 (52) | 5326 | 56 (50) | 1568 | 59 (53) | 377 | 49 (50) |
| 2013 | 5662 | 48 (48) | 4183 | 44 (50) | 1111 | 42 (48) | 401 | 52 (50) |
| Race | ||||||||
| White | 9930 | 84 (84) | 8331 | 88 (85) | 2237 | 84 (84) | 669 | 86 (81) |
| Black | 1366 | 12 (12) | 744 | 8 (10) | 337 | 13 (11) | 77 | 10 (13) |
| Hispanic | 131 | 1 (1) | 87 | 1 (1) | 16 | 1 (1) | <11 | NA† |
| Other | 450 | 4 (4) | 347 | 4 (4) | 89 | 3 (4) | >20 | NA† |
| Charlson index | ||||||||
| 0 | 7123 | 60 (60) | 6635 | 70 (62) | 1658 | 62 (60) | 498 | 64 (61) |
| 1 | 2780 | 23 (23) | 2002 | 21 (22) | 617 | 23 (24) | 157 | 20 (23) |
| ≥2 | 1974 | 17 (17) | 872 | 9 (15) | 404 | 15 (16) | 123 | 16 (16) |
| Region | ||||||||
| New England | 692 | 6 (6) | 452 | 5 (6) | 79 | 3 (6) | 31 | 4 (6) |
| Middle Atlantic | 1393 | 12 (12) | 819 | 9 (12) | 160 | 6 (12) | 194 | 25 (11) |
| East north central | 2018 | 17 (17) | 1554 | 16 (17) | 557 | 21 (17) | 81 | 10 (17) |
| West north central | 791 | 7 (7) | 866 | 9 (7) | 185 | 7 (7) | 36 | 5 (6) |
| South Atlantic | 2701 | 23 (23) | 1799 | 19 (21) | 737 | 28 (23) | 136 | 18 (25) |
| East south central | 792 | 7 (7) | 928 | 10 (6) | 264 | 10 (7) | 65 | 8 (6) |
| West south central | 1190 | 10 (10) | 1027 | 11 (10) | 198 | 7 (10) | 44 | 6 (11) |
| Mountain | 798 | 7 (7) | 656 | 7 (7) | 191 | 7 (7) | 109 | 14 (6) |
| Pacific/other | 1502 | 13 (13) | 1372 | 15 (16) | 297 | 12 (13) | 81 | 11 (13) |
| ADT use | ||||||||
| No | 11,877 | 100 (100) | 8495 | 89 (87) | 2259 | 84 (84) | 645 | 83 (80) |
| Yes | 0 | 0 (0) | 1014 | 11 (13) | 429 | 16 (16) | 133 | 17 (20) |
| State buy-in | ||||||||
| Partial/no | 11,190 | 94 (94) | 9188 | 97 (94) | 2567 | 96 (94) | 745 | 96 (95) |
| Full | 687 | 6 (6) | 321 | 3 (6) | 112 | 4 (6) | 33 | 4 (6) |
| Baseline GU incontinence | ||||||||
| No | 11,425 | 96 (96) | 9301 | 98 (96) | 2628 | 98 (97) | 761 | 98 (97) |
| Yes | 452 | 4 (4) | 208 | 2 (4) | 51 | 2 (3) | 17 | 2 (3) |
| Baseline GU bleeding/irritation | ||||||||
| No | 6827 | 58 (58) | 6030 | 63 (56) | 1647 | 62 (58) | 436 | 56 (56) |
| Yes | 5050 | 42 (42) | 3479 | 37 (44) | 1032 | 38 (42) | 342 | 44 (44) |
| Baseline GU obstruction/retention or stricture or fistula | ||||||||
| No | 9494 | 80 (80) | 7882 | 83 (79) | 2242 | 84 (80) | 623 | 80 (84) |
| Yes | 2383 | 20 (20) | 1627 | 17 (21) | 437 | 16 (20) | 155 | 20 (16) |
| Bowel toxicity | ||||||||
| No | 11,278 | 95 (95) | 9132 | 96 (95) | 2545 | 95 (95) | 741 | 95 (95) |
| Yes | 599 | 5 (5) | 377 | 4 (5) | 134 | 5 (5) | 37 | 5 (5) |
| Baseline erectile dysfunction | ||||||||
| No | 10,074 | 85 (85) | 8069 | 85 (83) | 2269 | 85 (84) | 645 | 83 (83) |
| Yes | 1803 | 15 (15) | 1440 | 15 (17) | 410 | 15 (16) | 133 | 17 (17) |
Abbreviations: ADT = androgen deprivation therapy; GU = genitourinary; SBRT = stereotactic body radiation therapy.
Unless otherwise indicated, data are reported as unadjusted (adjusted) percentages of patients.
Cell sizes <11 have been suppressed in accordance with Centers for Medicare and Medicaid Services privacy policies.
Total costs by treatment groups
Total raw mean costs by treatment group are presented in Figure 1A and Table 2. Adjusted total costs at 1 year after diagnosis were lowest in the AS group (mean $9687; 95% confidence interval [CI], $9528-$9848). Among the 3 radical treatment groups, the lowest 1-year cost was seen in the brachytherapy group ($19,980; 95% CI, $19,652-$20,313). The prostatectomy (mean $23,632; 95% CI, $23,243-$24,028) and SBRT (mean $26,895; 95% CI, $26,460-$27,337) groups had higher total 1-year costs than brachytherapy (both P < .001). A sensitivity analysis with multivariate adjustment for all available covariates in addition to inverse probability treatment weighted identified similar results.
Figure 1.
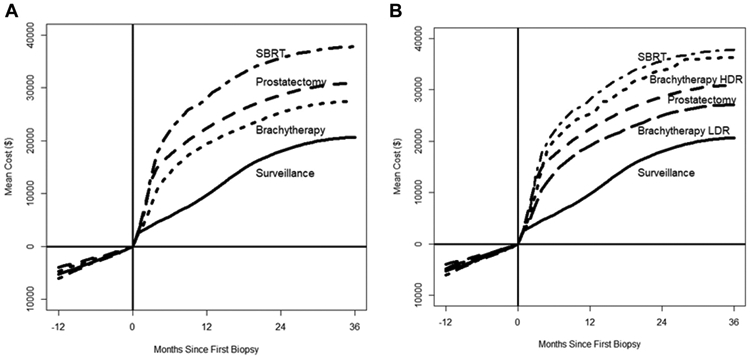
Mean patient cost after an initial diagnosis (time = 0) of prostate cancer. Negative time points (time <0) reflect costs before diagnosis. Brachytherapy is shown both as aggregated brachytherapy (A) and separated into high-dose-rate (HDR) and low-dose-rate (LDR) brachytherapy (B).
Table 2.
Mean total Medicare costs referenced to date of initial biopsy
| Group | Baseline (−1 to 0 y) cost |
0-1 y unadjusted cost |
0-1 y adjusted cost (95% CI) |
0-2 y unadjusted cost |
0-2 y adjusted cost (95% CI) |
|---|---|---|---|---|---|
| Active surveillance | $5283 | $9663, n = 11,877 | $9687 ($9528-$9848) | $17,972, n = 6,842 | $20,321 ($19,865-$20,787) |
| Prostatectomy | $3955 | $22,372, n = 9509 | $23,632 ($23,243 - $24,028) | $28,637, n = 5827 | $31,922 ($31,192 - $32,668) |
| Brachytherapy* | $4766 | $19,387, n = 2,679 | $19,980 ($19,652-$20,313) | $25,302, n = 1713 | $27,749 ($27,133-$28,380) |
| HDR | $5172 | $25,365, n = 153 | $26,019 ($24,316-$27,841) | $33,759, n = 84 | $40,105 ($36,338-$44,264) |
| LDR | $4784 | $19,127, n = 2479 | $19,723 ($19,386 - $20,065) | $24,941, n = 1594 | $27,162 ($26,535-$27,804) |
| SBRT | $6017 | $27,988, n = 778 | $26,895 ($26,460-$27,337) | $35,621, n = 413 | $35,140 ($34,343-$35,957) |
Abbreviations: CI = confidence interval; HDR = high-dose-rate brachytherapy; LDR = low-dose-rate brachytherapy; SBRT = stereotactic body radiation therapy.
Date of initial biopsy is used as a time reference and occurs at “0 y.”
HDR or LDR designation could not be assigned to 47 patients.
When brachytherapy was separated into LDR and HDR, the mean adjusted total 1-year cost for the HDR group was $26,019 (95% CI, $24,316-$27,841), which was significantly higher than that for the LDR group ($19,723; 95% CI, $19,386–$20,065; P < .001; Table 2, Fig 1B). At 2 years, a similar trend emerged (LDR mean: $27,162 vs HDR mean: $40,105, P < .001). Total 1-year costs for the HDR group were not significantly different from those for the most expensive treatment group, SBRT (P = .35). Finally, to estimate treatment-specific costs, the adjusted total 1-year cost in the AS cohort was subtracted from the 1-year cost in each of the active treatment cohorts: SBRT = $17,208, HDR brachytherapy = $16,332, prostatectomy = $13,945, and LDR brachytherapy = $10,036.
Overall costs by geographic region
Mean total costs 1 year after diagnosis stratified by US Census Bureau division is presented in Figure 2. In all regions except 2, costs were highest for the SBRT group (range, $21,504-$36,667). In the West South Central and Mountain regions, prostatectomy was the most expensive; however, in both regions the difference in total 1-year cost between SBRT and prostatectomy was minimal (<$1000). Furthermore, these 2 regions exhibited the lowest range in cost between active treatment modalities (Fig 2). In contrast, the New England and Middle Atlantic regions had the largest range in costs between radical treatments (Fig 2). In these 2 regions, the difference between the most expensive (SBRT) and least expensive (brachytherapy) radical treatments was $12,580 in New England and $16,286 in the Middle Atlantic. In all regions, brachytherapy had the lowest costs (range, $17,500-$21,648) of the 3 radical treatment groups.
Figure 2.
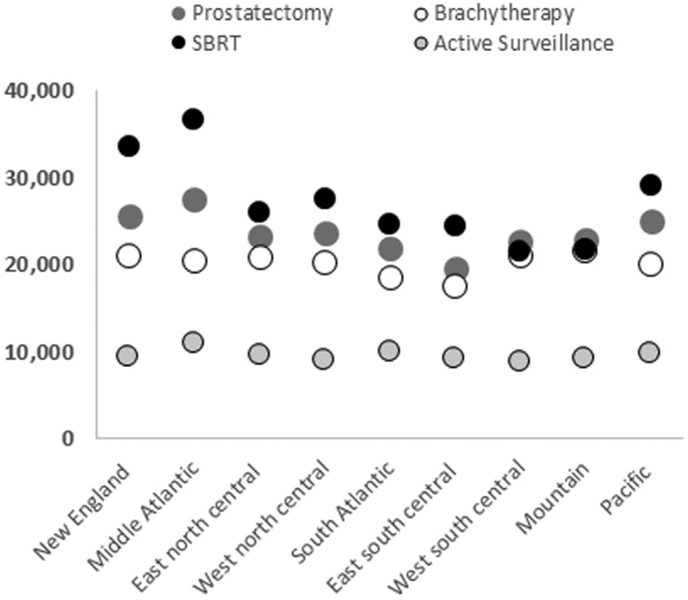
Mean patient costs at 1 year after diagnosis for each of the 4 treatment groups by geographic region. Abbreviation: SBRT = stereotactic body radiation therapy.
Treatment-associated toxic effects
Patients in the prostatectomy group had the highest rate of genitourinary (GU) toxicity (HR = 2.0 vs AS, 1-year incidence 80%), followed by brachytherapy (HR = 1-year incidence 74%) and SBRT (HR = 1.4, 1-year incidence 60%). Mean GU toxicity–associated costs reflected this trend, with the highest cost in the prostatectomy group ($2811; 95% CI, $2684-$2938) followed by the brachytherapy group ($1579; 95% CI, $1227-$1930; Table 3). Mean GU toxicity–associated costs at 1 year were similar to those of the AS group ($1113; 95% CI, $1015–$1210) and SBRT group ($1073; 95% CI, $786-$1360; Table 3). Urinary incontinence accounted for most of the GU toxicity in the prostatectomy group (HR = 1-year incidence 50%). In contrast, patients receiving radiation exhibited a higher frequency of bleeding/irritation and obstruction/retention. Comparing brachytherapy with SBRT, brachytherapy was associated with higher GU obstruction/retention (HR = 1.9 vs 1.1, 1-year prevalence 41% vs 21%, P < .001) and stricture (HR = 2.5 vs 1.3, 1-year prevalence 5% vs 2%, P < .001). Separating brachytherapy into HDR (n = 153) and LDR (n = 2479), the incidence and costs of GU toxicity was found to be substantially lower in patients receiving HDR (HR = 1.1 vs 1.8, 1-year incidence 55% vs 75%, P < .001; Table 3 and 4).
Table 3.
Model-derived mean toxicity costs 1 and 2 years after biopsy
| Variable | N at 1 y | 0-1 y adjusted cost (95% CI) | N at 2 y | 0-2 y adjusted cost (95% CI) |
|---|---|---|---|---|
| GU/GI/ED | ||||
| Active surveillance | 11,877 | $1303 ($1187-$1419) | 6842 | $2509 ($2279-$2740) |
| Prostatectomy | 9509 | $3500 ($3364-$3636) | 5827 | $4528 ($4293-$4764) |
| Brachytherapy | 2679 | $1847 ($1482-$2212) | 1713 | $2817 ($2139-$3496) |
| HDR | 153 | $355 ($176-$535) | 84 | $884 ($360-$1408) |
| LDR | 2479 | $1954 ($1559-$2349) | 1594 | $2933 ($2202-$3664) |
| SBRT | 778 | $1327 ($1017-$1636) | 413 | $2144 ($1727-$2560) |
| GU | ||||
| Active surveillance | 11,877 | $1113 ($1015-$1210) | 6842 | $2147 ($1942-$2353) |
| Prostatectomy | 9509 | $2811 ($2684-$2938) | 5827 | $3531 ($3317-$3745) |
| Brachytherapy | 2679 | $1579 ($1227-$1930) | 1713 | $2296 ($1641-$2951) |
| HDR | 153 | $280 ($123-$438) | 84 | $527 ($184-$871) |
| LDR | 2479 | $1676 ($1295-$2057) | 1594 | $2403 ($1697-$3109) |
| SBRT | 778 | $1073 ($786-$1360) | 413 | $1560 ($1199-$1920) |
| GI | ||||
| Active surveillance | 11,877 | $164 ($104-$223) | 6842 | $289 ($189-$389) |
| Prostatectomy | 9509 | $127 ($91-$162) | 5827 | $293 ($215-$370) |
| Brachytherapy | 2679 | $170 ($78-$262) | 1713 | $455 ($290-$620) |
| HDR | 153 | $9 ($0-$17) | 84 | $153 ($0-$439) |
| LDR | 2479 | $180 ($81-$279) | 1594 | $477 ($300-$654) |
| SBRT | 778 | $209 ($94-$324) | 413 | $473 ($280-$665) |
| ED | ||||
| Active surveillance | 11,877 | $81 ($64-$98) | 6842 | $164 ($135-$194) |
| Prostatectomy | 9509 | $888 ($831-$945) | 5827 | $1135 ($1042-$1229) |
| Brachytherapy | 2679 | $157 ($120-$193) | 1713 | $228 ($170-$287) |
| HDR | 153 | $97 ($1-$192) | 84 | $217 ($0-$524) |
| LDR | 2479 | $156 ($118-$194) | 1594 | $222 ($163-$281) |
| SBRT | 778 | $190 ($99-$281) | 413 | $238 ($138-$337) |
Abbreviations: AS = active surveillance; CI = confidence interval; ED = erectile dysfunction; GI = gastrointestinal; GU = genitourinary; HDR = high-dose-rate brachytherapy; LDR = low-dose-rate brachytherapy; SBRT = stereotactic body radiation therapy.
Table 4.
Model-derived incidence of toxic effects after initial diagnosis of prostate cancer
| Group | HR | 95% CI | P value | Model-derived incidence (%) |
|||
|---|---|---|---|---|---|---|---|
| 6 mo | 12 mo | 4 mo | 33 mo | ||||
| Any GU | |||||||
| AS | 1 | 38 | 48 | 62 | 69 | ||
| Prostatectomy | 2.0 | 1.9-2.1 | <.001 | 69 | 80 | 85 | 87 |
| Brachytherapy | 1.8 | 1.7-1.8 | <.001 | 59 | 74 | 82 | 86 |
| HDR | 1.1 | 1.0-1.3 | .025 | 39 | 55 | 69 | 9 |
| LDR | 1.8 | 1.8-1.9 | <.001 | 60 | 75 | 83 | 87 |
| SBRT | 1.4 | 1.4-1.4 | <.001 | 45 | 60 | 74 | 76 |
| Any GI | |||||||
| AS | 1 | 3 | 5 | 10 | 13 | ||
| Prostatectomy | 1.1 | 1.0-1.2 | .16 | 4 | 6 | 10 | 13 |
| Brachytherapy | 1.8 | 1.6-1.9 | <.001 | 5 | 8 | 17 | 22 |
| HDR | 1.2 | 0.9-1.5 | .3 | 3 | 4 | 9 | 16 |
| LDR | 1.8 | 1.7-2.0 | <.001 | 5 | 9 | 18 | 22 |
| SBRT | 1.8 | 1.3-1.9 | <.001 | 4 | 9 | 17 | 17 |
| Any ED | |||||||
| AS | 1 | 11 | 15 | 22 | 25 | ||
| Prostatectomy | 3.5 | 3.3-3.6 | <.001 | 31 | 49 | 58 | 60 |
| Brachytherapy | 1.4 | 1.3-1.5 | <.001 | 10 | 19 | 29 | 33 |
| HDR | 1.6 | 1.4-1.9 | <.001 | 11 | 20 | 29 | 34 |
| LDR | 1.4 | 1.3-1.5 | <.001 | 10 | 19 | 29 | 33 |
| SBRT | 1.4 | 1.3-1.5 | <.001 | 13 | 20 | 30 | 33 |
| GU incontinence | |||||||
| AS | 1 | 3 | 4 | 8 | 10 | ||
| Prostatectomy | 10.8 | 10.1-11.6 | <.001 | 35 | 50 | 57 | 59 |
| Brachytherapy | 1.8 | 1.6-1.9 | <.001 | 4 | 8 | 13 | 15 |
| HDR | 1.4 | 1.0-1.7 | .023 | 6 | 9 | 9 | 9 |
| LDR | 1.8 | 1.6-2.0 | <.001 | 4 | 8 | 13 | 16 |
| SBRT | 1.5 | 1.3-1.6 | <.001 | 3 | 5 | 10 | 10 |
| GU bleeding/irritation | |||||||
| AS | 1 | 31 | 40 | 53 | 60 | ||
| Prostatectomy | 1.3 | 1.2-1.3 | <.001 | 45 | 52 | 61 | 65 |
| Brachytherapy | 1.7 | 1.7-1.8 | <.001 | 47 | 62 | 73 | 77 |
| HDR | 1.2 | 1.1-1.3 | .002 | 17 | 21 | 21 | 21 |
| LDR | 1.8 | 1.7-1.8 | <.001 | 31 | 42 | 48 | 52 |
| SBRT | 1.5 | 1.5-1.6 | <.001 | 39 | 54 | 70 | 72 |
| GU obstruction/retention | |||||||
| AS | 1 | 16 | 21 | 29 | 35 | ||
| Prostatectomy | 1.2 | 1.1-1.2 | <.001 | 26 | 29 | 33 | 35 |
| Brachytherapy | 1.9 | 1.8-1.9 | <.001 | 31 | 41 | 47 | 51 |
| HDR | 0.8 | 0.7-1.0 | .011 | 17 | 21 | 21 | 21 |
| LDR | 2.0 | 1.9-2.1 | <.001 | 31 | 42 | 48 | 52 |
| SBRT | 1.1 | 1.0-1.1 | .037 | 16 | 21 | 29 | 34 |
| GU stricture | |||||||
| AS | 1 | 1 | 2 | 3 | 3 | ||
| Prostatectomy | 3.9 | 3.4-4.4 | <.001 | 6 | 8 | 9 | 12 |
| Brachytherapy | 2.5 | 2.2-2.8 | <.001 | 3 | 5 | 6 | 7 |
| HDR | NA* | ||||||
| LDR | 2.6 | 2.3-3.0 | <.001 | 4 | 5 | 7 | 8 |
| SBRT | 1.3 | 1.1-1.6 | <.001 | 2 | 2 | 2 | 2 |
| GU fistula | |||||||
| AS | 1 | 0.1 | 0.1 | 0.1 | 0.1 | ||
| Prostatectomy | 3.4 | 2.0-5.7 | <.001 | 0.4 | 0.4 | 0.5 | 0.5 |
| Brachytherapy | NA* | ||||||
| SBRT | NA* | ||||||
| GI bleeding/proctitis | |||||||
| AS | 1 | 3 | 5 | 9 | 12 | ||
| Prostatectomy | 1.1 | 1.0-1.2 | .037 | 4 | 6 | 10 | 12 |
| Brachytherapy | 1.9 | 1.7-1.9 | <.001 | 4 | 8 | 16 | 20 |
| HDR | 1.0 | 0.8-1.4 | .82 | 2 | 3 | 3 | 3 |
| LDR | 1.9 | 1.7-2.0 | <.001 | 4 | 8 | 17 | 21 |
| SBRT | 1.7 | 1.6-1.9 | <.001 | 3 | 8 | 15 | 15 |
| GI ulcer/stricture/fistula | |||||||
| AS | 1 | 0.2 | 0.3 | 0.5 | 0.5 | ||
| Prostatectomy | 1.0 | 0.7-1.5 | .83 | 0.3 | 0.4 | 0.5 | 0.5 |
| Brachytherapy | 2.7 | 2.0-3.7 | <.001 | 0.5 | 0.5 | 1.5 | 1.5 |
| HDR | NA* | ||||||
| LDR | 2.9 | 2.1-3.9 | <.001 | 0.4 | 0.4 | 1.6 | 1.6 |
| SBRT | 3.0 | 2.2-4.1 | <.001 | 0.7 | 0.9 | 0.9 | 0.9 |
| GI incontinence | |||||||
| AS | 1 | 0.2 | 0.3 | 0.7 | 0.9 | ||
| Prostatectomy | 0.8 | 0.6-1.1 | .17 | 0.1 | 0.3 | 0.6 | 0.6 |
| Brachytherapy | 1.9 | 1.5-2.5 | <.001 | 0.4 | 0.6 | 0.6 | 0.6 |
| HDR | NA* | ||||||
| LDR | 2.1 | 1.6-2.7 | <.001 | 0.4 | 0.4 | 0.4 | 0.4 |
| SBRT | NA* | ||||||
| GI proctectomy/hyperbaric oxygen | |||||||
| AS | 1 | ||||||
| Prostatectomy | 1.4 | 0.6-3.3 | .46 | 0 | 0.01 | 0.1 | 0.1 |
| Brachytherapy | NA* | ||||||
| SBRT | NA* | ||||||
Abbreviations: AS = active surveillance; CI = confidence interval; ED = erectile dysfunction; GI = gastrointestinal; GU = genitourinary; HDR = high-dose-rate brachytherapy; HR = hazard ratio; LDR = low-dose-rate brachytherapy; NA = not available; SBRT = stereotactic body radiation therapy.
All hazard ratios are expressed in relation to active surveillance.
Cell sizes <11 have been suppressed and marked as “NA” in accordance with Centers for Medicare and Medicaid Services privacy policies.
Erectile dysfunction (ED) was more prevalent among patients in the prostatectomy group compared with the AS cohort (HR = 3.5,1-year prevalence 49%). Compared with AS, the incidence of ED was also higher in patients receiving SBRT (HR = 1.4, 1-year prevalence 20%) and brachytherapy (HR = 1.4, 1-year prevalence 19%; Table 4). ED-associated costs followed this same ranking (Table 3). The prevalence of GI toxicity was generally low for all groups (1-year prevalence in all groups <10%). The highest rates were observed among patients in the brachytherapy group (HR = 1.8, 1-year prevalence 8%, P < .001) and the SBRT group (HR = 1.8, 1-year prevalence 9%, P < .001; Table 4). In contrast, prostatectomy was not associated with a difference in GI toxicity prevalence (HR = 1.1, 1-year prevalence 6%, P = .16). Separating brachytherapy into HDR and LDR, the incidence of GI toxicity in the HDR subgroup was substantially lower than that in the LDR group (HR = 1.2 vs 1.8, 1-year incidence 4% vs 9%). GI toxicity–associated costs at 1 year were similar among all treatment groups (Table 3).
Examination of early versus late toxicities revealed differences in the temporal patterns of each toxicity domain (Table E4). For GU toxicity, late incidence was similar among all radical treatment groups. For GU toxicity, the incidence of late toxicity was lower than that of early toxicity in all 3 groups (prostatectomy 52% vs 79%, brachytherapy 56% vs 73%, and SBRT 54% vs 57%), suggesting improvement with time. For ED, the incidence of early and late toxicity was equivalent (prostatectomy 47% vs 41%, brachytherapy 17 vs 22%, and SBRT 19 vs 20%), suggesting stabilization. Finally, for GI toxicity, late incidence was higher than early incidence in both the brachytherapy and SBRT groups (both 8% late vs 11% early), suggesting a worsening over time (Fig E1). Treatment-specific costs, calculated by subtracting toxicity costs from the 1-year unadjusted costs, identified the costs for SBRT, prostatectomy, brachytherapy, and AS to be following: $25,548, $20,132, $18,133, and $8284, respectively.
Discussion
Treatment costs are often presented in 2 ways: total cost over a specific timeframe or treatment-specific costs associated with specific Current Procedural Terminology codes. In the present analysis, the total adjusted 1-year cost for patients undergoing prostatectomy was $23,632 (95% CI , $23,243-$24,028) in 2015 dollars, which was similar to prior estimates of 1-year postdiagnosis costs estimated from SEER-Medicare data ($16,469-$29,988 in 2008 dollars).6 Furthermore, in the present analysis, total 1-year cost for patients who received prostatectomy was $13,945 more than that incurred by AS. This cost difference is attributable to oncologic care and is similar to estimates by Herrel et al ($14,614).10 With regard to SBRT, total adjusted 1-year SBRT cost in the present analysis was $26,895 (95% CI, $26,460-$27,337), which is similar to past estimates of 1-year postdiagnosis costs estimated from SEER-Medicare data ($27,145 in 2012 dollars).7 The adjusted 1-year SBRT cost in the present study was $17,208 more than that of the AS cohort and approximates cancer treatment–related costs identified by Yu et al for SBRT patients ($16,608 in 2011 dollars).9 Thus, there is excellent agreement between the presented costs and total and cancer-specific costs identified in external analyses. Of note, AS costs and toxicities are likely a mix of baseline health care utilization and those incurred by additional cancer monitoring.
Less is known about treatment costs associated with brachytherapy. To the best of our knowledge, this is the first report of HDR brachytherapy costs based on Medicare claims. Total adjusted 1-year brachytherapy costs were $19,980 (95% CI, $19,652-$20,313), an amount similar to the 1-year postdiagnosis costs identified by Nguyen et al ($17,076-$21,117) and Halpern et al ($17,183).6,7 This reduced cost of brachytherapy seems to be driven largely by LDR patients (total 1-year cost $19,723), who formed the bulk of this cohort (n = 2479, 93%). HDR brachytherapy was associated with higher cost, with a total 1-year cost of $26,019 (95% CI, $24,316-$27,841), a cost similar to that of SBRT. We thus estimate the treatment-specific costs to be $10,036 for LDR brachytherapy and $16,332 for HDR. For reference, IMRT and proton therapy for prostate cancer have been shown in external analyses to have significantly higher total (IMRT $31,574-$37,418, proton therapy $57,244) and treatment-specific (IMRT $21,023 and proton therapy $32,428) costs.6,8,9,15 The current RO-APM proposes a national base rate for prostate cancer radiation treatment to be $3228 for professional fees and $19,852 for technical fees,11 which approximates estimates of treatment-specific IMRT cost but is higher than the current estimate for time-efficient techniques and more than double current reimbursement for LDR brachytherapy.
Given the national scope of the Medicare claims data, the present analysis allowed a unique evaluation of regional cost variations. In most regions, the total 1-year cost range between definitive treatment modalities was approximately $6000 (Fig 2). The highest range was in the New England and Mid-Atlantic regions (>$10,000), and the lowest range was noted in the West South Central and Mountain regions, which showed nearly identical costs between modalities (range <$2000). Because the 1-year total costs for the AS cohort was uniform across regions at about $10,000 (Fig 2), it is unlikely that the observed regional variations can be fully attributed to differences in general population health and the cost of nononcologic care. Explanations for these observed variations include differences in the pattern and intensity of oncologic care, a finding that has been observed for diagnostic practices,20 and differences in the Medicare geographic adjustment factors, which is driven by cost of living, malpractice, and practice cost/expense. These findings are noteworthy because they suggest that cost-effectiveness analyses for prostate cancer treatment may reach divergent conclusions in different regions, and thus they underscore the challenge of determining which treatment(s) confer optimal value on a national level. For example, a formal cost-effectiveness analysis may conclude that SBRT as practiced and compensated in the mid-Atlantic region is not cost-effective at a certain societal willingness-to-pay threshold, whereas SBRT as practiced and compensated in the Mountain region could be highly cost-effective at the same threshold.
With regard to relative toxicity, the quality of life analysis from the Prostate Testing for Cancer and Treatment (ProtecT) phase 3 trial in addition to the 2 large recent quality-of-life registries identified prostatectomy as associated with worse sexual function and urinary incontinence and improved urinary and bowel irritative symptoms compared with radiation modalities.19, 21, 22 However, these analyses generally present limited comparisons among radiation modalities. The present series involved relatively large SBRT and brachytherapy cohorts to assess not only the frequency of toxicity-related encounters (Table 4) but also the toxicity-specific costs (Table 3). The present analysis identified the prostatectomy cohort as having a higher incidence of ED (HR = 3.5, P < .001) and GU incontinence (HR = 10.8, P < .001), with correspondingly high costs associated with ED (mean $888) and GU toxicity (mean $2811). Brachytherapy and SBRT patients had higher rates of any GI toxicity (HR = 1.8 for both, P < .001); however, given the relative infrequency of GI toxicity, the mean associated cost was only minimally higher (Table 3). Comparing radiation modalities, brachytherapy and SBRT had similar frequencies of GI toxicity (HR = 1.8 for both) and ED (HR = 1.4 for both), with similar associated toxicity costs (Tables 3 and 4). GU toxicity was both more frequent for brachytherapy than for SBRT (HR = 1.8 brachytherapy and HR = 1.4 SBRT) and more costly ($1579 and $1073), a finding consistent with past analyses.7 Compared with LDR, HDR brachytherapy is a newer technique in which radiation dose is modified based on catheter placement to reduce toxicity.23,24 The present data suggest that the use of HDR is limited nationally (n = 153), although HDR was associated with decreased toxicity across all domains (Tables 3 and 4). Notably, the toxicity-associated costs for HDR were substantially lower than those for AS (GU/GI/ED: $355 vs $1303), suggesting that the HDR cohort may represent highly selected patients with minimal baseline comorbidity.
Various limitations deserve mention. First, the analyzed data span a 4-year timeframe, and thus we are unable to assess truly long-term toxic effects.25 Second, SBRT and especially HDR brachytherapy represent newer techniques with which only limited numbers of selected patients have been treated. Thus, uncontrolled patient selection biases may exist when assessing this modality. Third, we chose to focus our analysis on total costs with comparison to a contemporary AS cohort. Although advantages to this approach exist, our approach may be influenced by differences in baseline health care utilization between cohorts. Fourth, although the utilization of national Medicare data allows for a more comprehensive view of national health care expenditures, unlike SEER-Medicare data, no information is available on patient stage. Fifth, patients were only included in this analysis if they were enrolled in fee-for-service Medicare, thus excluding patients from programs such as Medicare Advantage plans. Finally, analyses that examine time to incidence of first complication inherently bias against surgical procedures, in which toxicities occur early and patients eventually recover. To account for this, we performed secondary analyses assessing only late and early toxicities (Table E4).
Conclusions
Time-efficient prostate cancer treatment modalities offer trade-offs in terms of costs and toxicities. The estimated treatment-specific Medicare reimbursement of time-efficient treatments varies widely by geography but is generally less than the reimbursement rates proposed by the RO-APM. Additional data including personnel costs will be important for estimating institutional costs. Ultimately this analysis presents strong justification for prospective trials comparing time-efficient techniques, especially because these treatments may become further incentivized with bundled payment models.
Supplementary Material
Acknowledgments
Sources of support: Dr Tang is supported by a Radiation Oncology Institute (ROI) grant and Cancer Prevention & Research Institute of Texas (RP180140). Dr Smith is supported by NIH R01 CA207216-01 and the Cancer Prevention & Research Institute of Texas (RP160674), ROI, and is an Andrew Sabin Family Fellow. This work was also supported by The University of Texas MD Anderson Cancer Center under the Cancer Center Support Core Grant (CA016672).
Research data is available by application to the Centers for Medicare and Medicaid Services.
Disclosures: Dr Tang has received consulting fees from Reflexion unrelated to this manuscript. Dr Chapin has received consulting fees and/or research funding from Janssen pharmaceuticals and Blue Earth Diagnostics unrelated to this manuscript. Dr Frank has received consulting fees and/or research funding from Varian, C4 Imaging, Eli Lilly, Elekta, Hitachi, the National Comprehensive Cancer Network, Breakthrough Chronic Care, and Boston Scientific and is the founder of C4 Imaging unrelated to this manuscript. Dr Smith has received research funding from Varian and holds equity in Oncora unrelated to this article.
Footnotes
Supplementary data
Supplementary material for this article can be found at https://doi.org/10.1016/j.prro.2020.02.014.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Akinyemiju TF, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanders JW, Frank SJ, Kudchadker RJ, et al. Development and clinical implementation of seednet: A sliding-window convolutional neural network for radioactive seed identification in mri-assisted radiosurgery (mars). Magn Reson Med. 2019;81:3888–3900. [DOI] [PubMed] [Google Scholar]
- 4.Hamstra DA, Mariados N, Sylvester J, et al. Continued benefit to rectal separation for prostate radiation therapy: Final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017;97:976–985. [DOI] [PubMed] [Google Scholar]
- 5.Maenhout M, Peters M, Moerland MA, et al. MRI guided focal HDR brachytherapy for localized prostate cancer: Toxicity, biochemical outcome and quality of life. Radiother Oncol. 2018; 129:554–560. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29:1517–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halpern JA, Sedrakyan A, Hsu WC, et al. Use, complications, and costs of stereotactic body radiotherapy for localized prostate cancer. Cancer. 2016;122:2496–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan HY, Jiang J, Hoffman KE, et al. Comparative toxicities and cost of intensity-modulated radiotherapy, proton radiation, and stereotactic body radiotherapy among younger men with prostate cancer. J Clin Oncol. 2018;36:1823–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu JB, Cramer LD, Herrin J, et al. Stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: Comparison of toxicity. J Clin Oncol. 2014;32: 1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrel LA, Syrjamaki JD, Linsell SM, et al. Identifying drivers of episode cost variation with radical prostatectomy. Urology. 2016;97: 105–110. [DOI] [PubMed] [Google Scholar]
- 11.Department of Health and Human Services. Medicare program: Specialty care models to improve quality of care and reduce Book Medicare Program: Specialty Care Models to Improve Quality of Care and Reduce. Baltimore, Maryland: Department of Health and Human Services Centers for Medicare and Medicaid Service; 2019. [Google Scholar]
- 12.Modi PK, Kaufman SR, Qi J, et al. National trends in active surveillance for prostate cancer: Validation of Medicare claims-based algorithms. Urology. 2018;120:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollenbeck BK, Bierlein MJ, Kaufman SR, et al. Implications of evolving delivery system reforms for prostate cancer care. Am J Manag Care. 2016;22:569–575. [PMC free article] [PubMed] [Google Scholar]
- 14.Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu JB, Soulos PR, Herrin J, et al. Proton versus intensity-modulated radiotherapy for prostate cancer: Patterns of care and early toxicity. J Natl Cancer Inst. 2013;105:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sewell JM, Rao A, Elliott SP. Validating a claims-based method for assessing severe rectal and urinary adverse effects of radiotherapy. Urology. 2013;82:335–340. [DOI] [PubMed] [Google Scholar]
- 17.Bureau of Labor Statistics. Measuring price change for medical care in the CPI. Available at: https://www.resdac.org/articles/cms-cellsize-suppression-policy Accessed April 14, 2020.
- 18.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. [DOI] [PubMed] [Google Scholar]
- 19.Chen RC, Basak R, Meyer AM, et al. Association between choice of radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance and patient-reported quality of life among men with localized prostate cancer. JAMA. 2017;317:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song Y, Skinner J, Bynum J, et al. Regional variations in diagnostic practices. N Engl J Med. 2010;363:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barocas DA, Alvarez J, Resnick MJ, et al. Association between radiation therapy, surgery, or observation for localized prostate cancer and patient-reported outcomes after 3 years. JAMA. 2017;317:1126–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grills IS, Martinez AA, Hollander M, et al. High dose rate brachytherapy as prostate cancer monotherapy reduces toxicity compared to low dose rate palladium seeds. J Urol. 2004;171:1098–1104. [DOI] [PubMed] [Google Scholar]
- 24.Morgan TM, Press RH, Cutrell PK, et al. Brachytherapy for localized prostate cancer in the modern era: A comparison of patient-reported quality of life outcomes among different techniques. J Contemp Brachytherapy. 2018;10:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter GK, Reddy CA, Klein EA, et al. Long-term (10-year) gastrointestinal and genitourinary toxicity after treatment with external beam radiotherapy, radical prostatectomy, or brachytherapy for prostate cancer. Prostate Cancer. 2012;2012, 853487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


