Today, species richness is highest in the tropics and declines toward the poles. Although there are exceptions, this pattern is pervasive within both the terrestrial and marine realm and across taxonomic groups (1). This latitudinal diversity gradient (LDG) was first recognized by Alexander von Humboldt over two centuries ago. Despite this, understanding the mechanisms that underlie the LDG remains one of the great challenges of biodiversity science (1). Whether this is hyperbolic or not, this gradient is the first-order macroecological pattern that needs to be explained if we are to understand the broader question of what determines the distribution of biodiversity. In turn, this is critical to determining which geographical regions of Earth’s biosphere are most vulnerable to the ongoing climate emergency. Dozens of hypotheses have been proposed to explain the LDG, pertaining to a broad suite of climatic, environmental, geographical, and historical variables (2), but the answer remains elusive, in part because the proposed drivers covary in space today. However, the distribution patterns we see among living organisms provide only a snapshot of life: Earth’s geological record presents a unique window into the past, during which time these variables fluctuated substantially. Critically, this record also reveals the response of species to these changes. In PNAS, Song et al. (3) evaluated the evolution of the LDG in the marine realm from 254 to 201 Ma. Notably, this time span includes the most devastating of all mass extinctions, at the Permian/Triassic boundary, 252 Ma. Although Song et al. (3) recovered an LDG that is similar to the present-day pattern for much of their study interval, the distribution of biodiversity in the 5 My after the mass extinction event was characterized by a flat gradient, with no tropical peak. Song et al. (3) attributed this pattern to higher extinction rates in the tropics as a result of extreme warming and ocean anoxia, as well as increased origination and immigration rates at higher latitudes.
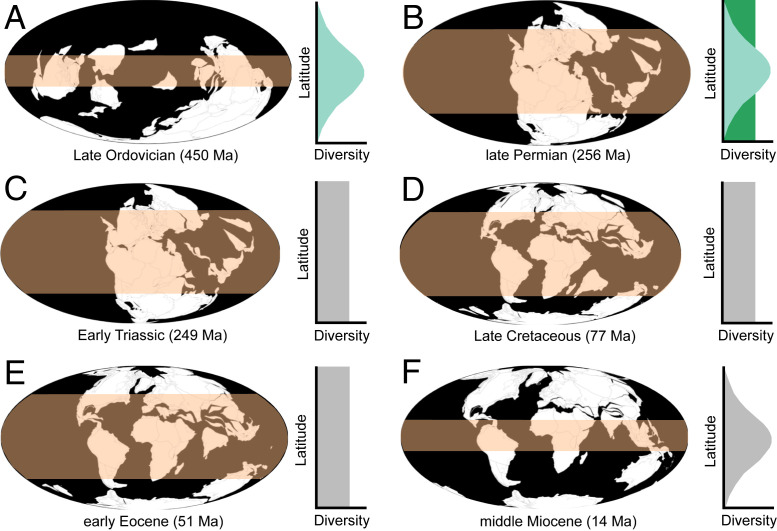
Interestingly, this is not the first time a flat LDG has been reconstructed in the immediate aftermath of a mass extinction. Rose et al. (4) recovered a similar pattern in North American terrestrial mammals following the Cretaceous/Paleogene (K/Pg) mass extinction, 66 Ma. However, subsequent work indicates that this is instead part of a longer-term pattern: Many terrestrial groups do not seem to have conformed to the present-day LDG either before or after the K/Pg event (Fig. 1), with flattened gradients or biodiversity peaks at higher latitudes characterizing their evolutionary histories (e.g., refs. 5–8). This even seems to be the case on land for most groups throughout the Permian–Triassic (9, 10), contrasting with the pattern in the marine realm recovered by Song et al. (3).
Fig. 1.
Schematic representation of the evolution of the LDG throughout the last 500 My: (A) Late Ordovician (450 Ma), (B) late Permian (256 Ma), (C) Early Triassic (249 Ma), (D) Late Cretaceous (77 Ma), (E) early Eocene (51 Ma), and (F) middle Miocene (14 Ma). For simplicity, the LDG is shown as either a steep or flat gradient, although its shape can be much more variable, with evidence for bimodal gradients (i.e., a tropical “trough”). Marine and terrestrial LDGs are shown in blue and green, respectively, whereas LDGs that are consistent between these two realms are shown in gray (depicted LDGs based on refs. 3–13, 18, 20, 23). The pink band represents an approximation of the extent of the tropical belt. Paleogeographic reconstructions modified from the Paleobiology Database Navigator interface (https://www.paleobiodb.org/navigator/), which is licensed under CC0 1.0.
This contrarian pattern is not restricted to the terrestrial realm (Fig. 1). Numerous analyses of marine organisms also recover flattened gradients during intervals of the past 500 My (e.g., refs. 11–14). Although the detailed analytical approaches vary between many of these deep-time studies, all of them account for the effect of sampling biases that can distort our reading of the fossil record (e.g., ref. 15). This contrasts with earlier work that often took a literal reading of the fossil record, leading to the perception that a tropical peak and poleward decline in biodiversity had been a largely consistent feature over the last 500 My (see reviews in refs. 5 and 6). As such, methodological issues are unlikely to be the source of differences between the results of Song et al. (3) and earlier studies that have failed to recover tropical biodiversity peaks in the geological record. As such, this suggests that the LDG was genuinely different at these times.
Work by Song et al. and others is starting to shed light both on the patterns and the underlying processes that have shaped the distribution of biodiversity.
Previous work noted that evidence for a steep, modern-type gradient is largely restricted to “icehouse” worlds (Fig. 1), which are characterized by steep environmental gradients (6, 12, 13, 16). By contrast, “greenhouse” worlds are characterized by LDGs and environmental gradients that are flattened (Fig. 1), in which tropical conditions extended into much higher latitudes (5, 6, 17, 18). However, the recovery of a modern-type gradient in the marine realm during the greenhouse world of the Middle to Late Triassic complicates this scenario. Song et al. (3) proposed a nuanced hypothesis, in which a modern-type gradient can also be produced in stable greenhouse worlds (see also ref. 19), whereas flattened LDGs might be restricted to extreme and variable greenhouse climates. This still leaves the question of why a modern-type gradient is not observed on land for most groups during this time interval. Do LDGs in the terrestrial and marine realms have different climatic constraints? Could variation in relative strengths of niche conservatism (i.e., the tendency of clades to retain their ecological niches over time) play a role in differences between environments? Do LDGs of taxonomic groups with contrasting thermophysiologies (i.e., “cold”- versus “warm”-blooded organisms) and/or body sizes respond differently to climatic changes? How does variation in dispersal ability between groups affect the evolution of LDGs (e.g., ref. 11)?
Returning to the present day, regardless of the underlying mechanism, today’s LDG is the result of higher diversification rates in the tropics, with the net rate of origination, extinction, and immigration exceeding that of higher latitudes (1, 20). As such, the tropics are often regarded as either a “cradle” (higher origination rates) or a “museum” (lower extinction rates) of biodiversity. Others have argued that it is more likely that the tropics are both a cradle and museum, and further complicated by dispersal, with species originating in the tropics and dispersing poleward while retaining their tropical presence (20). This paradigm has been assumed to hold true in deep time, too (e.g., ref. 19), although Song et al.’s (3) study demonstrates that severe latitudinal extinction selectivity has the ability to invert these patterns. During greenhouse intervals, the tropical belt expanded into higher latitudes (5): The flattened LDGs in the terrestrial realm, at least, presumably reflect this, with diversification rates largely consistent across a broad latitudinal band (18). Interestingly, the earliest known members of many of today’s “tropical” clades are from high-latitude localities during the last greenhouse world (e.g., ref. 17), which also suggests that the low-latitude tropics were not always a biodiversity cradle in the past (11, 18). We currently lack an understanding of why LDGs in the marine realm flattened after the Triassic (e.g., refs. 11 and 12), although the effect of the Triassic/Jurassic mass extinction, 201 Ma, has yet to be evaluated in this context. Notably, Song et al.’s (3) results indicate an important role for latitudinal variation in extinction selectivity on shaping gradients (see also refs. 21 and 22). The present-day LDG likely only began to form, or at least only steepened (12, 20), in the last 30 to 40 My in both the terrestrial and marine realms (6) and might still have varied substantially during this time (e.g., refs. 7 and 14). The timing of its inception might correspond to heightened extinction rates at high latitudes and increased dispersal into lower latitudes, resulting from latitudinal contraction of the tropics during the descent into the current icehouse world (18).
We are still at the formative stage of determining patterns of past biodiversity distribution, with many taxonomic groups, environments, and time intervals yet to be evaluated. However, work by Song et al. (3) and others is starting to shed light both on the patterns and the underlying processes (e.g., refs. 11, 17, 18, 23) that have shaped the distribution of biodiversity. These contributions have the potential to provide critical insights that supplement macroecological efforts, providing a framework for testing hypotheses otherwise based entirely on present-day data. The fossil record has a key role to play in answering many macroevolutionary and macroecological questions, especially when present-day data alone are consistent with a multitude of hypotheses (24).
Acknowledgments
P.D.M.’s research is supported by The Royal Society via a University Research Fellowship (UF160216) and grants RGF\R1\180020, RGF\EA\180318, and RGF\EA\201037. I thank Lewis Jones (Imperial College London) for providing insightful comments on an earlier draft.
Footnotes
The author declares no competing interest.
See companion article, “Flat latitudinal diversity gradient caused by the Permian–Triassic mass extinction,” 10.1073/pnas.1918953117.
References
- 1.Willig M. R., Kaufman D. M., Stevens R. D., Latitudinal gradients of biodiversity: Pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 34, 273–309 (2003). [Google Scholar]
- 2.Pontarp M., et al. , The latitudinal diversity gradient: Novel understanding through mechanistic eco-evolutionary models. Trends Ecol. Evol. (Amst.) 34, 211–223 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Song H., et al. , Flat latitudinal diversity gradient caused by the Permian–Triassic mass extinction. Proc. Natl. Acad. Sci. U.S.A. 117, 17578–17583 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose P. J., Fox D. L., Marcot J., Badgley C., Flat latitudinal gradient in Paleocene mammal richness suggests decoupling of climate and biodiversity. Geology 39, 163–166 (2011). [Google Scholar]
- 5.Archibald S. B., Bossert W. H., Greenwood D. R., Farrell B. D., Seasonality, the latitudinal gradient of diversity, and Eocene insects. Paleobiology 36, 374–398 (2010). [Google Scholar]
- 6.Mannion P. D., Upchurch P., Benson R. B. J., Goswami A., The latitudinal biodiversity gradient through deep time. Trends Ecol. Evol. (Amst.) 29, 42–50 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Marcot J. D., Fox D. L., Niebuhr S. R., Late Cenozoic onset of the latitudinal diversity gradient of North American mammals. Proc. Natl. Acad. Sci. U.S.A. 113, 7189–7194 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholson D. B., Holroyd P. A., Valdes P., Barrett P. M., Latitudinal diversity gradients in Mesozoic non-marine turtles. R. Soc. Open Sci. 3, 160581 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brocklehurst N., Day M. O., Rubidge B. S., Fröbisch J., Olson’s Extinction and the latitudinal biodiversity gradient of tetrapods in the Permian. Proc. Biol. Sci. 284, 20170231 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen B. J., Wignall P. B., Hill D. J., Saupe E. E., Dunhill A. M., The latitudinal diversity gradient of tetrapods across the Permo-Triassic mass extinction and recovery interval. Proc. Biol. Sci. 287, 20201125 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powell M. G., Moore B. R., Smith T. J., Origination, extinction, invasion, and extirpation components of the brachiopod latitudinal biodiversity gradient through the Phanerozoic Eon. Paleobiology 41, 330–341 (2015). [Google Scholar]
- 12.Fenton I. S., et al. , The impact of Cenozoic cooling on assemblage diversity in planktonic foraminifera. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20150224 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kröger B., Changes in the latitudinal diversity gradient during the Great Ordovician Biodiversification Event. Geology 46, 127–130 (2017). [Google Scholar]
- 14.Yasuhara M., et al. , Cenozoic dynamics of shallow-marine biodiversity in the Western Pacific. J. Biogeogr. 44, 567–578 (2017). [Google Scholar]
- 15.Close R. A., Benson R. B. J., Saupe E. E., Clapham M. E., Butler R. J., The spatial structure of Phanerozoic marine animal diversity. Science 368, 420–424 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Jones L. A., et al. , Coupling of palaeontological and neontological reef coral data improves forecasts of biodiversity responses under global climatic change. R. Soc. Open Sci. 6, 182111 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saupe E. E., et al. , Climatic shifts drove major contractions in avian latitudinal distributions throughout the Cenozoic. Proc. Natl. Acad. Sci. U.S.A. 116, 12895–12900 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meseguer A. S., Condamine F. L., Ancient tropical extinctions at high latitudes contributed to the latitudinal diversity gradient. Evolution, 10.1111/evo.13967 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Crame J. A., Early Cenozoic evolution of the latitudinal diversity gradient. Earth Sci. Rev. 202, 103090 (2020). [Google Scholar]
- 20.Jablonski D., Roy K., Valentine J. W., Out of the tropics: Evolutionary dynamics of the latitudinal diversity gradient. Science 314, 102–106 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Vilhena D. A., et al. , Bivalve network reveals latitudinal selectivity gradient at the end-Cretaceous mass extinction. Sci. Rep. 3, 1790 (2013). [Google Scholar]
- 22.Reddin C. J., Kocsis Á. T., Kiessling W., Climate change and the latitudinal selectivity of ancient marine extinctions. Paleobiology 45, 70–84 (2019). [Google Scholar]
- 23.Fraser D., Hassall C., Gorelick R., Rybczynski N., Mean annual precipitation explains spatiotemporal patterns of Cenozoic mammal beta diversity and latitudinal diversity gradients in North America. PLoS One 9, e106499 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louca S., Pennell M. W., Extant timetrees are consistent with a myriad of diversification histories. Nature 580, 502–505 (2020). [DOI] [PubMed] [Google Scholar]