Significance
Pandemic influenza continues to threaten public health across the world. Cross-species transmission of avian H5N1, H7N9, and H10N8 subtypes represent a potential threat to human as the viruses may acquire the ability to pass efficiently from human to human. In order to develop an effective influenza vaccine with protective immunity against a broad range of human and avian influenza viruses, we generated a monoglycosylated consensus cHA (cHAmg) from human H1N1 and avian H5N1 HAs. After immunization with the monoglycosylated consensus cHA vaccine, mice were found to generate a high level of antibodies with broader cross-reactive neutralizing activities, higher ADCC responses, and more memory CD8+ T cell response. This strategy may facilitate the development of a universal vaccine against influenza viruses.
Keywords: influenza vaccine, chimeric hemagglutinin, monoglycosylated, broad protection, CD4 and CD8 T cell responses
Abstract
Vaccination has been used to control the spread of seasonal flu; however, the virus continues to evolve and escape from host immune response through mutation and increasing glycosylation. Efforts have been directed toward development of a universal vaccine with broadly protective activity against multiple influenza strains and subtypes. Here we report the design and evaluation of various chimeric vaccines based on the most common avian influenza H5 and human influenza H1 sequences. Of these constructs, the chimeric HA (cHA) vaccine with consensus H5 as globular head and consensus H1 as stem was shown to elicit broadly protective CD4+ and CD8+ T cell responses. Interestingly, the monoglycosylated cHA (cHAmg) vaccine with GlcNAc on each glycosite induced more stem-specific antibodies, with higher antibody-dependent cellular cytotoxicity (ADCC), and better neutralizing and stronger cross-protection activities against H1, H3, H5, and H7 strains and subtypes. Moreover, the cHAmg vaccine combined with a glycolipid adjuvant designed for class switch further enhanced the vaccine efficacy with more IFN-γ, IL-4, and CD8+ memory T cells produced.
Vaccination has been used to control the spread of influenza infection (1), and most influenza vaccines used to date target mainly the viral surface hemagglutinin (HA). However, HA is easy to mutate through antigenic drift and reassortment, so the vaccine has to be updated annually (2). The traditional method for influenza vaccine production is to culture the virus in specific-pathogen-free (SPF) embryonated hens eggs, and the process often requires more than 6 months for mass production (3). However, some vaccine virus strains grow poorly in eggs, and people with allergy to chicken egg could cause safety concerns. New approaches based on cell culture of viruses have been developed to replace the egg-based method (4, 5); but the cell-culture method still has a risk of producing potentially hazardous viruses. To overcome these problems, alternative strategies have been explored and demonstrated that recombinant HA-based vaccines can induce neutralizing antibodies against influenza virus infection (6, 7). However, the antibodies induced by a specific influenza virus strain or subtype usually could not effectively neutralize other strains or subtypes. In addition, the vaccine has to be updated annually because of the constant mutation of the virus.
Therefore, recent efforts have been directed toward the development of universal influenza vaccines with broadly neutralizing activities against various influenza strains and subtypes; these include the monoglycosylated HA-based vaccine with improved hemagglutination inhibition and microneutralization activity (8), the ferritin-based nanoparticles expressing influenza virus HA with neutralizing activity toward H1N1 viruses from 1934 to 2007 (9), the HA-stem nanoparticles which elicited stem-specific antibodies with protective activity against H5N1 subtypes (10), the trimeric HA stem of H1N1 (A/Brisbane/59/2007) which elicited antibodies with cross-neutralization activities in both mice and nonhuman primates (11), the chimeric HAs with head domain from different strains for sequential vaccination to induce stalk-reactive antibodies (12), and the DNA vaccine of consensus H5 (pCHA5-II) which elicited broadly neutralizing antibodies against multiple influenza H5N1 viruses (13).
Since HA is the most immunogenic epitope of influenza virus, it has been an excellent target for vaccine design. However, HA could still easily mutate along with increasing glycosylation, rendering the virus evolved to escape from the host immune response. In order to develop more effective universal vaccines, we considered the evolution of avian flu to human flu through HA mutation and used the most common avian influenza hemagglutinin H5 and human influenza H1 to construct various chimeric forms from their consensus sequences and investigate their immunogenicity and cross-protection activities. Of these constructs, the chimeric HA vaccine (cHA) with the consensus sequence of H5 as globular head and the consensus sequence of H1 as stem showed most promising, especially the one with the glycans at all glycosites trimmed down to N-acetylglucosamine. This monoglycosylated chimeric HA (cHAmg) stills retains its trimeric form and exposes more of its peptide epitopes. Immunization of the cHAmg vaccine in mice was found to elicit more cross-reactive antibodies recognizing H1, H3, H5, and H7 subtypes with higher neutralization activity and provided greater protection in challenge studies against divergent strains of H1N1 and H5N1 viruses as compared to other HA-based vaccines. Furthermore, the cHAmg vaccine induced more stem-specific antibody, higher levels of antibody-dependent cellular cytotoxicity (ADCC) activities toward H1N1, H3N2, and H5N1 influenza-infected cells and stronger CD8+ T cell response.
Results
Preparation and Characterization of Monoglycosylated Chimeric HA.
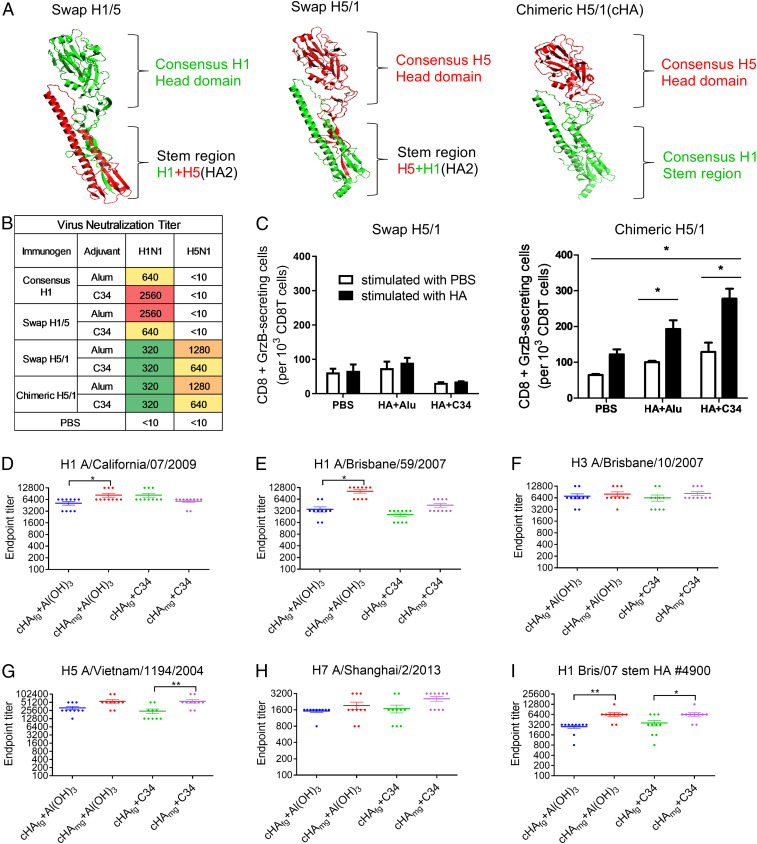
To design a universal vaccine, we first aimed to have a vaccine with broad protection against influenza A virus group 1 (H1 and H5 are the major subtypes while H2, H6, and H9 are minor). Therefore, the HA sequences from H1N1 viruses available from early 2009 to 2013 were used to create a consensus H1 sequence. The consensus H5 (13) and consensus H1 were then used as the templates for vaccine design. In influenza virus replication, the HA precursor (HA0) is proteolytically cleaved into two subunits, HA1 and HA2; the HA1 subunit carries the 5-N-acetylneuraminic acid (sialic acid) binding site, and the HA2 subunit is responsible for virus fusion with the host cellular membrane (SI Appendix, Fig. S1A). On the other hand, HA can be divided into two structural domains, globular head and stem, based on the three-dimensional (3D) structure. The stem region contains the HA2 domain, the N-terminal 36∼50 residues and a short stretch of the C terminus of the HA1 domain. We thus designed the vaccines based on various combinations of domains from H1 and H5. We first generated the swap H1/5 (H1 globular head and [H1+H5(HA2) stem], swap H5/1 (H5 globular head and [H5+H1(HA2) stem], and chimeric H5/1 (H5 globular head and H1 stem) for comparison (Fig. 1A and SI Appendix, Fig. S1A). The result indicated that immunization with consensus H1N1 and swap H1/5 did not induce cross-protective activities, but the swap H5/1 and chimeric H5/1 did elicit cross-neutralization activity against H1N1 and H5N1 viruses (Fig. 1B). We next investigated whether this cross-protection was contributed from the CD8+ T cell response, and found that granzyme B was more secreted in chimeric H5/1-immunized mice, suggesting that the chimeric H5/1 vaccine induced stronger CD8+ T cell response compared to the swap H5/1 vaccine (Fig. 1C).
Fig. 1.
The chimeric H5/1 construct with consensus H5 globular head and consensus H1 stem (cHA) and broadly cross-protective, stem-specific antibodies elicited by vaccination with cHAmg immunogens. (A) The constructs of swap H1/5 (H1 globular head and H1+H5[HA2] stem), swap H5/1 (H5 globular head and H5+H1[HA2] stem), and chimeric H5/1 (cHA: H5 globular head and H1 stem). (B) Neutralization activity against H1N1 California/07/2009 and H5N1 Vietnam/1194/2004 viruses. Titers of <400 (low) are shown in green, of 400 to 2,000 (medium) in yellow or orange, and >2,000 (high) in red. (C) The number of granzyme B (GrzB)-producing CD8+ T cells in splenocytes stimulated with HA (black bar) or PBS control (white bar) for 2 d in mice vaccinated with PBS (control), HA+Alu, or HA+C34 was evaluated by flow cytometric analysis. (D–I) The antibody titers from the mice vaccinated with cHAfg (blue) and cHAmg (red) adjuvanted with Al(OH)3 vs. cHAfg (green) and cHAmg (purple) adjuvanted with C34 were measured on day 42 by ELISA with the A/California/07/2009 H1N1 HA protein (D), A/Brisbane/59/2007 H1N1 HA protein (E), A/Brisbane/10/2007 H3N2 HA protein (F), A/Vietnam/1194/2004 H5N1 HA protein (G), A/Shanghai/2/2013 H7N9 HA protein (H), and the A/Brisbane/59/2007 (Bris/07) stem HA (no. 4900) protein (I) as the coating antigen. The endpoint antibody titer was defined as the last dilution of antisera to produce an absorbance 2.5 times higher than the optical absorbance produced by the negative control (preimmune serum). Data were examined by using Student’s t test and two-way ANOVA from Prism; differences were considered statistically significant at *P < 0.05; **P < 0.01. Data represent the mean ± SEM.
Effect of Glycosylation on the Immune Response of Chimeric H5/1 (cHA).
To explore the immunogenicity of the chimeric H5/1 (cHA) vaccine with different glycosylation states, monoglycosylated cHA (cHAmg) and fully glycosylated cHA (cHAfg) vaccine were compared (SI Appendix, Fig. S1). It is known that Endo-H is specific for high mannose but not complex-type glycans. The HA glycoprotein expressed in HEK293S cells, which are deficient in N-acetylglucosaminyltransferase I and produce glycoproteins with high-mannose-type N-glycans, was treated with Endo-H to cleave the N-glycans to a single GlcNAc residue. To generate cHAmg, cHA was produced from human cells (HEK293S) and the purified cHA with high-mannose glycans was treated with Endo-H to remove the outer parts of N-glycans to produce the HA with only one N-acetylglucosamine (GlcNAc) linked to the asparagine residue of each glycosite. After Endo-H treatment, the mixture was passed through gel filtration to separate Endo-H from trimeric cHAmg. After concentration, the cHAmg proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS/PAGE) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) analyses to ensure purity and glycan composition (SI Appendix, Fig. S1C). Since influenza HA exists as a trimer on the virus surface, gel filtration was performed to confirm that the cHAfg and cHAmg existed as a trimer (>200 kDa) (SI Appendix, Fig. S1D). We also generated another fully glycosylated cHAfg from human cells (HEK293T) for comparison (SI Appendix, Fig. S1B), and the cell culture yielded cHAfg with ∼6 mg/L.
The N-linked glycosylation sites and the glycan profile of recombinant cHAfg and cHAmg were analyzed by LC-MS/MS showing seven glycosylation sites (N28, N40, N171, N182, N292, N303, and N497); the N-glycans of cHAfg were mostly complex type and cHAmg could be obtained in ∼99% as a single glycoform with only GlcNAc at each of its N-glycosylation sites (SI Appendix, Fig. S1E and Table S1).
Cross-Reactivity of Antisera from Mice Immunized with Fully Glycosylated Chimeric H5/1 (cHAfg) and Monoglycosylated Chimeric H5/1 (cHAmg).
To evaluate the binding activity of antibody elicited by cHA constructs, BALB/c mice were immunized intramuscularly with 20 μg of cHAfg or cHAmg protein adjuvanted with Al(OH)3 or C34, an analog of α-galactosylceramide (α-GalCer) (14). The mice were immunized at weeks 0, 2, and 4, and HA-induced serum was obtained on days 28 and 42 and measured using enzyme-linked immunosorbent assay (ELISA) with various recombinant HAs (SI Appendix, Fig. S2). Comparing to the maximum dilution of antisera after two immunizations, three immunizations indeed produced antiserum with higher titers of HA-specific antibodies (Fig. 1 D–I and SI Appendix, Fig. S3), and vaccination with cHAmg induced better antibody response compared to cHAfg (Fig. 1 D, E, and G). In addition, the antiserum from cHAmg showed slightly better binding to H3 and H7 HA proteins (Fig. 1 F and H), and no significant differences were observed between Al(OH)3 and C34 adjuvants. These data indicate that the cHA vaccine could elicit cross-reactive antibodies recognizing the HA from H1N1, H3N2, H5N1, as well as H7N9 strains.
F10 is a broadly neutralizing IgG antibody known to target the stem region of HA, which is highly conserved among various subtypes of influenza viruses (15). To compare the binding of F10 to recombinant H1, H5, and cHA, the binding avidities of F10 to various HAs were measured, and the results showed that F10 could bind H1, H5, and cHA proteins (SI Appendix, Fig. S4). To investigate if F10-like antibodies were elicited by cHA vaccination, the binding of cHA-induced serum to HA stem no. 4900 (11) was measured by using ELISA. The results showed that the cHAmg vaccine can induce higher stem-specific antibody titer than cHAfg (Fig. 1I and SI Appendix, Fig. S3F), and better results were observed with C34-adjuvanted cHA vaccine (Fig. 1I), which induced more stem-specific antibodies.
Vaccination of Mice with cHAmg and Adjuvant C34 Elicits Strong CD4+ and CD8+ T Cell Responses and Antibody-Dependent Effector Functions, and Neutralizing Activities Against H1, H3, and H5 Viruses as Well as Their Subtypes.
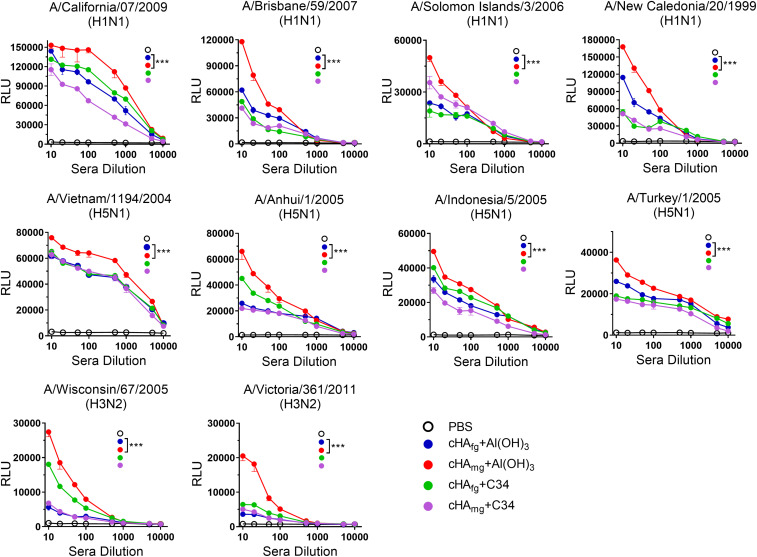
Besides antibody-mediated neutralization, Fc-mediated effector functions also play an important role in protection against influenza infection (16). We therefore examined whether the antibodies would induce Fc receptor-mediated immune response. The mouse-adapted ADCC assay was performed using Jurkat effector cells expressing FcγRIII to evaluate the ADCC activities of the sera from cHAfg- and cHAmg-immunized mice (Fig. 2). As expected, the serum from cHAfg- or cHAmg-vaccinated mice induced comparable levels of ADCC activities against H5N1 NIBRG14 (A/Vietnam/1194/2004), NIBRG23 (A/Turkey/1/2005), RG5 (A/Anhui/1/2005), or RG2 (A/Indonesia/5/2005) viruses. Interestingly, better ADCC activities were observed in the cHAmg group adjuvanted with Al(OH)3 (Fig. 2B), and similar results were observed in experiments against H1N1 A/California/07/2009, A/Brisbane/59/2007, A/Solomon Islands/3/2006, A/New Caledonia/20/1999 (Fig. 2A), H3N2 A/Wisconsin/67/2005, and A/Victoria/361/2011 viruses (Fig. 2C).
Fig. 2.
ADCC reporter assay of antisera from cHA-vaccinated mice against target cells expressing the HA of H1N1, H3N2, or H5N1 and subtypes. The antisera collected from mice immunized with cHAfg or cHAmg proteins adjuvanted with aluminum hydroxide or C34 were incubated with MDCK cells which were infected with (A) H1N1 virus (B) H5N1 virus, or (C) H3N2 virus for 30 min. Subsequently, the ADCC reporter assay was performed using Jurkat effector cells expressing mouse FcγRIII, and the relative luminescence unit (RLU) was measured and values are mean ± SEM. ***P < 0.001. The P value was calculated with Prism software using two-way ANOVAs.
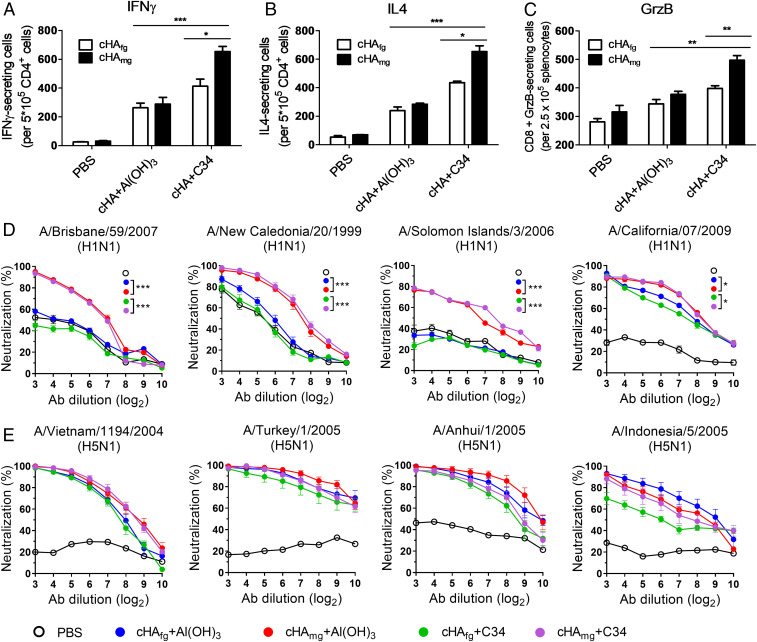
To evaluate the role of antigen-specific cytokine-secreting cells in cHA-immunized mice, the splenocytes were collected after two and three immunizations and the IFN-γ, IL-4, and granzyme B (GzB)-secreting cells were estimated by enzyme-linked immune absorbent spot (ELISpot) assays with specific peptides from HA for stimulation. As shown in Fig. 3, the cHAfg and cHAmg vaccines adjuvanted with Al(OH)3 produced similar levels of cytokine-secreting cells. However, more CD4+/IFN-γ+ Th1 cells (Fig. 3A), CD4+/IL-4+ Th2 (Fig. 3B), and CD8+ GzB-secreting cells (Fig. 3C) were elicited in cHAmg vaccination adjuvanted with C34 than with Al(OH)3. These results confirmed that cHAmg adjuvanted with C34 could stimulate more CD4+ T helper response and stronger CD8+ cytotoxicity effects compared to cHAfg.
Fig. 3.
More CD4+ and CD8+ T cell responses and broadly neutralizing antibodies were elicited to give broader cross-protection by cHAmg with adjuvant C34. BALB/c mice were immunized with cHAfg and cHAmg with adjuvant Al(OH)3 or C34; cells from spleens of immunized mice were obtained after three immunizations, and the IFN-γ (A), IL-4 (B), and GzB (C)-secreting cells were determined by ELISpot assay using specific peptides. The number of spot-forming cells (SFCs) is expressed as mean ± SEM. The neutralization activities of antisera from cHAfg- and cHAmg-vaccinated mice were assayed against (D) H1N1 virus, and (E) H5N1 virus. Data are presented as mean ± SEM. Results were calculated with Prism software using Student’s t test and two-way ANOVA; significant differences were marked as *P < 0.05; **P < 0.01; ***P < 0.001.
To evaluate the dose dependence of C34 on antibody titers and cell-mediated immunity, mice were immunized intramuscularly with cHAfg adjuvanted with three different doses of C34 at 0.5, 2, and 10 μg. The result indicated that cHAfg adjuvanted with 2 μg of C34 induced higher titers than with 0.5 and 10 μg of C34 after two or three immunizations (SI Appendix, Fig. S5). In addition, the cHAfg vaccine adjuvanted with 2 μg of C34 induced more IFN-γ than with 0.5 and 10 μg of C34 (SI Appendix, Fig. S6A) and 2 and 10 μg of C34 induced more IL-4 than with 0.5 μg of C34 after three immunizations (SI Appendix, Fig. S6B). On the other hand, there were no differences with regard to the increase in CD8+ GzB-secreting cells when the cHAfg vaccine was adjuvanted with 0.5, 2, or 10 μg of C34 after two and three immunizations (SI Appendix, Fig. S6C). Based on these observations, 2 μg of C34 were used throughout the experiments.
The neutralizing activities of cHA-induced antisera were further investigated. The antisera from cHAmg vaccination were shown to have better neutralization activities against the homologous viruses H1N1 A/California/07/2009 (Fig. 3D) and heterologous H5N1 NIBRG14 (A/Vietnam/1194/2004), NIBRG23 (A/Turkey/1/2005), RG5 (A/Anhui/1/2005), or RG2 (A/Indonesia/5/2005) (Fig. 3E). In addition, the antisera from mice vaccinated with cHAmg exhibit significant neutralizing activities against heterologous viruses H1N1 A/Brisbane/59/2007, A/New Caledonia/20/1999, and A/Solomon Islands/3/2006 (Fig. 3D). The antisera from cHA-immunized mice were clearly able to block the infection of H1N1 and H5N1 viruses, and the neutralizing activity of cHAmg was in general better than cHAfg, particularly against the heterologous viruses.
Vaccination of Mice with cHAmg/C34 Provides Cross-Protection Against H1N1 and H5N1 as Well as Their Subtypes in the Challenge Study.
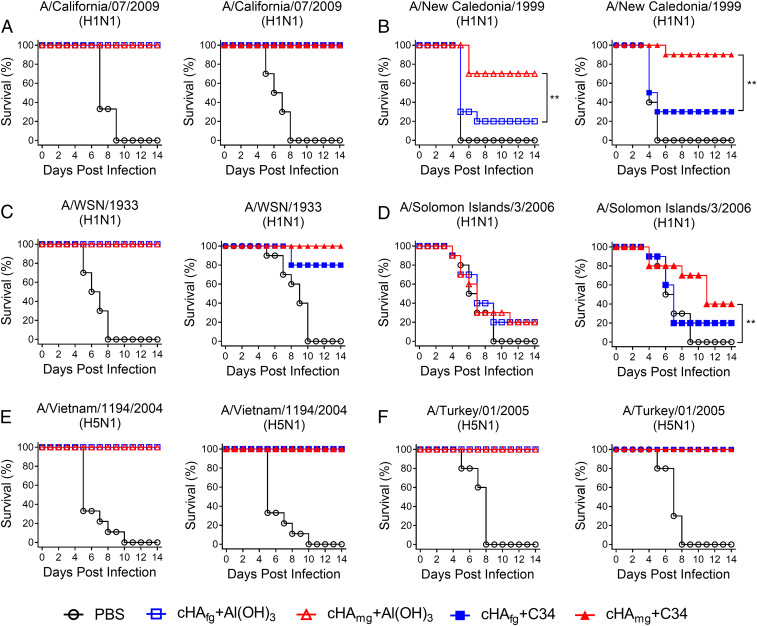
In order to assess whether cHAmg vaccination provides broadly cross-protective immunity against various H1N1 and H5N1 viruses, the vaccinated mice were challenged by intranasal inoculation with lethal doses of multiple H1N1 and H5N1 viruses, and the efficacy of vaccine protection was evaluated for 14 d by recording the survival rate and body weight change (Fig. 4 and SI Appendix, Fig. S7). For the mice challenged with H1N1 A/California/07/2009 viruses, all cHA vaccines offered 100% protection (Fig. 4A). In addition, the mice immunized with C34-adjuvanted cHAmg showed minimal amounts of weight loss compared with cHAfg (SI Appendix, Fig. S7A). The mice immunized with C34-adjuvanted cHAfg only gave 30% protection against A/New Caledonia/1999 challenges; however, the cHAmg vaccine adjuvanted with C34 offered 90% protection against cross-strain A/New Caledonia/1999 viruses, and similar results were observed in Al(OH)3-adjuvanted cHA vaccination (Fig. 4B). For the mice challenged with cross-strain A/WSN/1933 viruses, all mice immunized with Al(OH)3-adjuvanted cHA survived; however, the mice immunized with C34-adjuvanted cHAfg only gave 80% protection (Fig. 4C). The lethal challenges were also performed with A/Solomon Islands/03/2006. All mice immunized with Al(OH)3-adjuvanted cHA showed lower protection; however, the mice immunized with C34-adjuvanted cHAmg showed better protection against cross-strain A/Solomon Islands/03/2006 viruses (Fig. 4D). For the mice challenged with H5N1 NIBRG14 (A/Vietnam/1194/2004) and NIBRG23 (A/Turkey/1/2005), all immunized mice survived (Fig. 4 E and F). The body weight changes after viral challenges were also evaluated (SI Appendix, Fig. S7). The data showed that cHA was effective in eliciting a significant protective immunity against various H1N1 and H5N1 viruses, and cHAmg provides a broader cross-protection ability compared to cHAfg.
Fig. 4.
Cross-protective efficacy in mice challenged with lethal doses of H1N1 and H5N1 viruses. BALB/c mice were immunized with three doses of cHAfg and cHAmg with adjuvant Al(OH)3 or C34 at 2-wk intervals. The immunized mice were challenged with H1N1 A/California/07/2009 (A), H1N1 A/New Caledonia/1999 (B), H1N1 A/WSN/1933 (C), H1N1 A/Solomon Islands/03/2006 (D), H5N1 A/Vietnam/1194/2004/NIBRG14 (E), or H5N1 A/Turkey/1/2005/NIBRG23 (F), and the efficacy was evaluated by recording the survival rate for 14 d after infection. **P < 0.01. Significant differences in survival rate were analyzed by log-rank (Mantel–Cox) test.
Discussion
Development of universal influenza vaccine to provide protection against multiple strains and subtypes of influenza viruses is of current interest, and the epitopes used for universal vaccine development include the highly conserved ectodomain of M2 containing 24 nonglycosylated amino acids (17), the nucleoprotein NP (18), and the various HA constructs which have been shown to induce higher titers of broadly neutralizing antibodies to target the HA-stem region or block viral entry. For example, a soluble trimeric HA (mini-HA) vaccine with realigned stem subunit was shown to completely protect mice from lethal challenge by heterologous and heterosubtypic viruses (11), and a chimeric HA vaccination with DNA prime-protein boost and exposure to the same stem region and divergent exotic head domains was shown to elicit broadly protective stem-specific antibodies (12). However, the result showed that CD8+ T cells did not play a key role in the cross-protective activities. Although DNA vaccines are promising, they are still in the early stage of development (19). In this study, the cHA constructs that express the consensus H5 of globular head and the consensus H1 of stem region were designed to mimic the real status of influenza virus transmitting from avian virus to human. Both fully glycosylated cHAfg and monoglycosylated cHAmg were prepared for comparison, and the result showed that the cHAmg vaccine elicited higher titers of cross-reactive antibodies against H1, H3, H5, and H7 subtypes (Fig. 1 D–H) through CD4+ and CD8+ T cell responses (Fig. 3 A–C).
The glycosylation of HA was shown to play an important role in protein folding and stability and in modulating its biological activities (20), including shielding the antigenic sites from neutralizing antibodies to reduce the immunogenicity (21). In addition, hyperglycosylated HA was evolved to mask the antigenic sites in the highly variable head domain and the immune response was thus redirected toward the conserved stem region (22). In our results, the neutralization activities of the cHAmg antiserum were significantly superior to the cHAfg-induced antiserum, especially against the heterologous H1N1 A/Brisbane/59/2007, A/Solomon Islands/03/2006, and A/New Caledonia/20/1999 (Fig. 3D). The broader neutralizing activities of cHAmg vaccine is probably due its induction of more antibody variants as reported previously (8). IgG is the predominant antibody present in mouse and is the major subtype of HA-specific antibodies with high avidity to the FcγRIII receptor on immune cells to induce ADCC (23). We showed that immunization with cHAmg induced higher ADCC and more stem-specific antibodies with better protection activity (Figs. 1I and 2), consistent with the studies showing that ADCC is necessary for influenza protection in vivo (16, 24).
Aluminum hydroxide (Alum) was known to stimulate Th2 response and was approved by the FDA for use as vaccine adjuvant (25); however, its mode of action has not been well studied. The glycolipid C34 is a ligand for and presented by CD1d on dendritic cells to interact with a receptor on invariant natural killer T (iNKT) cells, leading to the stimulation of iNKT cells to produce Th1 cytokines (e.g., IFN-γ) with adjuvant effect and Th2 cytokines (e.g., IL-4) with class-switch activity (26). In our results, the number of IFN-γ (Th1 cytokine), IL-4 (Th2 cytokine)-secreting cells, and the granzyme B-producing CD8+ T cells were significantly increased by immunization with cHAmg adjuvanted with C34 than with Al(OH)3 (Fig. 3 A–C).
In summary, development of next-generation influenza vaccines with broad-protective immune responses is of current interest, and some promising results have been reported, making the development of a universal vaccine within reach (27–30). In an effort directed toward this goal, we have successfully demonstrated in this study a proof of principle that the monoglycosylated cHA vaccine with consensus H5 head and consensus H1 stem is an effective influenza vaccine exhibiting a broad protection activity against heterologous influenza viruses, including H1, H3, H5, and H7 viruses and subtypes in the neutralizing study and H1N1, H5N1, and subtypes in the challenge study. With the success in the development of a broadly protective vaccine against different strains and subtypes of influenza A virus, we aim to use the strategy developed in this study to design a broader universal vaccine against influenza A and B viruses and the work is ongoing.
Materials and Methods
The attenuated reassortant H5N1 influenza viruses A/Vietnam/1194/2004/NIBRG14, A/turkey/Turkey/01/2005/NIBRG23, H3N2 influenza viruses A/Wisconsin/67/2005, A/Victoria/361/2011, H1N1 influenza viruses A/California/07/2009, A/Brisbane/59/2007, A/Solomon Islands/03/2006, A/New Caledonia/20/1999, and A/WSN/1933 were obtained from the reference collection of the National Institute for Biological Standards and Control. A/Indonesia/5/2005/RG2 was obtained from the Center for Disease Control (CDC) in Indonesia. A/Anhui/1/2005/RG5 was provided by the US CDC. All viruses were inoculated into the allantoic cavities of 10-d-old embryonated SPF chicken eggs. After 2 d of incubation at 35 °C, the allantoic fluid was collected and stored at −80 °C. The viruses were titered in Madin–Darby canine kidney (MDCK) cells, and the titer was expressed as the 50% tissue culture infectious dose (TCID50). The 50% lethal dose (LD50) of virus in BALB/c mice was determined before experiments.
Mice studies were evaluated and approved by the Institutional Animal Care and Use Committee of Academia Sinica.
More details are provided in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Yung-Chieh Tseng, Ming-Liang Liu, and Chung-Hsuan Wu (Genomics Research Center, Academia Sinica) for technical assistance and Dr. Jia-Tsrong Jan and Hsiu-Hua Ma (Genomics Research Center, Academia Sinica) for virus strains. This research was supported by the Summit Program of Academia Sinica (AS-SUMMIT-108), Ministry of Science and Technology, Taiwan (MOST 107-0210-01-19-01, MOST 108-3114-Y-001-002).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2004783117/-/DCSupplemental.
Data Availability.
The authors confirm that the data supporting the findings of this study are available within the article and SI Appendix.
References
- 1.Osterholm M. T., Preparing for the next pandemic. N. Engl. J. Med. 352, 1839–1842 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Wong S. S., Webby R. J., Traditional and new influenza vaccines. Clin. Microbiol. Rev. 26, 476–492 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webby R. J., Webster R. G., Are we ready for pandemic influenza? Science 302, 1519–1522 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Hu A. Y. C. et al., Microcarrier-based MDCK cell culture system for the production of influenza H5N1 vaccines. Vaccine 26, 5736–5740 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Kistner O. et al., Cell culture (Vero) derived whole virus (H5N1) vaccine based on wild-type virus strain induces cross-protective immune responses. Vaccine 25, 6028–6036 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei C. J. et al., Comparative efficacy of neutralizing antibodies elicited by recombinant hemagglutinin proteins from avian H5N1 influenza virus. J. Virol. 82, 6200–6208 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safdar A., Cox M. M. J., Baculovirus-expressed influenza vaccine. A novel technology for safe and expeditious vaccine production for human use. Expert Opin. Investig. Drugs 16, 927–934 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Chen J. R. et al., Vaccination of monoglycosylated hemagglutinin induces cross-strain protection against influenza virus infections. Proc. Natl. Acad. Sci. U.S.A. 111, 2476–2481 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanekiyo M. et al., Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 499, 102–106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yassine H. M. et al., Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 21, 1065–1070 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Impagliazzo A. et al., A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 349, 1301–1306 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Krammer F., Pica N., Hai R., Margine I., Palese P., Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J. Virol. 87, 6542–6550 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen M. W. et al., Broadly neutralizing DNA vaccine with specific mutation alters the antigenicity and sugar-binding activities of influenza hemagglutinin. Proc. Natl. Acad. Sci. U.S.A. 108, 3510–3515 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu T. N. et al., Phenyl glycolipids with different glycosyl groups exhibit marked differences in murine and human iNKT cell activation. ACS Chem. Biol. 11, 3431–3441 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Sui J. et al., Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16, 265–273 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiLillo D. J., Tan G. S., Palese P., Ravetch J. V., Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat. Med. 20, 143–151 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiers W., De Filette M., Birkett A., Neirynck S., Min Jou W., A “universal” human influenza A vaccine. Virus Res. 103, 173–176 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Zheng M., Luo J., Chen Z., Development of universal influenza vaccines based on influenza virus M and NP genes. Infection 42, 251–262 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Laddy D. J., Weiner D. B., From plasmids to protection: A review of DNA vaccines against infectious diseases. Int. Rev. Immunol. 25, 99–123 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Tate M. D. et al., Playing hide and seek: How glycosylation of the influenza virus hemagglutinin can modulate the immune response to infection. Viruses 6, 1294–1316 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei C. J. et al., Cross-neutralization of 1918 and 2009 influenza viruses: Role of glycans in viral evolution and vaccine design. Sci. Transl. Med. 2, 24ra21 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eggink D., Goff P. H., Palese P., Guiding the immune response against influenza virus hemagglutinin toward the conserved stalk domain by hyperglycosylation of the globular head domain. J. Virol. 88, 699–704 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nimmerjahn F., Gordan S., Lux A., FcγR dependent mechanisms of cytotoxic, agonistic, and neutralizing antibody activities. Trends Immunol. 36, 325–336 (2015). [DOI] [PubMed] [Google Scholar]
- 24.DiLillo D. J., Palese P., Wilson P. C., Ravetch J. V., Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J. Clin. Invest. 126, 605–610 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bungener L. et al., Alum boosts TH2-type antibody responses to whole-inactivated virus influenza vaccine in mice but does not confer superior protection. Vaccine 26, 2350–2359 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Huang Y. L. et al., Carbohydrate-based vaccines with a glycolipid adjuvant for breast cancer. Proc. Natl. Acad. Sci. U.S.A. 110, 2517–2522 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erbelding E. J. et al., A universal influenza vaccine: The strategic plan for the national institute of allergy and infectious diseases. J. Infect. Dis. 218, 347–354 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sautto G. A., Kirchenbaum G. A., Ross T. M., Towards a universal influenza vaccine: Different approaches for one goal. Virol. J. 15, 17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estrada L. D., Schultz-Cherry S., Development of a universal influenza vaccine. J. Immunol. 202, 392–398 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J. et al., Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science 367, eaau0810 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and SI Appendix.