Significance
Cells from all domains of life sense and respond to mechanical and physical cues. In eukaryotes, mechanical signals such as adhesion and surface stiffness are important for regulating fundamental processes including cell differentiation during embryonic development. While mechanobiology is abundantly studied in eukaryotes, the role of mechanical and physical influences on prokaryotic biology remains underinvestigated. Here, we demonstrate that surface sensing mediated through obstruction of the dynamic extension and retraction of tight adherence (tad) pili stimulates cell differentiation and cell-cycle progression in the dimorphic α-proteobacterium Caulobacter crescentus. Our results demonstrate an important intersection between mechanical stimuli and the regulation of a fundamental aspect of cell biology.
Keywords: surface sensing, type IV pili, cell cycle, cellular differentiation, bacteria
Abstract
Cellular differentiation is a fundamental strategy used by cells to generate specialized functions at specific stages of development. The bacterium Caulobacter crescentus employs a specialized dimorphic life cycle consisting of two differentiated cell types. How environmental cues, including mechanical inputs such as contact with a surface, regulate this cell cycle remain unclear. Here, we find that surface sensing by the physical perturbation of retracting extracellular pilus filaments accelerates cell-cycle progression and cellular differentiation. We show that physical obstruction of dynamic pilus activity by chemical perturbation or by a mutation in the outer-membrane pilus secretin CpaC stimulates early initiation of chromosome replication. In addition, we find that surface contact stimulates cell-cycle progression by demonstrating that surface-stimulated cells initiate early chromosome replication to the same extent as planktonic cells with obstructed pilus activity. Finally, we show that obstruction of pilus retraction stimulates the synthesis of the cell-cycle regulator cyclic diguanylate monophosphate (c-di-GMP) through changes in the activity and localization of two key regulatory histidine kinases that control cell fate and differentiation. Together, these results demonstrate that surface contact and sensing by alterations in pilus activity stimulate C. crescentus to bypass its developmentally programmed temporal delay in cell differentiation to more quickly adapt to a surface-associated lifestyle.
In multicellular organisms, cellular differentiation is required for the formation of complex tissues and organs (1). In unicellular organisms, the ability to coordinate and control specialized cell morphologies and functions is critical for niche survival in diverse environments (2). Caulobacter crescentus exhibits a dimorphic life cycle where asymmetric division results in the production of a nonreproductive, motile swarmer cell and a reproductive, nonmotile stalked cell. In addition to their distinct reproductive states, each of these cell types possesses different polar structures. The swarmer cell is equipped with a single flagellum and multiple type IVc tight adherence (tad) pili at the same pole that are lost upon cellular differentiation into the stalked cell. Tad pili are subsequently replaced with a holdfast adhesin that mediates irreversible surface attachment and a thin cell-envelope extension called the stalk (3, 4).
Distinguishing characteristics between swarmer and stalked cells are partly due to the action of the master response regulator CtrA (4). In swarmer cells, CtrA is phosphorylated and binds strongly to chromosomal sites near the origin of replication, preventing the initiation of DNA replication and thus locking cells in a nonreproductive, arrested G1 phase. During differentiation from swarmer to stalked cell, CtrA is dephosphorylated and proteolytically cleaved to allow for entry into S phase and subsequent chromosome replication (4).
Regulatory control over differentiation is mediated by oscillating levels of cyclic diguanylate monophosphate (c-di-GMP), a ubiquitous secondary messenger molecule that coordinates bacterial behavior in diverse species (5). Newborn swarmer cells have low concentrations of c-di-GMP that slowly increase as they age. Between 20 and 40 min postdivision, a maximal level of c-di-GMP is observed, coinciding with holdfast synthesis and the transition from the motile to the sessile state. At the same time, a high level of c-di-GMP stimulates the dephosphorylation and deactivation of CtrA, allowing for chromosome replication as the swarmer cell differentiates (4).
C-di-GMP levels are controlled by the activity of the two histidine kinases PleC and DivJ, which localize at the swarmer and stalked pole of predivisional cells, respectively, and which dictate the distinct fates of the two progeny cells (6). Delocalization of PleC and localization of DivJ at the incipient stalked pole during cell differentiation mediate the activation of the diguanylate cyclase PleD by phosphorylation, resulting in an increase in c-di-GMP.
Although the signal transduction network governing the transition from swarmer to stalked cell has been well-described, whether surface attachment impacts this process is not known. Here, we demonstrate that inhibition of dynamic pilus activity stimulates c-di-GMP to initiate stalked cell development. We show that physical obstruction of pilus retraction and surface contact stimulates the initiation of DNA replication. We show that a mutation in the outer-membrane pilus secretin CpaC that partially disrupts pilus retraction stimulates holdfast synthesis and initiation of DNA replication in a PilA-dependent fashion. Finally, we show that physical obstruction of pilus retraction directly stimulates c-di-GMP synthesis by accelerating the delocalization of PleC and localization of DivJ at the incipient stalked pole, key steps in the activation of PleD and the production of c-di-GMP. Thus, by stimulating the synthesis of the holdfast (7) and cell differentiation, surface contact ensures that the permanently attached cell enters the stalked phase, which is best adapted for nutrient uptake on a surface (8).
Results
Obstruction of Pilus Retraction Stimulates DNA Replication Initiation.
Whether mechanical inputs can stimulate C. crescentus cell differentiation is unknown. Previous work has demonstrated that C. crescentus swarmer cells produce holdfast in response to surface contact independent of cell age (7, 9, 10), and recent findings suggest that this surface-stimulated holdfast synthesis is mediated by changes in type IVc tad pilus dynamic activity upon binding of pili to a surface (7). C. crescentus tad pili exhibit dynamic cycles of extension and retraction by polymerization and depolymerization of the major pilin subunit, PilA. Visualization of pili and their dynamic activity is achieved through a knockin cysteine mutation in PilA (Pil-cys) followed by the addition of thiol-reactive maleimide conjugates (7, 11, 12). Dynamic activity of pilus fibers can be obstructed by the addition of bulky maleimide conjugates like polyethylene glycol maleimide (PEG-mal) to Pil-cys strains. In C. crescentus, obstruction of dynamic pilus activity through this method stimulates holdfast synthesis in the absence of surface contact, suggesting that the tension on surface-bound, retracting tad pili stimulates bacterial surface sensing (7).
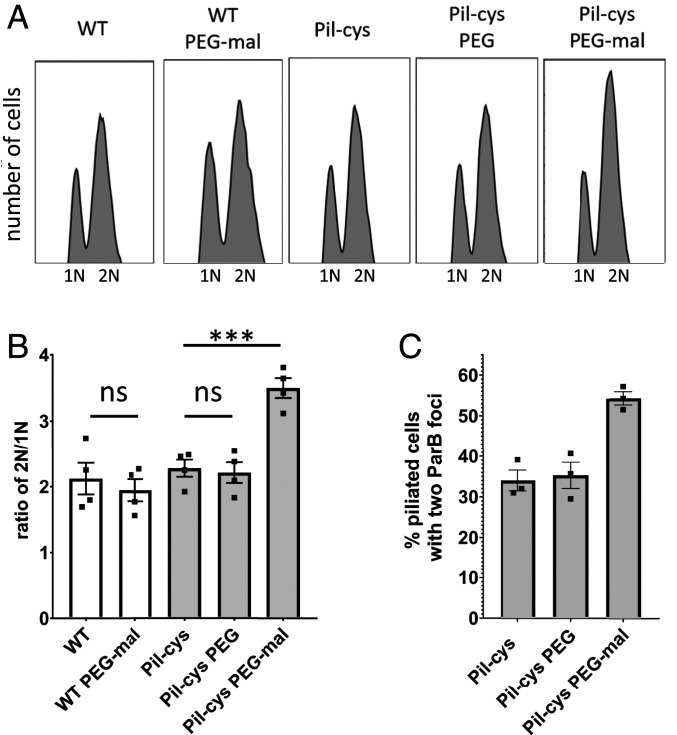
Because cell-cycle progression and cellular differentiation are concomitant with holdfast synthesis in planktonic cells, we hypothesized that surface contact may also accelerate the C. crescentus life cycle. A key marker for cell-cycle progression is the initiation of DNA replication. We reasoned that should surface sensing stimulate initiation of DNA replication, cells with obstructed pilus dynamics would have a higher DNA content compared with nonstimulated cells. To test this hypothesis, we incubated wild-type (WT) or Pil-cys cells with or without PEG-mal followed by rifampicin treatment to prevent new initiation of DNA replication while allowing for the completion of rounds of DNA replication that had already been initiated. We then labeled genomic DNA of treated cell cultures using SYTOX DNA-intercalating fluorescent dye and performed flow cytometry to quantify the DNA content of populations of cells. Swarmer cells arrested in G1 phase harbor a single chromosome (1N), whereas cells that initiate chromosome replication prior to rifampicin treatment possess two chromosomes (2N). WT populations and untreated populations of the Pil-cys strain exhibited an ∼2-fold ratio of 2N:1N chromosome content. In contrast, the Pil-cys population treated with PEG-mal exhibited an ∼3-fold ratio of 2N:1N chromosome content (Fig. 1 A and B). Importantly, Pil-cys cells treated with polyethylene glycol lacking the thiol-reactive maleimide group (PEG) exhibited a ratio of 2N:1N genomic content similar to WT cells and untreated Pil-cys cells, and PEG-mal treatment did not cause cell aggregation (SI Appendix, Fig. S1). These results suggest that obstruction of pilus dynamics stimulates the initiation of DNA replication.
Fig. 1.
Obstruction of pilus retraction stimulates DNA replication initiation. (A) Representative flow cytometry plots showing chromosome content of cells quantified in B. (B) Ratio of cells with two chromosomes to cells with one chromosome determined by flow cytometry analysis of genomic content. Bar graph shows the mean ± SEM of three independent biological replicates. Flow cytometry experiments were performed with holdfast synthesis mutants to prevent cell–cell aggregation. (C) Quantification of the percent of piliated cells with two ParB-mCherry foci. Maleimide-positive cells were labeled as “piliated” as the fluorophore entered the cells via pilus retraction. Bar graph shows the mean ± SEM of three independent biological replicates. A minimum of 100 cells was quantified for each replicate. Statistical comparisons were made using Sidak’s multiple-comparisons test. ***P < 0.001; ns, not significant.
To confirm the above results, we tracked chromosome replication at the single-cell level. During S phase, the chromosomal partitioning system parABS in C. crescentus is involved in chromosome segregation. ParB dimers bind parS sequences adjacent to the origin of replication and subsequent interactions with cytoplasmic ParA help to physically migrate the ParB–parS DNA complex across the length of the cell (13). To determine whether obstruction of pilus dynamics stimulates the initiation of DNA replication, we tracked the localization of ParB in cells obstructed for pilus retraction with PEG-mal. For cells in G1 phase, a single ParB focus is observed at the flagellar pole where the origin of replication is localized. After initiation of DNA replication, a second ParB focus appears as newly synthesized parS sites are bound by ParB dimers and translocated to the opposite cell pole. We thus examined the percentage of piliated cells with two ParB foci as a marker for cells that had initiated DNA replication. Because cells that lack pili containing the Pil-cys mutation are unaffected by PEG-mal treatment (Fig. 1 A and B and Table 1), we focused on piliated cells to assess how obstruction of pilus retraction affected ParB duplication. When treated with PEG-mal, the Pil-cys strain exhibited a 20% increase in the number of piliated cells with two ParB foci as compared with untreated and PEG-treated cells (Fig. 1C and Table 1). Taken together, these results suggest that obstruction of pilus dynamics stimulates entry into the cell cycle.
Table 1.
Percent of piliated cells and cells harboring two ParB foci in piliated or unpiliated cells
| Category | Untreated | PEG | PEG-mal |
| Total cells with labeled pili | 18.8 ± 3.4 | 19.9 ± 6.1 | 19.9 ± 7.5 |
| Total cells with two ParB foci | 79.9 ± 4.8 | 76.9 ± 13.4 | 86.2 ± 4.7 |
| Piliated cells with two ParB foci | 33.5 ± 1.7 | 34.6 ± 1.1 | 56.6 ± 2.1 |
| Unpiliated cells with two ParB foci | 90.5 ± 4.4 | 86.8 ± 13.7 | 93.5 ± 2.1 |
Mean ± SD is shown.
A Mutation in the Outer-Membrane Pilus Secretin That Disrupts Pilus Retraction Stimulates Holdfast Synthesis and Initiation of DNA Replication.
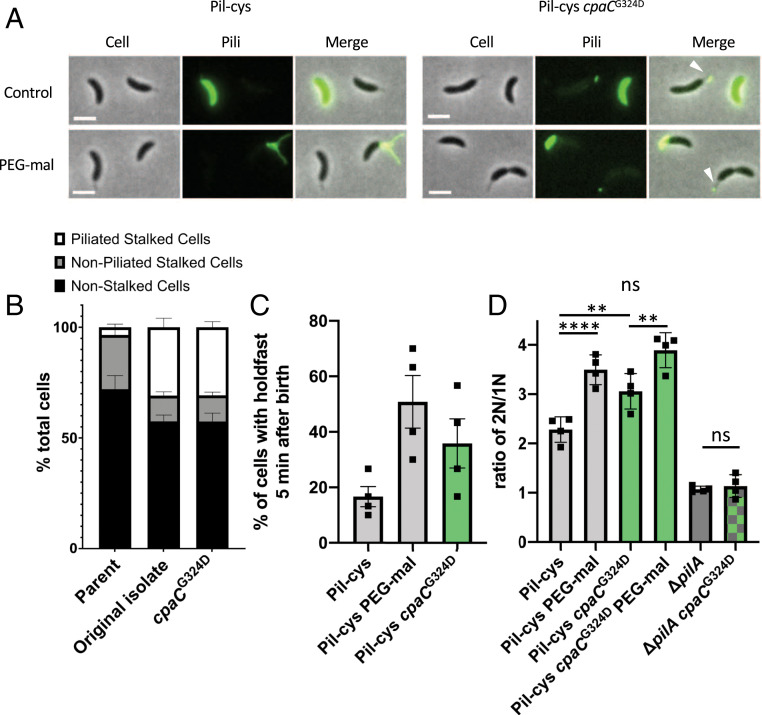
Because chemical obstruction of pilus retraction through the addition of PEG-mal stimulates initiation of DNA replication, we reasoned that some mutants genetically deficient in pilus retraction would exhibit a similar phenotype. Since pili are terminally retracted prior to cellular differentiation, we hypothesized that stalked cells of a retraction mutant would exhibit an increase in the number of cells with pili localized at the tips of stalks where the outer-membrane secretin CpaC remains after stalk synthesis (14). Because retraction mutants in several species are hyperpiliated and hyperpiliation results in increased surface attachment, we performed an unbiased genetic screen to enrich for mutants that attach more efficiently to surfaces. We then screened the enriched cell population for changes in pilus-dependent ΦCbK phage sensitivity because we assumed that a mutant deficient in pilus dynamics would be more resistant to pilus-dependent phage infection. From this screen, we isolated a mutant that harbored pili at the tips of stalked cells, indicative of obstructed pilus retraction and a failure to terminally retract its pili prior to cellular differentiation (Fig. 2 A and B). Whole-genome sequencing revealed a mutation that mapped to the outer-membrane pilus secretin gene cpaCG324D. Movement of this mutation into the parent Pil-cys strain by allelic replacement resulted in cells harboring pili at the tips of stalks, confirming that the identified cpaCG324D mutation was responsible for the observed phenotype (Fig. 2 A and B).
Fig. 2.
Mutation in the CpaC outer-membrane pilus secretin partially obstructs pilus retraction and stimulates cell-cycle progression and cellular differentiation. (A) Representative images of Pil-cys parent and strain containing the cpaCG324D mutation. Arrowheads indicate stalks with labeled pilus fibers attached to them. (Scale bars, 2 µm.) (B) Quantification of the piliated stalk phenotype shown in A. Data are from four independent biological replicates and the bar graph shows the mean ± SEM. (C) Percent of synchronized cells that have made a holdfast by the start of the imaging experiment 5 min after birth. Bar graph shows the mean ± SEM. Data are from four independent biological replicates (n = 30 cells per replicate). (D) Ratio of cells with two chromosomes to cells with one chromosome determined by flow cytometry analysis of genomic content. Bar graph shows the mean ± SEM of four independent biological replicates. Flow cytometry experiments were performed with holdfast synthesis mutants to prevent cell–cell aggregation. Statistical comparisons were made using Sidak’s multiple-comparisons test. **P < 0.005, ****P < 0.0001.
To test whether the obstruction of pilus retraction mediated by the cpaCG324D mutation stimulates cell-cycle progression similar to physical obstruction by PEG-mal treatment, we first quantified holdfast synthesis in mutant populations. In the cpaCG324D mutant, ∼36% of synchronized cells produced a holdfast within 5 min of birth as compared with 17% in cells with the wild-type allele of cpaC (Fig. 2C). By comparison, 51% of cells obstructed for pilus retraction by the addition of PEG-mal synthesize a holdfast within 5 min of birth. These results suggest that the cpaCG324D mutant is partially stimulated for surface sensing. Interestingly, the cpaCG324D mutant appears only partially obstructed for pilus retraction as evidenced by fluorescent cell bodies (Fig. 2A). Indeed, we have previously shown that cell-body fluorescence in Pil-cys cells labeled with fluorescent maleimide is dependent upon pilus retraction and dispersal of labeled pilins into an inner-membrane pilin pool (7). As the cpaCG324D mutant exhibits both cell-body fluorescence as well as pili at the tips of stalks, we infer that there is only partial obstruction of pilus retraction.
To test whether the cpaCG324D mutant had an increase in DNA replication initiation similar to cells physically obstructed for pilus retraction, we measured the DNA content of cpaCG324D mutants. We found that the cpaCG324D mutant had an intermediate increase in the number of cells harboring two chromosomes compared with the PEG-mal–treated Pil-cys strain and WT, indicative of accelerated cell-cycle progression (Fig. 2D). Importantly, a cpaCG324D pilA double mutant lacking the major pilin subunit exhibited the same phenotype as a pilA mutant alone, demonstrating that cell-cycle acceleration of the cpaCG324D mutant is dependent on the presence of PilA. Notably, the cpaCG324D mutant is further stimulated for DNA replication upon PEG-mal treatment, reaching a similar 2N:1N ratio as Pil-cys cells treated with PEG-mal (Fig. 2D). As discussed above, cpaCG324D appears only partially obstructed for pilus retraction, and PEG-mal treatment likely obstructs the remaining retraction activity. These results suggest that obstruction of pilus dynamics by the cpaCG324D mutation stimulates both holdfast synthesis and entry into the cell cycle.
Surface Contact Stimulates Cell-Cycle Progression.
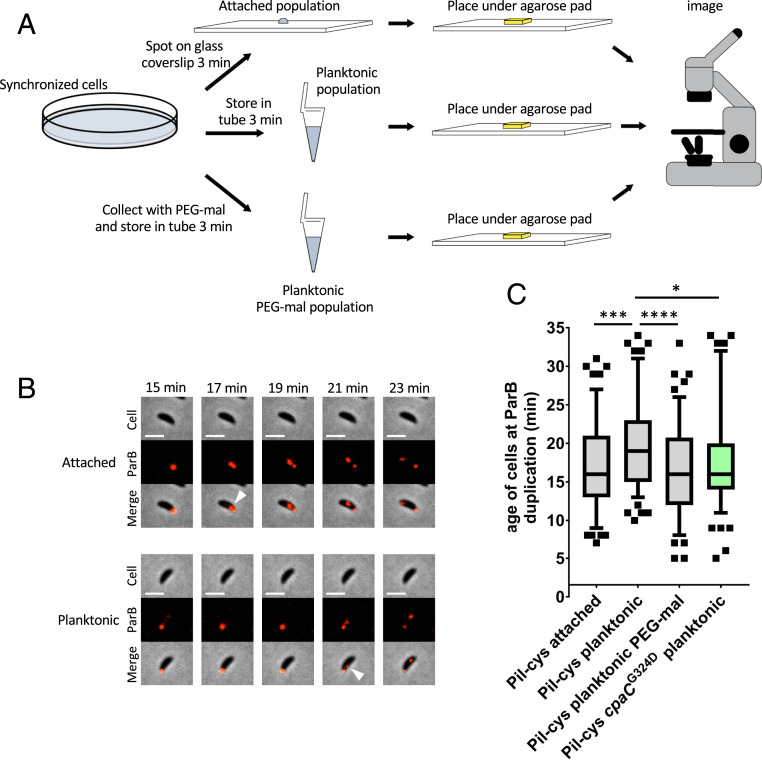
While physical obstruction of pilus retraction with PEG-mal or by cpaC mutation is inferred to simulate surface contact in the absence of a surface, we sought to directly test whether surface contact stimulates cell-cycle entry. Because cultures of C. crescentus harbor a mixture of undifferentiated swarmer cells, stalked cells, and predivisional cells at various stages of replication, we synchronized cultures of cells using a plate synchrony method to isolate newborn swarmer cells. We then tracked the timing of ParB duplication in surface-attached, planktonic, and PEG-mal–treated planktonic populations (Fig. 3A). Attached cells and planktonic cells treated with PEG-mal displayed similar ParB duplication times averaging 17 and 16.4 min after birth, respectively, while untreated planktonic cells displayed a delay in ParB duplication an average of 19.7 min after birth (Fig. 3 B and C). Notably, the cpaCG324D mutant that is genetically obstructed for pilus retraction exhibited ParB duplication an average of 17.6 min after birth, similar to both attached and PEG-mal–treated cells. These results show that surface contact accelerates the C. crescentus swarmer cell life cycle by an approximate 15%.
Fig. 3.
Surface contact stimulates cell-cycle progression. (A) Schematic of experimental setup. (B) Representative time-lapse images of data shown in C. (Scale bars, 2 µm.) White arrowheads indicate ParB duplication event. (C) Box and whisker plots show 5 to 95% CI. Data are compiled from four independent biological replicates (n = 30 cells per replicate). Statistical comparisons were made using Sidak’s multiple-comparisons test. *P < 0.05, ***P < 0.001, ****P < 0.0001.
Taken together, our results indicate that swarmer cells that contact a surface, planktonic swarmer cells physically obstructed for pilus retraction, and planktonic swarmer cells with a mutation that obstructs pilus retraction differentiate earlier than planktonic swarmer cells. We next sought to determine the mechanism by which obstruction of pilus dynamics stimulates entry into the cell cycle.
Obstruction of Pilus Retraction Stimulates C-di-GMP Synthesis by Altering the Activity of Developmental Regulators.
The initiation of DNA replication and polar differentiation are tightly coupled during swarmer cell differentiation. This coupling is mediated in part by the histidine kinases PleC and DivJ, which antagonistically regulate the phosphorylation state of the single-domain response regulator DivK in order to control entry into the cell cycle and the phosphorylation of PleD to stimulate c-di-GMP synthesis (15). It was previously demonstrated that PleD is important for surface-contact stimulation of holdfast synthesis (7), suggesting an increase of c-di-GMP upon surface sensing. We thus measured c-di-GMP concentrations of cell populations after obstruction of pilus activity (Fig. 4A). WT cells lacking the Pil-cys mutation were unaffected by PEG-mal treatment while the Pil-cys strain treated with PEG-mal exhibited 144% of the c-di-GMP concentration of untreated cells upon obstruction of pilus retraction. Of note, these measurements were performed in mixed populations containing unpiliated stalked or predivisional cells, and piliated swarmer cells (∼30% of the population). Because only piliated swarmer cells harboring the Pil-cys mutation are affected by PEG-mal treatment, the fold change in c-di-GMP concentrations is likely much higher in individual cells obstructed for pilus retraction than in the measured population average. These results suggest that surface sensing by obstruction of pilus retraction is sufficient to stimulate the production of c-di-GMP.
Fig. 4.
Obstruction of pilus retraction stimulates c-di-GMP synthesis by altering the activity of developmental regulators. (A) Quantification of intracellular c-di-GMP concentrations by mass spectrometry of populations of wild-type and Pil-cys strains with PEG-mal treatment. Bar graph shows the mean ± SEM of three independent biological replicates. Statistical comparisons were made using Sidak’s multiple-comparisons test. *P < 0.05. (B) Percent of piliated cells with delocalized PleC at each time point. Error bars indicate the mean ± SEM of three independent biological replicates (n = at least 30 cells per replicate per time point). (C) Percent of piliated cells with localized DivJ at each time point. Error bars indicate the mean ± SEM of four independent biological replicates (n = at least 30 cells per replicate per time point). (D) Representative microscopy images of cells from data shown in B. The green arrowhead represents delocalized PleC at the piliated pole. (E) Representative microscopy images of cells at the 25-min time point of data shown in C. Blocked pili in Pil-cys PEG-mal–treated samples appear as puncta due to shearing of filaments. The blue arrowhead indicates DivJ localization in a piliated cell. White arrowheads indicate piliated cells. (Scale bars, 2 μm.) Maleimide-positive cells were labeled as piliated as the fluorophore entered the cells via pilus retraction.
C-di-GMP synthesis by PleD is spatially and temporally controlled by PleC and DivJ. Conveniently, the subcellular localization of PleC and DivJ correlates with their activity (4, 15). PleC is localized at the flagellar pole in swarmer cells, where it acts as a phosphatase for DivK and PleD. PleC delocalizes and switches to a kinase during differentiation from the swarmer to stalked cell. During differentiation, DivJ interacts with its localization and activation factor SpmX to localize to the incipient stalked pole, where it phosphorylates DivK and PleD to start the cell cycle and stimulate cell differentiation (4). To determine if surface sensing regulates this key regulatory switch, we tracked changes in PleC and DivJ localization as a proxy for their activity in cells with obstructed pilus retraction (Fig. 4). Strains containing PleC-YFP (yellow fluorescent protein) or DivJ-CFP (cyan fluorescent protein) were treated with maleimide dye with or without PEG-mal, and piliated cells were tracked for changes in protein localization over time. Notably, the PleC-YFP mutant strain exhibited only partial functionality as evidenced by a delay in cell-cycle progression, and accordingly only ∼20% of untreated Pil-cys or the PEG-treated control cells had delocalized PleC by 60 min. In contrast, ∼60% of cells treated with PEG-mal delocalized PleC by the same time, demonstrating an acceleration in the PleC switch from phosphatase to kinase activity upon disruption of pilus dynamics (Fig. 4 B and D). DivJ also localized to the incipient stalked pole up to 20 min earlier in PEG-mal–treated samples in comparison with untreated or PEG-treated cells, showing that DivJ kinase activation is triggered by obstruction of pilus retraction (Fig. 4 C and E). These results demonstrate that the PleC-DivJ cell-differentiation switch is stimulated by the obstruction of pilus retraction in addition to DNA replication and holdfast synthesis. Thus, surface contact stimulates differentiation of swarmer cells into stalked cells, which are better adapted for nutrient uptake on a surface (8).
Discussion
It is becoming clear that mechanical signals from the environment have a substantial impact on cell biology (16–18). The mechanobiology of bacteria is an emerging field where we know little about the processes that can be modulated by mechanical and physical signals and about how the signals are sensed and transduced. Here, we demonstrate that the physical cue of surface contact stimulates bacterial cell-cycle progression and cell differentiation. We show that perturbation of pilus dynamic activity through surface contact, physical obstruction, or mutation of the pilus outer-membrane secretin stimulates DNA replication initiation and that physical obstruction of pilus dynamics causes a spike in c-di-GMP synthesis. These results suggest that surface contact causes an increase of c-di-GMP as a consequence of perturbation of pilus dynamics and that this increase in c-di-GMP stimulates cell-cycle progression and cell differentiation. It was previously shown that PleD is the main diguanylate cyclase responsible for the increase of c-di-GMP during swarmer to stalked cell differentiation (15). C-di-GMP production by PleD stimulates the ShkA-TacA phosphorylation cascade, ultimately creating a positive feedback loop that results in increased PleD activity and ensures irreversible commitment to cell differentiation (19). The activity of PleD is modulated by the histidine kinases PleC and DivJ, whereby PleC dephosphorylates PleD to inhibit its activity and DivJ phosphorylates PleD to activate it (15). Delocalization of PleC and localization of DivJ at the incipient stalked pole result in an increase in PleD phosphorylation and c-di-GMP levels, which triggers cell differentiation. PleC and DivJ similarly antagonistically modulate the phosphorylation state of the single-domain response regulator DivK to ultimately control CtrA activity and chromosome replication (15). We show that obstruction of pilus dynamics accelerates delocalization of PleC and localization of DivJ at the incipient stalked pole, which is expected to increase c-di-GMP and thereby stimulate entry into the cell cycle and cell differentiation (Fig. 5). At the same time, surface contact also stimulates holdfast synthesis through flagellum motor interference and obstruction of pilus dynamics, causing a spike in c-di-GMP that allosterically activates the holdfast polysaccharide glycosyltransferase HfsJ to stimulate holdfast synthesis (7, 9, 10).
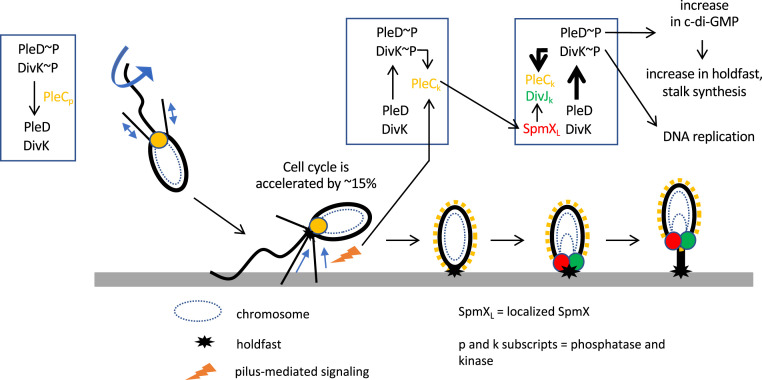
Fig. 5.
Model of cell-cycle acceleration upon surface contact. Surface sensing through alterations in pilus retraction upon surface binding stimulates the switch in PleC activity from phosphatase to kinase, concomitant with its delocalization from the piliated pole. This in turn stimulates the localization of SpmX, which recruits the kinase DivJ. DivJ phosphorylates PleD and DivK, resulting in the production of c-di-GMP and the stimulation of DNA replication, respectively. Increased c-di-GMP production from phosphorylated PleD results in more holdfast synthesis and stalk growth.
When newborn swarmer cells swim to a surface, the DivJ kinase is not yet localized nor active (20) and PleC is localized at the pole bearing pili and the flagellum, where it acts as a phosphatase to dephosphorylate PleD, preventing its activation and localization (15). The accelerated delocalization of PleC and its concomitant switch to a kinase are therefore likely to be the major step in the stimulation of PleD activity. Furthermore, the elevation of DivK∼P concentration stimulates DivJ kinase activity, causing a positive feedback loop between PleC and DivJ that leads to an increase in both DivK∼P and PleD∼P (15). This synergy is also likely potentiated by PleC’s positive action on the localization and activation of DivJ by SpmX (20). The colocalization of PleC with the pili and flagellum suggests that there may be cross talk between the two surface-contact sensory systems and PleC, providing an integration of holdfast synthesis, initiation of DNA replication, and cell differentiation upon surface contact. In support of this model, data from a parallel study by Del Medico et al. (21) suggest that a PilA signal sequence is involved in stimulating c-di-GMP synthesis to trigger cell-cycle progression through the PleC-PleD signaling cascade. Interestingly, recent data suggest that an additional, unknown kinase may initiate PleD phosphorylation as an important step in the G1/S-phase switch, and it is possible that this unknown kinase plays a role in the surface-contact response pathway in addition to the major cell-cycle regulator PleC (19).
Taken together, data from Del Medico et al. and our experiments suggest at least two models for how surface sensing through pili could be achieved. In the first, tension on retracting, surface-bound pili could result in a conformational change in the pilus fiber that the cell senses as a signal for surface contact. Indeed, type IV pili have been shown to stretch under increased load force, revealing hidden epitopes that are otherwise obscured (22). It is thus possible that conformational changes in surface-bound, retracting pili may reveal pilin peptides, such as the PilA-signaling sequence reported by Del Medico et al. that is important for surface sensing. Alternatively, it is also possible that cells may sense changes in local pilin concentration upon altered pilus dynamics after surface binding to mediate surface sensing and cell-cycle progression. While both of these models could explain how cells sense physical barriers in the environment, there is some debate about whether a system where pilin concentration is sensed can be termed “mechanosensing.” It has been suggested that to fit the criteria of mechanosensing, changes in mechanical load must be directly sensed (23), and it remains to be determined whether pilus-dependent surface sensing in C. crescentus fits this definition.
There remain some unknowns about the surface-sensing mechanisms found in C. crescentus. It has been shown that under flow conditions, the flagellum plays a role in surface contact-stimulated holdfast synthesis (10), while the same does not appear to be true under static conditions (7). As the experiments described here were performed under static conditions lacking environmental flow, the flagellum is less likely to play a critical role. However, recent work suggests a complementary role for the flagellum and pili in transmitting surface-contact sensory feedback whereby pilus retraction is promoted at a peak c-di-GMP concentration that is reached after flagellum-mediated surface attachment (24). In conjunction with data showing that flagellar mutants have alterations in c-di-GMP–dependent holdfast synthesis in low-nutrient medium (25), it is possible that c-di-GMP provides a platform for cross talk between these two systems that is important under certain environmental conditions to coordinate surface attachment and cell-cycle progression. Indeed, this idea is supported by another report that flagellar mutants have reduced pilus synthesis, which may be a result of altered local concentrations of c-di-GMP promoting retraction in those mutants (26). It is likely that both flagella and pili play a role in environmental surface sensing, and that cross talk between them may be important for modulating surface attachment and cell-cycle progression in response to varied environmental contexts.
From an ecological perspective, accelerated cellular differentiation after surface contact and permanent attachment likely benefits C. crescentus by activating the pathway that stimulates stalk synthesis. Indeed, the synthesis of a thin stalk is thought to improve nutrient uptake capacity in the diffusion-limited environment of a surface, and its synthesis is dependent on PleD activation (8, 27). A recent study demonstrated that some bacteria can sense and respond to changes in liquid flow rates (17), and accelerated stalk synthesis may also provide an advantage to surface-associated cells by allowing better access to environmental flow conditions (28). Furthermore, accelerated swarmer cell differentiation and DNA replication initiation may reduce interdivision time and therefore increase fitness, which may be even more pronounced in low-nutrient conditions where the G1 phase is developmentally prolonged.
Finally, our results are an important milestone in understanding how cells sense and respond to their environments by highlighting that physical cues can influence the hardwired circuitry of cellular differentiation and reproduction. Elucidating how cells sense and transduce the inputs from physical stimuli will be critical for determining how the physical environment influences intracellular processes.
Materials and Methods
Bacterial Strains, Plasmids, and Growth Conditions.
Bacterial strains and primers used in this study are listed in SI Appendix, Table S1. C. crescentus strains were grown at 30 °C in peptone yeast extract (PYE) medium (29). Escherichia coli DH5α (Bioline) were used for cloning and grown in lysogeny broth medium at 37 °C supplemented with 25 µg/mL kanamycin when appropriate.
Plasmids were transferred to C. crescentus by electroporation, transduction with ΦCr30 phage lysates, or conjugation with S-17 E. coli strains as described previously (30). In-frame allelic substitutions were made by double-homologous recombination using pNPTS-derived plasmids as previously described (31). Briefly, plasmids were introduced into C. crescentus and then two-step recombination was performed using sucrose and kanamycin resistance or sensitivity as a selection for each step. Mutants were verified through a combination of sequencing and microscopy phenotyping.
For construction of the pNPTS-derived plasmids, ∼500-bp flanking regions of DNA on either side of the desired mutations were amplified from C. crescentus genomic DNA. The cpaCG324D point mutation was built into the UpR (reverse) and DownF (forward) primers used to build the pNPTS-derived plasmid as indicated in SI Appendix, Table S1. Upstream regions were amplified using UpF and UpR primers while downstream regions were amplified using DownF and DownR primers. The resulting DNA was purified (QIAquick; Zymo Research) and assembled in pNPTS138 that had been digested with the restriction enzyme EcoRV (New England Biolabs) using HiFi Assembly Master Mix (New England Biolabs). For construction of pNPTS138hfsA+, the entire hfsA gene and ∼500 bp of both up- and downstream flanking DNA were amplified from strain FC764 (32) and cloned into pNPTS138 as described above for use in restoring holdfast synthesis in NA1000 strains.
C-di-GMP Quantification.
C-di-GMP was quantified as described previously (25). Briefly, strains were grown to early-log growth phase (OD600 0.15 to 0.25) in PYE medium. One milliliter of culture was centrifuged for 5 min at 21,000 × g and the supernatant was removed. Cell pellets were resuspended in 200 µL of cold extraction buffer (1:1:1 mix of methanol, acetonitrile, and distilled H2O + 0.1 M formic acid) and incubated at −20 °C for 30 min. Samples were then centrifuged at 21,000 × g to pellet cell debris, and the supernatant was transferred to a fresh tube and stored at −80 °C until use. Experimental extraction solutions were desiccated overnight in a SpeedVac, resolubilized in 100 µL of ultrapure water, briefly vortexed, and centrifuged for 5 min at 21,000 × g to pellet insoluble debris. The clarified extract solutions were transferred to sample vials and analyzed by ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) in negative ion-mode electrospray ionization with multiple-reaction monitoring using an Acquity Ultra Performance LC System (Waters) coupled with a Quattro Premier XE mass spectrometer (Waters) over an Acquity UPLC BEH C18 column (130 Å, 1.7 µm, 2.1 × 50 mm). C-di-GMP was identified using precursor > product masses of 689.16 > 344.31 with a cone voltage of 50.0 V and collision energy of 34.0 V. Quantification of c-di-GMP in sample extracts was determined using a standard curve generated from chemically synthesized c-di-GMP (AXXORA). The standard curve solutions were prepared using twofold serial dilutions of c-di-GMP (1.25 µM to 19 nM) in ultrapure water that were further diluted 1:10 into biological extracts (final c-di-GMP concentrations 125 to 1.9 nM) from a low c-di-GMP strain of C. crescentus lacking several diguanylate cyclases (c-di-GMP0) described previously (5) which had been grown, harvested, extracted, desiccated, and solubilized in ultrapure water in tandem with the experiment samples described above. General UPLC buffer preparations, chromatographic gradients, and MS/MS parameters were performed using a previously published method (33). Intracellular concentrations of c-di-GMP were calculated as described previously (34) assuming a C. crescentus average cellular volume of 6.46 × 10−16 L. The total number of cells present in each extraction was calculated by normalizing OD600 for each sample to the average colony-forming units (CFUs) found for NA1000 cultures grown to an OD600 of 0.2 (2 × 109 CFUs per milliliter).
Quantification of Piliated Cells with Two ParB Foci.
Bacterial cultures were grown to an OD600 of 0.2 to 0.4 and labeled for pili as described previously (7). Briefly, 100-µL cultures were labeled with 25 µg/mL Alexa Fluor 488 C5 maleimide dye (AF488-mal) (Thermo Fisher) for 5 min at room temperature. To block pilus retraction, cells were incubated simultaneously with AF488-mal and 500 µM methoxypolyethylene glycol maleimide (5,000 Da) (PEG-mal) (Sigma) for 5 min at room temperature. Cells were centrifuged at 5,200 × g for 1 min, the supernatant was discarded, and the pellet was then washed with 100 µL PYE and centrifuged again. The supernatant was removed, and the cells were concentrated in 5 to 8 µL PYE. One microliter of washed, labeled cells was spotted onto a 60 × 22-mm glass coverslip and imaged under a 1% agarose (SeaKem) pad made with sterile distilled water before imaging. Imaging was performed on an inverted Nikon Ti-2 microscope using a Plan Apo 60× objective, GFP/DsRed filter cube, Hamamatsu ORCAFlash4.0 camera, and Nikon NIS Elements Imaging software. Quantification of piliated cells and number of ParB foci was performed manually using NIS Elements Analysis software. For Table 1 data collection, the ParB-mCherry mutation caused some unpiliated cells to exhibit division defects as evidenced by filamentous morphology and more than two ParB foci (∼15% cells within the total population). These cells were excluded from the analysis. No piliated cells exhibited this defect, and thus none were excluded from the analysis.
Quantification of Genomic Content in Populations of Cells.
Bacterial cultures were grown to an OD600 of 0.2 to 0.4 and left untreated or treated with either 500 µM PEG5000-mal or 500 µM polyethylene glycol (∼5,000 Da) (Sigma). After pilus treatment, cells were incubated with 15 µg/mL rifampicin for 3 h to prevent new cycles of DNA initiation; 1.5 mL of culture was concentrated into 180 µL phosphate-buffered saline (PBS) and fixed with 420 µL 100% ethanol at 4 °C for 1 h. After fixation, cells were centrifuged at 5,200 × g and washed once with 600 µL PBS. Cells were finally resuspended in 600 µL PBS and 2.5 µM SYTOX Green Nucleic Acid Stain (Thermo Fisher) was added preceding stationary incubation overnight at 4 °C. Fluorescence intensity and light scattering were quantified by flow cytometry using the FACSCalibur at the Indiana University Bloomington Flow Cytometry (IUB FACS) Core Facility and data were analyzed using FlowJo software.
Quantification of PleC and DivJ Localization Patterns.
Pili were labeled with AF594-mal (Thermo Fisher) and treated with either PEG-mal or PEG as described above. For tracking DivJ-CFP localization after pilus treatment, cells were placed in a static, 30 °C incubator. Every 10 min, 1 µL of sample was spotted onto a glass coverslip and imaged using DsRed/CFP filter settings under 1% agarose pads made with sterile distilled water as described above. For tracking PleC delocalization, cells were spotted onto a glass coverslip and placed under a 1% agarose pad made with PYE and an initial image was taken using DsRed filter settings to identify piliated cells. Cells were then imaged using YFP filter settings every 2 min to track PleC-YFP delocalization. Quantification of piliated cells with delocalized PleC or localized DivJ was performed manually using NIS Elements Analysis software.
Identification of Mutant Deficient in Pilus Retraction.
A subculture-based forward genetic screen was performed to enrich for mutants efficient in holdfast-independent surface attachment. Ten replicates of a parent Pil-cys strain lacking the holdfast-synthesizing genes (ΔhfsDAB) were grown in 5 mL of PYE in glass tubes to stationary phase. Cultures were then dumped and lightly washed with PYE to remove loosely bound cells. The tubes were then refilled with 5 mL of PYE and again grown to stationary phase, and this was repeated until turbid growth was observed after overnight growth (23 h). Cultures were then streaked out onto PYE agar plates to isolate individual mutants. Isolates were then tested for changes in phage sensitivity to the pilus-dependent phage ΦCbK, and those exhibiting an alteration from wild-type sensitivity were sequenced to identify mutations. Whole-genome sequencing and mutant identification were performed as described previously (35) with the exception that sequencing reads were mapped to the genome of C. crescentus NA1000 (NC_011916.1).
Phage Sensitivity Assays.
Phage sensitivity assays were performed as described previously (26). Briefly, 5 µL of ΦCbK phage dilutions was spotted onto lawns of growing C. crescentus strains. Lawns were made by adding 200 µL of stationary-phase cultures to 3 mL of melted top agar (0.5% agar in PYE) and spread over 1.5% PYE agar plates. After the top agar solidified, 5 µL of phage dilutions in PYE was spotted on top. Plates were grown for 2 d at 30 °C before imaging.
Cell Synchronization and Surface Stimulation Experiments.
Cells were synchronized as described previously (7) with some modifications. Briefly, 50 mL of PYE in a 15-cm polystyrene Petri dish was inoculated with 1 mL of overnight culture of the indicated holdfast-synthesizing strain expressing ParB-mCherry and incubated for 16 h at room temperature at 70 rpm on an orbital shaker. Four hours prior to experiments, the Petri dish was washed with 50 mL of sterile distilled water; 50 mL of PYE medium was added to the Petri dish and incubated at room temperature shaking for an additional 4 h. Just before use, the Petri plate was washed twice with 100 mL of distilled water, and then 1 mL of PYE (containing 500 μM PEG-mal where indicated) was added to the Petri plate and harvested after 1 min to collect newborn swarmer cells. For planktonic populations, the 1 mL of PYE containing newborn swarmer cells was added to a 1.7-mL centrifuge tube and left stationary at room temperature for 3 min before 1 μL was spotted onto a coverslip and imaged under a 1% agarose pad made with PYE and containing 0.5 μg/mL AF488-WGA to label holdfasts. For surface-attached cells, 1 μL of the harvested newborn swarmer cells was spotted onto a glass coverslip and left stationary at room temperature for 3 min before the addition of the 1% agarose pad. Agarose pads do not stimulate surface-contact responses as reported elsewhere (25), and we found that allowing cells to attach to the glass coverslip for 3 min before the addition of the pad was critical for observing a surface-stimulated response. Time-lapse images of ParB-mCherry foci and holdfasts labeled with AF488-WGA in the agarose pad were captured once per minute over 35 min using the same settings described above. Holdfast and ParB-mCherry duplication events were quantified manually using NIS Elements Analysis software.
Statistical Analysis.
All statistics used throughout the study were performed using Prism 8 software. Specifically, one-way ANOVA was used throughout followed by post hoc Sidak’s tests to determine statistical differences between samples.
Data and Materials Availability.
All data are available in the main text or SI Appendix.
Supplementary Material
Acknowledgments
We thank A. Dalia, C. Berne, and members of the Gitai laboratory for helpful discussions regarding the manuscript. We thank the Center for Genomics and Bioinformatics at Indiana University for whole-genome sequencing and single-nucleotide polymorphism mutant identification. We also thank the FACS Core Facility at IUB for training and assistance in flow cytometry experiments. We thank D. Kysela for construction of strain YB7341. This study was supported by Grant R35GM122556 from the NIH and by a Canada 150 Research Chair in Bacterial Cell Biology (to Y.V.B.), Grant R01GM109259 from the NIH (to C.M.W.), and NSF Fellowship 1342962 (to C.K.E.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1920291117/-/DCSupplemental.
References
- 1.Sánchez Alvarado A., Yamanaka S., Rethinking differentiation: Stem cells, regeneration, and plasticity. Cell 157, 110–119 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro L., Agabian-Keshishian N., Bendis I., Bacterial differentiation. Science 173, 884–892 (1971). [DOI] [PubMed] [Google Scholar]
- 3.Toh E., Kurtz H. D. Jr., Brun Y. V., Characterization of the Caulobacter crescentus holdfast polysaccharide biosynthesis pathway reveals significant redundancy in the initiating glycosyltransferase and polymerase steps. J. Bacteriol. 190, 7219–7231 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtis P. D., Brun Y. V., Getting in the loop: Regulation of development in Caulobacter crescentus. Microbiol. Mol. Biol. Rev. 74, 13–41 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abel S. et al., Bi-modal distribution of the second messenger c-di-GMP controls cell fate and asymmetry during the Caulobacter cell cycle. PLoS Genet. 9, e1003744 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkpatrick C. L., Viollier P. H., Decoding Caulobacter development. FEMS Microbiol. Rev. 36, 193–205 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Ellison C. K. et al., Obstruction of pilus retraction stimulates bacterial surface sensing. Science 358, 535–538 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner J. K., Setayeshgar S., Sharon L. A., Reilly J. P., Brun Y. V., A nutrient uptake role for bacterial cell envelope extensions. Proc. Natl. Acad. Sci. U.S.A. 103, 11772–11777 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li G. et al., Surface contact stimulates the just-in-time deployment of bacterial adhesins. Mol. Microbiol. 83, 41–51 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hug I., Deshpande S., Sprecher K. S., Pfohl T., Jenal U., Second messenger-mediated tactile response by a bacterial rotary motor. Science 358, 531–534 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Ellison C. K. et al., Retraction of DNA-bound type IV competence pili initiates DNA uptake during natural transformation in Vibrio cholerae. Nat. Microbiol. 3, 773–780 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellison C. K., Dalia T. N., Dalia A. B., Brun Y. V., Real-time microscopy and physical perturbation of bacterial pili using maleimide-conjugated molecules. Nat. Protoc. 14, 1803–1819 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shebelut C. W., Guberman J. M., van Teeffelen S., Yakhnina A. A., Gitai Z., Caulobacter chromosome segregation is an ordered multistep process. Proc. Natl. Acad. Sci. U.S.A. 107, 14194–14198 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viollier P. H., Sternheim N., Shapiro L., Identification of a localization factor for the polar positioning of bacterial structural and regulatory proteins. Proc. Natl. Acad. Sci. U.S.A. 99, 13831–13836 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul R. et al., Allosteric regulation of histidine kinases by their cognate response regulator determines cell fate. Cell 133, 452–461 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Persat A. et al., The mechanical world of bacteria. Cell 161, 988–997 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanfilippo J. E. et al., Microfluidic-based transcriptomics reveal force-independent bacterial rheosensing. Nat. Microbiol. 4, 1274–1281 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berne C., Ellison C. K., Ducret A., Brun Y. V., Bacterial adhesion at the single-cell level. Nat. Rev. Microbiol. 16, 616–627 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Kaczmarczyk A. et al., Precise timing of transcription by c-di-GMP coordinates cell cycle and morphogenesis in Caulobacter. Nat. Commun. 11, 816 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radhakrishnan S. K., Thanbichler M., Viollier P. H., The dynamic interplay between a cell fate determinant and a lysozyme homolog drives the asymmetric division cycle of Caulobacter crescentus. Genes Dev. 22, 212–225 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Medico L., Cerletti D., Schächle P., Christen M., Christen B., The type IV pilin PilA couples surface attachment and cell-cycle initiation in Caulobacter crescentus. Proc. Natl. Acad. Sci. U.S.A. 117, 9546–9553 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biais N., Higashi D. L., Brujić J., So M., Sheetz M. P., Force-dependent polymorphism in type IV pili reveals hidden epitopes. Proc. Natl. Acad. Sci. U.S.A. 107, 11358–11363 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chawla R., Gupta R., Lele T. P., Lele P. P., A skeptic’s guide to bacterial mechanosensing. J. Mol. Biol. 432, 523–533 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sangermani M., Hug I., Sauter N., Pfohl T., Jenal U., Tad pili play a dynamic role in Caulobacter crescentus surface colonization. MBio 10, e01237-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berne C. et al., Feedback regulation of Caulobacter crescentus holdfast synthesis by flagellum assembly via the holdfast inhibitor HfiA. Mol. Microbiol. 110, 219–238 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellison C. K., Rusch D. B., Brun Y. V., Flagellar mutants have reduced pilus synthesis in Caulobacter crescentus. J. Bacteriol. 201, e00031-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner J. K., Brun Y. V., Out on a limb: How the Caulobacter stalk can boost the study of bacterial cell shape. Mol. Microbiol. 64, 28–33 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Klein E. A. et al., Physiological role of stalk lengthening in Caulobacter crescentus. Commun. Integr. Biol. 6, e24561 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poindexter J. S., Biological properties and classification of the Caulobacter group. Bacteriol. Rev. 28, 231–295 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ely B., Genetics of Caulobacter crescentus. Methods Enzymol. 204, 372–384 (1991). [DOI] [PubMed] [Google Scholar]
- 31.Ried J. L., Collmer A., An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in gram-negative bacteria by marker exchange-eviction mutagenesis. Gene 57, 239–246 (1987). [DOI] [PubMed] [Google Scholar]
- 32.Marks M. E. et al., The genetic basis of laboratory adaptation in Caulobacter crescentus. J. Bacteriol. 192, 3678–3688 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Severin G. B., Waters C. M., “Spectrophotometric and mass spectroscopic methods for the quantification and kinetic evaluation of in vitro c-di-GMP synthesis” in c-di-GMP Signaling, (Humana Press, New York, NY, 2017), Vol. 1657. [DOI] [PubMed] [Google Scholar]
- 34.Massie J. P. et al., Quantification of high-specificity cyclic diguanylate signaling. Proc. Natl. Acad. Sci. U.S.A. 109, 12746–12751 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellison C. K. et al., A bifunctional ATPase drives tad pilus extension and retraction. Sci. Adv. 5, eaay2591 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.