Significance
Variation in individual cognition affects how animals learn about and communicate information to others. We provide evidence that differences in how individual honey bees learn influences the collective foraging dynamics of a colony. By creating colonies of distinct learning phenotypes, we evaluated how bees make foraging choices in the field. Colonies containing individuals that learn to ignore unimportant information preferred familiar food locations; however, colonies of individuals that are unable to ignore familiar information visit novel and familiar feeders equally. Colonies with a 50/50 mix of these phenotypes prefer familiar food locations because individuals who learn the familiar location recruit nestmates by dancing more intensely. Our results reveal that cognitive variation among individuals nonlinearly shapes collective behavioral outcomes.
Keywords: collective behavior, cognition, learning, latent inhibition, honey bee
Abstract
Individual differences in learning can influence how animals respond to and communicate about their environment, which may nonlinearly shape how a social group accomplishes a collective task. There are few empirical examples of how differences in collective dynamics emerge from variation among individuals in cognition. Here, we use a naturally variable and heritable learning behavior called latent inhibition (LI) to show that interactions among individuals that differ in this cognitive ability drive collective foraging behavior in honey bee colonies. We artificially selected two distinct phenotypes: high-LI bees that ignore previously familiar stimuli in favor of novel ones and low-LI bees that learn familiar and novel stimuli equally well. We then provided colonies differentially composed of different ratios of these phenotypes with a choice between familiar and novel feeders. Colonies of predominantly high-LI individuals preferred to visit familiar food locations, while low-LI colonies visited novel and familiar food locations equally. Interestingly, in colonies of mixed learning phenotypes, the low-LI individuals showed a preference to visiting familiar feeders, which contrasts with their behavior when in a uniform low-LI group. We show that the shift in feeder preference of low-LI bees is driven by foragers of the high-LI phenotype dancing more intensely and attracting more followers. Our results reveal that cognitive abilities of individuals and their social interactions, which we argue relate to differences in attention, drive emergent collective outcomes.
Collective behavior allows animals to undertake tasks that they could not accomplish alone. Individuals utilize local information to adjust to ecological changes as a collective. Local information is implicitly or explicitly communicated among group members to form a collective response (1–3). Individuals within a group differ in their cognitive performance. At an individual level, cognition involves synthesis and internal representation of acquired information from past experiences to solve novel problems (4). Collective behavior emerges from the interactions among individuals working together to solve a task that could not be accomplished as effectively by an individual (1, 5). Many of the basic rules that explain collective behavioral dynamics come from theoretical models, which emphasize the importance of variation in perception and cognition among individuals within a social group (6). For example, leaders can emerge in computer simulations to guide uninformed group members to a resource. However, both informed and uninformed individuals are needed to effectively move in the correct direction (7). Although individual variation in responsiveness and cognitive performance is recognized as critical for the emergence of collective behavior, empirical work on the mechanisms by which variation in individual cognition and the interaction between these individuals scale to the collective is rare.
One way in which animals differ from one another in their cognitive abilities is the way in which they learn information (8). These differences may be driven by several cognitive properties, including the ability to perceive stimuli and internally represent information. This ability has important ecological and evolutionary consequences (9). For example, learning is the foundation of the evolution of aposematic coloration (10). Humans who are able to quickly learn important information report increased productivity compared with individuals who cannot focus on pertinent information (11–13). Naturally, collective groups of organisms will consist of individuals that differ in how they learn information. Here we ask how variation in learning among individuals shapes the way in which individuals share ecological information with group members to determine collective outcomes.
While foraging, honey bees (Apis mellifera) must learn various aspects about the location of food sources, such as landmarks, odors, and direction (14–16). Honey bee foragers then return to the colony to communicate information about food sources to colony members at the nest via their recruitment dances (14). Learning in honey bees has been extensively studied in the laboratory (17), where many features of the learning context can be controlled and neural and molecular mechanisms can be more extensively investigated (18–21). One such study involves investigation of how honey bees exhibit heritable variation in learning to ignore unimportant information, such as unrewarding odors, which is a form of learning called latent inhibition (LI) (22, 23). LI has been studied in vertebrates (24–28) and is correlated with attention disorders in humans (11). Foraging honey bees vary in their expression of LI. After familiarizing bees to an odor and then evaluating their ability to learn the familiar versus a novel odor in the laboratory, some individuals tend to slowly learn familiar odors and quickly learn a novel odor, exhibiting high LI. Other bees learn familiar and novel odors equally well, exhibiting low LI (20). Ecologically, LI may facilitate the process of novelty seeking in exploration behavior (29) by focusing distributed attention across foragers (27, 30–32) to resources that are important for the colony. Heritable, natural variation observed in foragers from the same colony (33) implies that this variation has some function for the fitness of the collective. Despite our knowledge of variation among individuals in LI (20, 34, 35) and its effects on predator avoidance (24, 25, 36), it is unknown whether or how this variation affects ecologically relevant decisions in social systems.
We provide empirical evidence that the interaction of individuals that differ in their cognitive abilities drives collective foraging dynamics. Using the genetic heritability of LI, we created two distinct genetic learning lines of high-LI and low-LI workers from singly inseminated queens with the same LI drones. From these lines we created 24 colonies composed of single cohorts of only low, only high, 50/50 mixed high- and low-LI workers, and age-matched nonselected control bees. To evaluate colony foraging preference for novel or familiar food sources, we used a novelty-seeking field assay on freely foraging bees (29). We then characterized behavioral mechanisms that drive collective foraging behavior by quantifying the round recruitment dance of high- and low-LI bees as they foraged on novel or familiar food sources. These experiments allowed us to simultaneously quantify how social groups differ in performing cognitive tasks as a result of the composition of the individuals of the group and how cognitively distinct individuals interact to shape collective outcomes.
Results
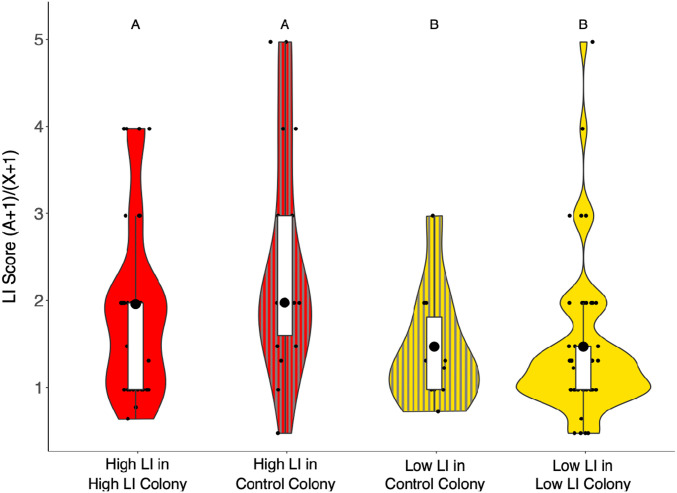
To determine whether workers in different social environments exhibited the predicted heritable LI phenotype, we evaluated the LI score of foragers after 21 d in either their natal colony or a control colony. We marked 1,000 individuals from each selected line (high or low LI) on the day of emergence. We then placed 500 individuals back into their natal colony and 500 individuals into an established control colony of equal size with an open mated queen, i.e., a colony containing workers that exhibit different learning phenotypes. We monitored the colonies until marked bees began to make foraging flights (∼21 d). We then collected marked foragers as they returned to the colony and brought them into the laboratory to evaluate LI. We avoided pollen foragers as they tend to exhibit different learning behavior compared to nectar foragers (37). We found that foragers retained the expected LI based on the LI of their parents, regardless of whether they were housed with same or with a range of learning phenotypes. Foragers from the high-LI and low-LI lines differed in expression of LI as expected (generalized linear model [GLM]: χ2 = 4.84, df = 1, P = 0.027, Fig. 1). We did not detect an effect of the identity of the colony in which the bees were housed on the LI phenotype (χ2 = 3.28, df = 2, P = 0.193, Fig. 1).
Fig. 1.
Social environment does not alter expression of heritable latent inhibition. LI scores of individuals from high-LI lines that spent their adult life either in high-LI-only colonies (red, n = 36) or in a control colony with a variety of LI phenotypes from an open mated queen (red with gray vertical lines, n = 18); individuals from low LI lines that spent their adult life either in low-LI-only colonies (yellow, n = 52) or in control colonies (yellow with gray vertical lines, n = 10). LI score is calculated by dividing the number of responses to the novel odor (A) + 1 by the number of responses to the familiar odor (X) + 1. An LI score closer to 1 indicates similar responses to novel and familiar odors and qualifies a bee as low-LI, while an LI score over 1.33 qualifies a bee as high LI, having fewer responses to familiar odor and more responses to the novel odor. Here, high-LI bees had a median LI score of 2 in both treatments, and low-LI bees had a median of 1.25 when in low-LI colonies and 1.29 in control colonies. Different letters above violins indicate statistically significant differences according to a post hoc Tukey test. In this and subsequent figures, the large black dot is the median, the white box is the interquartile range (IQR), whiskers extend to 1.5*IQR, and the small points beyond the whiskers are outliers. The violin shapes show the distribution of the data. Here, and in all following figures, yellow indicates low-LI colonies and individuals, gray indicates control colonies and individuals, and red indicates high-LI colonies and individuals.
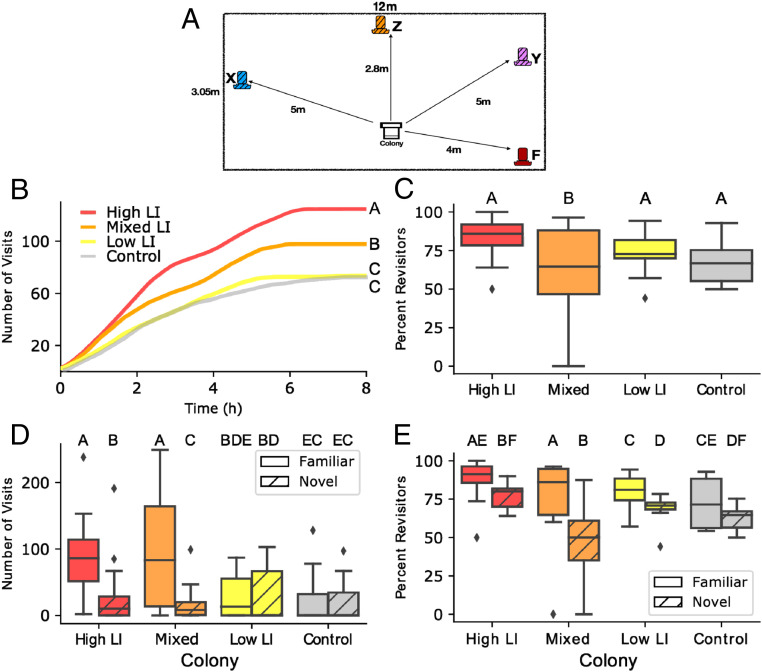
To determine how the learning phenotypes influenced colony-level foraging behavior, we placed small single-cohort (same age bees) colonies into a flight cage and monitored foraging activity. We evaluated four colony types each week: one control colony consisting of ∼1,300 age-matched bees from open mated queens; one colony consisting of 650 workers from high-LI queens, and 650 age-matched control bees; one colony consisting of 650 workers from low-LI queens and 650 aged-matched control bees; and one 50/50 mixed colony with 325 workers from each LI line and 650 aged-matched control bees. In the last three colony types, the supplemented 650 age-matched bees from open mated queens were used to ensure a small but functioning colony, as we did not have enough workers from the single-drone–inseminated queens, and colonies of just 650 individuals would be too weak to forage. Honey bee division of labor is largely influenced by worker age, so we used age-matched bees to control for influence that age may have on foraging propensities. On day 1, we trained bees to a feeder inside the flight cage containing 1 M sucrose, a pink color, and an odor, which became the “familiar” feeder. During the subsequent 3 d, in addition to the familiar feeder, we introduced a single novel feeder each day with a different odor and color, but with the same sugar concentration as the familiar feeder (Fig. 2A). To evaluate the collective ability of the colony to find a new feeder, we recorded the number of visits to each feeder by bees from each selected line, identified according to the color of paint on the bees’ thorax. We further marked bees with a feeder-specific color on their abdomen when they visited the feeder for the first time to determine if bees revisited that feeder. We repeated this procedure for 6 wk on six colonies of each group type.
Fig. 2.
Colonies constructed from different genetic lines selected for high or low latent inhibition exhibited differences in collective foraging behavior. (A) The experimental setup illustrating the location of feeders in relation to the location of the colony (Center, white) within the experimental flight cage arena (represented here as a large rectangle that encompasses the feeder and colony diagram). The familiar feeder F (red) was provided on day 1 and on all subsequent days. Novel feeder X (blue) was presented on day 2, novel feeder Y (purple) on day 3, and novel feeder Z (orange) on day 4. See SI Appendix, Table S2, for associated odors. Visits to all novel feeders were combined for statistical analysis. (B) Cumulative number of visits of bees to all feeders over time by colony type. Different letters to the right of the lines indicate statistically significant differences according to a post hoc Tukey test. For further illustration of visitation by each colony on each day, see SI Appendix, Fig. S1. (C) Percentage of revisits out of the total number of visits to all feeders by colony type. Here and in all following panels, different letters above boxes indicate statistically significant differences according to a post hoc Tukey test. (D) Number of all visits to the familiar feeder (solid boxes) and a novel feeder (hatched boxes) for each type of colony, when both novel and familiar feeders were presented simultaneously (days 2 to 4). (E) Percentage of revisits out of the total number of visits to either the familiar or the novel feeder by type of colony when both novel and familiar feeders were presented simultaneously (days 2 to 4). In C–E, horizontal lines are the median, the boxes are the IQR, whiskers extend to 1.5*IQR, and the small points beyond the whiskers are outliers. n = 24 colonies, six colonies per group type, and 6,172 total visits.
Colony composition strongly influenced the overall number of visits to the food locations (n = 6 colonies in each line, 24 total, 6,172 total visits; GLM: χ2 = 1,270, df = 3, P < 0.0001, Fig. 2B). High-LI colonies performed significantly more visits to all food locations compared to low-LI colonies (Tukey post hoc: Z = 25.5, P < 0.0001, Fig. 2A), mixed colonies (Z = 5.18, P < 0.0001), and controls (Z = 26.6, P < 0.0001). Mixed-LI colonies also performed significantly more visits compared to low-LI colonies (Z = 20.7, P < 0.0001) and controls (Z = 21.8, P < 0.0001). Low-LI and control colonies performed the fewest total visits and were not significantly different from each other (Z = −1.38, P = 0.50).
Foraging in the high, low, and control colonies was largely performed by bees revisiting the feeders (GLM: χ2 = 22.32, df = 3, P < 0.0001, Fig. 2C). However, the mixed-LI colonies had a significantly lower proportion of revisiting foragers compared to the low-LI (Tukey post hoc: Z = −4.2, P = 0.0002), high-LI (Z = −3.1, P = 0.01), and control colonies (Z = −3.33, P = 0.004). We did not detect significant differences among the other colony types (SI Appendix, Table S3).
A colony’s LI phenotype composition determined its preference between the novel and familiar feeders (GLM: Feeder*Colony, χ2 = 473.64, df = 3, P < 0.0001; Fig. 2D). High-LI and mixed colonies preferred the familiar feeder over the novel one (Tukey post hoc: High Familiar:Novel: Z = 20.2, P < 0.0001; Mixed Familiar:Novel: Z = 25.6, P < 0.0001). Low-LI and control colonies did not show a strong preference for either feeder, visiting them equally (Low Familiar:Novel: Z = −1.24, P = 0.92; Control Familiar:Novel: Z = 2.03, P = 0.46). For full pairwise comparisons, see SI Appendix, Table S4).
The number of revisits to the novel and familiar feeders was different across colony compositions (Fig. 2E: Colony*Feeder χ2 = 53.67, P < 0.0001). All colonies had a higher proportion of revisits to the familiar feeder compared to the novel feeder. However, the mixed-LI colonies had a much lower proportion of revisitation to the novel feeders than the other colony types (SI Appendix, Table S5). Thus, new foragers in the mixed colonies that visited the novel feeder were less likely to return to it compared to foragers that visited the novel feeders in other colonies.
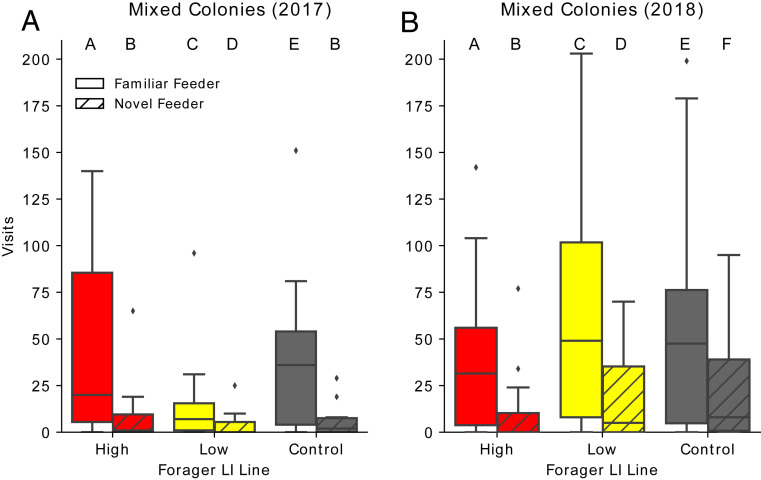
To determine why the mixed colonies showed a preference for the familiar feeder (Fig. 2D), we examined the visitation of individuals from each line in mixed colonies to each feeder (Fig. 3). In 2017, we tested mixed colonies placed in a flight cage. In 2018, we reselected lines and then placed mixed colonies into two-frame observation hives to evaluate recruitment dances along with visitation to the feeders in the flight cages. We found that there was a significant year effect (SI Appendix, Table S6), likely due to reselection and different environmental conditions. We therefore statistically analyzed each year separately to focus on the within-year differences between the selected lines.
Fig. 3.
Visits of individuals from different genetically selected lines when in a mixed colony. Daily visits to the familiar (solid) and novel (hatched) feeders by individual bees in mixed colonies from low-LI parents (yellow), high-LI parents (red) or open-mated queens (gray) in (A) in 2017, n = 6 mixed colonies, 2,347 overall visits and in (B) in mixed colonies from lines that were reselected in 2018, n = 6 colonies, 6,272 overall visits. The horizontal line in the box is the median, the box is 25 to 75% of the data, whiskers represent 95% of the data, and diamonds show outliers beyond 95%. Different letters above boxes indicate statistically significant differences according to a post hoc Tukey test.
Low-LI and control individuals shift their preference to the familiar feeder when mixed with high-LI individuals. This trend occurred across 2 y. In 2017, we found a significant interaction between the selected line and the feeder that foragers visited (GLM: χ2 =7.79, df = 2, P = 0.02; Fig. 3A). Low LI and control individuals preferred to visit the familiar feeder in the mixed colonies (GLM: Low Familiar:Novel: Z = 13.28, P < 0.0001; Control Familiar:Novel: Z = 18.32, P < 0.0001; Fig. 3A). High-LI individuals showed a preference for familiar feeders in the mixed group, as they did in the uniform groups (GLM: Familiar:Novel: Z = 22.03, P < 0.0001; Fig. 2E). We found a similar trend in 2018, when there were more visits to all feeders by all genetic lines. There was again a significant interaction between selected line and feeder (GLM: χ2 = 85.27, df = 2, P < 0.0001; Fig. 3B), with low-LI, high-LI, and control individuals showing preference to the familiar feeder over the novel feeder (GLM: Low Familiar:Novel: Z = 25.05, P < 0.0001; High LI Familiar:Novel: Z = 18.48, P < 0.0001, Control Familiar:Novel: Z = 13.90, P < 0.0001; Fig. 3B). For full contrasts from 2017, see SI Appendix, Table S7; for 2018, see SI Appendix, Table S8.
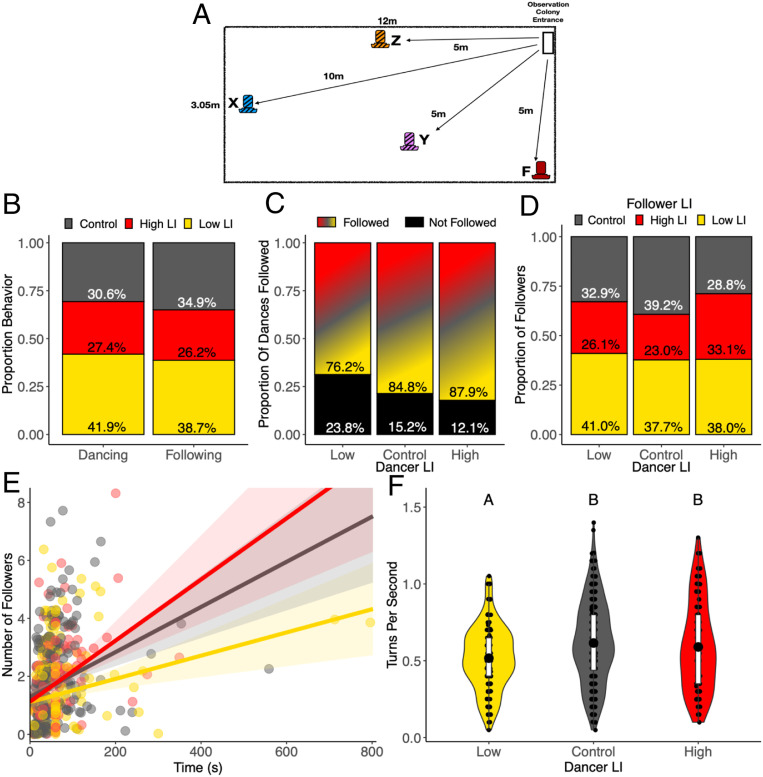
To uncover the behavioral mechanisms that underlie the switching of low-LI individuals from having no feeder preference when in a uniform colony composition to preferring the familiar feeder when in a mixed colony, we examined the round dance, the modified waggle dance which is used at short distances (38). We observed individuals from each selected line in mixed colonies as they returned from foraging. Using observation hives with glass walls, we videorecorded bees performing the round dance to recruit other individuals in the colony to forage. To determine which selected line recruited to each feeder, we noted the selected line of the dancer (high or low LI) according to the paint marks on the individuals’ thorax and whether the dancer had visited a feeder according to the paint marks on abdomens. We did not record dancers without abdominal marks as they were likely collecting from and recruiting to unmonitored water sources. To determine to which bee the information about a feeder was communicated, we counted the number of followers of each dancer and the selected line of the followers. To quantify the dance intensity, we recorded the duration of the dance and the number of turns the dancer made during the first 20 s of the dance.
Individuals from the lines differed in their likelihood to perform a round dance (χ2 test: χ2 = 26.61, df = 2, P < 0.0001; Fig. 4B). Low-LI individuals were significantly more likely to perform a dance compared to high-LI individuals (pairwise χ2 test: P = 0.0001) and controls (pairwise χ2 test: P = 0.004). High-LI individuals were just as likely to perform a dance as controls (P = 0.36). Individuals differed in their likelihood to follow a dance based on their selected line (χ2 test: χ2 = 28.26, df = 2, P < 0.0001; Fig. 4B). Low-LI individuals were significantly more likely to follow a dance compared to high-LI bees (pairwise χ2 test: P < 0.0001) and controls (pairwise χ2 test: P < 0.003). High-LI and control individuals were equally likely to follow a dance (pairwise χ2 test: P = 0.240).
Fig. 4.
Recruitment dances facilitate integration of information from different genetically selected lines. (A) The experimental setup illustrating the location of feeders in relation to the location of the colony entrance (Top Right, white) within the experimental arena (large rectangle). The familiar feeder F (red) was provided on day 1 and on all subsequent days. Novel feeder X (blue) was presented on day 2, novel feeder Y (purple) on day 3, and novel feeder Z (orange) on day 4. See SI Appendix, Table S2, for associated odors. Visitations to novel feeders were combined for statistical analysis. (B) Proportion of dances (n = 667) or follows (n = 1,201) across six colonies performed by bees from each line, relative to their abundance in the mixed colony (350 high LI, 350 low LI, 700 control). We accounted for the difference in abundance of each selected line by dividing the number of observed control dancers by 2 before calculating these proportions. (C) Proportion of dances performed per LI line type that were either followed by at least one individual (colored) or not followed by any other bees (black). (D) Proportion of dances by LI line type that were followed (from B) broken down by LI of the follower. (E) Relationship between number of followers and duration of a dance by line. Point and line colors indicate LI of dancer. Best-fit line represents the GLM, and shaded area represents the 95% confidence interval. (F) Rate of turns per second in a dance by line. The large black dots in the violin shape is the mean, the white box in the violin shape is 25 to 75% of the data, and whiskers represent 95% of the data. The violin shapes illustrate distribution of the data. Different letters above violins indicate statistically significant differences according to a post hoc Tukey test.
Although the high-LI individuals danced less often, dances by high-LI individuals had significantly more followers compared to low-LI and control bees (χ2 test: χ2 = 13.93, df = 2, P < 0.001; Fig. 4C). Low-LI bees performed more dances that had no followers compared to dances by high-LI and control bees. We did not detect a statistically significant difference between the proportion of individuals from each line that followed each line of dancer (χ2 test: χ2 = 7.05, df = 4, P = 0.13, Fig. 4D). Low-LI individuals spent more time dancing; however, they attracted fewer followers than high-LI and control dancers, indicated by the significant interaction between the LI of the dancer and the dance duration when predicting the number of followers (GLM): χ2 = 6.42, df = 2, P = 0.04; Fig. 4E).
The relative attraction of dances of high-LI bees could be due to the intensity of the dance. High-LI bees performed more turns per second during their dances (ANOVA: χ2 = 12.8, df = 2, P = 0.002; Fig. 4F). High-LI dancers performed an average of 0.59 turns per second, significantly higher than low-LI dancers that performed an average of 0.52 turns per second (Tukey: t = −3.13, P = 0.005). Control bees also performed more turns per second than low-LI bees (Tukey: t = −2.5, P = 0.03), but not significantly different from high-LI bees at an average 0.62 turns per second (Tukey: t = −0.7, P = 0.7).
Discussion
By combining techniques from experimental psychology and behavioral ecology, we have developed a system for investigating how variation among individuals in learning behavior drives collective behavior. We demonstrate that a naturally variable, heritable learning behavior nonlinearly shapes the collective performance of a honey bee colony in foraging tasks. We found that high-LI colonies preferred a familiar feeder over novel feeders, while low-LI colonies visited all feeders equally. In mixed colonies low-LI bees switched to be more like high-LI bees and preferred familiar feeders. This change was facilitated by the high-LI bees performing the round dance more vigorously, garnering the attention of more followers and driving them to their preferred food location.
In the laboratory, high-LI honey bees learn to ignore familiar odors that they experienced without reinforcement, while readily learning novel odors. When a novel stimulus is rewarding, high-LI bees exhibit increased attention to that stimulus. One interpretation of reduced learning to a familiar, unrewarding stimulus is that pre-exposure reduces attention to that stimulus, diminishing its associability with reinforcement. This interpretation is an extension of conditioned attention theory (31, 39), which proposes that latent inhibition is induced by allowing animals to focus their attention on important information (30, 31, 40). The novelty-seeking field assay clarified what information high-LI bees focus on: food. Our observations of behavior in the field of high-LI individuals and colonies are consistent with this interpretation, whereby high-LI individuals have stronger attention capacities to food compared to low-LI individuals, regardless of the familiarity of associated stimuli. Once high-LI individuals have found a food location, they continue to revisit it, attending more strongly to reinforced feeders over new ones. The increased impact of the resource on these bees translates into stronger, more vigorous dances.
In contrast, low-LI colonies learn and visit both known and new feeders equally, dividing their attention across resources. This is reflected in their learning abilities in the laboratory, where they readily learn novel and familiar odors equally well, translating into the ability to continue to visit known food sources while exploring the landscape for other locations. This learning phenotype translates to a more generalist foraging phenotype when in a colony of other low-LI individuals. However, when mixed with an equal proportion of high-LI individuals, low-LI individuals were more likely to follow round dances compared to high-LI bees. This broadened attention by low-LI individuals may therefore make them the perfect audience for the high-LI dancers, driving them to prefer feeders that high-LI individuals preferentially visit.
When balancing whether to explore for new resources or exploit known ones, solitary individuals can accomplish only one of these tasks at a time and therefore must switch strategies over time. Social groups are able to balance exploration and exploitation over time and space, with many individuals performing both strategies at the same time (21, 41). This may be advantageous to complex social groups like honey bees, which must track locations of several different food sources as they change daily and through seasonal shifts (42, 43). To effectively balance which strategy to employ at the collective level, some individuals may be cognitively predisposed to focus on one resource, urging other individuals to focus their attention on a rewarding resource while it is reliable via the recruitment dance. Under natural conditions, where queens mate with many different drones, most colonies would possess both types of learners (44). In fact, our unselected control colonies most closely resembled the low-LI colonies, indicating that there may be a collective equilibrium in between 50/50 high and low and 100% low LI that reflects an ecologically relevant collective phenotype (32).
Individual cognition and attention may also play a role in how a colony balances nutritional needs for the colony. Colonies must balance carbohydrates and protein coming into the colony as nectar and pollen, respectively. Foragers evaluate the needs of the colony based on how much nectar and pollen are stored, as well as by the increased protein demands of the developing brood (45). Latent inhibition may play a role in what information foragers pay attention to, driving what they evaluate as necessary for the colony and in turn influencing what food they collect. In fact, pollen foragers exhibit higher latent inhibition than nectar foragers (37). Independent QTL analyses identified the same region on the genome that strongly correlates with influencing both pollen foraging and latent inhibition (46, 47). Pollen foragers may exhibit higher latent inhibition, both at the colony and while out foraging. Cognitive and foraging phenotypes may be influenced pleiotropically by the same genes or gene networks (48).
Attention is critical for many individual behaviors, including finding the correct mate or prey (49). Aspects of cognition covary to form characteristics of individual behavioral phenotypes, behavioral syndromes, and personality (8, 50). We show here that cognition drives collective behavioral traits as well. Future studies can expand on this work by identifying how individuals differ in their communication of information, such as food type and distance, by investigating the waggle dance, as well as by exploring the transcriptomic and physiological mechanisms that drive such differences. We propose that this diversity-of-attention aspect of individual cognitive phenotypes may enhance the overall efficacy with which a group finds and exploits resources, particularly when social groups may be in need of a specific resource.
Materials and Methods
Obtaining Queens and Drones and LI Testing.
To obtain reproductive bees (queens and drones) for producing selected lines of a specific LI behavior, we performed the LI assay on mature virgin queens 10 d after emergence and mature drones returning from unsuccessful mating flights. We tested queens and drones from at least eight different colonies (SI Appendix, Tables S9 and S10).
Tested queens were placed into individually labeled queen cages and returned to a queenless colony until insemination, which typically occurred within a week of testing. To obtain fertile drones, we collected them from their returning unsuccessful mating flights at the entrance of colonies. We placed them into cages overnight in a queenless colony for LI testing the next day.
To evaluate LI, we collected the individual bees and placed them into a 2.5-cm plastic harness and then secured the bee with masking tape, repeating for 30 individuals. To ensure that bees would respond to sucrose for later learning assays, we tested the bees by touching their antenna with a small droplet of 1 M sucrose solution from a syringe but did not feed them. We proceeded only with bees that had a positive response to this test. To allow the bee to acclimate to the harness, we placed 16 individuals into a quiet, dark cabinet for 1 h. To familiarize bees to an odor, we placed them on an automated odor exposure station which puffs scented air at the bees for 4 s every 5 min for a total of 40 exposures. We then used the proboscis extension reflex (PER) to test their ability to learn to associate a food reward with the familiar versus a novel odor. During the PER evaluation, we placed a single bee in a Plexiglas testing arena, allowed the bee to acclimate for 20 s, puffed either the familiar or a novel odor at the bee (1-octanone or 2-hexanol, constituent simple floral odors) for 4 s. To elicit proboscis extension, we touched the antenna of the bee with 1.5 M sucrose solution in the last second of the odor exposure and then allowed the bee to consume 0.4 μL of sucrose solution. For a more in-depth explanation of the LI training and PER testing, see refs. 20, 34.
After testing, drones were marked for individual identification and placed into a cage in a queen bank for no longer than 3 d until inseminations occurred.
Queen Inseminations.
We used instrumental insemination to inseminate a queen with sperm from a single drone. We inseminated a high-LI queen with a high-LI drone and a low-LI queen with a low-LI drone (51, 52). We ensured that every queen was inseminated with a drone from a different colony to ensure genetic variation (SI Appendix, Tables S9 and S10). LI varies across individuals. However, for this behavioral selection, we used the highest and lowest LI scoring individuals to create the high and low colonies, respectively. We then introduced queens to small queenless colonies and allowed the queens to produce workers for 1 mo. Colonies were checked weekly to eliminate the possibility of supersedure.
Cohoused Worker Preparation and Testing.
To test the LI of foragers from each LI line, we placed frames of capped pupae from three high-LI and three low-LI colonies into 34 °C incubators for 18 h. After 18 h, we used water-based acrylic paint pens (Montana) to mark the thorax of the eclosed bees with a color indicating their natal colony. Half of the bees were then returned to their natal colony and half were placed into an established control colony of an open-mated queen. Fewer bees were recovered for testing from the established control colony as many were recognized as nonnestmates and rejected. After 2 wk, colonies were monitored every day until marked bees began to forage, ∼21 d after emergence. Returning nectar foragers were collected and tested for LI.
Field Colony Experimental Setup.
To explore the colony-level foraging behavior of the LI lines, we set up four treatment colonies for each of the colony types: a high-LI colony, a low-LI colony, a 50/50 mixed colony, and a control colony. We ran weekly field experiments for 6 wk. High-LI and low-LI colonies consisted of bees from three different selected colonies, while mixed colonies consisted of these same three colonies for both the high-LI and low-LI lines for a total of six selected colonies. Hi-LI, low-LI, and mixed colonies came from a stock of eight different high-LI colonies and seven different low-LI colonies in 2017 and eight high-LI and eight low-LI colonies in 2018. We attempted to use all of these stock colonies equally; however, we used some stock colonies more often because they tended to produce more brood. For ease of identification, we always marked individuals from high-LI colonies red, orange, and pink, and individuals from low-LI colonies green, blue, yellow, and white. We continued to mark emerging bees from the same frames until we had 650 bees to form a colony, which took typically 2 to 3 d. To achieve conditions for typical honey bee behavior, we supplemented workers from three unselected colonies (control bees) that were not marked. For colony setup, see SI Appendix, Tables S1 and S11–S18. Bees were then placed into four different treatment colonies consisting of ∼1,300 bees: high-LI plus controls, low-LI plus controls, 50/50 mixed high-LI/low-LI plus controls, and only the control colony. Bees were provided a honeycomb and remained inside for 5 d before being placed for field experimentation. We then placed nucleus colonies into outdoor flight cages (3.05 × 12 m) and replaced the honeycomb frame with an empty frame to induce foraging the night before the experiment. Water was provided as needed. We ran high-LI, low-LI, mixed, and control colonies concurrently in four different tents.
We used a familiar and novel feeder foraging assay to characterize colony-level foraging behavior (29). This assay is typically used to assess novelty seeking that is associated with exploration and exploitation. Although the LI assay in the laboratory involves familiarizing individuals to an odor without a food reward, familiarization without reward or in the absence of important cues (such as trying to familiarize to odors while inside the colony) is difficult to accomplish in the field for colonies (22). The novelty-seeking assay effectively maps onto ecologically relevant exploration behavior that latent inhibition likely facilitates.
We placed a feeder with 1 M sucrose on day 1, which remained in the same location all week and became the familiar feeder (Fig. 2). We then placed one novel feeder in different locations each day (day 2 [X], day 3 [Y], and day 4 [Z]). Feeders had unique colors and unique odors and remained consistent throughout the experiment (SI Appendix, Table S2).
Mixed-Colony Round Dance Preparation and Data Collection.
To evaluate round-dance behavior of each of the selected lines, we created six 50/50 mixed colonies as detailed above and in SI Appendix, Tables S17 and S18. To induce foraging behavior, we placed the colonies in a climate-controlled indoor room for 10 d to allow bees to age, which increases foraging behavior. After 10 d, we then placed all bees from each colony into a two-frame observation hive with glass walls. All comb surfaces were visible. We videorecorded round-dance behavior using a Panasonic HC-WXF991K, starting the recording 15 min before feeders were placed in the flight cage. For distances from the colony entrance, see Fig. 4A. We followed the same feeder placement pattern across 4 d, from Monday to Thursday, in Fig. 4A. Round-dance data were then extracted visually by watching videos. We recorded the LI line of the dancer according to the color marking on her thorax, the feeder she visited according to the color mark on her abdomen (which also distinguished her as having visited a feeder), the duration of the round dance, the LI line of the round-dance followers, and the number of turns in a dance during the first 20 s of the dance or less if the dance ended before 20 s had elapsed. We identified a follower bee as a bee that moved with the dancer so that the follower’s head was oriented toward the dancer through at least one turn of her dance (16). As the feeders were less than 12 m away from the colony, bees performed round dances, which are on the continuum of the waggle dance and contain directional information (53). In addition to the information encoded in the dance, the liquid sucrose food from each feeder contained a unique odor, which is communicated during the dance (14, 54, 55) and allows the followers to orient to a specific feeder. Video observers were blind to the thorax and abdomen color associations between LI line and feeder visitation, respectively.
Data Analysis.
To test whether bees exhibited a similar LI score as their parents regardless of where they were housed after emergence, we used a GLM. We used LI score as the response variable, which fit a log-linear distribution, so we used a Gaussian family with a log link. Our fixed predictor variables were the line from which the bees originated (high LI or low LI) and the colony type in which they were placed after emergence (either their natal colony or a control colony).
To evaluate the effect of colony composition on colony-level foraging behavior to novel and familiar feeders, we performed a general linear model with a Gaussian error distribution on the number of visits, with selected line and feeder as fixed predictor variables, as well as the interaction between line and feeder. We performed a GLM with a binomial error distribution with a logit link function on percentage of revisitation, as it was a proportion comparing the number of revisits divided by the total number of visits. Line and feeder were fixed predictor variables, as well as the interaction between the line and feeder.
To explore whether the selected LI line of a forager bee influenced which feeder it visited while in the mixed colony, we used a general linear model with a Gaussian error distribution on the number of visits, with year, selected line, and feeder as a fixed predictor variables, as well as the interactions between these three variables. We found a significant three-way interaction between year, selected line, and feeder, which we present in SI Appendix, Table S6. Because of the significant difference between years, we treated years independently and performed two different GLMs with selected line and feeder as our fixed predictor variables. Several factors likely account for differences between years. In 2018, queens and drones were reselected, which introduced genetic variation. Colonies also differed in housing conditions: in 2017, colonies were housed outside in nucleus Langstroth hive boxes, and in 2018 they were housed indoors in glass-sided observation colonies.
To compare the round-dance behavior among the selected lines, we examined the effect of the selected LI of the dancer on the duration of the round dance, intensity of dancing, number of turns by the dancer, and number of followers of each dance using GLMs. To assess whether the duration of the round dance differed across the learning lines, we used a GLM with a gaussian family and a log link. The predictor variable was the LI of the dancer, and the response variable was the duration of the dance, which fit a log-normal distribution. To evaluate which lines attracted more dancers, we used a χ2 test to compare the proportion of dances that attracted no followers across the lines. To evaluate whether there were differences in the number of turns that the dancers from each line performed, we used a linear model because the response variable—the number of turns per second—was normally distributed. Finally, we used a negative binomial regression model using the package MASS (56) to understand how duration of a dance and the LI of the dancer interacted to predict the number of followers. A total of 159 of 908 dances had no followers, requiring the use of a zero-inflated model. We analyzed only bees that had paint marks on their abdomens, ensuring that they had visited a feeder.
We used an Analysis of Deviance Wald χ2 test using the function ANOVA in the MASS (56) package to further evaluate the overall effect of each fixed predictor variable and interaction. We used the lme4 package (57) to perform these tests unless otherwise noted. Post hoc tests were performed to determine the relationships between the different levels of fixed predictor variables and their interactions using the package emmeans (58). We used R (59) for all analyses.
Ethical Compliance.
Honey bees (A. mellifera) were used in this study. Queens (reproductive females) and drones (males) were behaviorally selected using lab assays to create selected lines of colonies. Worker honey bees (nonreproductive females) were tested in the field and in the lab. All colonies were kept following typical honey bee practices. There was no ethics committee involved in approving the animal husbandry protocol.
Supplementary Material
Acknowledgments
We thank S. Ohrt, E. Sezen, N. Kulkarni, and A. Phillips for help with field data collection and J. Bautista, H. Lei, and S. MacKnight for collecting video data. We thank C. Kwapich & D. Charbonneau for omments on drafts of this manuscript. This work was funded by NIH National Institute of General Medical Sciences (NIGMS) Grant R01GM113967 (to B.H.S., J.G., and N.P.-W.) and by NIH NIGMS Grant F32GM126728 (to C.N.C.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: Data have been deposited on Figshare at https://doi.org/10.6084/m9.figshare.9775955.v6.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1920554117/-/DCSupplemental.
Data Availability.
Data are available on Figshare (60).
References
- 1.Sumpter D. J., Collective Animal Behavior, (Princeton University Press, 2010). [Google Scholar]
- 2.Camazine S. et al., Self-Organization in Biological Systems, (Princeton University Press, 2003). [Google Scholar]
- 3.Couzin I. D., Krause J., “Self-organization and collective behavior in vertebrates” in Advances in the Study of Behavior, Slater P. J. B., Rosenblatt J. S., Snowdon C. T., Roper T. J., Eds. (Elsevier, 2003), pp. 1–75. [Google Scholar]
- 4.Perry C. J., Barron A. B., Chittka L., The frontiers of insect cognition. Curr. Opin. Behav. Sci. 16, 111–118 (2017). [Google Scholar]
- 5.Couzin I. D., Collective cognition in animal groups. Trends Cognit. Sci. 13, 36–43 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Del Mar Delgado M. et al., The importance of individual variation in the dynamics of animal collective movements. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20170008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couzin I. D. et al., Uninformed individuals promote democratic consensus in animal groups. Science 334, 1578–1580 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Sih A., Del Giudice M., Linking behavioural syndromes and cognition: A behavioural ecology perspective. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 2762–2772 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dukas R., Cognitive Ecology: The Evolutionary Ecology of Information Processing and Decision Making, (University of Chicago Press, 1998). [DOI] [PubMed] [Google Scholar]
- 10.ten Cate C., Rowe C., Biases in signal evolution: Learning makes a difference. Trends Ecol. Evol. (Amst.) 22, 380–387 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Schachar R. et al., Restraint and cancellation: Multiple inhibition deficits in attention deficit hyperactivity disorder. J. Abnorm. Child Psychol. 35, 229–238 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Dayan P., Kakade S., Montague P. R., Learning and selective attention. Nat. Neurosci. 3 (suppl.), 1218–1223 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Mitchell C. J., Le Pelley M. E., Attention and Associative Learning: From Brain to Behaviour, (Oxford University Press, 2010). [Google Scholar]
- 14.Von Frisch K., Chadwick L. E., The Dance Language and Orientation of Bees, (Belknap Press of Harvard University Press, Cambridge, MA, 1967). [Google Scholar]
- 15.Seeley T. D., Mikheyev A. S., Pagano G. J., Dancing bees tune both duration and rate of waggle-run production in relation to nectar-source profitability. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 186, 813–819 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Biesmeijer J. C., Seeley T. D., The use of waggle dance information by honey bees throughout their foraging careers. Behav. Ecol. Sociobiol. 59, 133–142 (2005). [Google Scholar]
- 17.Hammer M., Menzel R., Learning and memory in the honeybee. J. Neurosci. 15, 1617–1630 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menzel R., Müller U., Learning and memory in honeybees: From behavior to neural substrates. Annu. Rev. Neurosci. 19, 379–404 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Smith B. H., Huerta R., Bazhenov M., Sinakevitch I., “Distributed plasticity for olfactory learning and memory in the honey bee brain” in Honeybee Neurobiology and Behavior, Galizia C. G., Eisenhardt D., Giurfa M., Eds. (Springer Netherlands, 2012), pp. 393–408. [Google Scholar]
- 20.Cook C. N. et al., Individual differences in learning and biogenic amine levels influence the behavioural division between foraging honeybee scouts and recruits. J. Anim. Ecol. 88, 236–246 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemanski N. J., Cook C. N., Smith B. H., Pinter-Wollman N., A multiscale review of behavioral variation in collective foraging behavior in honey bees. Insects 10, 370 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abramson C. I., Bitterman M. E., Latent inhibition in honeybees. Anim. Learn. Behav. 14, 184–189 (1986). [Google Scholar]
- 23.Ferguson H. J., Cobey S., Smith B. H., Sensitivity to a change in reward is heritable in the honeybee, Apis mellifera. Anim. Behav. 61, 527–534 (2001). [Google Scholar]
- 24.Mitchell M. D., McCormick M. I., Ferrari M. C. O., Chivers D. P., Friend or foe?: The role of latent inhibition in predator and non-predator labelling by coral reef fishes. Anim. Cogn. 14, 707–714 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Ferrari M. C. O., Chivers D. P., Latent inhibition of predator recognition by embryonic amphibians. Biol. Lett. 5, 160–162 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lubow R. E., Latent inhibition. Psychol. Bull. 79, 398–407 (1973). [DOI] [PubMed] [Google Scholar]
- 27.Lubow R. E., Latent inhibition as a measure of learned inattention: Some problems and solutions. Behav. Brain Res. 88, 75–83 (1997). [DOI] [PubMed] [Google Scholar]
- 28.Lubow R. E., Latent inhibition: Effects of frequency of nonreinforced preexposure of the CS. J. Comp. Physiol. Psychol. 60, 454–457 (1965). [DOI] [PubMed] [Google Scholar]
- 29.Liang Z. S. et al., Molecular determinants of scouting behavior in honey bees. Science 335, 1225–1228 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Lubow R. E., Latent Inhibition and Conditioned Attention Theory, (Cambridge University Press, 1989). [Google Scholar]
- 31.Lubow R. E., Weiner I., Schnur P., “Conditioned attention theory (Academic Press, 1981), pp. 1–49” in The Psychology of Learning and Motivation: Advances in Research and Theory, Bower G. H., Ed. (Academic Press, 1981), pp. 1–49. [Google Scholar]
- 32.Mosqueiro T. et al., Task allocation and site fidelity jointly influence foraging regulation in honeybee colonies. R. Soc. Open Sci. 4, 170344 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhagavan S., Benatar S., Cobey S., Smith B. H., Effect of genotype but not of age or caste on olfactory learning performance in honey bee, Apis mellifera. Anim. Behav. 48, 1357–1369 (1994). [Google Scholar]
- 34.Chandra S. B. C., Hosler J. S., Smith B. H., Heritable variation for latent inhibition and its correlation with reversal learning in honeybees (Apis mellifera). J. Comp. Psychol. 114, 86–97 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Brandes C., Menzel R., Common mechanisms in proboscis extension conditioning and visual learning revealed by genetic selection in honeybees (Apis mellifera). J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 166, 545–552 (1990). [Google Scholar]
- 36.Ferrari M. C. O., Chivers D. P., Learning about non-predators and safe places: The forgotten elements of risk assessment. Anim. Cogn. 14, 309–316 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latshaw J. S., Smith B. H., Heritable variation in learning performance affects foraging preferences in the honey bee (Apis mellifera). Behav. Ecol. Sociobiol. 58, 200–207 (2005). [Google Scholar]
- 38.Waddington K. D., Honey bee foraging profitability and round dance correlates. J. Comp. Physiol. 148, 297–301 (1982). [Google Scholar]
- 39.Pavlov P. I., Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Ann. Neurosci. 17, 136–141 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gershman S. J., Blei D. M., Niv Y., Context, learning, and extinction. Psychol. Rev. 117, 197–209 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Hills T. T., Todd P. M., Lazer D., Redish A. D., Couzin I. D.; Cognitive Search Research Group , Exploration versus exploitation in space, mind, and society. Trends Cognit. Sci. 19, 46–54 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Couvillon M. J. et al., Honey bee foraging distance depends on month and forage type. Apidologie (Celle) 46, 61–70 (2015). [Google Scholar]
- 43.Steffan-Dewenter I., Kuhn A., Honeybee foraging in differentially structured landscapes. Proc. Biol. Sci. 270, 569–575 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mattila H. R., Burke K. M., Seeley T. D., Genetic diversity within honeybee colonies increases signal production by waggle-dancing foragers. Proc. Biol. Sci. 275, 809–816 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dreller C., Page R. E. Jr., Fondrk M. K., Regulation of pollen foraging in honeybee colonies: Effects of young brood, stored pollen, and empty space. Behav. Ecol. Sociobiol. 45, 227–233 (1999). [Google Scholar]
- 46.Chandra S. B. C., Wright G. A., Smith B. H., Latent inhibition in the honey bee, Apis mellifera: Is it a unitary phenomenon? Anim. Cogn. 13, 805–815 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Hunt G. J., Page R. E. Jr., Fondrk M. K., Dullum C. J., Major quantitative trait loci affecting honey bee foraging behavior. Genetics 141, 1537–1545 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith B. H., Cook C. N., Experimental psychology meets behavioral ecology: What laboratory studies of learning polymorphisms mean for learning under natural conditions, and vice versa. J. Neurogenet. 34, 178–183 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Dukas R., Behavioural and ecological consequences of limited attention. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357, 1539–1547 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griffin A. S., Guillette L. M., Healy S. D., Cognition and personality: An analysis of an emerging field. Trends Ecol. Evol. (Amst.) 30, 207–214 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Cobey S. W., Tarpy D. R., Woyke J., Standard methods for instrumental insemination of Apis mellifera queens. J. Apic. Res. 52, 1–18 (2013). [Google Scholar]
- 52.Harbo J. R., “Propagation and instrumental insemination” in Bee Breeding and Genetics, Rinderer T. E., Ed. (Academic Press, 1986), pp. 361–389. [Google Scholar]
- 53.Gardner K. E., Seeley T. D., Calderone N. W., Do honeybees have two discrete dances to advertise food sources? Anim. Behav. 75, 1291–1300 (2008). [Google Scholar]
- 54.Grüter C., Balbuena M. S., Farina W. M., Informational conflicts created by the waggle dance. Proc. Biol. Sci. 275, 1321–1327 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Díaz P. C., Grüter C., Farina W. M., Floral scents affect the distribution of hive bees around dancers. Behav. Ecol. Sociobiol. 61, 1589–1597 (2007). [Google Scholar]
- 56.Ripley B., et al. , Package “mass”, (Cran R, 2013). [Google Scholar]
- 57.Bates D. M., Mäechler M., Bolker B., Walker S., Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 58.Lenth R., Lenth M. R., Package “lsmeans”. Am. Stat. 34, 216–221 (2018). [Google Scholar]
- 59.R Core Team , R: A Language and Environment for Statistical Computing, (R Foundation for Statistical Computing, Vienna, Austria, 2017). [Google Scholar]
- 60.Cook C. N., et al. , Individual learning phenotypes drive collective behavior dataset. Figshare. 10.6084/m9.figshare.9775955.v5. Deposited 20 May 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on Figshare (60).