Significance
The persistence of large carnivores in human-dominated landscapes will become increasingly challenging as the human footprint expands. Here, we bring together long-term demographic and behavioral data on one of the worlds’ most conflict-prone species, the brown bear, to quantify the mechanisms facilitating human–carnivore coexistence. We found that human-dominated landscapes are highly lethal, especially to young bears, until they learn to adapt to people. As bears age, they avoid times when people are most active but do not strongly avoid where people live. To sustain human–carnivore coexistence under high rates of mortality requires the influx of animals from areas with low human presence (i.e., demographic rescue). Paradoxically, our work demonstrates that connectivity leads to both coexistence and conflict.
Keywords: source-sink, wilderness, coadaptation, grizzly bear, demography
Abstract
With a shrinking supply of wilderness and growing recognition that top predators can have a profound influence on ecosystems, the persistence of large carnivores in human-dominated landscapes has emerged as one of the greatest conservation challenges of our time. Carnivores fascinate society, yet these animals pose threats to people living near them, resulting in high rates of carnivore death near human settlements. We used 41 y of demographic data for more than 2,500 brown bears—one of the world’s most widely distributed and conflict-prone carnivores—to understand the behavioral and demographic mechanisms promoting carnivore coexistence in human-dominated landscapes. Bear mortality was high and unsustainable near people, but a human-induced shift to nocturnality facilitated lower risks of bear mortality and rates of conflict with people. Despite these behavioral shifts, projected population growth rates for bears in human-dominated areas revealed a source-sink dynamic. Despite some female bears successfully reproducing in the sink areas, bear persistence was reliant on a supply of immigrants from areas with minimal human influence (i.e., wilderness). Such mechanisms of coexistence reveal a striking paradox: Connectivity to wilderness areas supplies bears that likely will die from people, but these bears are essential to avert local extirpation. These insights suggest carnivores contribute to human–carnivore coexistence through behavioral and demographic mechanisms, and that connected wilderness is critical to sustain coexistence landscapes.
Human coexistence with large carnivores poses one of the greatest conservation challenges of our time. From tiger (Panthera tigris) and leopard (Panthera pardus) attacks in rural Asian villages (1), shark-attack hotspots (2), to brown bear (Ursus arctos) conflicts in urban areas of North America and Europe (3, 4), carnivores pose real and perceived threats to human life, livelihoods, and property (3, 4). As a result, humans kill carnivores either preemptively or in retaliation, making human-dominated areas highly lethal for many animals (5). Carnivores also profoundly influence ecosystem dynamics (6), provide socioeconomic benefits to society (7), and receive disproportionate attention in conservation and the media (8). This juxtaposition of fascination and lethal force toward carnivores exposes a profound tension in conservation: How can people and carnivores coexist?
Historically, carnivore populations have been suppressed in many areas, with remnant populations persisting in areas of minimal human influence (hereafter wilderness) (5, 9). However, a no-analog scenario of large carnivores purportedly coexisting in more heavily human-dominated landscapes is developing. Multiple-use landscapes—composed of cities, highways, and rural communities within a patchwork of remaining natural habitats—are now being recolonized by carnivores across the globe [e.g., Western Europe (10), East Africa (11), Midwest United States (12), and Southeast Asia (13)]. This emerging pattern of cooccurrence has led to the suggestion that coexistence—persisting wildlife presence in human-dominated landscapes that facilitate life requisites, such as reproduction, via coadaptation between wildlife and people (as in the sense of refs. 14 and 15)—no longer requires wilderness areas to maintain viable populations of large carnivores (16). However, views in support of a diminished role of wilderness in the conservation of large carnivores are not universal (17, 18).
When coexistence occurs, success is often attributed to the role of people taking action to improve connectivity and reduce human-caused mortality (10, 16, 19). However, animals are not passive actors and may be actively shaping coexistence landscapes themselves. For example, carnivores are known to reduce their home range extent in human-dominated areas (20) and to increase their activity at night to avoid people (21, 22). In the absence of evidence linking behavior to a demographic response, it is not clear if the behavioral responses of carnivores to people are a signal of coexistence or a portend of extirpation. For example, depressed vagility could have fitness consequences that lead to population declines, or it could be an adaptive response to avoid lethal encounters with people, thereby enhancing fitness (23). A mechanistic link between behavioral adaptation of carnivores and population persistence is needed to better understand how coexistence arises.
Demographic or behavioral mechanisms can lead to coexistence if at least one of the four following, nonexclusive mechanisms facilitates population persistence. First, carnivores can increase their survival through spatial avoidance of human-dominated areas. Second, carnivores may overlap in space with people, but increase their survival through temporal avoidance of humans [e.g., nocturnality (21, 24)]. Third, high mortality could be compensated by high rates of reproduction by surviving animals (i.e., density dependence). Finally, high mortality could be subsidized by immigration from areas with lower human-caused mortality, thus sustaining coexistence through connectivity [i.e., source-sink dynamics (25–29)].
To evaluate these mechanisms, we focused on brown bears, one of the world’s most widely distributed and conflict-prone carnivores (3, 5, 30). Brown bear conflicts with, and attacks on, humans are increasing (30). Conflicts between people and bears generate more media coverage than those with any other terrestrial or aquatic predator (30). Such intense conflicts and associated lethal removal by humans threaten coexistence for this species in nonwilderness areas. To understand how bears persist near people, we compiled data on the mortality rates, movement, habitat use, and demography of 2,669 brown (grizzly) bears over a 378,191-km2 area in North America. Our data were collected in and around British Columbia, Canada, over a 41-y period (1979 to 2019) and included 808 bear years of individual demographic monitoring, 474,222 telemetry relocations, and 5,867 genetic detections of marked animals. We integrated individual and population-level responses with satellite-derived measures of landscape productivity and human influence (Fig. 1A and SI Appendix, sections 1.1 and 1.2). These data revealed that, despite high rates of bear mortality in human-dominated areas, coexistence is possible via a combination of individual behavioral shifts and connectivity to wilderness areas that rescue bears from extirpation. These mechanisms, paired with shifting social attitudes toward the tolerance of—and coadaptation with—carnivores (15, 31, 32), have facilitated brown bear recolonization, persistence, and increasing densities in many human-dominated landscapes across western North America (33–37).
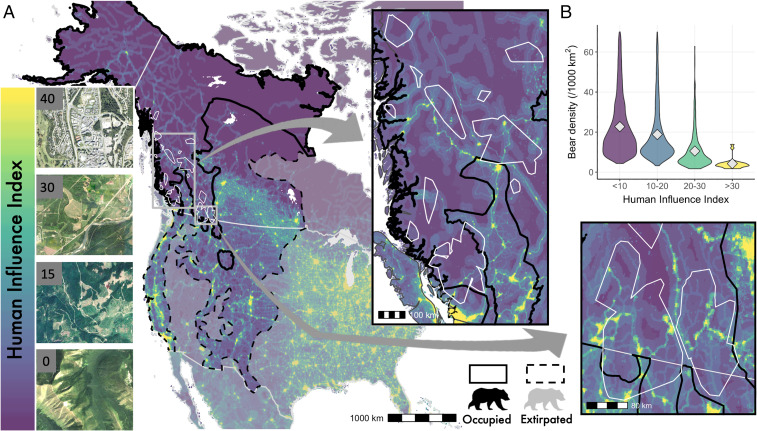
Fig. 1.
(A) Study extents (white polygons) for each of 12 telemetry and 29 genetic tagging studies on brown bear. Human Influence Index (HII) depicted with satellite images from across brown bear range on left. HII is a composite index derived by combining human population density, human land use and infrastructure (built-up areas, nighttime lights, land use/land cover), and human access (coastlines, roads, railroads, navigable rivers) (48). The index ranges from 0 (lowest human impact) to 64 (the most human-dominated category). For our purposes, we consider the range from 0 to 40 as brown bears generally don’t use—or survive in—habitats exceeding HII of 40 (SI Appendix, section 1.3). National borders in gray. Inset maps show the variation in human influence within and among studies. (B) Relationship between brown bear population density and HII within the study extents.
Bear density was negatively correlated with the Human Influence Index (HII) (Fig. 1B), a satellite-derived aggregated measure of the human footprint (SI Appendix, section 2.1). This negative correlation was driven by lower survival due to elevated human-caused mortality, rather than lowered reproduction (Fig. 2A and SI Appendix, sections 2.2 and 2.4). Compared to adults, subadults faced a 7.5× higher mortality risk in the highest human-dominated areas where bears occur (Fig. 2A). Humans were the dominant cause of mortality for bears, especially in regions where HII > 12 (Fig. 2C).
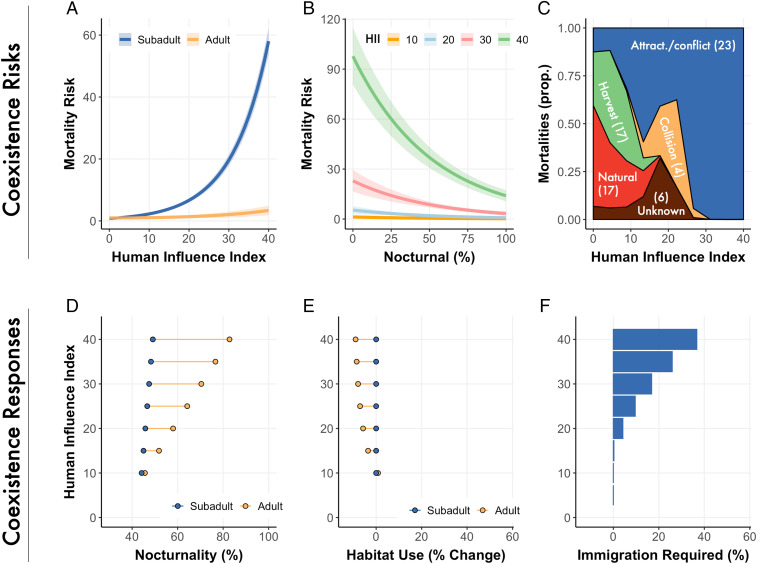
Fig. 2.
Per capita risk of annual mortality predicted from Cox proportional hazard model for subadult (3 to 6 y old) and adult (>6 y old) bears across HII gradients (A) and for HII and nocturnality (B). See SI Appendix, Section 2.2 for further details on hazard models. Uncertainty shown as SE. (C) Proportion of mortalities by cause by HII for animals > 2 y old; 76% of recorded mortalities were human-caused. Number of observed mortalities by cause is shown in brackets. Attract./conflict = mortality due to an attractant or conflict issue. Relationship between HII and (D) nocturnality (percent) between age classes, and (E) change in habitat use between age classes, indicating the degree to which animals changes their use of HII as they aged. For example, where HII = 20, this shows the change in habitat use as animals moved from subadults to adults when the area used by the subadult had an average HII area of 20, and (F) immigration required (percent of population) to sustain stable brown bear populations (population growth = 1).
Although there was a high mortality rate for bears living near people, some bears shifted to a nocturnal activity pattern as they aged (Fig. 2 B and D). Bears in human-dominated areas increased their nocturnality by 2 to 3% per year past the age of 3, which led to a 2 to 3% increase in survival per year (SI Appendix, section 2.3). In wilderness areas, we detected no significant, age-related shift in brown bear nocturnality (Fig. 2D), suggesting that humans are inducing the shift of bears toward nocturnality and that nocturnality is not an inherent expression of bears in wild areas. In the rural areas that characterize coexistence landscapes (i.e., HII = 25), it takes 14 y for a bear to become a successful coexistor (i.e., attain survival rates similar to adults in wilderness areas: i.e., >90% annual survival) (SI Appendix, section 2.5). These coexisters will generally have to attain nocturnality levels exceeding 75% to survive (see, for example, Fig. 3B). For every bear that lives to 14 y, there will be about 29 other bears in the cohort that will die, while only 4 bears will die during the same time in a wilderness area.
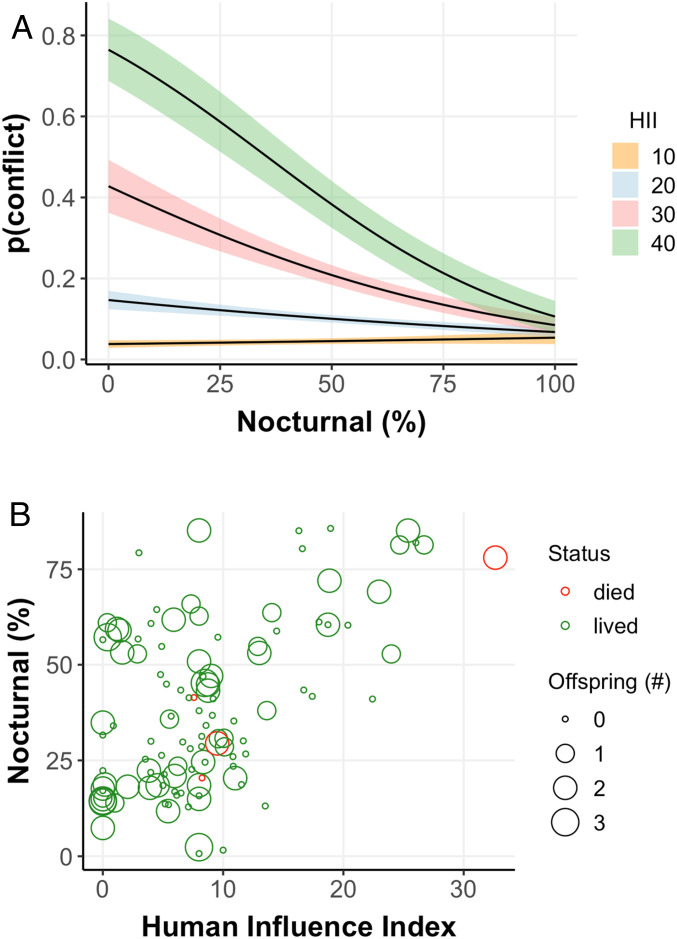
Fig. 3.
(A) the relationship between probability of conflict and nocturnality across human influence gradients (SI Appendix, Analyses, Section 2.8). Uncertainty shown as SE. (B) annual number of dependent offspring observed (0 to 2 y old) per female (>5 y old), whether the female survived that year, annual measures of nocturnality, and use of human-dominated landscapes. We did not detect evidence for variation the number of offspring observed for females across the human influence gradient (effect of HII on offspring [n] = −0.018 [95% CI: 0.015 to −0.05], P = 0.29; but see SI Appendix, Analyses, Section 2.4 for more information on cub reproduction across HII gradients).
Increased nocturnality not only enhances bear survival but reduces conflicts with people. We examined 1,848 brown bear conflict records and associated these with the HII of the surrounding landscape (SI Appendix, section 2.8). We found that the number of conflicts increased by one incident with every 3.5 unit increase in HII. We also maintained records of conflicts with 45 individual GPS-collared bears. The probability of a conflict occurring with one of these bears at least once in a year was ∼71% lower if bears were more active at night than the day (Fig. 3A and SI Appendix, section 2.8). Thus, the shift to nocturnality increases brown bear survival, reduces conflict for people, and facilitates coexistence.
Despite the lower risk of mortality in wilderness areas, individual bears did not “learn” to strongly avoid spaces used by people; we found little to no spatial avoidance of human-dominated areas as bears aged (Fig. 2E and SI Appendix, section 2.3). This weak avoidance suggests that once a bear establishes a home range following dispersal, there is limited behavioral plasticity to avoid areas used by people. Consequently, there are two outcomes for young animals in landscapes of coexistence: Adapt to people by becoming more nocturnal (Fig. 2D) or die because of people (Fig. 2A).
Even though a majority of adult female bears in human-dominated landscapes have shifted to a nocturnal activity pattern and are breeding successfully (Fig. 3B), their mortality rates are far too great to maintain current densities (Fig. 1B) via recruitment alone. The persistence of bears outside of wilderness areas requires increasing amounts of immigration as HII increases. For every unit increase in HII (past an HII of 12, where population growth becomes negative), 1 to 2% more of the population must be replaced by immigrants to maintain population stability (Fig. 4A). These immigrants are supplied via dispersal. Male and female bears disperse an average of 42 km (maximum 160 km) and 14 km (maximum 53 km), respectively (38) (SI Appendix, section 2.6). Dispersal from wilderness areas offsets the survival deficit from human-caused mortality, creating source-sink dynamics (26). Such dynamics extend human influence to areas well beyond the disturbance footprint of towns and roads, creating a wilderness-subsidized basin of coexistence surrounding human-dominated areas (27) (Fig. 5). Using a conservative dispersal distance of 20 km, we estimate that the basin of coexistence (the main sources supplying immigrants to the sink, plus the sink area) encompasses 19% of the North American brown bear range and ∼1.2 million people. The basin occupies 46% of the southern range margin of brown bears (south of 60° latitude) and is home to 0.46 million people (Fig. 4A and SI Appendix, section 2.7). In the southern range, there are an estimated 4,158 (95% CI: 3,950 to 4,365) brown bears living completely, or partially, within sink areas, representing 16% of the bear population.
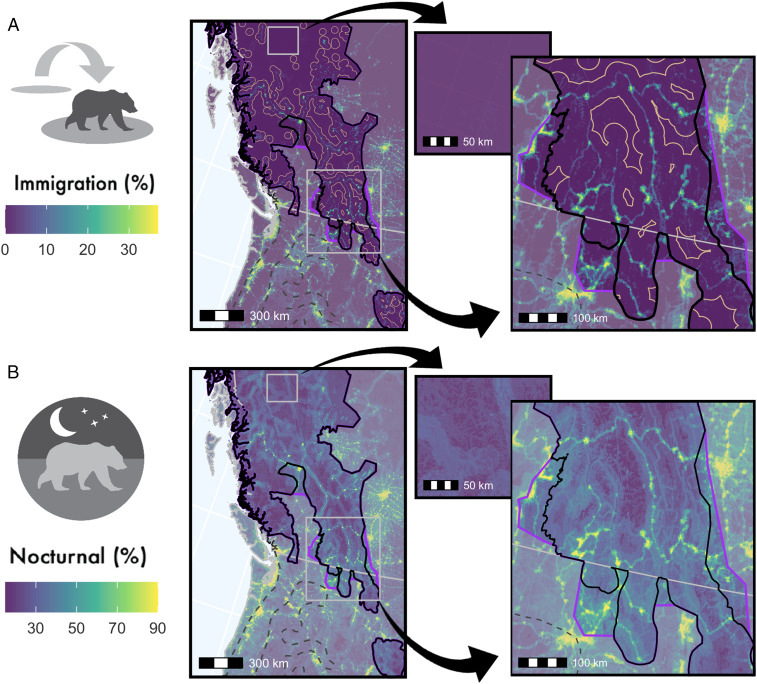
Fig. 4.
Spatial depiction of the landscape of coexistence and what sustains it across the southern range margin of brown bears. Black lines represent the current extent of the brown bear distribution and purple represents the contemporary recolonization frontier. (A) Percent immigration required to sustain the population, calculated by as the difference between a population that can sustain itself without immigration (in situ population growth = 1) and the observed in situ population growth rates for these areas (often <1). Tan lines represent a conservative extent of influence from localized sink areas (HII > 12) on the larger population (20-km buffer on sinks). (B) Estimated percent nocturnality displayed by adult bears (15 y old) across the landscape. Inset maps depict the coexistence landscape in a wilderness area, and in an area of high human influence and recolonization at the international brown bear range margin.
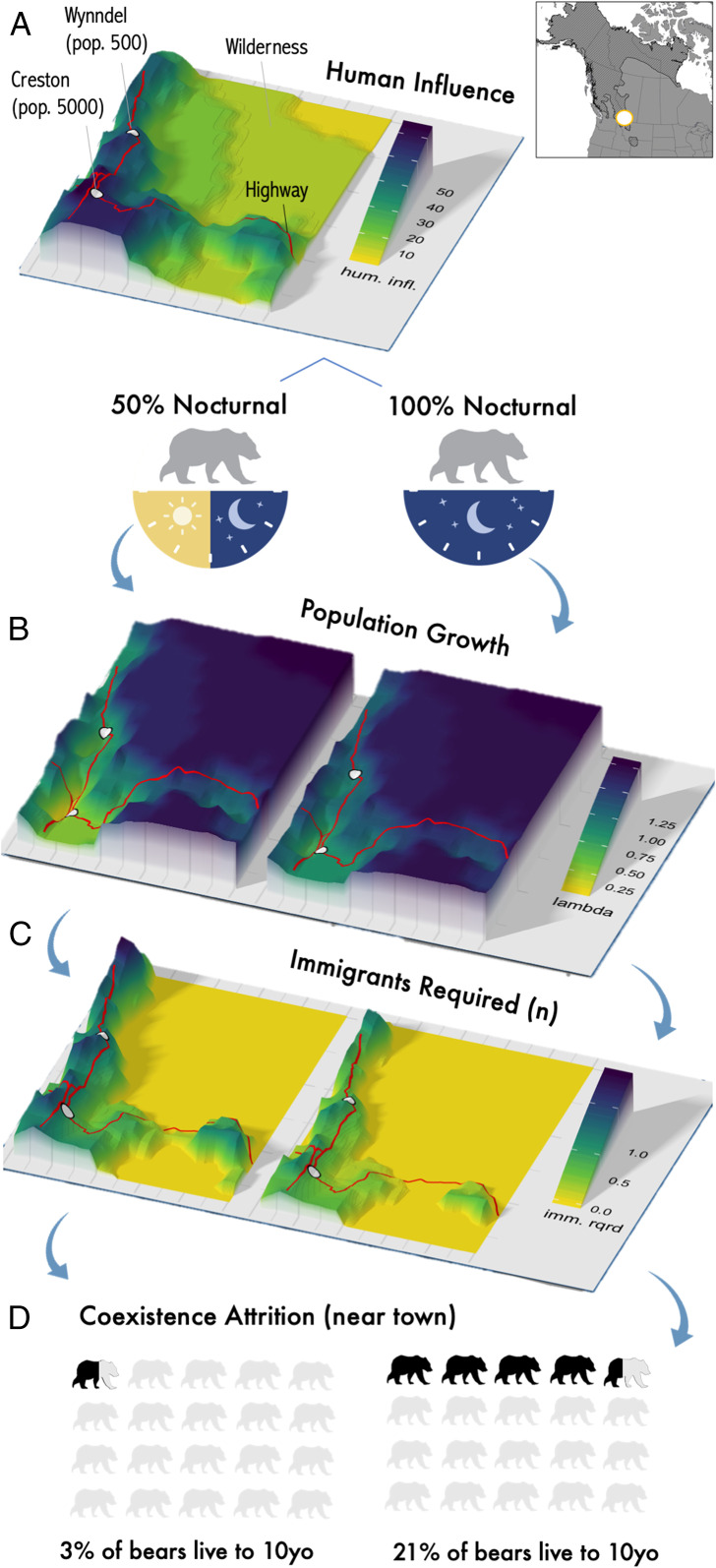
Fig. 5.
A basin of coexistence near Creston, British Columbia, Canada showing (A) human influence, towns, highways, and wilderness, demographic responses under two modeled scenarios of nocturnality (50% and 100%) for the following parameters: (B) projected population growth of brown bears, (C) number of immigrants (per 25 km2 per decade) to support coexistence, and (D) survival to 10 y old (10yo).
Although sink habitats elevate mortality for bears, these areas can provide nutritional subsidies. For example, clearing of land for logging, roads, and urban development, reduces overstory cover and increases production of bears’ preferred forage (27, 39). Furthermore, many human-derived food sources—highway roadkill, fruit trees, livestock, and garbage—simply do not occur in wilderness (27, 33, 40, 41). Ecological trap dynamics, a special case of source-sink dynamics where attractive habitat decouples evolutionary habitat–fitness links, has been shown to contribute to the source-sink dynamics within our study system (27, 29, 41). In addition, the foraging efficiency of bears in sink habitats needs further study. Bears require daylight to forage on berries, their preferred food during late summer (42). The nocturnality required for bears to persist near people may thus reduce foraging efficiency, with potential impacts on body condition and reproduction. Nevertheless, nocturnal female bears in human-dominated areas were observed with slightly more dependent offspring (ages 0 to 2: 1.33 per year) compared to females in wilderness areas (0.87 per year), but the difference was not significant (P = 0.21). These data suggest that female bears were repeatedly able to find sufficient nutrition in human-dominated landscapes and produced at least as many offspring as females in wilderness; however, the poor survival of animals in human-dominated areas often counteracted any differences in fecundity (Fig. 3B and SI Appendix, section 2.4).
Given the intense human-induced pressure on brown bear survival, it is possible that differential survival between coexistence phenotypes (e.g., earlier ontogenetic shifts to nocturnality, or shyer animals) could lead to microevolution. In other cases of human-induced “unnatural selection” (e.g., harvesting targeted at size of horn, tusk, or body), microevolution only emerged when populations were sufficiently closed to genetic swamping by immigrants that were not under such selection (43). In contrast, brown bears in coexistence landscapes are sustained by immigrants, weakening the capacity for microevolution to take place. Connectivity is critical for demographic rescue and to supply enough “learners” to human-dominated areas that can adapt to risk of mortality from people. If connectivity were impeded to allow for the accumulation of coexistence genotypes, populations would be extirpated well before (10 to 20 y, where HII > 12) sufficient microevolution could take place (SI Appendix, sections 2.4 and 2.5). Thus, it is unlikely that bears in human-dominated landscapes will evolve a more positive genotype for coexistence as a direct result of unnatural selection. However, cultural transmission of behavior from mothers to offspring may represent a means to spread coexistence behavior (44).
Our findings expose a striking paradox of coexistence: The mobility of brown bears averts extirpation through demographic rescue, yet these same animals face considerable risk once they arrive near people. Along with bears’ adaptive responses to people, we show that connectivity to wilderness is a critical mechanism of coexistence. Efforts to protect intact wilderness areas for carnivores, maintain and enhance connectivity, and reduce human-caused mortality will allow carnivores to be more active participants in coexistence (45). Bear density in human-dominated landscapes often remains an order-of-magnitude lower than in wilderness areas (Fig. 1B) and would rapidly be extirpated without continual immigration, highlighting the importance of maintaining, and in some cases restoring, intact wilderness. On the human side, social tolerance for carnivores, and creative solutions for coexistence, are increasing (15). Reducing human influences at night can restore carnivore movement (46), and highway crossing structures can increase carnivore survival and connectivity without interfering with human transportation (47). The behavioral adaptation and demographic processes of large carnivores will support global large carnivore coexistence efforts (21, 22), provided that there is sufficient wilderness connected to these areas for demographic rescue.
Methods
Data.
Remotely sensed habitat information.
We used the HII as our measure of human impact and risk on the landscape (SI Appendix, section 1.1) (48). This dataset is a global grid of 1-km cells, created from nine global data layers covering human population pressure (population density), human land use and infrastructure (built-up areas, nighttime lights, land use/land cover), and human access (coastlines, roads, railroads, navigable rivers). We followed the approach of Tucker et al. (20) and used an index of habitat productivity (Normalized Difference Vegetation Index, NDVI) (SI Appendix, section 1.2). The NDVI provides a measure of vegetative abundance, which has been widely used in animal ecology (49) and to describe brown bear diet (50) and population density (51).
Brown bear demography, habitat use, and conflict.
We compiled data from 29 genetic tagging projects (SI Appendix, section 1.4), wherein 2,200 bears were detected 5,867 times between 1996 and 2017. We used GPS and VHF telemetry data collected on 469 brown bears across British Columbia between 1979 and 2019 (SI Appendix, section 1.3). These data consist of telemetered animals of known sex and age, monitored for 808 bear years, with 75 mortalities, 661 reproductive intervals, and 474,222 relocations spread across multiple ecosystems and 12 research projects. For individuals with a relocation frequency that was sufficient to assess patterns of nocturnality (fix rates ≤ 8 h), we calculated percent nocturnal as [nightly movement rate/(daily movement rate + nightly movement rate) × 100]. We defined “day” as after dawn and before dusk, and “night” as after dusk but before dawn. We used 1,848 brown bear conflict records collected across British Columbia between 2014 and 2019 to estimate conflict patterns.
Analyses.
All analyses were conducted in program R (52). For all models we tested competing models using Akaike Information Criterion (AIC)c, and model-averaged results by model weight when the top model has less than 90% of the overall weight.
Spatial capture–recapture.
We conduct a spatially explicit capture–recapture (SCR) (SI Appendix, section 2.1) analysis with the genetic tagging data to parameterize a density∼HII relationship while controlling for habitat productivity. We followed the SCR analysis methods of Lamb et al. (35) and fit models with the “oSCR” package (53).
Mortality hazard analysis.
We parameterized spatially explicit mortality risk models using the telemetry relocations as the live location and contrasted these against the HII at mortality locations (SI Appendix, section 2.2). We only considered independent animals (≥3 y old) and the active (nondenning) season between April and November. We used Cox-proportional hazard models with Bear ID as a clustered effect to account for clusters of correlated observations (i.e., monthly sampling of the same individual).
Risk mitigation strategies: Spatial and temporal risk avoidance.
We explored spatial and temporal risk avoidance by bears in response to the mortality risk of human-dominated areas (SI Appendix, section 2.3). We used generalized linear mixed-effects models with a random effect for individual, and month. We assessed changes in the values of HII as an indicator of spatial avoidance of human influence, and changes in daily timing of use of HII as an indicator of temporal avoidance of human influence. The random-effects model allows individuals to have different baseline values (intercept) for a parameter, but the model tests if variable coefficients (slopes) are different from 0 within each individuals time monitored. Thus, we could test if individuals were behaviorally adapting to risk as they aged. For spatial avoidance we measured the individual change as deviation from their habitat use as subadults, and assessed the degree to which the HII values used as an adult differed.
Immigration required.
We calculated projected population growth (i.e., population trajectory without immigration and emigration) across human influence and productivity gradients (SI Appendix, section 2.4). This measure of population growth is distinct from realized population growth (i.e., the observed change in population trajectory [N/Nt−1], which includes birth, death, immigration, and emigration). By calculating the projected population growth we could disentangle the unmeasured and confounding influences of immigration in sustaining brown bears in human-dominated areas.
We estimated age-specific survival and reproduction across human influence and productivity gradients, and created spatially explicit Leslie matrices. We then projected population growth for each of these matrices using the dominant eigenvalue of the matrix. If the projected growth rate was <1, (i.e., the population could not intrinsically sustain itself) we calculated the percentage of immigrants required to sustain the population as (1−population growth) × 100.
Conflict.
We investigated the relationship between a bear’s nocturnal behavior and its probability of being in conflict with people using a subset of 45 GPS-collared bears for which we have maintained records of conflict incidents (40 animals with no conflict, 5 with conflict, as reported by the British Columbia Government). We modeled the probability of animals having a conflict, at least once in a year, as a function of HII, NDVI, and percent nocturnality using logistic regression (SI Appendix, section 2.8).
Supplementary Material
Acknowledgments
The data in this paper were augmented by generous data sharing from the British Columbia Ministry of Forests, Lands, and Natural Resource Operations, Clayton Apps, Grant MacHutchon, Francis Iredale, Stefan Himmer, Tony Hamilton, Deb Wellwood, Karen Diemart, and Michelle McLellan. We thank Ullas Karanth, Jon Swenson, Mark Hebblewhite, Andrew Derocher, Mark Boyce, and Robert Serrouya for their insights on earlier versions of this work; the First Nations, within whose traditional territories we studied bears; all the field staff that helped collect the data used here; Laura Smit and Grant MacHutchon for painstakingly preparing much of the data for these analyses; and Kate Broadley and Fuse Consulting, who were integral in the graphic design and layout of our figures. This work would not have been possible without the generous support of the following groups: Government of British Columbia, Habitat Conservation Trust Foundation, Vanier Canada Graduate Scholarship, Liber Ero Fellowship Program, Canada Research Chairs program, National Science Engineering and Research Council, Wildlife Conservation Society, British Columbia Conservation Foundation, Fish and Wildlife Compensation Program, Forest Enhancement Society of British Columbia, Teck Coal, Columbia Basin Trust, Okanagan Nation Alliance, Ktunaxa Nation, Margo Supplies, Counter Assault, Wildsight, Nature Conservancy of Canada, Wildlife Conservation Society, Yellowstone to Yukon Conservation Initiative, Safari Club International, Sparwood and District Fish and Wildlife Association, Elkford Rod and Gun Club, Grizzly Bear Foundation, all the South Rockies Grizzly Bear Project volunteers, Ministry of Transport and Infrastructure, Outdoor Research, and the British Columbia Conservation Officer Service.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: All analyses are posted on Github, https://github.com/ctlamb/CarnivoreCoexistence-brownbear-2020. All code and accessible data are deposited in a publicly available Figshare repository, except the raw brown bear genetic tagging and collar relocation data, as these data include sensitive location data that we are unable to share publicly. Interested parties can contact us directly to request these data.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1922097117/-/DCSupplemental.
Data Availability.
Open access code that reproduces our analyses is available at https://github.com/ctlamb/CarnivoreCoexistence-brownbear-2020. All code and accessible data from this work are available at the publicly available Figshare repository, except the raw brown bear genetic tagging and collar relocation data, as these data include sensitive location data that we are unable to share publicly. Interested parties may contact us directly to request these data.
References
- 1.Dhanwatey H. S. et al., Large carnivore attacks on humans in central India: A case study from the Tadoba-Andhari Tiger Reserve. Oryx 47, 221–227 (2013). [Google Scholar]
- 2.Chapman B. K., McPhee D., Global shark attack hotspots: Identifying underlying factors behind increased unprovoked shark bite incidence. Ocean Coast. Manage. 133, 72–84 (2016). [Google Scholar]
- 3.Bautista C. et al., Patterns and correlates of claims for brown bear damage on a continental scale. J. Appl. Ecol. 54, 282–292 (2017). [Google Scholar]
- 4.Bombieri G. et al., Patterns of wild carnivore attacks on humans in urban areas. Sci. Rep. 8, 17728 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf C., Ripple W. J., Range contractions of the world’s large carnivores. R. Soc. Open Sci. 4, 170052 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estes J. A. et al., Trophic downgrading of planet Earth. Science 333, 301–306 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Gilbert S. L. et al., Socioeconomic benefits of large carnivore recolonization through reduced wildlife-vehicle collisions. Conserv. Lett. 10, 430–438 (2017). [Google Scholar]
- 8.Martín-López B., Montes C., Ramírez L., Benayas J., What drives policy decision-making related to species conservation? Biol. Conserv. 142, 1370–1380 (2009). [Google Scholar]
- 9.Ripple W. J. et al., Status and ecological effects of the world’s largest carnivores. Science 343, 1241484 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Chapron G. et al., Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346, 1517–1520 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Woodroffe R., Demography of a recovering African wild dog (Lycaon pictus) population. J. Mammal. 92, 305–315 (2011). [Google Scholar]
- 12.LaRue M. A. et al., Cougars are recolonizing the midwest: Analysis of cougar confirmations during 1990-2008. J. Wildl. Manage. 76, 1364–1369 (2012). [Google Scholar]
- 13.Wikramanayake E. et al., A landscape-based conservation strategy to double the wild tiger population. Conserv. Lett. 4, 219–227 (2011). [Google Scholar]
- 14.Carter N. H., Linnell J. D. C., Co-adaptation is key to coexisting with large carnivores. Trends Ecol. Evol. (Amst.) 31, 575–578 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Lute M. L., Carter N. H., Are we coexisting with carnivores in the American West? Front. Ecol. Evol. 8, 1–13 (2020). [Google Scholar]
- 16.Lopez-Bao J. V. et al., Carnivore coexistence: Wilderness not required. Science 348, 871–872 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Gilroy J. J., Ordiz A., Bischof R., Carnivore coexistence: Value the wilderness. Science 347, 382 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Karanth K. U. et al., Sinks as saviors: Why flawed inference cannot assist tiger recovery. Proc. Natl. Acad. Sci. U.S.A. 110, E110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawaya M. A., Kalinowski S. T., Clevenger A. P., Genetic connectivity for two bear species at wildlife crossing structures in Banff National Park. Proc. Biol. Sci. 281, 20131705 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tucker M. A. et al., Moving in the Anthropocene: Global reductions in terrestrial mammalian movements. Science 359, 466–469 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Gaynor K. M., Hojnowski C. E., Carter N. H., Brashares J. S., The influence of human disturbance on wildlife nocturnality. Science 35, 1232–1235 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Carter N. H., Shrestha B. K., Karki J. B., Pradhan N. M. B., Liu J., Coexistence between wildlife and humans at fine spatial scales. Proc. Natl. Acad. Sci. U.S.A. 109, 15360–15365 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lima S. L., Dill L. M., Behavioral decisions made under the risk of predation: A review and prospectus. Can. J. Zool. 68, 619–640 (1990). [Google Scholar]
- 24.MacHutchon G. A., Himmer S., Davis H., Gallagher M., Temporal and spatial activity patterns among coastal bear populations. Ursus 10, 539–546 (1998). [Google Scholar]
- 25.Doak D. F., Source-sink models and the problem of habitat degradation: General models and applications to the Yellowstone grizzly. Conserv. Biol. 9, 1370–1379 (1995). [Google Scholar]
- 26.Pulliam H., Sources, sinks, and population regulation. Am. Soc. Nat. 132, 652–661 (1988). [Google Scholar]
- 27.Lamb C. T., Mowat G., McLellan B. N., Nielsen S. E., Boutin S., Forbidden fruit: Human settlement and abundant fruit create an ecological trap for an apex omnivore. J. Anim. Ecol. 86, 55–65 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Pease C. M., Mattson D. J., Demography of the Yellowstone grizzly bears. Ecology 80, 957–975 (1999). [Google Scholar]
- 29.Nielsen S. E., Stenhouse G. B., Boyce M. S., A habitat-based framework for grizzly bear conservation in Alberta. Biol. Conserv. 130, 217–229 (2006). [Google Scholar]
- 30.Bombieri G. et al., Brown bear attacks on humans: A worldwide perspective. Sci. Rep. 9, 8573 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruskotter J. T. et al., Modernization, risk, and conservation of the world’s largest carnivores. Bioscience 67, 646–655 (2017). [Google Scholar]
- 32.Manfredo M. J., Urquiza-Haas E. G., Don Carlos A. W., Bruskotter J. T., Dietsch A. M., How anthropomorphism is changing the social context of modern wildlife conservation. Biol. Conserv. 241, 108297 (2020). [Google Scholar]
- 33.Lamb C. T. et al., Genetic tagging in the Anthropocene: Scaling ecology from alleles to ecosystems. Ecol. Appl. 29, e01876 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Proctor M. F. et al., Conservation of threatened Canada-USA trans-border grizzly bears linked to comprehensive conflict reduction. Human–Wildlife Interact. 12, 348–372 (2018). [Google Scholar]
- 35.Lamb C. T. et al., Effects of habitat quality and access management on the density of a recovering grizzly bear population. J. Appl. Ecol. 55, 1406–1417 (2018). [Google Scholar]
- 36.Morehouse A. T., Boyce M. S., Grizzly bears without borders: Spatially explicit capture recapture in southwestern Alberta. J. Wildl. Manage. 80, 1152–1166 (2016). [Google Scholar]
- 37.Schwartz C. C. et al., Temporal, spatial, and environmental influences on the demographics of grizzly bears in the Greater Yellowstone ecosystem. Wildl. Monogr. 161, 29941–30008 (2006). [Google Scholar]
- 38.Proctor M., McLellan B. N., Strobeck C., Barclay R., Gender-specific dispersal distances of grizzly bears estimated by genetic analysis. Can. J. Zool. 1118, 1108–1118 (2004). [Google Scholar]
- 39.Nielsen S., Stenhouse G. B., Beyer H. L., Huettmann F., Boyce M. S., Can natural disturbance-based forestry rescue a declining population of grizzly bears? Biol. Conserv. 141, 2193–2207 (2008). [Google Scholar]
- 40.Can O. E., D’Cruze N., Garshelis D. L., Beecham J., Macdonald D. W., Resolving human-bear conflict: A global survey of countries, experts, and key factors. Conserv. Lett. 7, 501–513 (2014). [Google Scholar]
- 41.Battin J., When good animals love bad habitats: Ecological traps and the conservation of animal populations. Conserv. Biol. 18, 1482–1491 (2004). [Google Scholar]
- 42.McLellan M. L., McLellan B. N., Effect of season and high ambient temperature on activity levels and patterns of grizzly bears (Ursus arctos). PLoS One 10, e0117734 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coltman D. W. et al., Undesirable evolutionary consequences of trophy hunting. Nature 426, 655–658 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Morehouse A. T., Graves T. A., Mikle N., Boyce M. S., Nature vs. nurture: Evidence for social learning of conflict behaviour in grizzly bears. PLoS One 11, e0165425 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamb C. T., Festa-Bianchet M., Boyce M. S., Invest long term in Canada’s wilderness. Science 359, 1002 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Whittington J., Low P., Hunt B., Temporal road closures improve habitat quality for wildlife. Sci. Rep. 9, 3772 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ford A. T., Barrueto M., Clevenger A. P., Road mitigation is a demographic filter for grizzly bears. Wildl. Soc. Bull. 41, 712–719 (2017). [Google Scholar]
- 48.Wildlife Conservation Society and Center for International Earth Science Information Network at Columbia University , Last of the Wild Project, Version 2, 2005 (LWP-2): Global Human Influence Index (HII) Dataset, (Geographic, 2005). [Google Scholar]
- 49.Pettorelli N. et al., The Normalized Difference Vegetation Index (NDVI): Unforeseen successes in animal ecology. Clim. Res. 46, 15–27 (2011). [Google Scholar]
- 50.Bojarska K., Selva N., Spatial patterns in brown bear Ursus arctos diet: The role of geographical and environmental factors. Mammal Rev. 42, 120–143 (2012). [Google Scholar]
- 51.Mowat G., Heard D. C., Schwarz C. J., Predicting grizzly bear density in western North America. PLoS One 8, e82757 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.R Core Team , R: A Language and Environment for Statistical Computing, (Version 3.5.1, R Foundation for Statistical Computing, Vienna, Austria, 2018). [Google Scholar]
- 53.Sutherland C., Royle J. A., Linden D. W., oSCR: A spatial capture–recapture R package for inference about spatial ecological processes. Ecography 42, 1459–1469 (2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Open access code that reproduces our analyses is available at https://github.com/ctlamb/CarnivoreCoexistence-brownbear-2020. All code and accessible data from this work are available at the publicly available Figshare repository, except the raw brown bear genetic tagging and collar relocation data, as these data include sensitive location data that we are unable to share publicly. Interested parties may contact us directly to request these data.