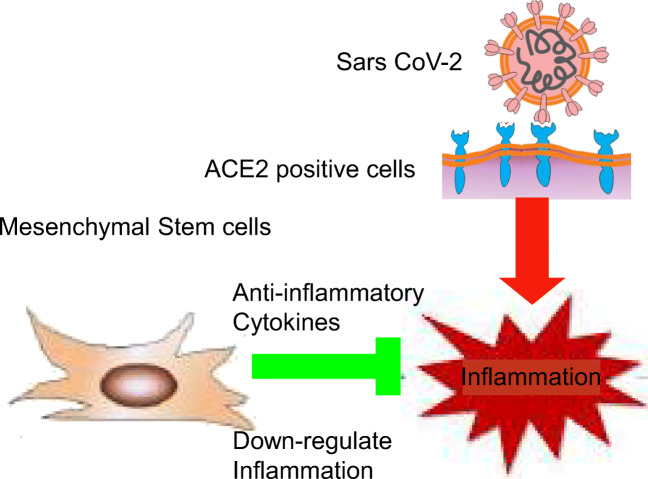
Abstract
An unfortunate emergence of a new virus SARS-CoV-2, causing a disease known as COVID-19, has spread all around the globe and has caused a pandemic. It primarily affects the respiratory tract and lungs in some cases causing severe organ damage and pneumonia due to overwhelming immune responses. Clinical reports show that the most commons symptoms are fever, dry cough, and shortness of breath, along with several other symptoms. It is thought that an immense cytokine dysregulation in COVID-19 patients is caused following the virus infection. Notably, if patients present with pre-existing specific comorbidities like diabetes or high blood pressure, rates of COVID-19 induced complications and deaths are escalated. Mesenchymal stem cell (MSC) therapy has been shown to alleviate pneumonia and acute respiratory syndrome (ARDS) symptoms, through their immunomodulatory activities in COVID-19 patients. Although more research studies and clinical trial results are needed to elucidate the exact mechanism by which MSCs provide relief to COVID-19 infected patients. Results from clinical trials are encouraging as patients treated with MSCs, regain lung functions and have restored levels of cytokines and trophic factors underscoring the fact that stem cell therapy can be, at least, a complementary therapy to alleviate sufferings in COVID-19 patients. This review discusses the possible therapeutic uses of MSCs for treating COVID-19.
Graphical Abstract
Keywords: COVID-19, Immunomodulation, Mesenchymal stem cells (MSCs), MSCs' immunomodulatory properties, Lymphocytes, SARS-CoV-2
Introduction
By late 2019, a novel coronavirus outbreak was recorded in Wuhan, Hubei Province, China. In the beginning, the virus was called 2019-nCoV which was subsequently renamed by the International Committee on Taxonomy of Viruses (ICTV) as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus disease 2019 (now more commonly known by the acronym “COVID-19”). This virus primarily affects the respiratory tract and lungs causing dry cough, fever, and shortness of the breath [1]. Nucleic acid sequence analyses from the lower respiratory tract samples indicated that the series of pneumonia cases of unknown cause reported during December 2019, were consequences of this novel virus [2].
Although most of the human coronavirus infections are mild, two major epidemic events caused by the members of coronavirus family have been reported in the last two decades itself. Severe acute respiratory syndrome coronavirus (SARS-CoV) emerged in Guangdong Province, China in 2003. The infection transmitted human to human and spread to 37 different countries and infected more than 8,000 people, of which 9% of the cases were fatal [3–5]. After the genetic identification of the pathogenic agent which was named SARS-CoV, it was hypothesized that it could have emerged from an animal host traded in the live-animal markets in China. Although horseshoe bats have been found to be a natural reservoir of the SARS-CoV-like virus, evidence suggests that the origin of the epidemic was from masked palm civets. However, it is still uncertain whether these animals were hosts of the virus in wild conditions or got infected during trading in the animal market [6]. Fortunately, due to the implementation of quarantining of infected persons, and restrictions on air travels the spreading of this particular virus was controlled in the same year and no human infections have been reported since [7].
Later in 2012, another viral outbreak of MERS-CoV was reported from the Kingdom of Saudi Arabia. In the last two decades, it had caused more than 10,000 cases, with mortality rates of 37% [2]. It is believed that bats are the reservoir for MERS-CoV also because closely related two other known bat viruses (BtCoV-HKU4 and BtCoV-HKU5) have been recorded. It is worth keeping in consideration that a virus containing the full sequence of MERS-CoV has not been isolated from any bat source so far [7, 8]. It is likely that there are other animal reservoirs of this particular virus, while the infections to humans were communicated via dromedary camels [9, 10].
The pandemic caused by coronavirus (SARS-CoV-2) has emerged as a global threat. According to the World Health Organization (WHO), the outbreak of the coronavirus infections has already been recorded in almost all countries of the world. By the end of May, 2020 a total of more than 5.7 million positive cases and more than 357,000 deaths (https://covid19.who.int/) have been recorded and the numbers are continuously increasing. Quarantining of confirmed non-critical cases and home quarantining of their established contacts have been ongoing important preventive practices. Currently, no specific drugs are available to cure the COVID-19 patients neither are vaccines to immunize healthy uninfected individuals. Hence, vigorous efforts are needed for safe and effective treatments for COVID-19, especially for severe cases. An approach of regenerative medicine by infusion of stem cells for treating lung diseases has been reported with promising results [11–13]. The immense capacity of mesenchymal stem cells (MSCs) in altering the activity of most of the components of immune system, along with their pro-angiogenic and cytoprotective effects by directly interacting with injured tissue, and by releasing a number of extracellular vesicles loaded with cytoprotective factors, cytokines, are now being perceived as a potential therapeutic agent for treating a number of diseases including lung diseases [13, 14]. In this review, an attempt has been made to discuss the immunomodulatory properties of MSCs, and how these properties make them useful for the treatment of patients suffering from SARS-CoV-2.
Similarities in the Pathogenicity of SARS-CoV-2 and COVID-19 SARS-CoV Viruses
SARS-CoV-2 virus belongs to the family Coronaviridea. Viruses belonging to this family have a single-stranded positive-sense RNA genome ranging from 26 to 32 kilobases in length [15]. Their genetic material is enveloped in a spherical capsid bearing club-shaped glycoprotein projections giving it a crown-like appearance after which the family is named. Reports based on phylogenetic evidence suggest that bats could be the natural reservoir of SARS-CoV-2, since bat-derived coronaviruses which also fall in basal positions in the subgenus Sarbecovirus. This virus is most closely related to bat-SL-CoVZC45 and bat-SL-CoVZXC21 viruses [16]. There is a possibility that the COVID-19 virus might have jumped from bats to humans but scientific proves indicating this fact are not conclusive and the probability that it could have been transmitted to humans from some other currently undecided wild animal/s sold at the Hunan seafood market is still there [17].
In order to infect a host cell, the S1 unit of the spike protein (S), present on the capsid of the virus binds to the angiotensin I converting enzyme 2 receptor (ACE2) after being primed by a protease called TMPRSS2 [18–20]. There are numerous ACE2 receptors on the surface of human cells, especially of the alveolar type II cells (AT2) and cells of capillary endothelium [21]. Genetic material replication takes place in the cytoplasm of the host cells which involves coordinated processes of continuous and discontinuous RNA synthesis facilitated by the replicase [22].
In the first clinical publication by China-based clinicians reported that most of the patients often presented with fever, dry cough and about one third of them had shortness of breath. Other common symptoms were muscle ache, headache, confusion, chest pain, and diarrhea. Even though further investigation is needed to ascertain all of the vehicles of disease transmission, there is a strong evidence of human-to-human transmission as all death cases were inline of early warning model for predicting mortality in viral pneumonia [23]. Findings based on MuLBSTA scoring system, comprised of six parameters routinely recorded in hospitals (multilobular infiltration, lymphopenia, bacterial co-infection, smoking history, hypertension, and age) [24] show that the presence of comorbidities like hypertension or other cardiovascular diseases, cerebro-vascular diseases, diabetes, hepatitis B infections, chronic obstructive pulmonary disease, chronic kidney diseases, malignancy and immunodeficiency significantly escalated the risk of poor prognosis. The prognoses became grimmer in patients with two or more comorbidities when compared to that in persons who had one or none [25].
Clinical profiles showed that in most cases level of lymphocytes, especially T lymphocytes were reduced. After the infection, virus particles spread through the respiratory mucosa inducing a cytokine storm in the body which caused changes in the population of peripheral white blood cells including lymphocytes [23]. Other laboratory parameters frequently recorded in patients indicated that besides lymphopenia, there were increased values in levels of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), lactate dehydrogenase (LDH) and D-dimer, and had low levels of serum albumin and hemoglobin [26]. Extremely high concentrations of IL-6, GCSF, IP10, MCP-1, MIP1A, CRP and TNF-α have been recorded in COVID-19 suffering patients [27]. The formations of microthrombi have been recorded in the patients [2, 28, 29] which could be formed in any organ of the body or could migrate to any organ and thus affect adversely. This could be the most likely reason to cause severe organ damages and death in high numbers [29].
Although, little is known about the pathology of the disease, due to the recent emergence of the virus, reports indicate that the lungs are the most affected organ as pneumonia-like symptoms occurred in most of the cases. A few other reports indicated other pathological indications like edema, proteinaceous exudates, vascular congestion, and inflammatory clusters with fibrinoid material and multinucleated giant cells [30]. Histopathological findings showed extensive pulmonary interstitial fibrosis and pulmonary hemorrhagic infarcts along with alveolar edema accompanied by the inflammatory injury of epithelial cells [31].
Stem Cell Therapy
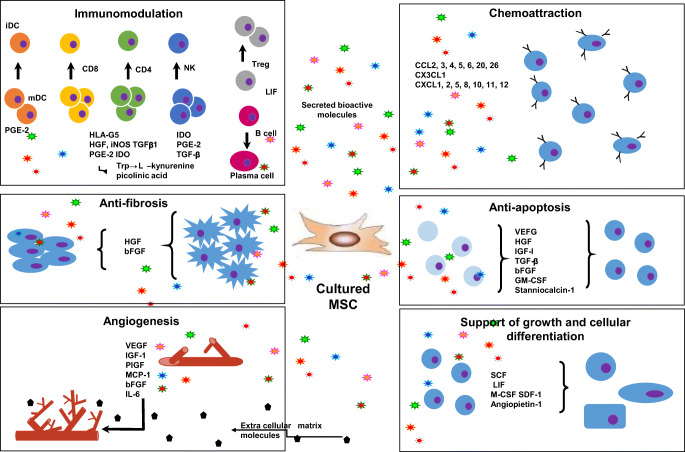
Stem cells are either totally undifferentiated or partially differentiated cells that can divide indefinitely to produce more cells of the same type and can differentiate into various types of cells. Because of many ethical and legal limitations, usage of the adult stem cells is more favored in clinical practices. Both Hematopoietic stem cells (HSCs), isolated from bone marrow, and Mesenchymal stem cells (MSCs), isolated from adipose tissues, are adult stem cells which have an excellent capacity to repair tissues since they can proliferate for extended periods while maintaining undifferentiated state and differentiate into various types of cells. MSCs can differentiate into endodermal and ectodermal lineages such as hepatocytes, cardiomyocytes, neurons, and astrocytes [32–34]. MSCs can be present in different niches in perivascular spaces in almost every tissue of the body [35, 36]. In general the number of stem cells decrease with age but the stemness has been thought to stay throughout the life. However, studies demonstrate that physical activity increases the quality of stem cells on one hand while the stemness and quality of stem cells decreases drastically with age in obese and diabetic individuals on the other [37, 38]. The most common type of MSCs used for therapy in lung diseases are from bone marrow, adipose tissue, umbilical cord blood, and endothelial progenitor cells [39, 40]. The MSCs can potentially serve as a promising tool in the cell-based therapy for treating COVID-19 patients due to their virtually unlimited proliferative and regenerative capability in addition to their ability to modulate immune responses of the recipient. Generally, MSCs can accelerate a number of processes by affecting a number of activities like immunomodulation, antiapoptosis, angiogenesis, supporting the growth and differentiation of local stem and progenitor cells, anti-scarring, and chemo-attraction (Fig. 1). Immunomodulatory properties of MSCs have been reported to be one of the major elements imparting the therapeutic benefits during the process of lung repair and regeneration in many pathological conditions such as bronchopulmonary dysplasia, asthma, acute lung injury, chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis. A number of active efforts is being carried out in this pursuit [39–41].
Fig. 1.
MSCs, in general, accelerate healing processes by acting at the level of immunomodulation, antiapoptosis, angiogenesis, supporting the growth and differentiation of local stem and progenitor cells, antiscarring, and chemoattraction. BFGF - basic fibroblast growth factor; CCL - CC chemokine ligand; CXCL - chemokine (C-X-C motif) ligand; ECM - extracellular matrix; GM-CSF - granulocyte-macrophage colony-stimulating factor; HGF - hepatocyte growth factor; iDC - invasive ductal carcinoma; IGF-1 - insulin growth factor-1; LIF - leukaemia-inhibitory factor; M-CSF - macrophage colony-stimulating factor; mDC - macrophage-derived chemokine; NK - natural killer cells; PGE2 - prostaglandin E2; SCF - stem cell factor; SDF-1 - stromal cell-derived factor 1; TGF-β - transforming growth factor-β; VEGF - vascular endothelial growth factor [42]
Mechanism of Stem Cell Action
During inflammation T-cells are activated in a series of cell signaling steps. The first signal that activates lymphocytes is mediated by specific antigen-specific T cell receptors (TCR), while the second step, called co-stimulation is independent of the TCR but is crucial for facilitating the full activation of immune response and avoiding anergy [43]. MSCs can exert immunosuppression by inhibiting T-cells at both primary and secondary activation steps by signaling through soluble factors like cytokines and growth factors, and by mechanisms involving direct cell to cell contacts [44]. Exposure of MSCs to interferon-ƴ (IFN-ƴ) stimulates indoleamine 2,3-dioxygenase (IDO) activity in MSC through the JAK/STAT signaling pathway [45, 46] which converts tryptophan to kynurenine that inhibits in a variety of cells and suppresses inflammatory response. Apoptosis of pro-inflammatory T-cells was observed after inducing IDO activity through tryptophan starvation via a generalized reduction in cellular energetics which was evident by increased generation and accumulation of secreted kynurenines [47]. Recorded T-cell specific inhibitory effects of IDO are thought to be because of the local accumulation of tryptophan metabolites rather than by tryptophan depletion [48]. Interestingly, more than 30 soluble factors have been reported to induce the immunomodulation capacity of MSCs to regulate activation and proliferation of T-cells. Contrary to other species, human MSCs produce IDO to suppress T-cell proliferation [49] and induce T cell anergy which is characterized by absence of their proliferation, and decrease in cytokine production, [50]. In vitro studies showed that T-cell IFN-ƴ production was elevated when activities of MSCs were eliminated. These findings underscore the fact that the major immune- inhibitory effect of MSCs takes place at the level of T-cell proliferation [44].
Cell-to-cell contacts also play crucial roles in MSC-mediated immunomodulation. Even after exposure to inflammatory signals, MSCs do not express co-stimulatory molecules, such as CD40, CD80, CD86, CD134, and CD252, which are key molecules for inducing the inflammatory process [51, 52]. On the contrary, in vitro studies suggest that after cell to cell interaction expression of Hes1 and Hey1 genes are increased in CD34+HPCs (Haemopoietic progenitor cells), which could prevent terminal differentiation into dendritic cells (DC) via the activation of Notch signaling [53, 54]. Overall, during the activation of the inflammation process, MSCs might have increased expression of CD274 for counteracting the regulatory effects because of up-regulation of CD40, and thus may suppress the induced immune response [52]. In addition, MSCs can suppress T-cell proliferation via the expression of CD39 and by the production of adenosine, which activates the adenosine A2 receptor (ADORA2A) on the surface of lymphocytes [55].
It is an interesting fact worth mention that MSCs from different sources do not have comparable immunoregulatory capacity. In a comparative study on MSCs from different sources, Warton Jelly derived MSCs were found to be most effective in immunosuppression [52]. Studies suggest that activation of Toll-like receptors (TLRs) play very significant roles in immunomodulation mediated by cell-to-cell contact, and also by MSC-secreted soluble factors. TLR family has been found to play important role in the innate immune system for the recognition of pathogen-associated molecular patterns (PAMPs), initiating primary responses against pathogens [56]. Eleven TLRs in human help recognize bacteria, viruses, protozoa, and fungi; and are commonly associated with chronic inflammatory and autoimmune diseases also [57]. MSCs, in general, are reported to express TLR 2, 3, 4, 5, 6, and 9, and the type of TLR activated during cell culture may affect their in vivo behavior after being transplanted [58]. TLR3 and TLR4 ligation, in the absence of IFN-ƴ, has been proved to enhance the immunosuppressive properties of MSCs by increasing tryptophan degradation leading to increased kynurenine production which is known to be catalyzed IDO and activated by autocrine interferon-β [59]. MSCs, depending upon the exposure to the type of extracellular signals, can secrete pro-inflammatory cytokines as well which may enhance innate immunity. In the presence of specific agonists, activated TLRs lead to the expression of inflammatory cytokines or co-stimulatory molecules. These agonists include a wide array of exogenous molecules like microbial components (LPS), lipoproteins and peptidoglycans, viral RNA, bacterial and viral and methylate CpG-DNA and endogenous molecules shed by dying cells [60, 61]. MSCs can be polarized by downstream TLR signaling into two homogenously phenotypes, known as MSC1 and MSC2. For MSC1, low-level exposure to TLR4 agonists polarizes them toward the pro-inflammatory behavior; through some mechanisms not fully understand yet. In contrast, for phenotype 2, MSCs can be polarized via TLR3 activation, and thus MSCs suppress the immune response [61]. Therefore, in order to avoid deleterious consequences while using MSCs as anti-inflammatory therapy, it has been suggested to activate specific TLRs in culture conditions itself before using the cells in vivo [62].
MSCs express low levels of human leukocyte antigen (HLA) class I molecules. This property of stem cells, in general, helps them evade the killings by natural killers (NK) cells. Also, MSCs and other stem cells do not express HLA class II molecules or costimulatory molecules like CD40, CD40L, CD80, and CD86, which are involved in the activation of T-lymphocyte-mediated immune responses [12, 49, 50, 63]. This property of the stem cells is very important from the clinical point of view as it takes care of concerns of host’s immune response. Accordingly, clinical trials have demonstrated the efficacy and safety in using allogenic MSCs, as neither toxicity nor adverse effects provoking serious concerns have been reported [41]. MSCs having the “immune-privileged” characteristics in addition to being non-immunogenic that is they do not trigger a rejection response because of less intense pro-inflammatory IFN-ƴ-induced HLA-II expression make them a very attractive clinical tool for treating COVID-19 [64].
In addition, it has been reported that MSCs express several growth factors which are involved in the regulation of proliferation, apoptosis, and differentiation. Signaling by TGF-β family ligands plays important roles in cell differentiation, proliferation, as well as in the maintenance of pluripotency in stem cells [65]. Moreover, evidence suggests that fibroblast growth factors (FGF) signaling mediated by the activation of P13K- and MAPK- pathway promotes repair and lung regeneration [66]. Vascular endothelial growth factor (VEGF) is another well-described pro-angiogenic factor with important implications in all organs, including the pulmonary system [67]. VEGF can act like a pneumotrophic factor and, thus help in tissue repair during the recovery process from lung injury [68]. Epidermal growth factor (EGF) also plays important roles in lung development, epithelial maturation, and regeneration. Lung epithelial cells including alveolar Type II cells are very rich in content of EGF receptors. Numerous studies have shown that EGF regulates the growth of various cellular components of the lung parenchyma after any injury [69]. Similarly, hepatocyte growth factor (HGF) also supports the growth in epithelial and endothelial cells by inducing mitogenic process, and by enhancing the survival of pulmonary endothelial and alveolar type II epithelial cells by blocking apoptosis process [70].
MSC-therapy in Patients Infected with COVID-19
The unfortunate outbreak of SARS-CoV-2 has imposed an urgent demand from scientists and clinicians around the globe to find effective therapeutic agents to alleviate sufferings in the COVID-19 patients on one hand while enforcing a demand for effective vaccines to mitigate its spread in future and spared or unaffected present populations on the other. Cell therapy and gene therapy at present are advanced fields of science in treating many diseases. It has been reported that umbilical cord-derived MSCs (hUCMSCs) have the potential to treat the H5N1 infection induced acute lung injury. It is worth keeping in consideration that inflammatory cytokine profiles induced by H5N1 and COVID-19 are similar [71] like extremely high concentrations of IL-6, GCSF, IP10, MCP-1, MIP1A, and TNF-α, likely to cause severe organ damages and death in high numbers [2, 28]. There are very limited reports available on MSC therapy for COVID-19. In a recent clinical report from Beijing, China [72], 7 patients with COVID-19 pneumonia were treated with human MSCs by intravenous administration of 1 × 06 cells per kilogram of body weight. Patients received one single dose of cells in addition to standard care and then were monitored for the following 14 days. On the other hand for placebo control, 3 patients were treated with the standard regimen of drugs. The results from MSC-treated patients showed a dramatic reduction in levels of serum proinflammatory cytokines and chemokines which attracted fewer mononuclear/ macrophages such as CXCR3+CD4+, CXCR+CD8+ T cells and CXCR3+ NK cells to the lungs. On the other hand, MSCs induced more regulatory dendritic cells (CD14+CD11c+CD11) to the inflamed areas [72]. In comparison with placebo control patients, MSC-recipients showed significantly decreased levels of TNF-α, a strong pro-inflammatory cytokine, and, elevated levels of IL-10, an anti-inflammatory cytokine. When administrated intravenously, a fraction of the MSCs settle in the lungs also, which could improve the pulmonary microenvironment, and thereby, reduce the over-activation of the immune system and support regeneration of affected lung tissues [72, 73]. RNA-seq analysis of transplanted MSCs revealed high expression of anti-inflammatory and trophic factors such as TGF-β, HGF, LIF, GAL, NOA1, FGF, VEGF, EGF, BDNF, and NGF [72]. While the immunosuppressive properties of the MSCs help fend off the cytokine storm, growth factors released by them might participate in lung regeneration activities. These findings indicate the possibility that the administration of exogenous growth factors, such as EGF might help in a more rapid regeneration which has been recorded in experimental animal models [69].
In another case study reported from Baoshan, China, a critically ill patient infected with COVID-19 was treated with allogeneic human umbilical cord-derived MSCs. The patient had severe pneumonia, acute respiratory distress, moderate anemia, hypertension, type 2 diabetes, and electrolyte disturbance. Laboratory analysis showed high levels of white blood cells and neutrophil percentage (92.4%), and had severe multi-organ damage. The patient received three intravenous administrations of 5 × 107 cells at intervals of 3 days (days 1st, 4th, and 7th) in addition to other standard treatments like thymosin α1 and antibiotics to prevent infections. After the second dose of MSC administration, levels of white blood cells, neutrophil and lymphocyte as well as the counts of CD3+, CD4+, CD8+ T-cells returned back to normal range. The finding demonstrates that the treatment of COVID-19 infections with MSC therapy alone or in combination with other immune-modulating agents like thymosin α1 offers a very promising therapeutic option to COVID-19 patients [74]. Many clinical trials undergoing in the USA are listed on the website https://clinicaltrials.gov/ct2/home.
Cytokine dysregulations have also been reported for influenza pathogenesis caused by several subtypes of influenza A viruses. Pneumonia and acute respiratory distress syndrome are among the most common pathogenesis observed in these viral infection conditions. Intravenous administrations of MSCs in patients suffering from influenza and similar pulmonary diseases have shown positive outcomes in restoring lung functions and reversing the lung functional and structural losses induced by the cytokine storm brought about by the infection. Therefore, it is logical to believe that MSC therapy can help patients suffering from COVID-19 via immunomodulatory pathways [75]. MSC therapy is emerging as a feasible therapeutic option in the treating COVID-19 patients because of the therapeutic abilities of MSCs like suppression of immune response. Their ability to differentiate into type II alveolar epithelial (AT2) cells, demonstrated in vitro, also may help further [76]. Lung injury may lead to altered signals of BMP4-NFATc1-TSP1 signaling pathway and could trigger aberrant alveolar progenitor function. MSC-secreted factor TSP1 may restore the disrupted vascular signals and facilitate epithelial repair [77]. Therefore, prevention of cytokine storm is believed as the major step in treatment of COVID-19. MSCs, owing to their powerful immunomodulatory abilities, may have beneficial effects for preventing the cytokine storm altogether or attenuating it and help regenerate the damaged lung tissues /other organs.
Conclusions
The novel virus SARS-CoV-2, causes the disease known as COVID-19, which primarily affects the respiratory tract and lungs, pneumonia leading to damaged organs. These viruses provoke intense immune response resulting in excessive cytokine secretion which could be termed as “cytokine storm”. Genetic analyses proved this virus is closely related to SARS-CoV and most likely harbor in wild bats. Due to the continuous interaction with wildlife in markets, it is likely the zoonotic process caused the outbreak.
Despite the fact that the pandemic erupted only a few months ago, a lot of genetic and phylogenetic information about the causative agent has already been reported. SARS-CoV-2, like other previously described coronaviruses, infects host cells by binding to ACE2 receptor and it is closely resembles with bat-SL-CoVZC45 and bat-SL-CoVZXC21, both found on wild populations of bats [16].
MSC therapy has shown promising results on many lung diseases [12] because of the remarkable immunomodulatory properties exhibited by stem cells. Even in patients ill with influenza A viruses, MSC therapy has proved to alleviate symptoms of pneumonia and acute respiratory syndrome [75, 78]. Still more research and clinical trial results are needed to elucidate the exact participation of MSCs in COVID-19 infected patients. Patients treated with MSCs regain lung function and have restored levels of cytokines and growth factors. Recommendation of MSC therapy along with other immune-modulating agents may help control the COVID-19 induced symptoms more efficiently [72, 74].
Author Contributions
Idea conception: AS; Writing-original draft: D.E.; writing reviewing and editing: R.M., P.S., and A.S.; Proof-reading: RM, RS and AM.
Compliance with Ethical Standards
Conflict of Interest
The authors declare no conflict of interest.
Footnotes
This article belongs to the Topical Collection: Special Issue on COVID-19 Pandemic and Stem Cells
Guest Editor: Mariusz Z. Ratajczak
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lupia T, Scabini S, Mornese Pinna S, Di Perri G, De Rosa FG, Corcione S. 2019 novel coronavirus (2019-NCoV) outbreak: A new challenge. Journal of Global Antimicrobial Resistanc. 2020;21:22–27. doi: 10.1016/j.jgar.2020.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 Novel Coronavirus in Wuhan, China. The Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W. A novel coronavirus associated with severe acute respiratory syndrome. The New England Journal of Medicine. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 4.Kuiken T, Fouchier RA, Schutten M, Rimmelzwaan GF, van Amerongen G, van Riel D, Laman JD, de Jong T, van Doornum G, Lim W, et al. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. The Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Chinese SARS Molecular Epidemiology Consortium Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science. 2004;303:1666–1669. doi: 10.1126/science.1092002. [DOI] [PubMed] [Google Scholar]
- 6.Shi, Z., & Hu, Z. A. (2008). Review of studies on animal reservoirs of the SARS coronavirus. Virus Research, 133, 74–87. [DOI] [PMC free article] [PubMed]
- 7.Coleman, C. M., Frieman, M. B., & Coronaviruses (2014). Important emerging human pathogens. Journal of Virology, 88, 5209–5212. [DOI] [PMC free article] [PubMed]
- 8.van Boheemen, S., de Graaf, M., Lauber, C., Bestebroer, T. M., Raj, V. S., Zaki, A. M., Osterhaus, A. D. M. E., Haagmans, B. L., Gorbalenya, A. E., Snijder, E. J., et al. (2012). Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio, 3, e00473–e00471. [DOI] [PMC free article] [PubMed]
- 9.Reusken, C. B. E. M., Haagmans, B. L., Müller, M. A., Gutierrez, C., Godeke, G.-J., Meyer, B., Muth, D., Raj, V. S., Smits-De Vries, L., Corman, V. M., et al. (2013). Middle east respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: A comparative serological study. The Lancet, 13, 859–866, S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed]
- 10.Haagmans BL, Dhahiry SHSA, Reusken CBEM, Raj VS, Galiano M, Myers R, Godeke G-J, Jonges M, Farag E, Diab A, et al. Middle east respiratory syndrome coronavirus in dromedary camels: An outbreak investigation. The Lancet. 2014;14:140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotton DN. Next-generation regeneration. American Journal of Respiratory and Critical Care Medicine. 2012;185:1255–1260. doi: 10.1164/rccm.201202-0228PP. [DOI] [PubMed] [Google Scholar]
- 12.Weiss DJ. Concise review: Current status of stem cells and regenerative medicine in lung biology and diseases. Stem Cells. 2014;32:16–25. doi: 10.1002/stem.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruz FF, Rocco PRM. Stem-Cell extracellular vesicles and lung repair. Stem Cell Investigation. 2017;4:1–11. doi: 10.21037/sci.2017.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruz FF, Rocco PRM. The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Review of Respiratory Medicine. 2020;14:31–39. doi: 10.1080/17476348.2020.1679628. [DOI] [PubMed] [Google Scholar]
- 15.Su, S., Wong, G., Shi, W., Liu, J., Lai, A. C. K., Zhou, J., Liu, W., Bi, Y., Gao, G. F., & Epidemiology (2016). Genetic recombination, and pathogenesis of coronaviruses. Trends in Microbiology, 24, 490–502. [DOI] [PMC free article] [PubMed]
- 16.Hu, D., Zhu, C., Ai, L., He, T., Wang, Y., Ye, F., Yang, L., Ding, C., Zhu, X., Lv, R., et al. (2018). Genomic characterization and infectivity of a novel SARS-like coronavirus in Chinese bats. Emerging Microbes & Infections, 7, 1–10. [DOI] [PMC free article] [PubMed]
- 17.Lu, R., Zhao, X., Li, J., Niu, P., Yang, B., Wu, H., Wang, W., Song, H., Huang, B., Zhu, N., et al. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. The Lancet, 395, 565–574. [DOI] [PMC free article] [PubMed]
- 18.Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., Schiergens, T. S., Herrler, G., Wu, N.-H., Nitsche, A., et al. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell, 181, 1–10. [DOI] [PMC free article] [PubMed]
- 19.Li, W., Moore, M. J., Vasilieva, N., Sui, J., Wong, S. K., Berne, M. A., Somasundaran, M., Sullivan, J. L., Luzuriaga, K., Greenough, T. C., et al. (2003). Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Letters to nature, 426, 450–454. [DOI] [PMC free article] [PubMed]
- 20.Mousavizadeh, L., & Ghasemi, S. (2020). Genotype and phenotype of COVID-19: Their roles in pathogenesis. Journal of Microbiology, Immunology and Infection. 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed]
- 21.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. Journal of Pathology. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sola I, Almazán F, Zúñiga S, Enjuanes L. Continuous and discontinuous RNA synthesis in coronaviruses. Annual Review of Virology. 2015;2:265–288. doi: 10.1146/annurev-virology-100114-055218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen N, Zhou M, Dong X, Qu J, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. The Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo L, Wei D, Zhang X, Wu Y, Li Q, Zhou M, Qu J. Clinical features predicting mortality risk in patients with viral pneumonia: The MuLBSTA score. Frontiers in Microbiology. 2019;10:1–10. doi: 10.3389/fmicb.2019.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan, W., Liang, W., Zhao, Y., Liang, H., Chen, Z., Li, Y., Liu, X., Chen, R., Tang, C., Wang, T., et al. (2020). Comorbidity and its impact on 1590 patients with Covid-19 in China: A nationwide analysis. European Respiratory Journal , in press. 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed]
- 26.Lippi, G., Plebani, M. (2020). Laboratory abnormalities in patients with COVID-2019 infection. Clinical Chemistry and Laboratory Medicine, in press. 10.1515/cclm-2020-0198. [DOI] [PubMed]
- 27.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 28.Wang D, Hu B, Hu C, Zhu F, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klok, F. A., Kruip, N. J. M., der Meer, M. S., Arbous, D. A. M., et al. (2020). Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Research, 2020, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7146714/. [DOI] [PMC free article] [PubMed]
- 30.Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, Chen H, Wang D, Liu N, Liu D, et al. Characteristics of COVID-19 infection in Beijing. Journal of Infection. 2020;80:401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo, W., Yu, H., Gou, J., Li, X., Sun, Y., Li, J., & Liu, L. Clinical pathology of critical patient with novel coronavirus pneumonia (COVID-19). Preprints,2020, 2020020407.
- 32.Lee, K. D., Kuo, T. K., Whang-Peng, J.; et al. (2004). In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology, 40, 1275–1284. [DOI] [PubMed]
- 33.Tropel P, Platet N, Platel JC, et al. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:2868–2876. doi: 10.1634/stemcells.2005-0636. [DOI] [PubMed] [Google Scholar]
- 34.Ratajczak MZ, Marycz K, Poniewierska-Baran A, Fiedorowicz A, Zbucka-Kretowskae M, Moniuszkof M. Very small embryonic-like stem cells as a novel developmental concept and the hierarchy of the stem cell compartment. Advances in Medical Sciences. 2014;59:273–280. doi: 10.1016/j.advms.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Kolf CM, Cho E, Tuan RS. Biology of adult mesenchymal stem cells: Regulation of niche, self-renewal and differentiation. Arthritis Research & Therapy. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glenn JD, Whartenby KA. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World Journal of Stem Cells. 2014;6:526. doi: 10.4252/wjsc.v6.i5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marędziak M, Śmieszek A, Chrząstek K, Basinska K, Marycz K. Physical Activity increases the total number of bone-marrow-derived mesenchymal stem cells, enhances their osteogenic potential, and inhibits their adipogenic properties. Stem Cells International. 2015 doi: 10.1155/2015/379093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marędziak, M., Marycz, K., Tomaszewski, K., Kornicka, K. A., K., and Henry, B. M. B. (2016). The influence of aging on the regenerative potential of human adipose derived mesenchymal stem cells. Stem Cells International2016, Article ID# 2152435. [DOI] [PMC free article] [PubMed]
- 39.Yang, J., & Jia, Z. (2014). Cell-based therapy in lung regenerative medicine. Regenerative Medicine Research, 2, 1–7. [DOI] [PMC free article] [PubMed]
- 40.Behnke J, Kremer S, Shahzad T, Chao C-M, Böttcher-Friebertshäuser E, Morty RE, Bellusci S, Ehrhardt H. MSC based therapies—New perspectives for the injured lung. Journal of Clinical Medicine. 2020;9:682. doi: 10.3390/jcm9030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss, D. J. (2010). Mesenchymal stem cells for lung repair and regeneration. In M. Rojas (Ed.), Stem cells in the respiratory system (pp. 25–42). New York: Springer Science & Business Media.
- 42.da Silva Meirelles L, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of Mesenchymal stem cells. Cytokine & Growth Factor Reviews. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Frauwirth KA, Thompson CB. Activation and inhibition of lymphocytes by Costimulation. Journal of Clinical Investigation. 2002;109:295–299. doi: 10.1172/JCI14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glennie S, Soeiro I, Dyson PJ, Lam E W-F, Dazzi F. Bone marrow Mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 45.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. Journal of Cell Science. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 46.Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase–mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 47.Mbongue J, Nicholas D, Torrez T, Kim N-S, Firek A, Langridge W. The role of indoleamine 2, 3-dioxygenase in immune suppression and autoimmunity. Vaccines. 2015;3:703–729. doi: 10.3390/vaccines3030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-γ does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clinical and Experimental Immunology. 2007;149:353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haddad R, Saldanha-Araujo F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: What do we know so far? BioMed Research International. 2014;2014:1–14. doi: 10.1155/2014/216806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stagg J. Immune regulation by mesenchymal stem cells: Two sides to the coin. Tissue Antigens. 2007;69:1–9. doi: 10.1111/j.1399-0039.2006.00739.x. [DOI] [PubMed] [Google Scholar]
- 51.Briones J, Novelli S, Sierra J. T.-cell costimulatory molecules in acute-graft-versus host disease: Therapeutic implications. Journal of Bone Marrow Research. 2011;2011:1–7. doi: 10.1155/2011/976793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Najar M, Raicevic G, Kazan HF, De Bruyn C, Bron D, Toungouz M, Lagneaux L. Immune-related antigens, surface molecules and regulatory factors in human-derived mesenchymal stromal cells: The expression and impact of inflammatory priming. Stem Cell Reviews and Reports. 2012;8:1188–1198. doi: 10.1007/s12015-012-9408-1. [DOI] [PubMed] [Google Scholar]
- 53.Li Y-P, Paczesny S, Lauret E, Poirault S, Bordigoni P, Mekhloufi F, Hequet O, Bertrand Y, Ou-Yang J-P, Stoltz J-F. Human mesenchymal stem cells license adult CD34 + hemopoietic progenitor cells to differentiate into regulatory dendritic cells through activation of the notch pathway. Journal of Immunology. 2008;180:1598–1608. doi: 10.4049/jimmunol.180.3.1598. [DOI] [PubMed] [Google Scholar]
- 54.Qu G, Xie X, Li X, Chen Y, Isla ND, Huselstein C, Stoltz J-F, Li Y. Immunomodulatory function of mesenchymal stem cells: Regulation and application. Journal of Cellular Immunotherapy. 2018;4:1–3. [Google Scholar]
- 55.Saldanha-Araujo F, Ferreira FIS, Palma PV, Araujo AG, Queiroz RHC, Covas DT, Zago MA, Panepucci RA. Mesenchymal Stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Research. 2011;7:66–74. doi: 10.1016/j.scr.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR, Zipori D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109:1422–1432. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- 57.Lombardo E, DelaRosa O, Mancheño-Corvo P, Menta R, Ramírez C, Büscher D. Toll-like receptor–mediated signaling in human adipose-derived stem cells: Implications for immunogenicity and immunosuppressive potential. Tissue Engineering. 2009;15:1579–1589. doi: 10.1089/ten.tea.2008.0340. [DOI] [PubMed] [Google Scholar]
- 58.Hwa Cho H, Bae YC, Jung JS. Role of toll-like receptors on human adipose-derived stromal cells. Stem Cells. 2006;24:2744–2752. doi: 10.1634/stemcells.2006-0189. [DOI] [PubMed] [Google Scholar]
- 59.Opitz CA, Litzenburger UM, Lutz C, Lanz TV, Tritschler I, Köppel A, Tolosa E, Hoberg M, Anderl J, Aicher WK, et al. Toll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-β and protein kinase R. Stem Cells. 2009;27:909–919. doi: 10.1002/stem.7. [DOI] [PubMed] [Google Scholar]
- 60.Sabroe, I., Read, R. C., Whyte, M. K. B., Dockrell, D. H., Vogel, S. N., & Dower, S. K. (2003). Toll-Like Receptors in Health and Disease: Complex Questions Remain. J Immunol, 171, 1630–1635. [DOI] [PubMed]
- 61.Bunnell BA, Betancourt AM, Sullivan DE. New concepts on the immune modulation mediated by mesenchymal stem cells. Stem Cell Research & Therapy. 2010;1:34. doi: 10.1186/scrt34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raicevic G, Rouas R, Najar M, Stordeur P, Id Boufker H, Bron D, Martiat P, Goldman M, Nevessignsky MT, Lagneaux L. Inflammation modifies the pattern and the function of toll-like receptors expressed by human mesenchymal stromal cells. Human Immunology. 2010;71:235–244. doi: 10.1016/j.humimm.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Rasmusson I. Immune modulation by mesenchymal stem cells. Experimental Cell Research. 2006;312:2169–2179. doi: 10.1016/j.yexcr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 64.Gao F, Chiu SM, Motan DAL, Zhang Z, Chen L, Ji H-L, Tse H-F, Fu Q-L, Lian Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death & Disease. 2016;7:e2062–e2062. doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.James D, Levine AJ, Besser D, Hermmati-Brivanlou A. TGFβ/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- 66.Chu, X., Chen, C., Chen, C., Zhang, J.-S., Bellusci, S., & Li, X. Evidence for Lung Repair and Regeneration in Humans: Key Stem Cells and Therapeutic Functions of Fibroblast Growth Factors. Frontiers of Medicine, 2019, 1–11. [DOI] [PMC free article] [PubMed]
- 67.Sakurai MK, Lee S, Arsenault DA, Nose V, Wilson JM, Heymach JV, Puder M. Vascular endothelial growth factor accelerates compensatory lung growth after unilateral pneumonectomy. The American Journal of Physiology-Lung C. 2007;292:L742–L747. doi: 10.1152/ajplung.00064.2006. [DOI] [PubMed] [Google Scholar]
- 68.Medford, A. R. L., Millar, A. B. (2006). Vascular Endothelial Growth Factor (VEGF) in Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS): Paradox or Paradigm? Thorax, 61, 621–626. [DOI] [PMC free article] [PubMed]
- 69.Kaza AK, Laubach VE, Kern JA, Long SM, Fiser SM, Tepper JA, Nguyen RP, Shockey KS, Tribble CG, Kron IL. Epidermal growth factor augments postpneumonectomy lung growth. The Journal of Thoracic and Cardiovascular Surgery. 2000;120:916–922. doi: 10.1067/mtc.2000.110460. [DOI] [PubMed] [Google Scholar]
- 70.Panganiban RAM, Day RM. Hepatocyte growth factor in lung repair and pulmonary fibrosis. Acta Pharmaceutica Sinica. 2011;32:12–20. doi: 10.1038/aps.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Darwish I, Mubareka S, Liles WC. Immunomodulatory therapy for severe influenza. Expert Review of Anti-infective Therapy. 2011;9:807–822. doi: 10.1586/eri.11.56. [DOI] [PubMed] [Google Scholar]
- 72.Leng Z, Zhu R, Hou W, Feng Y, et al. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging and Disease. 2020;11:216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shetty AK. Mesenchymal stem cell infusion shows promise for combating coronavirus (COVID-19)- induced pneumonia. Aging and Disease. 2020;11:462. doi: 10.14336/AD.2020.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liang, B., Chen, J., Li, T., Wu, H., Yang, W., Li, Y., Li, J., Yu, C., Nie, F., Ma, Z., et al. (2020). Clinical remission of a critically Ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells. ChinaXiv, 202002.00084. [DOI] [PMC free article] [PubMed]
- 75.Chen, J., Hu, C., Chen, L., Tang, L., Zhu, Y., Xu, X., Chen, L., Gao, H., Lu, X., Yu, L., et al. (2020). Clinical Study of Mesenchymal Stem Cell Treatment for Acute Respiratory Distress Syndrome Induced by Epidemic Influenza A (H7N9) Infection: A Hint for COVID-19 Treatment. Engineering, in press. 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed]
- 76.Ma N, Gai H, Mei J, Ding F, Bao C, Nguyen DM, Zhong H. Bone marrow mesenchymal stem cells can differentiate into type II alveolar epithelial cells in vitro. Cell Biology International. 2011;35:1261–1266. doi: 10.1042/CBI20110026. [DOI] [PubMed] [Google Scholar]
- 77.Leeman KT, Pessina P, Lee J-H, Kim CF. Mesenchymal stem cells increase alveolar differentiation in lung progenitor organoid cultures. Scientific Reports. 2019;9:1–10. doi: 10.1038/s41598-019-42819-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilson, J. G., Liu, K. D., Zhuo, N. J., Caballero, L., McMillan, M., Fang, X. H., et al. (2015). Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respiratory Medicine, 3, 24–32. [DOI] [PMC free article] [PubMed]