Abstract
Proliferative pathologic lesions of parathyroid glands encompass a spectrum of entities ranging from benign hyperplastic processes to malignant neoplasia. This review article outlines the pathophysiologic classification of parathyroid disorders and describes histologic, immunohistochemical, and molecular features that can be assessed to render accurate diagnoses.
Keywords: Parathyroid, Primary hyperplasia, Parathyroid adenoma, Parathyroid carcinoma, Parafibromin
OVERVIEW
Proliferative parathyroid disease is diagnostically challenging, because many disease entities within this group of disorders require supplementary clinical information and are not readily diagnosed with a light microscope and a good eye alone. In addition, for most pathologists, parathyroid specimens represent only a small percentage of case volume. Although the recognition and accurate diagnosis of the parathyroid lesions can be challenging because of limitations in conventional morphologic approaches, advances in radiological imaging techniques and rapid intraoperative parathyroid hormone assay combined with immu- nohistochemistry and molecular studies are progressively reducing diagnostic uncertainties and resolving diagnostic dilemmas.
EMBRYOLOGY AND ANATOMY OF THE PARATHYROID GLAND
Parathyroid glands develop as epithelial thickenings of the dorsal endoderm of the third and fourth branchial pouches around the fifth week of intrauterine life and are histologically visible only after 14 weeks.1,2 The fourth pouch gives rise to the superior parathyroid glands, whereas the inferior parathyroid glands are derived from the third branchial pouch. By the end of their migration, approximately 80% of superior parathyroid glands are found near the posterior edge of the thyroid gland at the junction of the superior and central aspects of each thyroid lobe (Fig. 1).3,4 The normal anatomic location of the inferior parathyroid glands is more variable than that of their superior counterparts. During embryogenesis and intrauterine development, the inferior parathyroid glands travel caudally in the neck and come to rest within 1 cm from the intersection of the inferior thyroid artery and the recurrent laryngeal nerve.4
Fig. 1.
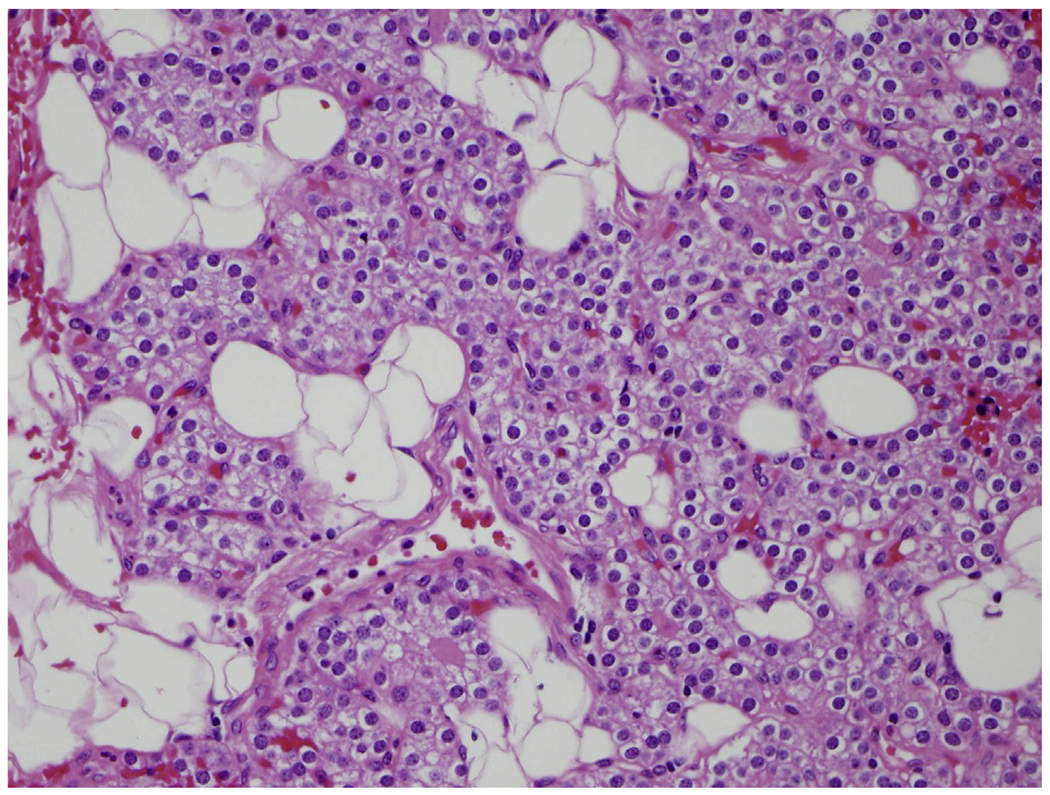
Normal parathyroid gland composed mainly of chief cells and adipocytes with thin fibrous septa dividing gland into lobules (hematoxylineosin [H&E], original magnification ×400).
Ectopic or variant locations of superior and inferior parathyroids are determined by migration routes. Ectopic superior parathyroid glands are uncommon but, when present, may be located in the central to posterior aspects of the mediastinum or as far as the developmental aortopulmonary window.3,5 Larger glands may travel down in the tracheoesophageal groove and come to rest below the inferior parathyroid glands.6 About 1% of the superior parathyroid glands may be observed in the paraesophageal or retroesophageal space.6,7 In contrast, the end anatomic location of inferior parathyroid glands tends to be more variable because of their longer migratory route. It is estimated that 15% to 50% of inferior parathyroid glands descend to the thymus.3,8 Additional unusual sites also include the skull base, angle of the mandible, or even above the superior parathyroid glands. The frequency of intrathyroidal glands is approximately 2% (Fig. 2).3,4,9
Fig. 2.
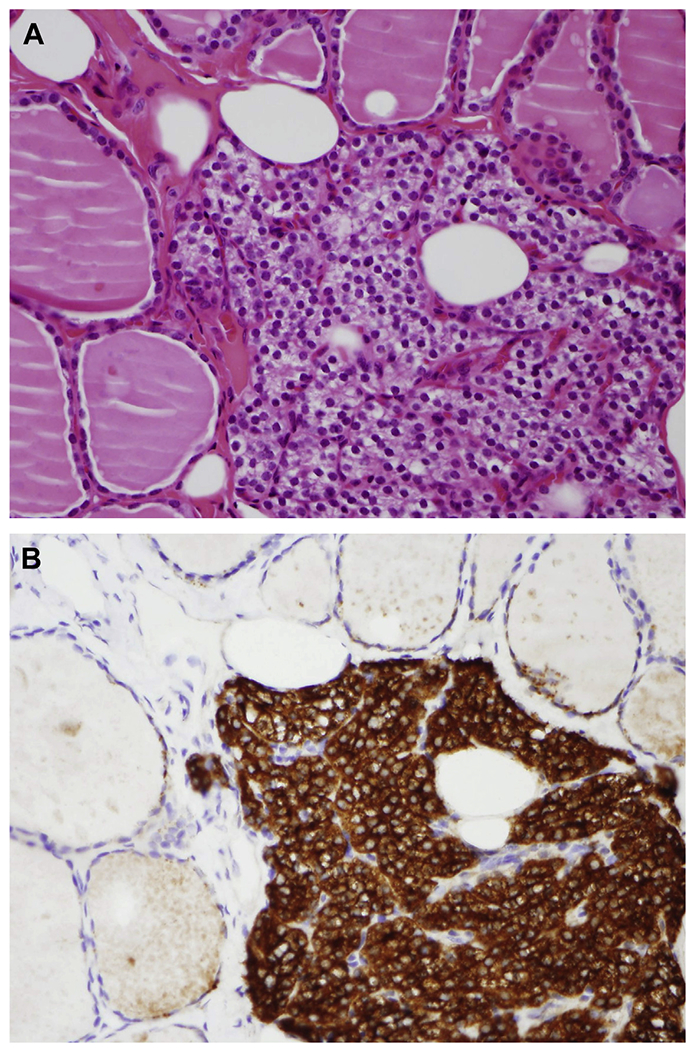
(A) Incidental finding of ectopic intrathyroidal parathyroid tissue (H&E, original magnification ×400). (B) Parathyroid hormone (PTH) by immunohistochemistry highlights parathyroid chief cells, allowing proper identification (PTH, original magnification ×400).
DISORDERS ARISING IN THE PARATHYROID GLAND
Abnormalities of the parathyroid glands are the most common causes of hypercalcemia. Pathologists facilitate the appropriate assessment and diagnosis of the underlying pathologic condition. The spectrum of parathyroid proliferative disorders includes parathyroid hyperplasia, parathyroid adenoma (PA), atypical PA, and parathyroid carcinoma (PC).
IMAGING
Normal parathyroid glands are small, with overall dimensions typically averaging 5 × 3 × 1 mm and weighing less than 50 mg, challenging radiologic detection.5,10 Parathyroid proliferative disorders result in the enlargement of 1 or several glands, increasing the likelihood of lesional detection. The main purpose of imaging patients with hyperparathyroidism is to identify those who are suitable for minimally invasive surgery by correctly identifying the location of the enlarged gland or glands.11,12
Although several imaging modalities may be used preoperatively, dual-phase protocol 99mTc- sestamibi scintigraphy is currently the most widely used technique for visualization of parathyroid disease.13–15 Sestamibi is a lipophilic cationic isonitrile derivative that accumulates in mitochondriarich oxyphil cells, which are present in parathyroid tissue.16 Sestamibi is washed out less rapidly from parathyroid tissue than from adjacent thyroid tissue. The scan sensitivity is limited in cases with atypical washout rates, such as rapid parathyroid washout or delayed thyroid washout. Rapid parathyroid washout is associated with parathyroid hyperplasia and other disorders.11,16 Variation in the scanning protocols makes the reported sensitivity of 99mTc-sestamibi scintigraphy range from 80% to 100%.5,17 Other modalities, such as contrast- enhanced computed tomography (CT) and MRI, are less commonly used for preoperative localization but may be of benefit in cases of failed parathyroidectomy for the localization of ectopic glands.11,18
HYPERPLASIA AND HYPERPARATHYROIDISM
Parathyroid hyperplasia is defined as an absolute increase in parenchymal cell mass, which occurs from the proliferation of chief cells, oncocytes, and transitional oncocytes in multiple parathyroid glands.10,19 In more than 50% of cases, the enlargement of glands is symmetric.5 When asymmetric, the distinction between hyperplasia and adenoma may be challenging by standard morphologic criteria alone. Hyperparathyroidism is divided into primary, secondary, and tertiary hyperparathyroidism.
PRIMARY HYPERPARATHYROIDISM
From 80% to 85% of primary hyperparathyroidism is caused by PA, followed by primary parathyroid hyperplasia (15%) and PC (<5%).12,20 Ectopic locations of hyperplastic parathyroid tissue have also been documented.21 Patients with primary hyperparathyroidism have abnormal regulation of serum parathyroid hormone secretion. Primary hyperparathyroidism is characterized by increased serum calcium level in the setting of increased parathyroid hormone levels.22,23 Clinically, most patients are asymptomatic or show nonspecific symptoms such as fatigue, mild depression, or cognitive impairment. It is with longstanding increased parathyroid hormone levels that symptomatic hypercalcemia occurs. In these cases, patients may present with a litany of associated comorbidities, from debilitating renal disorders (including nephrolithiasis and renal deficiency) to gastrointestinal issues (including nausea/vomiting, peptic ulcer disease, and pancreatitis) and skeletal complications (including bone pain, secondary fractures, and osteitis fibrosa cystica).23–25 Neuropsychiatric disturbances, including lethargy, psychosis, and coma, may also arise in the setting of severe hypercalcemia.24,26
Grossly, chief cell hyperplasia is characteristically a proliferative disorder involving all 4 glands. Often, the process is insidious and progressive, with variable gland size and weight.14 Hyperplastic glands are usually round to oval and vary from red to brown. The cut surface is usually homogeneous and occasionally slightly nodular. Histologic assessment reveals that each nodule is composed of sheets, cords, or an acinar arrangement of parenchymal cells with reduced stromal fat (Fig. 3). These nodules may be separated by fibrotic bands and septa, mimicking a fibrous capsule. The chief cells may reveal mild to marked nuclear pleomorphism and atypia. Degenerative features, including cystification, hemorrhage, hemosiderin deposition, and fibrosis, may be observed. When the hyperplastic parenchymal tissue lacks precise circumscription and involves the surrounding tissue, this finding may be called parathyromatosis, typically resulting from prior trauma.27
Fig. 3.
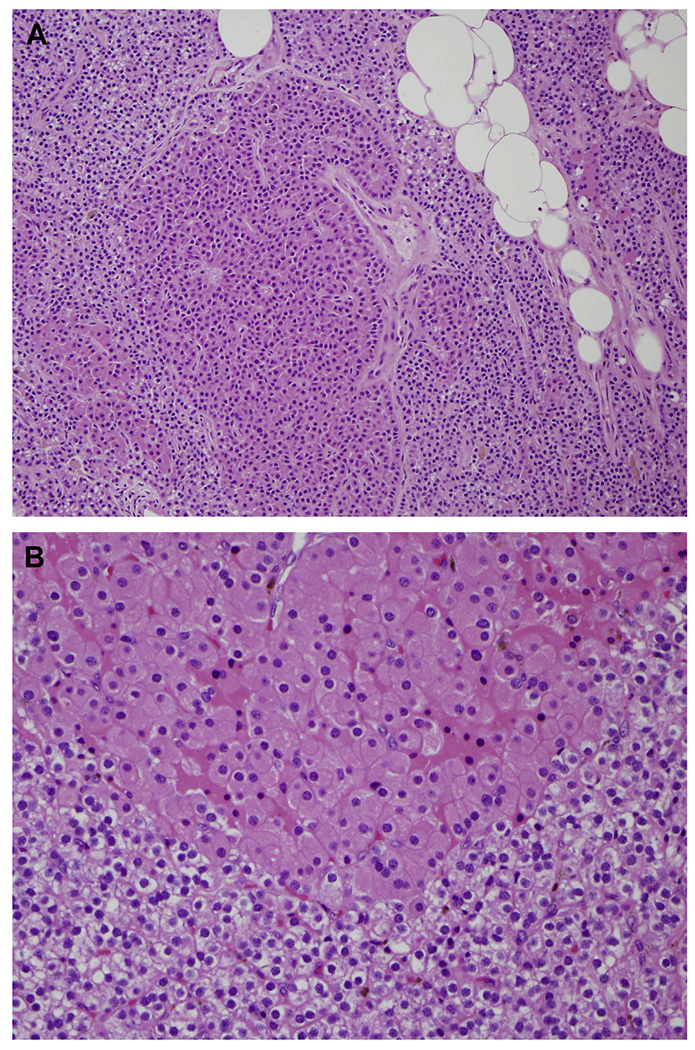
(A) Parathyroid hyperplasia is characterized by chief cell proliferation involving all 4 glands (H&E, original magnification ×200). (B) Nodules of oncocytic cells are common (H&E, original magnification ×400).
SECONDARY HYPERPARATHYROIDISM
Most parathyroid hyperplasia is the result of secondary hyperparathyroidism caused by chronic kidney disease (CKD), malabsorption syndrome, and chronic inadequate sunlight exposure. Impaired renal function leads to downregulation of the parathyroid vitamin D and calciumsensing receptors, which negatively affect mineral metabolism and result in high serum phosphate level, low serum calcium level, and vitamin D deficiency.28 Parathyroid hyperplasia, cardiovascular disease, and concomitant bone disorders are the most common clinical complications.29
TERTIARY HYPERPARATHYROIDISM
A significant proportion of patients with CKD and secondary hyperparathyroidism maintain increased levels of parathyroid hormone following kidney transplant. This state of hyperparathyroidism is known as tertiary hyperparathyroidism. Without appropriate management and treatment, tertiary hyperparathyroidism can lead to kidney allograft rejection and decreased patient survival.30
ADENOMA
PAs are responsible for approximately 85% of cases of primary hyperparathyroidism.31 PA can occur among all age groups, with a peak incidence in the fifth and sixth decades and with a slight female predilection and a 2:1 female/male ratio.19,32 PA tends to be located more frequently in the lower glands than in the upper glands, although studies may be conflicting.33 Furthermore, these benign neoplasms may arise in any ectopic or supernumerary parathyroid gland, with described cases occurring in the retroesophageal space, thymus, vestigial aortopulmonary window, mediastinum, and thyroid gland.3,34–39 PA weights range from approximately 300 mg to several grams, with sizes ranging from a few millimeters to, in some cases, more than 10 cm.40,41 Grossly, these lesions are characterized as well- circumscribed, smooth, red-brown nodules, occasionally encapsulated. Larger lesions often replace the nonlesional parathyroid tissue and may show areas of hemorrhage and cystic degeneration.42 On histology, PAs are typically well circumscribed or encapsulated by a thin, fibrous capsule and composed of chief cells (round nucleus, scant cytoplasm) arranged within a delicate capillary network. Lobules and nodules inside the adenoma can be observed, with some revealing oncocytic cell change, prominent pink cytoplasm of variable granularity. Adenomas composed entirely of oxyphilic or oncocytic cells occur and may be functional (Fig. 4).43–46 This variant is uncommon and accounts for less than 6% of PA.47 If not absent, stromal fat is usually sparse. A rim of normal or atrophic parathyroid tissue is typically identified adjacent to the adenoma in more than half of cases, but it may be harder or impossible to detect in larger lesions. The absence of a normocellular rim does not preclude the diagnosis of PA, because large adenomas may have outgrown the preexisting parathyroid or the rim may have simply been lost during sectioning.3,41 In large tumors, areas of fibrosis, calcification, cholesterol clefts, and/or hemorrhage with hemosiderin deposition may be seen. Most cells in PA are small, uniform, and bland with central, hyperchromatic nuclei. However, areas of marked endocrine nuclear atypia, including cells with enlarged, smudged, irregular nuclei or multiple nuclei, may be observed.48 PA are not mitotically active but may be mildly proliferative, usually showing less than 1 mitosis per 10 high-power fields.49 Increased mitotic activity and/or necrosis are worrisome features that should raise the possibility of malignancy.50–53
Fig. 4.
(A) Oncocytic PA with residual normocellular parathyroid tissue identified outside the adenoma capsule (H&E, original magnification ×200). (B) Microcystic or cystic architecture may be observed inside the adenoma (H&E, original magnification ×400).
Other unusual variants of PA consist of lipoade- noma and water-clear cell adenoma. Parathyroid lipoadenomas are benign tumors made up of both stromal and parenchymal elements, with mature adipocytes comprising more than 50% of the tumor (Fig. 5).54–56 Small nests of parathyroid parenchymal cells, mainly chief cells and infrequently chief and oncocytic cells, are found scattered throughout the tumor.57 Lipoadenomas deviate from the typical distribution of fat noted in hyperplasia and neoplasia of the parathyroid. Water-clear cell adenomas are rare tumors with few cases reported in literature.58–62 These adenomas are composed of intermediate to large cells with clear cytoplasm containing small vesicles and glycogen.58,60 Lipoadenoma and water-clear cell adenoma are occasionally associated with primary hyperparathyroidism.54,59,60,63,64
Fig. 5.
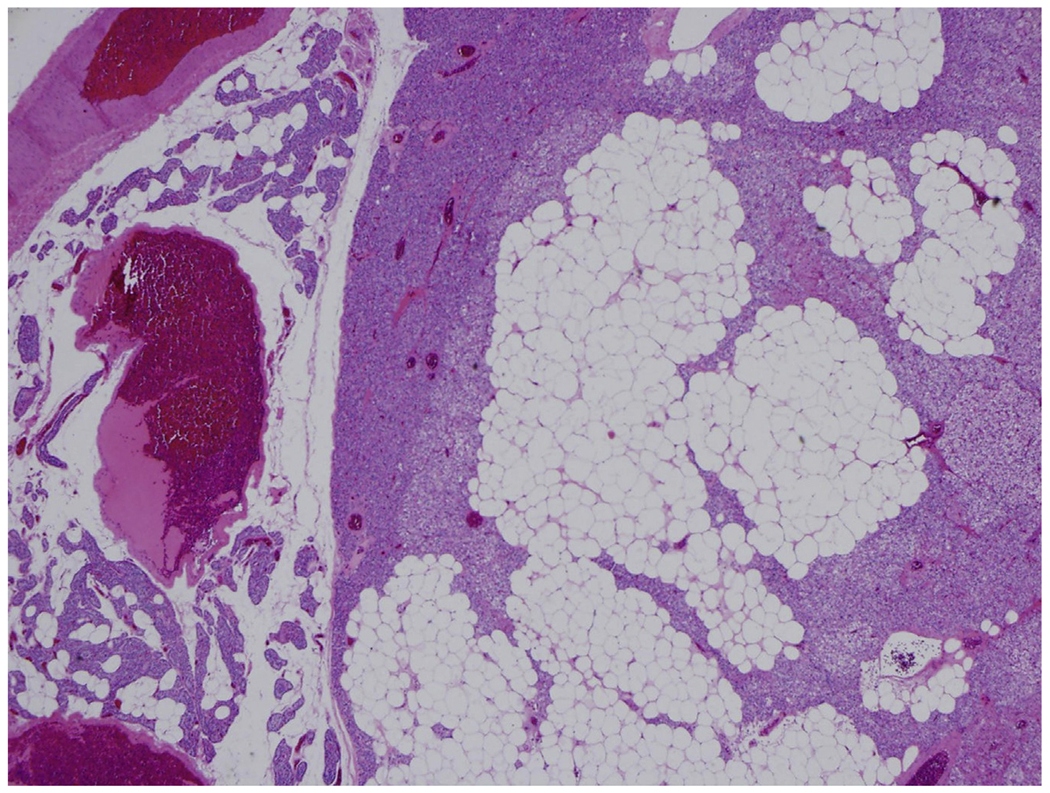
Lipoadenoma is a rare variant of PA composed of mature adipose tissue and chief cells (H&E, original magnification ×200).
Over the years, the diagnosis of double PA has lost favor among the medical community.65 Many patients diagnosed with double PA eventually return with recurrent hyperparathyroidism associated with residual glands. Nonetheless, the diagnosis of double adenoma requires the identification of certain criteria. Initially, the patient needs to present with 2 enlarged and histologically abnormal hypercellular parathyroid glands. The remaining 2 glands must be structurally and serologically normal. After the excision of both abnormal glands, long-term follow-up should remain uneventful because the patient should be cured from hyperparathyroidism. In addition, the patient should have a negative family history of parathyroid disease. With those stringent criteria, only a handful of definitive cases of double PA are described in the literature.66–69
A mutational genetic profile has been identified for PA that encompasses several tumor suppressors and oncogenes. Among others, mutations in CCND1, cyclin D1, multiple endocrine neoplasia (MEN) type 1, ZFX, EZH2, and many CDKN genes have been linked to the development of both sporadic and familial PA.70–78
ATYPICAL ADENOMA
Some parathyroid neoplasms show concerning features for malignancy, including broad bands of fibrosis, nuclear atypia, conspicuous mitotic figures, necrosis, and a desmoplastic stromal response. However, unequivocal features of malignancy, including direct infiltration of adjacent tissue, vascular invasion, or neural involvement, are not identified.14,27,48,79–81 These borderline tumors do not fulfill the histologic requirements for a diagnosis of carcinoma and are generally classified as atypical adenomas (AAs), tumors considered to be of uncertain malignant potential. Studies evaluating parafibromin immunoreactivity have proved valuable to predict the potential for recurrence in AAs.82–86 Among the parafibromin (CDC73)-deficient group, 10% of AAs recurred, whereas none of the parafibromin (CDC73)-positive group did.82 However, more long-term studies to assess for malignant biological potential and to determine the risk for metastatic disease among these lesions are needed.27,87–89 In the meantime, patients with AA should benefit from close clinical followup. It is important not to make a diagnosis of carcinoma without unequivocal evidence of malignancy. Treatment (ie, surgery plus clinical followup) is often equivalent for AA and a localized PC, and, provided appropriate clinical follow-up, there is little need to emotionally traumatize the patient in borderline cases.
CARCINOMA
Carcinoma arising in a parathyroid gland is rare and accounts for less than 5% of primary hyperparathyroidism cases.90–92 Unlike in PA and hyperplasia, the incidence of PC among women seems to predominate. Patients are typically young and almost always show symptoms related to increased serum calcium levels, which may reach as high as 15 mg/dL (normal typically 8.5–10.2 mg/dL). Most PC occurs in a sporadic setting, but cases in patients with familial endocrinopa- thies are well documented.53,93–99
PCs tend to be large, with an average weight of about 12 g (vs typical normal weight of 50 mg).94 Preoperatively, carcinomas may show adherence to adjacent soft tissue, thyroid tissue, or even esophagus. Morphologically, there is a constellation of histologic findings to look for to confirm the diagnosis of PC. Per World Health Organization criteria, a diagnosis of PC requires unequivocal lymphovascular or perineural invasion, or invasion into adjacent structures, or metastatic disease. Characteristically, PC are hypercellular neoplasms with trabecular growth, thick fibrous bands, and a thick fibrous tumor capsule. Mitoses, virtually absent in both PA and hyperplasia, may be frequent and atypical in PC. Importantly, the presence of mitotic figures is not pathognomonic for malignancy but should at least lead to suspicion for malignancy in such neoplasms.49 A similar concept applies to capsular invasion. Capsular invasion by neoplastic processes has been observed in PAsthat have undergone hemorrhagic degeneration followed by fibrosis and entrapment of tumor cells within the capsule (Fig. 6).100 Other features reported among PCs include tumor necrosis, tumor cell spindling, prominent macronucleoli, and atypical mitotic figures. Bondeson and colleagues101 suggested that the histologic triad of macronucleoli, more than 5 mitoses per 50 high-power fields, and necrosis is associated with recurrent disease, but this system is not widely used. The rarity of PCs has made it difficult to establish a definitive system to risk stratify these tumors.
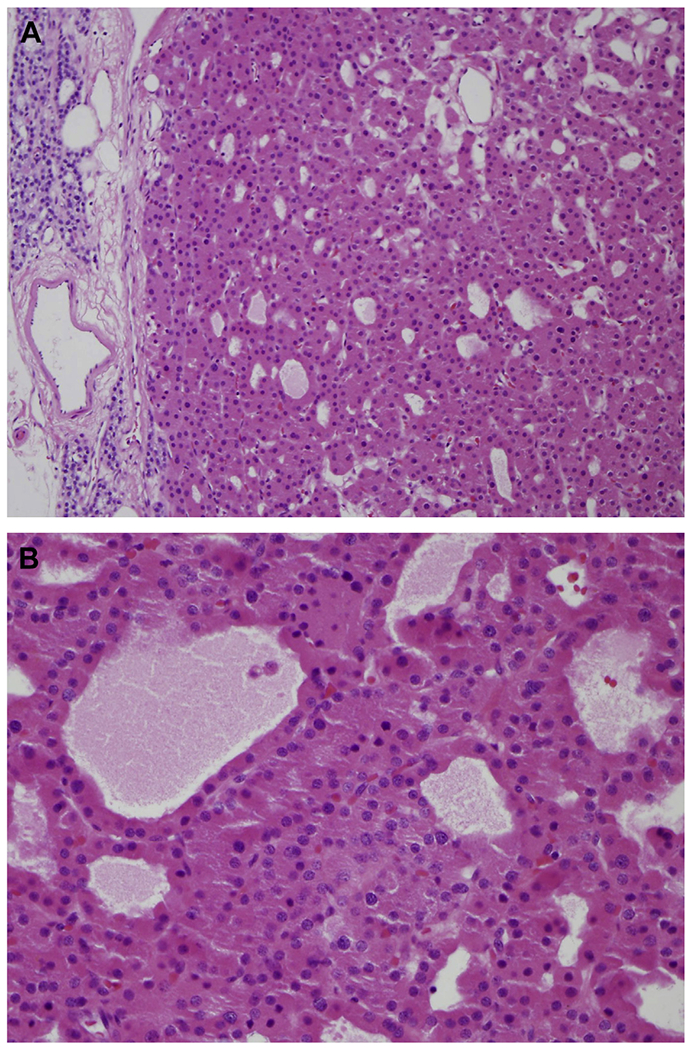
Fig. 6.
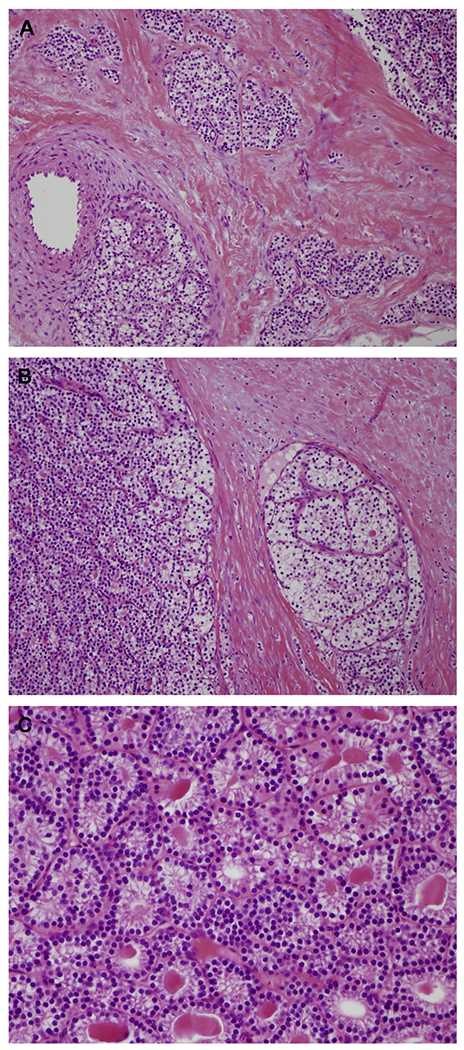
(A) Thick fibrotic capsule with parathyroid cells infiltrating surrounding soft tissue associated with (B) true vascular invasion are key features of PC (H&E, original magnification ×200). (C) Microcystic growth pattern in PC with unremarkable bland cytologic features (H&E, original magnification ×400).
The development of PC is usually a sporadic, 1-gland condition. PC arising in the setting of 4-gland hyperplasia or as part of secondary hyperparathyroidism is rare but has been docu- mented.102 Certain familial endocrine disorders related to CDC73 gene mutations, namely hyperparathyroidism–jaw tumor syndrome, familial isolated hyperparathyroidism, and sporadic PC with germline CDC73 mutations, are responsible for the occurrence of some PCs.94,95,103–105 As a result, genetic counseling should be considered for patients with PC. CDC73, also known as HRPT2, is a tumor suppressor gene located on chromosome 1q31.2, which has been documented as a driver in both familial and sporadic PC. In its normal state, CDC73 protein, parafibro- min, regulates both gene expression and transcription, inhibiting cell proliferation and maintaining the cellular structural framework. Its inactivation, whether resulting from sporadic or germline mutation, drives tumorigenesis, although the complex mechanisms of action are not well understood.70,85,106 Next-generation sequencing of several PCs has confirmed the presence of additional candidates as putative drivers of parathyroid carcinogenesis, including CCND1, PRUNE2, PIK3CA, HMT2D, ADCK1, MTOR, THRAP3, and CDKN2C, although, again, the mechanisms of action are not well documented because of the rarity of mutations and low incidence of PC.40,78,107
Although metastases are unusual at the time of diagnosis, metastatic disease has been reported in more than 30% of cases and is commonly found in regional lymph nodes, bone, lung, and liver. In advanced metastatic disease, the severity of symptoms is directly proportional to tumor burden, which is concordant with parathyroid hormone levels produced. The overall prognosis for such disease is usually favorable, with an estimated 5-year overall survival of 78% to 85%.91 It is common for patients to experience multiple disease recurrence over a course of 15 to 20 years.91,100,108–112
IMMUNOHISTOCHEMISTRY
In general, immunohistochemical studies are not needed for the diagnosis of parathyroid disease. Parathyroid tissue, whether as part of a normal gland or abnormal hyperplastic or neoplastic process, is immunoreactive with antibodies to chro- mogranin and parathyroid hormone, often useful when attempting to differentiate parathyroid tissue from thyroid tissue, a common conundrum. Use of MIB1 Immunohisochemical use of the Ki-67 proliferative index to distinguish PA from hyperplasia has been attempted, but has had limited success.68 The primary role for immunohistochemistry is for identification of parathyroid tissue. Secondarily, immunohistochemistry has been used to attempt differentiation between adenoma, AA, and PC. In some circumstances, parathyroid neoplasms have histologic features suspicious for malignancy, but the full spectrum of findings needed to make the diagnosis are not present. For these problematic cases, some studies have suggested the use of a broad panel of immunohistochemical markers, including bcl-1, Ki-67, and p27,48,113 although most laboratories do not have several of these markers, and their diagnostic utility, as a panel, is modest at best. Importantly, the protein product of CDC73, parafibromin, is expressed in the nuclei of benign parathyroid tissue, adenomas, and some AAs, except for those arising in the setting of hyperparathyroidism–jaw tumor syndrome.14,114 Studies have further shown that loss of nuclear expression of parafibromin may be seen in atypical PAs and carcinomas. However, this immunostain is notoriously difficult to properly titrate and, because it is in such infrequent use clinically, it is not very helpful as a validated marker for malignancy.14
MULTIPLE ENDOCRINE NEOPLASIA SYNDROMES
MEN type 1 and 2 are hereditary cancer syndromes. They are characterized by the emergence of various benign and malignant neoplasms. MEN1 is associated mainly with parathyroid, pituitary, and pancreatic tumors, whereas MEN2 is more likely associated with medullary thyroid carcinoma, pheochromocytoma, and parathyroid disorders.14,115 Although parathyroid tumors are found in both MEN1 and MEN2, patients with MEN1 most commonly have parathyroid proliferative disorder as part of their syndrome, with nearly 90% of them diagnosed with parathyroid hyperplasia.40 PA and carcinoma can also be seen as part of these syndromes. The possibility of MEN syndrome should always be kept in mind when evaluating these patients.
SUMMARY
The parathyroid glands are unique organs responsible for maintaining calcium homeostasis through parathyroid hormone secretion and end-organ/tissue response. Parathyroid dysfunction alters this fragile homeostasis, primarily through hyperparathyroidism, a common endocrine disorder. Primary hyperparathyroidism includes a wide spectrum of parathyroid proliferative lesions, such as parathyroid hyperplasia, PA, atypical PA, and PC. Proper classification of the pathologic spectrum of parathyroid disease is essential for effective clinical management and facilitating appropriate patient discussions regarding morbidity and long-term prognosis.
Key points.
Proliferative parathyroid disorders represent the most common cause of hyperparathyroidism.
From 80% to 85% of primary hyperparathyroidism is caused by parathyroid adenoma, followed by primary parathyroid hyperplasia (15%) and parathyroid carcinoma (5%).
Parathyroid carcinoma key histologic features include vascular invasion, perineural invasion, invasion into adjacent structures, and metastatic disease.
Parathyroid carcinoma is typically sporadic but may arise as part of familial endocrine disorders: multiple endocrine neoplasia, hyperparathyroidism–jaw tumor syndrome, and familial isolated hyperparathyroidism.
Parafibromin immunohistochemistry may be a helpful diagnostic aid in distinguishing parathyroid carcinoma from parathyroid atypical adenoma.
Footnotes
Conflict of interest: There are no conflicts of interest to report by any of the authors.
Ethical approval: This article does not contain any studies with human participants performed by the authors.
REFERENCES
- 1.Mallik S, Aggarwal P, Singh I, et al. A study on development and morphogenesis of parathyroid gland in the developing human embryo. J Med Soc 2017;31(3):195–200. [Google Scholar]
- 2.Grevellec A, Tucker AS. The pharyngeal pouches and clefts: development, evolution, structure and derivatives. Semin Cell Dev Biol 2010;21(3): 325–32. [DOI] [PubMed] [Google Scholar]
- 3.Akerstrom G, Malmaeus J, Bergstrom R. Surgical anatomy of human parathyroid glands. Surgery 1984;95(1):14–21. [PubMed] [Google Scholar]
- 4.Fancy T, Gallagher D, Hornig JD. Surgical anatomy of the thyroid and parathyroid glands. Otolaryngol Clin North Am 2010;43(2):221–7. [DOI] [PubMed] [Google Scholar]
- 5.Åkerström G, Rudberg C, Grimelius L, et al. Histologic parathyroid abnormalities in an autopsy series. Hum Pathol 1986;17(5):520–7. [DOI] [PubMed] [Google Scholar]
- 6.Phitayakorn R, McHenry CR. Incidence and location of ectopic abnormal parathyroid glands. Am J Surg 2006;191(3):418–23. [DOI] [PubMed] [Google Scholar]
- 7.LoPinto M, Rubio GA, Khan ZF, et al. Location of abnormal parathyroid glands: lessons from 810 parathyroidectomies. J Surg Res 2017;20(7):22–6. [DOI] [PubMed] [Google Scholar]
- 8.Uno N, Tominaga Y, Matsuoka S, et al. Incidence of parathyroid glands located in thymus in patients with renal hyperparathyroidism. World J Surg 2008;32(11):2516–9. [DOI] [PubMed] [Google Scholar]
- 9.Goodman A, Politz D, Lopez J, et al. Intrathyroid parathyroid adenoma: Incidence and location - The case against thyroid lobectomy Otolaryngol Head Neck Surg 2011;144(6):867–71. [DOI] [PubMed] [Google Scholar]
- 10.MacKenzie-Feder J, Sirrs S, Anderson D, et al. Primary hyperparathyroidism: an overview. Int J Endocrinol 2011;2011(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel CN, Salahudeen HM, Lansdown M, et al. Clinical utility of ultrasound and99mTc sestamibi SPECT/CT for preoperative localization of parathyroid adenoma in patients with primary hyperparathyroidism. Clin Radiol 2010;65(4):278–87. [DOI] [PubMed] [Google Scholar]
- 12.Ruda JM, Hollenbeak CS, Stack BC. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol - Head Neck Surg 2005; 132(3):359–72. [DOI] [PubMed] [Google Scholar]
- 13.Piciucchi S, Barone D, Dubini A, et al. Primary hyperparathyroidism: imaging to pathology. J Clin Imaging Sci 2012;2(1):59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delellis RA. Parathyroid tumors and related disorders. Mod Pathol 2011;24(Suppl 2):S78–93. [DOI] [PubMed] [Google Scholar]
- 15.Elgazzar A, Alenezi S, Asa’ad S. Scintigraphic parathyroid imaging: concepts and new developments. Res Rep Nucl Med 2015;2015(5):9–18. [Google Scholar]
- 16.O’Doherty MJ, Kettle AG, Wells P, et al. Parathyroid imaging with technetium-99m-sestamibi: preoperative localization and tissue uptake studies. J Nucl Med 1992;33(3):313–8. [PubMed] [Google Scholar]
- 17.Mitchell BK, Merrell RC, Kinder BK. Localization studies in patients with hyperparathyroidism. Surg Clin North Am 1995;75(3):483–98. [DOI] [PubMed] [Google Scholar]
- 18.Beland MD, Mayo-Smith WW, Grand DJ, et al. Dynamic MDCT for localization of occult parathyroid adenomas in 26 patients with primary hyperparathyroidism. Am J Roentgenol 2011. ;196(1): 61–5. [DOI] [PubMed] [Google Scholar]
- 19.Silva BC, Cusano NE, Bilezikian JP. Primary hyperparathyroidism. Best Pract Res Clin Endocrinol Metab 2018;32(5):593–607. [DOI] [PubMed] [Google Scholar]
- 20.Molinari AS, Irvin GL, Deriso GT, et al. Incidence of multiglandular disease in primary hyperparathyroidism determined by parathyroid hormone secretion. Surgery 1996;120(6):934–6. [DOI] [PubMed] [Google Scholar]
- 21.Souza ÉRV, Scrignoli JA, Bezerra FC, et al. Devastating skeletal effects of delayed diagnosis of complicated primary hyperparathyroidism because of ectopic adenoma. J Clin Rheumatol 2008;14(5):281–4. [DOI] [PubMed] [Google Scholar]
- 22.Khan AA, Hanley DA, Rizzoli R, et al. Primary hyperparathyroidism: review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus. Osteoporos Int 2017;28(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pallan S, Khan A. Primary hyperparathyroidism: Update on presentation, diagnosis, and management in primary care. Can Fam Physician 2011; 57(2):184–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician 2003;67(9): 1959–66. [PubMed] [Google Scholar]
- 25.Barakat MT, Ashrafian H, Todd JF, et al. Severe hypercalcaemia from secretion of parathyroid hormone-related peptide. Lancet Oncol 2004; 5(10):633–5. [DOI] [PubMed] [Google Scholar]
- 26.Metzger R, Milas M. Inherited cancer syndromes and the thyroid: An update. Curr Opin Oncol 2014;26(1):51–61. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Ranvier GG, Khanafshar E, Jensen K, et al. Parathyroid carcinoma, atypical parathyroid adenoma, or parathyromatosis? Cancer 2007; 110(2):255–64. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: Pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol 2011;6(4):913–21. [DOI] [PubMed] [Google Scholar]
- 29.Tomasello S Secondary hyperparathyroidism and chronic kidney disease. Diabetes Spectr 2008; 21(1):19–25. [Google Scholar]
- 30.Dulfer RR, Franssen GJH, Hesselink DA, et al. Systematic review of surgical and medical treatment for tertiary hyperparathyroidism. Br J Surg 2017; 104(7):804–13. [DOI] [PubMed] [Google Scholar]
- 31.Marcocci C, Cetani F. Clinical practice. Primary hyperparathyroidism. N Engl J Med 2011;365(25): 2389–97. [DOI] [PubMed] [Google Scholar]
- 32.Fraser W. Hyperparathyroidism. Lancet 2009; 374(9684):145–58. [DOI] [PubMed] [Google Scholar]
- 33.Debruyne F, Ostyn F, Delaere P. Distribution of the solitary adenoma over the parathyroid glands. J Laryngol Otol 1997;111(5):459–60. [DOI] [PubMed] [Google Scholar]
- 34.Birdas TJ, Keenan RJ. Mediastinal parathyroid adenoma [8]. Ann Thorac Surg 2005;97(4):259–61. [DOI] [PubMed] [Google Scholar]
- 35.lihara M, Suzuki R, Kawamata A, et al. Thoracoscopic removal of mediastinal parathyroid lesions: Selection of surgical approach and pitfalls of pre-operative and intraoperative localization. World J Surg 2012;36(6):1327–34. [DOI] [PubMed] [Google Scholar]
- 36.Pawlik TM, Richards M, Giordano TJ, et al. Identification and management of intravagal parathyroid adenoma. World J Surg 2001;25(4):419–23. [DOI] [PubMed] [Google Scholar]
- 37.Arnault V, Beaulieu A, Lifante JC, et al. Multicenter study of 19 aortopulmonary window parathyroid tumors: the challenge of embryologic origin. World J Surg 2010;34(9):2211–6. [DOI] [PubMed] [Google Scholar]
- 38.Spinelli C, Liserre J, Pucci V, et al. Primary hyperparathyroidism: fifth parathyroid intrathymic adenoma in a young patient. J Pediatr Endocrinol Metab 2012;25(7):781–4. [DOI] [PubMed] [Google Scholar]
- 39.Stalberg P, Grodski S, Sidhu S, et al. Cervical thymectomy for intrathymic parathyroid adenomas during minimally invasive parathyroidectomy. Surgery 2007;141(5):626–9. [DOI] [PubMed] [Google Scholar]
- 40.Wieneke JA, Smith A. Parathyroid adenoma. Head Neck Pathol 2008;2(4):305–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Summers GW. Parathyroid update: a review of 220 cases. Ear Nose Throat J 1996;75(7):434–9. [PubMed] [Google Scholar]
- 42.Van der Walt J Pathology of the parathyroid glands. Diagn Histopathol 2012;18(6):221–33. [Google Scholar]
- 43.Giorgadze T, Stratton B, Baloch ZW, et al. Oncocytic parathyroid adenoma: Problem in cytological diagnosis. Diagn Cytopathol 2004;31(4):276–80. [DOI] [PubMed] [Google Scholar]
- 44.Erickson L, Jin L, Papotti M. Oxyphil parathyroid carcinomas: a clinicopathological and immunohis- tochemical study of 10 cases. Am J Surg Pathol 2002;26(3):344–9. [DOI] [PubMed] [Google Scholar]
- 45.Paul A, Villepelet A, Lefevre M, et al. Oncocytic parathyroid adenoma. Eur Ann Otorhinolaryngol Head Neck Dis 2015;132(5):301–3. [DOI] [PubMed] [Google Scholar]
- 46.Paker I, Yilmazer D, Yandakci K, et al. Intrathyroidal oncocytic parathyroid adenoma: a diagnostic pitfall on fine-needle aspiration. Diagn Cytopathol 2010; 38(11):833–6. [DOI] [PubMed] [Google Scholar]
- 47.Howson P, Kruijff S, Aniss A, et al. Oxyphil cell parathyroid adenomas causing primary hyperparathyroidism: a clinico-pathological correlation. Endocr Pathol 2015;26(3):250–4. [DOI] [PubMed] [Google Scholar]
- 48.Stojadinovic A, Hoos A, Nissan A, et al. Parathyroid neoplasms: Clinical, histopathological, and tissue microarray-based molecular analysis. Hum Pathol 2003;34(1):54–64. [DOI] [PubMed] [Google Scholar]
- 49.Snover D, Foucar K. Mitotic activity in benign para-thyroid disease. Am J Clin Pathol 1981;75(2): 345–7. [DOI] [PubMed] [Google Scholar]
- 50.Chang YJ, Mittal V, Remine S, et al. Correlation between clinical and histological findings in parathyroid tumors suspicious for carcinoma. Am Surg 2006;72(5):419–26. [PubMed] [Google Scholar]
- 51.Szender B, Farid P, Vegso G. Apoptosis and P53, Bcl-2 and Bax gene expression in parathyroid glands of patients with hyperparathyroidism. Pathol Oncol Res 2004;10(1):98–103. [DOI] [PubMed] [Google Scholar]
- 52.DeLellis RA, Mazzaglia P, Mangray S. Primary hyperparathyroidism: a current perspective. Arch Pathol Lab Med 2008;132(8):1251–62. [DOI] [PubMed] [Google Scholar]
- 53.DeLellis RA. Parathyroid carcinoma: an overview. Adv Anat Pathol 2005;12(2):53–61. [DOI] [PubMed] [Google Scholar]
- 54.Chow L, Erickson L, Abu-Lebdeh H, et al. Parathyroid lipoadenomas: a rare cause of primary hyperparathyroidism. Endocr Pract 2006;12(2):131–6. [DOI] [PubMed] [Google Scholar]
- 55.Johnson N, Serpell JW, Johnson WR, et al. Parathyroid lipoadenoma. ANZ J Surg 2015;85(6):489–90. [DOI] [PubMed] [Google Scholar]
- 56.Hyrcza MD, Sargin P, Mete O. Parathyroid Lipoadenoma: a clinicopathological diagnosis and possible trap for the unaware pathologist. Endocr Pathol 2016;27(1):34–41. [DOI] [PubMed] [Google Scholar]
- 57.Cetani F, Torregrossa L, Marcocci C. A large functioning parathyroid lipoadenoma. Endocrine 2016; 53(2):615–6. [DOI] [PubMed] [Google Scholar]
- 58.Murakami K, Watanabe M, Nakashima N, et al. Water-clear cell adenoma associated with primary hyperparathyroidism: report of a case. Surg Today 2014;44(4):773–7. [DOI] [PubMed] [Google Scholar]
- 59.Kodama H, lihara M, Okamoto T, et al. Water-clear cell parathyroid adenoma causing primary hyperparathyroidism in a patient with neurofibromatosis type 1:Report of a case. Surg Today 2007;37(10): 884–7. [DOI] [PubMed] [Google Scholar]
- 60.Piggott RP, Waters PS, Ashraf J, et al. Water-clear cell adenoma: a rare form of hyperparathyroidism. Int J Surg Case Rep 2013;4(10):911–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arik D, Dundar E, Yilmaz E, et al. Water-clear cell adenoma of the mediastinal parathyroid gland. Turk Patoloji Derg 2017;1(1):1–5. [DOI] [PubMed] [Google Scholar]
- 62.Chou YH, Jhuang JY, Hsieh MS. Water-clear cell parathyroid adenoma in a patient with acute pancreatitis. J Formos Med Assoc 2014;113(11): 872–3. [DOI] [PubMed] [Google Scholar]
- 63.Bansal R, Trivedi P, Sarin J, et al. Lipoadenoma of the parathyroid gland - a rare cause of hyperparathyroidism. Gulf J Oncolog 2012;3(11):63–5. [PubMed] [Google Scholar]
- 64.Ozden S, Guresci S, Saylam B, et al. A rare cause of primary hyperparathyroidism: Parathyroid lipoadenoma. Auris Nasus Larynx 2018;45(6):1245–8. [DOI] [PubMed] [Google Scholar]
- 65.Harness J, Ramsburg S, Nishiyama R. Multiple adenomas of the parathyroid: do they exist? Arch Surg 1979;114(5):468–74. [DOI] [PubMed] [Google Scholar]
- 66.Tezelman S, Shen W, Shaver J. Double parathyroid adenomas. Clinical and biochemical characteristics before and after parathyroidectomy. Ann Surg 1993;218(3):300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou W, Katz MH, Deftos LJ, et al. Metachronous double parathyroid adenomas involving two different cell types: chief cell and oxyphil cell. Endocr Pract 2003;9(6):522–5. [DOI] [PubMed] [Google Scholar]
- 68.Bergson EJ, Heller KS. The clinical significance and anatomic distribution of parathyroid double adenomas. J Am Coll Surg 2004;198(2):185–9. [DOI] [PubMed] [Google Scholar]
- 69.Ogus M, Mayir B, Dinckan A. Mediastinal, cystic and functional parathyroid adenoma in patients with double parathyroid adenomas: a case report. Acta Chir Belg 2006;106(10):736–8. [DOI] [PubMed] [Google Scholar]
- 70.Costa-Guda J, Arnold A. Genetic and epigenetic changes in sporadic endocrine tumors: Parathyroid tumors. Mol Cell Endocrinol 2014;386(1–2):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Costa-Guda J, Soong CP, Parekh VI, et al. germline and somatic mutations in cyclin-dependent kinase inhibitor genes CDKN1A, CDKN2B, and CDKN2C in sporadic parathyroid adenomas. Horm Cancer 2013;4(5):301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Imanishi Y, Hosokawa Y, Yoshimoto K, et al. Primary hyperparathyroidism caused by parathyroid- targeted overexpression of cyclin d1 in transgenic mice. J Clin Invest 2001;107(9):1093–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chandrasekharappa SC, Guru SC, Manickam P, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 1997; 276(5311):404–7. [DOI] [PubMed] [Google Scholar]
- 74.Pellegata NS, Quintanilla-Martinez L, Siggelkow H, et al. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci U S A 2006;103(42): 15558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cromer MK, Starker LF, Choi M, et al. Identification of somatic mutations in parathyroid tumors using whole-exome sequencing. J Clin Endocrinol Metab 2012;97(9):E1774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soong C- P, Arnold A. Recurrent ZFX mutations in human sporadic parathyroid adenomas. Oncoscience 2014;1(5):360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iolascon A, Faienza MF, Coppola B, et al. Analysis of cyclin-dependent kinase inhibitor genes (CDKN2A, CDKN2B, andCDKN2C) in childhood rhabdomyosarcoma. Genes Chromosomes Cancer 1996;15(4):217–22. [DOI] [PubMed] [Google Scholar]
- 78.Pandya C, Uzilov AV, Bellizzi J, et al. Genomic profiling reveals mutational landscape in parathyroid carcinomas. JCI Insight 2017;2(6):61–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guiter G, Delellis R. Risk of recurrence or metastasis in atypical parathyroid adenoma. Mod Pathol 2002;15(1):115A. [Google Scholar]
- 80.Ippolito G, Palazzo FF, Sebag F, et al. Intraoperative diagnosis and treatment of parathyroid cancer and atypical parathyroid adenoma. Br J Surg 2007; 94(5):566–70. [DOI] [PubMed] [Google Scholar]
- 81.Levin KE, Galante M, Clark OH. Parathyroid carcinoma versus parathyroid adenoma in patients with profound hypercalcemia. Surgery 1987; 101(6):649–60. [PubMed] [Google Scholar]
- 82.Kruijff S, Sidhu SB, Sywak MS, et al. Negative parafibromin staining predicts malignant behavior in atypical parathyroid adenomas. Ann Surg Oncol 2014;21(2):426–33. [DOI] [PubMed] [Google Scholar]
- 83.Tan MH, Morrison C, Wang P, et al. Loss of parafibromin immunoreactivity is a distinguishing feature of parathyroid carcinoma. Clin Cancer Res 2004; 10(19):6629–37. [DOI] [PubMed] [Google Scholar]
- 84.Cetani F, Ambrogini E, Viacava P, et al. Should parafibromin staining replace HRTP2 gene analysis as an additional tool for histologic diagnosis of parathyroid carcinoma? Eur J Endocrinol 2007;156(5): 547–54. [DOI] [PubMed] [Google Scholar]
- 85.Newey PJ, Bowl MR, Thakker RV. Parafibromin - functional insights. J Intern Med 2009;266(1): 84–98. [DOI] [PubMed] [Google Scholar]
- 86.Kim HK, Oh YL, Kim SH, et al. Parafibromin immunohistochemical staining to differentiate parathyroid carcinoma from parathyroid adenoma. Head Neck 2012;34(2):201–6. [DOI] [PubMed] [Google Scholar]
- 87.Goshen O, Aviel-Ronen S, Dori S, et al. Brown tumour of hyperparathyroidism in the mandible associated with atypical parathyroid adenoma. J Laryngol Otol 2000;114(4):302–4. [DOI] [PubMed] [Google Scholar]
- 88.Wani S, Hao Z. Atypical cystic adenoma of the parathyroid gland: case report and review of literature. Endocr Pract 2005;11(2):389–93. [DOI] [PubMed] [Google Scholar]
- 89.Yener S, Saklamaz A, Demir T. Primary hyperparathyroidism due to atypical parathyroid adenoma presenting with peroneus brevis tendon rupture. J Endocrinol Invest 2007;30(5):442–4. [DOI] [PubMed] [Google Scholar]
- 90.Duan K, Mete O. Parathyroid carcinoma: diagnosis and clinical implications. Turk Patoloji Derg 2015; 31(Suppl 1):80–97. [DOI] [PubMed] [Google Scholar]
- 91.Asare EA, Sturgeon C, Winchester DJ, et al. Parathyroid carcinoma: an update on treatment outcomes and prognostic factors from the national cancer data base (NCDB). Ann Surg Oncol 2015; 22(12):3990–5. [DOI] [PubMed] [Google Scholar]
- 92.Kassahun WT, Jonas S. Focus on parathyroid carcinoma. Int J Surg 2011;9(1):13–9. [DOI] [PubMed] [Google Scholar]
- 93.Centani F, Pardi E, Marcocci C. Parathyroid Carcinoma. Front Horm Res 2019;51:63–76. [DOI] [PubMed] [Google Scholar]
- 94.Vaswani N The parathyroids: basic and clinical concepts. JAMA 1995;273(9):753–62. [Google Scholar]
- 95.Howell VM, Haven CJ, Kahnoski K, et al. HRPT2 mutations are associated with malignancy in sporadic parathyroid tumours. J Med Genet 2003; 40(9):657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Betea D, Potorac I, Beckers A. Parathyroid carcinoma: challenges in diagnosis and treatment. Ann Endocrinol (Paris) 2015;26(6):1221–38. [DOI] [PubMed] [Google Scholar]
- 97.Wei CH, Harari A. Parathyroid carcinoma: update and guidelines for management. Curr Treat Options Oncol 2012;13(1):11–23. [DOI] [PubMed] [Google Scholar]
- 98.Gill AJ. Understanding the genetic basis of parathyroid carcinoma. Endocr Pathol 2014;25(1):30–4. [DOI] [PubMed] [Google Scholar]
- 99.Adam MA, Untch BR, Olson JA Jr. Parathyroid carcinoma: current understanding and new insights into gene expression and intraoperative parathyroid hormone kinetics. Oncologist 2010;15(1):61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kameyama M, Fujii H, Konishi M. Parathyroid carcinomas: can clinical outcomes for parathyroid carcinomas be determined by histologic evaluation alone? Endocr Pathol 2002;13(1):135–9. [DOI] [PubMed] [Google Scholar]
- 101.Bondeson L, Sandelin K, Grimelius L. Histopathological variables and DNA cytometry in parathyroid carcinoma. Am J Surg Pathol 1993;17(8):820–9. [DOI] [PubMed] [Google Scholar]
- 102.Nasrallah MP, Fraker DL, LiVolsi VA. Parathyroid carcinoma in the setting of tertiary hyperparathyroidism after renal transplant. Endocr Pathol 2014;25(4):433–5. [DOI] [PubMed] [Google Scholar]
- 103.Shattuck TM, Valimaki S, Obara T, et al. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N Engl J Med 2003; 349(18):1722–9. [DOI] [PubMed] [Google Scholar]
- 104.Bradley KJ, Cavaco BM, Bowl MR, et al. Parafibromin mutations in hereditary hyperparathyroidism syndromes and parathyroid tumours. Clin Endocri nol 2006;64(3):299–306. [DOI] [PubMed] [Google Scholar]
- 105.Bricaire L, Odou MF, Cardot-Bauters C, et al. Frequent large germline HRPT2 deletions in a french national cohort of patients with primary hyperparathyroidism. J Clin Endocrinol Metab 2013. 10.1210/jc.2012-2789. [DOI] [PubMed] [Google Scholar]
- 106.Costa AG, Bilezikian JP. Bone turnover markers in hyperparathyroidism. J Clin Densitom 2013;16(1): 22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kasaian K, Wiseman SM, Thiessen N, et al. Complete genomic landscape of a recurring sporadic parathyroid carcinoma. J Pathol 2013;230(3): 249–60. [DOI] [PubMed] [Google Scholar]
- 108.Kebebew E, Arici C, Duh QY, et al. Localization and reoperation results for persistent and recurrent parathyroid carcinoma. Arch Surg 2001;136(8): 878–85. [DOI] [PubMed] [Google Scholar]
- 109.Brown J, Mohamed H, Williams-Smith L. Primary hyperparathyroidism secondary to simultaneous bilateral parathyroid carcinoma. Ear Nose Throat J 2002;2(2):393–8. [PubMed] [Google Scholar]
- 110.Wiseman SM, Rigual NR, Hicks WL, et al. Parathyroid carcinoma: a multicenter review of clinicopath ologic features and treatment outcomes. Ear Nose Throat J 2004;83(7):491–4. [PubMed] [Google Scholar]
- 111.Busaidy NL, Jimenez C, Habra MA, et al. Parathyroid carcinoma: a 22-year experience. Head Neck 2004;26(8):716–26. [DOI] [PubMed] [Google Scholar]
- 112.Harari A, Waring A, Fernandez-Ranvier G, et al. Parathyroid carcinoma: a 43-year outcome and survival analysis. J Clin Endocrinol Metab 2011; 96(12):3679–86. [DOI] [PubMed] [Google Scholar]
- 113.Vargas MP, Vargas HI, Kleiner DE, et al. The role of prognostic markers (MiB-1, RB, and bcl-2) in the diagnosis of parathyroid tumors. Mod Pathol 1997;10(1):12–7. [PubMed] [Google Scholar]
- 114.Howell VM, Gill A, Clarkson A, et al. Accuracy of combined protein gene product 9.5 and parafibro- min markers for immunohistochemical diagnosis of parathyroid carcinoma. J Clin Endocrinol Metab 2009;94(2):434–41. [DOI] [PubMed] [Google Scholar]
- 115.Pacheco MC. Multiple endocrine neoplasia: a genetically diverse group of familial tumor syndromes. J Pediatr Genet 2016;5(2):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]


