Abstract
As the number of people infected with the newly identified 2019 novel coronavirus (SARS-CoV2) is continuously increasing every day, development of potential therapeutic platforms is vital. Based on the comparatively high similarity of receptor-binding domain (RBD) in SARS-CoV2 and SARS-CoV, it seems crucial to assay the cross-reactivity of anti-SARS-CoV monoclonal antibodies (mAbs) with SARS-CoV2 spike (S)-protein. Indeed, developing mAbs targeting SARS-CoV2 S-protein RBD could show novel applications for rapid and sensitive development of potential epitope-specific vaccines (ESV). Herein, we present an overview on the discovery of new CoV followed by some explanation on the SARS-CoV2 S-protein RBD site. Furthermore, we surveyed the novel therapeutic mAbs for targeting S-protein RBD such as S230, 80R, F26G18, F26G19, CR3014, CR3022, M396, and S230.15. Afterwards, the mechanism of interaction of RBD and different mAbs were explained and it was suggested that one of the SARS-CoV-specific human mAbs, namely CR3022, could show the highest binding affinity with SARS-CoV2 S-protein RBD. Finally, some ongoing challenges and future prospects for rapid and sensitive advancement of therapeutic mAbs targeting S-protein RBD were discussed. In conclusion, it may be proposed that this review may pave the way for recognition of RBD and different mAbs to develop potential therapeutic ESV.
Keywords: Corona virus, spike protein, receptor binding domain, antibodies, epitope-specific vaccines (ESV)
1. Introduction
The coronavirus (CoV) has various strains, the most prominent of which is severe acute respiratory syndrome (SARS), middle east respiratory syndrome (MERS), and newly identified 2019 novel CoV (SARS-CoV2) [1,2]. SARS-CoV is known as an infectious disease with high transmissibility that caused the global epidemic following the early cases of Guangdong, China [3]. Within weeks, SARS-CoV reached almost all countries and peaked in March and April 2003 within a short time [4,5]. At first, the cause of the disease was unknown, but due to the rapid spread of the disease, it was considered as a potential infectious agent [6,7]. With reports announced by the US Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO), a viral agent belonging to the CoV group but with completely different genetic features than other CoVs, was isolated from infected patients [8]. Finally, at the end of 2003, the epidemic was controlled [4]. Given that the disease is a newly emerging pandemic and is likely to recur, understanding suspected clinical and laboratory symptoms, the treatment process, its transmission, some strategies for controlling the infection, and essential recommendations before traveling to and from infected areas were shown to be necessary [9,10].
Nearly ten years later in 2012, the CoV caused a SARS-like illness in Saudi Arabia that, unlike SARS-CoV, affected not only the respiratory system but also other vital organs such as the kidney and liver [11,12]. As a result, its mortality rate was more than SARS-CoV and was named MERS-CoV [13,14]. The clinical features of MERS-CoV infection were varied and ranged from asymptomatic or mild to acute respiratory syndrome and multiple organ failure and could lead to death, especially in people with underlying disease [15,16]. There is no specific drug for the treatment of MERS and preventive intensive care are needed to prevent the virus from spreading to health care centers [17].
SARS-CoV2 is known as a new variant of the CoV that has not been previously reported in humans [18,19]. There is still much to be learned about how CoV diseases-19 (COVID-19) spreads, its severity, and other features of the virus. Indeed, epidemiological and clinical research are ongoing to determine the prevalence of CoV-19 infections among people as a public health concern. Bioinformatical and biophysical researches are underway to examine a number of drugs available to find the potential drug to inhibit the CoV protease. Therefore, there is a great deal of interest in identifying potential drugs among the numerous compounds available in the treatment of viral or other diseases through the bioinformatics and biophysical approaches. Drugs that have the ability to bind the RBD can be considered as potential inhibitors of the CoV-19 protease .
Rapid access to CoV genomic data made it possible to produce first-generation homologous models for 3CLpro cysteine protease [20]. This enzyme is critical for CoV replication and has previously been studied as a target for antiviral therapies in the treatment of SARS-CoV [20,21]. This version shows that although the viral genome closely resembles the bat CoV, the protease most closely resembles the corona protease of the SARS-CoV, indicating that the virus has entered the human population through another animal (civets) [22].
Huang, et al. [23] used crystallographic and biophysical methods to identify the structural and functional properties of HKU9 (a bat CoV not transmitted to humans). The main reason for these studies was that β-CoV (a type that includes SARS-CoV and MERS-CoV) should be well characterized if they lead to the next global epidemic. After comparing the RBD of the virus with the existing structures of SARS-CoV, MERS-CoV, and HKU4-CoV (bat CoV), it was found that the recognition of receptor-ligand interaction is difficult despite the knowledge of the evolutionary history of RNA viruses [24].
Extensive research is ongoing to find disinfectants for previously known CoV as well as SARS-CoV2. A review study by Liu, et al. [25] discusses the possible prevention and treatment options for SARS-CoV2. There are four important enzymes that are essential for the pathogenesis: the S- protein that facilitates virus entry through the angiotensin-converting enzyme 2 (ACE2) to the host cell surface receptor, the major protease of CoV 3CLpro, and the papain-like protease (PLpro) involved in the assembly of new viruses, and RNA-dependent RNA polymerase (RdRp) that facilitates CoV RNA genome replication [25].
2. Discovery of new CoV
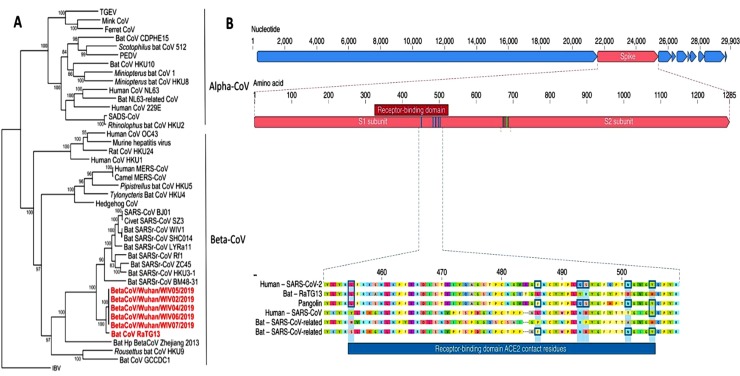
A new variant of the CoV spread in Wuhan, China, last year, followed by Chinese authorities reporting an outbreak of pneumonia of unknown origin. CoVs belong to the family Coronaviridae and α and β viruses normally infect mammals, while γ and δ CoVs usually infect birds and fish [26,27]. Canine CoV, which can cause mild diarrhea, and cat CoV, which can cause infectious catfish peritoneal inflammation, are both α-CoVs. Until the appearance of SARS-CoV2, which belongs to β-CoV (Fig. 1 A), only six known CoV were capable of infecting humans and causing respiratory disease, including SARS and MERS. The new CoV is genetically more related to SARS-CoV than MERS-CoV, but both are β-Cov, originating from bats. While we don't know for sure if this virus will behave like SARS-CoV and MERS-CoV, we can use the information from both previous viruses for development of potential anti-viral agents [1,26,28].
Fig. 1.
(A) The phylogenetic tree related to SARS-CoV2 [26]. (B) S- protein characterization in human SARS-CoV2 and related CoV [31,32]. Reprinted with permission from Refs. [26,31,32].
3. RBD of SARS-CoV2
The RBD in the S-protein has been shown to be the most flexible segment of the CoV structure [26,29]. Six RBD residues have been demonstrated to be crucial for interaction of S-protein with ACE2 as a receptor and for identifying the host range of SARS-CoV-mimicking viruses (Table 1 ) [30,31].
Table 1.
Six RBD residues of S-protein which are critical for the interaction of SARS-CoV and SARS-CoV2 with ACE2 [29,31].
| SARS-CoV | SARS-CoV2 | Status |
|---|---|---|
| Tyr-442 | Leu-445 | Different |
| Leu-472 | Phe-486 | Different |
| Asn-479, | Gln-493 | Different |
| Asp-480 | Ser-494 | Different |
| Thr-487 | Asn-501 | Different |
| Tyr-491 | Tyr-505 | Similar |
As tabulated in Table 1 and Fig. 1(B), only one of these six amino acids are similar between SARS-CoV2 and SARS-CoV.
While this data revealed that SARS-CoV2 may interact with human ACE2 with a high binding affinity, theoretical investigations demonstrated that the formation of this complex is not favorable and the RBD sequence is not similar with that in SARS-CoV to be potential for interaction with receptor [30, 33].
S-proteins present on the surface of the virus are a key factor in the detection of receptors and play an important role in the mechanism of membrane infiltration and infection. Researchers have found that S-protein from β-CoV originates from a common ancestor and evolves in the outer region of the RBD and this determines the virus's potential for transmission between species. An atomic model of a CoV S-protein has been introduced that facilitates the entry of CoV into host cells [34]. A number of strategies for development of ESV using only certain domains of the S-protein as antigen have been proposed through the analysis of this model. These human viruses, which contain S-proteins, are responsible for one-third of the typical cold-like pseudo-pneumonia. The epidemic nature of deadly pneumonia reveals that animal CoV are capable of being transmitted from animals to humans. Currently, it was assumed that a limited number of people could be infected by animal CoV; however, animals are infected by a large number of these kinds of viruses. The recent animal CoV outbreak is due to the overcoming inter-species barriers by this virus. This indicates the inevitability of the new CoV with the potential to be contagious. Antiviral treatments and approved ESV are not yet available for SARS-CoV2.
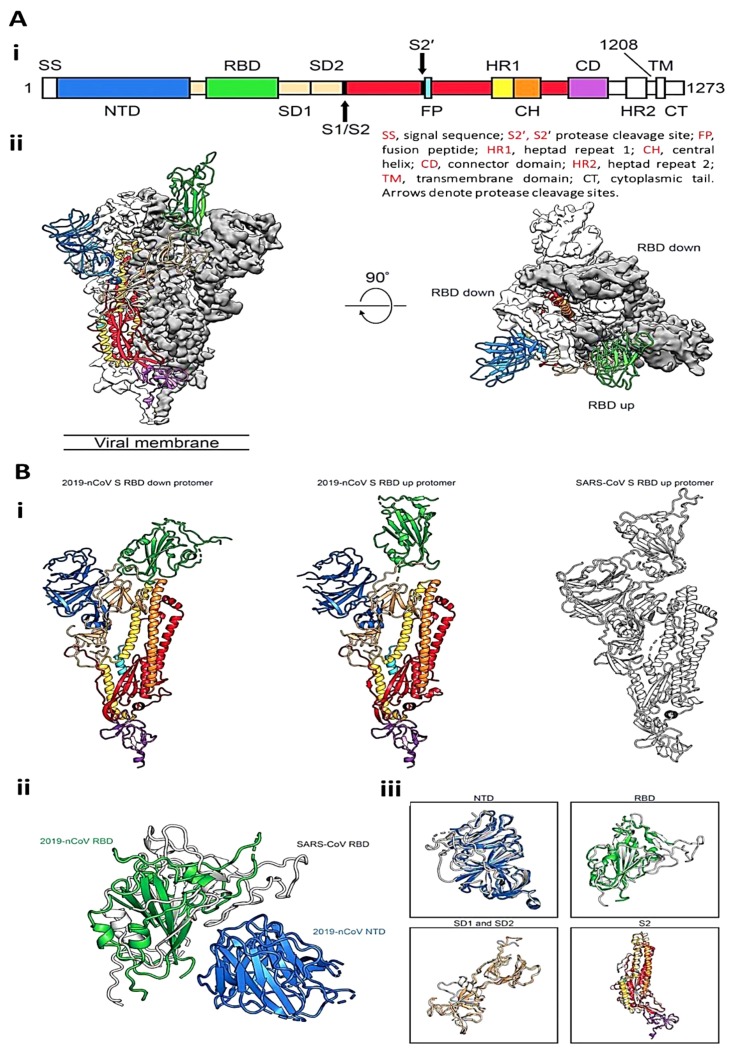
S-proteins with a transparent membrane help the CoV attach to specific cells and eventually enter them. The cryo-electron microscope and supercritical analysis has revealed the structure of the SARS-CoV2 trimeric S-protein. Fig. 2 A(i) demonstrates the domain of the expression construct. After supercritical analysis, three-dimensional (3D) conformation of a trimer with a single RBD was obtained in the up conformation (Fig. 2A (ii)). The observation in SARS-CoV2 S-protein and SARS-CoV suggested that RBD provides the similar mechanism of binding that is believed to be conserved among the different CoVs. The general conformation of SARS-CoV2 S-protein is similar to that of SARS-CoV S-protein (Fig. 2B (i)). One of the minor differences between these two S-proteins is the location of the RBDs in their related down-state configurations. The SARS-CoV RBD in the down-state configuration folds substantially against the N-terminal domain (NTD), whereas, the SARS-CoV2 S-protein RBD in the down-state configuration is moved to the central domain (Fig. 2B (ii)). However, the structural homology showed a high degree of similarity between the two S-proteins (Fig. 2B (iii)) [34].
Fig. 2.
(A) Structure of SARS-CoV2 S-protein, (i) Schematic of SARS-CoV2 S-protein primary structure, (ii) Side- and top- views of the prefusion structure of the SARS-CoV2 S-protein. (B) Conformational comparison between SARS-CoV2 S-protein and SARS-CoV S-protein, (i) Single protomer of SARS-CoV2 S-protein with the RBD, (ii) RBDs of SARS-CoV2 and SARS-CoV and the NTD of SARS-CoV2, (iii) Structural alignments of different domains from SARS-CoV2 S-protein and SARS-CoV S-protein [34]. Reprinted with permission from Ref. [34].
4. Therapeutic antibodies for COVID-19 targeting S-protein RBD
4.1. S230
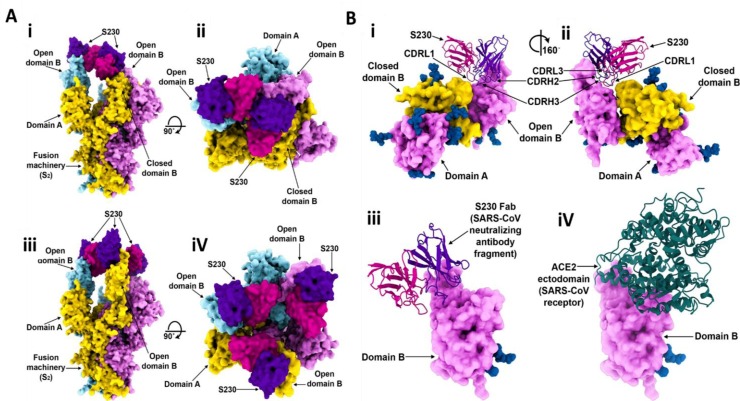
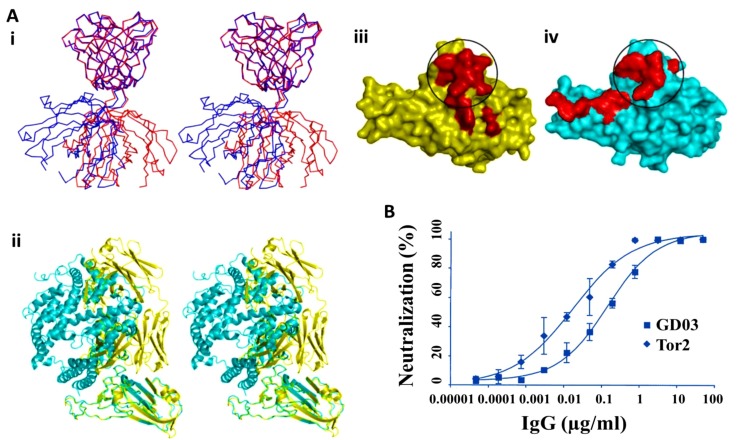
Walls, et al. [35] showed that as both SARS-CoV and SARS-CoV2 has similar host cell surface binding domain, promising blocking compounds or approaches assayed to block SARS-CoV entry could be explored against SARS-CoV2. They studied two Abs isolated from human survivors targeting the S-protein RBD (Fig. 3 A, B). They found that only S230 mAb stimulated fusogenic structural alterations through receptor bio-functional mimetics. The S230 mAb was purified from B cells of SARS-CoV-contaminated patients and effectively neutralized several SARS-CoV isolates [[36], [37], [38]]. Cryo-EM characterization of the SARS-CoV S-protein upon interaction with the S230 Fab disclosed that protein could be in the close and open states (Figs. 3 A(i-iv)). Docking study of S230 and S-protein revealed that some segments of mAb interact with B domain (Figs. 3B (i, ii)), which also mediates the interaction of S-protein and human ACE2 [39]. Comparison of the SARS-CoV S-protein-S230 complex conformation with the SARS-CoV B domain-ACE2 complex revealed that these proteins would compete upon interaction with SARS-CoV S (Figs. 3B (iii, iv)) [35].
Fig. 3.
(A) Cryo-EM structure of the SARS-CoV S-protein upon interaction with S230 mAb, (i and ii) Orthogonal views of the S-protein structure with one closed B domain, (iii and iv) Orthogonal views of the S-protein structure with three open B domains. (B) The interaction of S230 with the SARS-CoV S-protein, (i and ii) Different views of the S230 mAb binding site to an open B domain, (iii) S230 and (iv) ACE2 compete in the attachment to SARS-CoV S-protein. Reprinted with permission from Re.
f. [35].
4.2. 80R
Sui, et al. [40] probed the different recombinant single-chain variable region fragments (scFvs) against the S1 domain of S- protein of the SARS-CoV. Mapping of the 80R epitope demonstrated it binds the N-terminal residues of S-protein and relative to ACE2, they bind to S1 domain with high affinity. Sui, et al. [40] also investigated the antiviral activity of 80R mAb on SARS-CoV S-protein in vivo. They found that 80R mAb at levels therapeutically permissible in humans, significantly decreased the virus infection. They suggested that the crucial core domain of S-protein needed for 80R binding acts as a structurally sensitive segment to overlap the receptor ACE2-binding site [41].
4.3. F26G18 and F26G19
Berry, et al. [42] reported the selectivity and affinity of chimeric versions of recombinant mAbs. They showed that the F26G18 and F26G19 chimeric mAbs bind to the different domains of S-proteins [42]. Although, F26G18 mAb binds to the linear epitope (460-476) on the S1 segment of SARS-CoV, F26G19 mAb binds to the different conformational epitope on the S1 segment of SARS-CoV. This data suggested that the specificity of chimeric mAbs to the S-protein is based on the interaction of mAb with residues 318–510 in the RBD.
4.4. CR3014 and CR3022
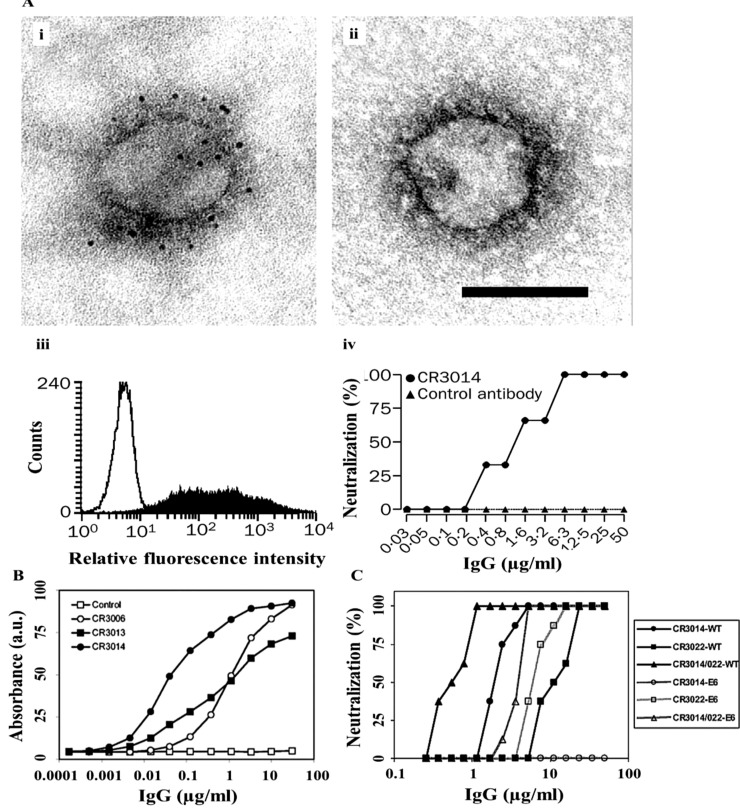
Ter Meulen, et al. [43] reported that prophylactic administration of the CR3014 mAb decreased replication of SARS-CoV and completely blocked the development of SARS-CoV-stimulated lung pathology and reduced shedding of CoV in pharyngeal secretions (Fig. 4 A (i-iv)). This data showed that administration of a human mAbs might serve as a potential prophylaxis for the blocking of human SARS-CoV outbreak. The epitope mapping employing recombinant S segments with some naturally appearing mutations, demonstrated the dominance of residue N479 for the binding of the most efficient neutralizing MAb, namely CR3014 (Fig. 4B) [44].
Fig. 4.
(A) Binding of CR3014 mAb to (i, ii) Viral peplomers of SARS-CoV, (iii) HEK293 T cells with S-protein, (iv) In vitro neutralisation of SARS-Cov [43]. (B) Titration of IgGs CR3006, CR3013, CR3014, and negative control IgG (Control) in an S565 fragment-coated ELISA [44]. (C) Wild-type SARS-CoV and a CR3014-neutralization escape variant (E6) with combined CR3014 and CR3022 mAbs [45]. Reprinted with permission from Refs. [[43], [44], [45]].
The combination of two mAbs CR3014 and CR3022 promisingly regulate the immune escape mechanism and also the potential synergistic effect between these mAbs may result in a lower mAb level to be used for passive immune prophylaxis of CoV infection (Fig. 4C) [45]. The combination of mAbs demonstrated neutralization of SARS-CoV in a synergistic mode by screening various epitopes on the S-protein RBD (Fig. 4C). Dose reduction indices (DRI) of 4.5 and 20.5 were determined for CR3014 and CR3022, respectively. Since increase of SARS-CoV infection by subneutralizing mAb levels is of great drawbacks, it can be assumed that anti-SARS-CoV mAbs do not result in the conversion of the abortive infection of macrophages by SARS-CoV into a high-yielding agent [45].
4.5. M396 and S230.15
Prabakaran, et al. [46] reported the structure of SARS-CoV RBD interacted with m396 mAb (Fig. 5 (i-iv)). They found that there is a remarkable alteration in the elbow angle between the unliganded and RBD-bound conformations of the Fab 396 (Fig. 5i). The unliganded Fab, depicted in red, shows an undeviating elbow angle, whereas the RBD-interacted Fab, depicted in blue, is significantly turned. The mAb and the ACE2 bind to common residues within RBD consisting Thr-484, Thr-486, Thr-487, Gly-488, Tyr-491, Arg-426. Hence, this segment is crucial for the interaction of RBD to both the mAb and the ACE2. Fig. 5(ii) displays a superimposed illustration of the RBD binding with mAb, shown in yellow and with ACE2, shown in cyan. Fig. 5(iii-iv) presents the binding domain of the mAb and the ACE2, respectively [46].
Fig. 5.
(A) Interaction of m396 and ACE2 with RBD, (i) Superimposed illustration of RBD-unliganded (red) and RBD-interacted Fab (blue), (ii) Comparison of the RBD-Fab and the RBD-ACE2 interaction, (iii, iv) Conformational footprints of the mAb and ACE2 RBD, respectively demonstrated as red balls on the RBD surface [46]. (B) M396 considerably neutralizes viruses pseudotyped with S-protein [47]. Reprinted with permission from Refs. [46,47].
Zhu, et al. [47] reported that two human mAbs, m396 and S230.15, effectively neutralized related isolates from the SARS-CoV infection, namely Urbani, Tor2 and from palm civets, namely SZ3, SZ16. Both mAbs competed with ACE2 for interaction with the S-protein RBD (Fig. 5B). Two recognized crucial amino acids in the RBD, namely lle-489 and Tyr-491 were determined in the SARS-CoV S-protein that probably play an important role in the interaction of m396 and S-protein RBD. These amino acids are potentially conserved in the SARS-CoV S-protein, revealing prospective m396 cross-reactivity.
Some other mAbs targeting the same region of ACE2 on the surface of RBD of SARS-CoV S-protein are 9F7, 10E7, 12B11, 18C2, 24H8, 26E1, 29G2, 32H5, 20E7, 26A4, 27C1, 31H12, 30E10, 13B6, 11E12, 18D9, 19B2, 28D6, 30F9, 35B5, 24F4, 33G4, 38D4, and 26E1 [48,49].
5. Comparison between the mAbs and the mechanism of interaction
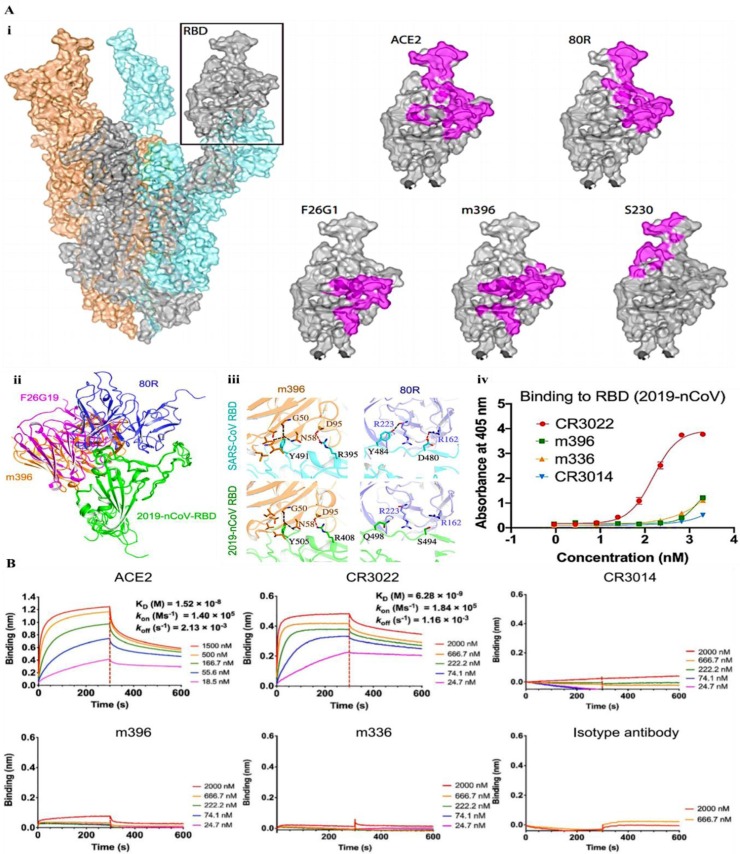
Coughlin and Prabhakar [50] studied a number of human mAbs binding the S-protein RBD. The interaction of SARS-CoV S-protein and mAbs are demonstrated in Fig. 6 A(i). As both SARS-CoV and SARS-CoV2 used ACE2 as a receptor, promising blocking compounds or approaches assayed to inhibit SARS-CoV entry could be explored against SARS-CoV2 [51].
Fig. 6.
(A) The interaction of different mAbs with S-protein RBD, (i) The docking of SARS-CoV-RBD binding to specific mAbs [35,46,51,[53], [54], [55]], (ii) The docking of SARS-CoV2 S-protein RBD binding to specific mAbs, (iii) The comparison of the residues involved in the interaction of two mAbs with SARS-CoV-RBD and SARS-CoV2 S-protein RBD, (iv) Binding of mAbs to SARS-CoV2 S-protein RBD examined by ELISA [52]. (B) Binding representation of SARS-CoV2 S-protein RBD to ACE2 and mAbs [52]. Reprinted with permission form Refs [35,46,[51], [52], [53], [54], [55]].
Indeed, the S-protein RBD with 193 residue length is the crucial site for interaction of neutralizing mAbs. Several mAbs interact with various epitopes on RBD; e.g. the SARS-CoV neutralizing mAbs, namely CR3014 and CR3022 interacted with the SARS-CoV RBD in a noncompetitive manner and neutralized the CoV in a synergistic mode [41]. Tian, et al. [52] modeled the structure of SARS-CoV2 S-protein RBD and reported its interaction with a number of neutralizing mAbs, and displayed that the molecular dynamic outcomes complete the binding of SARS-CoV2 S-protein RBD and some SARS-CoV mAbs (Fig. 6A(ii)). For example, amino acids in RBD of SARS-CoV that contribute in the formation of polar forces with a neutralizing mAb m396 are firmly conserved in SARS-CoV2 S-protein RBD (Fig. 6A(iii)). Afterwards ELISA assay was done to examine the binding ability of SARS-CoV-specific mAbs to SARS-CoV2 S-protein RBD (Fig. 6A(iv)). Interestingly, it was shown that most of these mAbs failed to bind SARS-CoV2 S-protein RBD [52].
Based on the comparatively high identity of RBD in SARS-CoV2 and SARS-CoV, it is assumed to assay the cross-reactivity of anti-SARS-CoV mAbs with SARS-CoV2 S-protein RBD, which could show outstanding applications for rapid and sensitive advancement of ESV-based therapeutic systems against SARS-CoV2. It was revealed that SARS-CoV-specific human mAbs, namely CR3022, could potentially interact with SARS-CoV2 S-protein RBD (Fig. 6B) [52]. As the epitope of this mAb does not overlap with the ACE2 binding domain, it may be indicated that CR3022 show the potential to be used as a potential candidate therapeutic, alone or in integration with other mAbs, for the inhibiting and treatment of COVID-19. Surprisingly, a number of the most efficient SARS-CoV-specific neutralizing mAbs such as S230, m396, CR3014 that bind the ACE2 binding domain do not show an ability to bind SARS-CoV2 S-protein, indicating that dissimilarity in the RBD of SARS-CoV and SARS-CoV2 shows a crucial effect for the cross-reactivity of neutralizing mAbs, and that it is still important to design and develop potential mAbs that could interact specifically and selectively to SARS-CoV2 S-protein RBD.
6. Ongoing challenges and future prospects
Scientists around the world have stepped up their research to tackle CoV and have suggested some potential approaches to develop potential ESV and related treatments. As soon as Chinese researchers released the genetic information of the SARS-CoV2, scientists quickly began working on the development of ESV and drugs to treat COVID-19. It is believed that the previously developed SARS-CoV and MERS-CoV ESV would be sufficiently useful in the case of overlapping sequences and structures. This is because like SARS-CoV and MERS-CoV, the SARS-CoV2 as a new virus uses RNA as a genetic material and shows the same protein and ligand interactions [1,2,56].
However, in-depth analysis has shown that the vaccine is not working for SARS-CoV2 and cannot stop the outbreak and infection of this virus [52]. Therefore, the development of potential ESV against SARS-CoV2 has received a great deal of interest in preventing CoV infection. The conventional vaccines are currently produced from weak or inactivated toxic of the virus or parts of the virus, including proteins, and the immune system recognizes the virus as an invader at the time of injection and generates Abs to prevent subsequent invasions. However, the point is that growing enough viruses or purifying enough proteins to vaccinate millions of people against SARS-CoV2 can take months or even years. Therefore, some other promising strategies should be considered to develop vaccines against SARS-CoV2 in a less labor and time-intensive manner. One approach can be done by transformation of the virus's RNA (SARS-CoV or MERS-CoV ESV) into DNA and determining some regions of the virus based on molecular simulations, which potentially stimulate the immune system to produce Abs [57]. Those identified segments of DNA are then introduced into the bacteria to produce large amounts of protein fragments for development of ESV. This method dramatically shortens the time needed to produce a potential ESV against SARS-CoV2.
Another approach is to develop a mRNA that stimulates the body to produce vaccine components [58]. Scientists have selected parts of SARS-CoV2 that may trigger a severe immune response against the virus. The mRNA vaccine dictates the human cells which viral proteins should be biosynthesized. Since the body biosynthesizes protein by vaccine mRNA, researchers can bypass the time-consuming and costly process of producing vaccine proteins.
This strategy can be used to design vaccines against future viruses or other infectious and contagious diseases. Meanwhile, other mRNA vaccines against SARS-CoV2 and other diseases are still being tested.
The mRNA vaccine can be ready for initial immunization testing within the next few months [58]. However, researchers must find some pharmaceutical companies to produce the large amounts of mRNA that are essential for the general public. Indeed, the experience with MERS vaccine is an example of how long it typically takes to make sure a vaccine is safe and effective. The company conducted initial safety testing of the MERS vaccine in a phase I clinical trial from February 2016 to May 2017 [59]. The vaccine was switched to Phase II testing in August 2018 to test the safety of a larger number of people and to determine whether the vaccine stimulates the immune system to produce protective Abs [60]. Even if everything goes smoothly, the MERS vaccine must pass the phase III safety and efficacy test before being approved by the US Food and Drug Administration, and vaccines and medicines will then become publicly available [60]. Using some well-developed devices, researchers can perform experiments over several weeks. The laboratory device may be improved in the next few weeks. Indeed, the emergence of the SARS-CoV2 has forced the researchers to look into speeding up their efforts. However, we estimate that it will take at least a year to prepare the SARS-CoV2 ESV.
Although, vaccines help people avoid becoming infected with pathogens, it may not help as soon as they become infected. However, protective mAbs can both prevent and treat the infection. Abs in the blood of people who have recovered from infection and become resistant to the virus or bacteria that cause the disease often stay in the body for several years or decades, and when mAbs later become infected, they can protect the person. However, the important thing is that these mAbs can protect the individual against other pathogens as well. It takes weeks to months for the ESV to stimulate the immune system to produce a protective level of mAbs. For example, Ebola vaccines take at least a week to stimulate Ab production, but in the end, they provide immediate protection. The companies are now producing potential mAbs to fight the SARS-CoV2 based on the information that they have learned from the SARS-CoV and MERS-CoV projects.
7. Conclusion
Most natural and synthetic compounds are involved in enhancing vaccine efficacy, but toxicity is the most important problem in introducing them for use in living organisms. Therefore, the design and development of potential platforms is based on their safety and efficacy, knowledge of the functional pathways, the activation of innate and acquired immune system, and the ability to induce strong memory immunity. Cellular proteins that induce immune responses expressed extensively in host body tissues are promising options for development of ESV. Despite extensive studies in the prevention and control of COVID-19 to increase public health due to the variable nature of this virus, the co-circulation of different subtypes, the emergence of multiple antigenic shifts and the ability of new strains to adapt to new hosts, the effort to develop a comprehensive ESV continues with the minimum side effects.
Authors' contributions
All authors have made a considerable, direct and intellectual contribution to the work, and approved it for publication.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgment
This research was made possible by the grants NPRP10-120-170-211from Qatar National Research Fund (QNRF) under Qatar Foundationand GCC-2017-005 under the GCC collaborative research Program from Qatar University.
References
- 1.Lee P.-I., Hsueh P.-R. Emerging threats from zoonotic coronaviruses-from SARS and MERS to SARS-CoV2. Journal of Microbiology, Immunology and Infection. 2020 doi: 10.1016/j.jmii.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz D.A., Graham A.L. Potential maternal and infant outcomes from (Wuhan) coronavirus SARS-CoV2 infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12(2):194. doi: 10.3390/v12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong N., Zheng B., Li Y., Poon L., Xie Z., Chan K., Li P., Tan S., Chang Q., Xie J. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. The Lancet. 2003;362(9393):1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson R.M., Fraser C., Ghani A.C., Donnelly C.A., Riley S., Ferguson N.M., Leung G.M., Lam T.H., Hedley A.J. Epidemiology, transmission dynamics and control of SARS: the 2002–2003 epidemic, Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1447;359(2004):1091–1105. doi: 10.1098/rstb.2004.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heymann D.L. The international response to the outbreak of SARS in 2003, Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1447;359(2004):1127–1129. doi: 10.1098/rstb.2004.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith R.D. Responding to global infectious disease outbreaks: lessons from SARS on the role of risk perception, communication and management. Social science & medicine. 2006;63(12):3113–3123. doi: 10.1016/j.socscimed.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilder-Smith A. The severe acute respiratory syndrome: impact on travel and tourism. Travel medicine and infectious disease. 2006;4(2):53–60. doi: 10.1016/j.tmaid.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arguin P.M., Navin A.W., Steele S.F., Weld L.H., Kozarsky P.E. Health communication during SARS. Emerging infectious diseases. 2004;10(2):377. doi: 10.3201/eid1002.030812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau Y.-L. SARS: future research and vaccine. Paediatric respiratory reviews. 2004;5(4):300–303. doi: 10.1016/j.prrv.2004.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauch C.T., Lloyd-Smith J.O., Coffee M.P., Galvani A.P. Dynamically modeling SARS and other newly emerging respiratory illnesses: past, present, and future. Epidemiology. 2005:791–801. doi: 10.1097/01.ede.0000181633.80269.4c. [DOI] [PubMed] [Google Scholar]
- 11.Gastanaduy P.A. Update: severe respiratory illness associated with Middle East respiratory syndrome coronavirus (MERS-CoV)—worldwide, 2012–2013. MMWR. Morbidity and mortality weekly report. 2013;62(23):480. [PMC free article] [PubMed] [Google Scholar]
- 12.Alsahafi A.J., Cheng A.C. The epidemiology of Middle East respiratory syndrome coronavirus in the Kingdom of Saudi Arabia, 2012–2015. International Journal of Infectious Diseases. 2016;45:1–4. doi: 10.1016/j.ijid.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Momattin H., Mohammed K., Zumla A., Memish Z.A., Al-Tawfiq J.A. Therapeutic options for Middle East respiratory syndrome coronavirus (MERS-CoV)–possible lessons from a systematic review of SARS-CoV therapy. International Journal of Infectious Diseases. 2013;17(10):e792–e798. doi: 10.1016/j.ijid.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Tawfiq J.A., Zumla A., Memish Z.A. Travel implications of emerging coronaviruses: SARS and MERS-CoV. Travel medicine and infectious disease. 2014;12(5):422–428. doi: 10.1016/j.tmaid.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omrani A.S., Matin M.A., Haddad Q., Al-Nakhli D., Memish Z.A., Albarrak A.M. A family cluster of Middle East Respiratory Syndrome Coronavirus infections related to a likely unrecognized asymptomatic or mild case. International Journal of Infectious Diseases. 2013;17(9):e668–e672. doi: 10.1016/j.ijid.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang K., Ki M., Lee E.G., Lee S.Y., Yoo B., Choi J.H. MERS epidemiological investigation to detect potential mode of transmission in the 178th MERS confirmed case in Pyeongtaek, Korea. Epidemiology and health. 2015;37 doi: 10.4178/epih/e2015036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T., Scott D., Cihlar T., Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proceedings of the National Academy of Sciences. 2020;117(12):6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novel C.P.E.R.E. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua liu xing bing xue za zhi= Zhonghua liuxingbingxue zazhi. 2020;41(2):145. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoermer M. 2020. Homology Models of Coronavirus SARS-CoV2 3CLpro Protease. [Google Scholar]
- 21.Jo S., Kim S., Shin D.H., Kim M.-S. Inhibition of SARS-CoV 3CL protease by flavonoids. Journal of enzyme inhibition and medicinal chemistry. 2020;35(1):145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paraskevis D., Kostaki E.G., Magiorkinis G., Panayiotakopoulos G., Sourvinos G., Tsiodras S. Full-genome evolutionary analysis of the novel corona virus (SARS-CoV2) rejects the hypothesis of emergence as a result of a recent recombination event, Infection. Genetics and Evolution. 2020;79 doi: 10.1016/j.meegid.2020.104212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C., Qi J., Lu G., Wang Q., Yuan Y., Wu Y., Zhang Y., Yan J., Gao G.F. Putative receptor binding domain of bat-derived coronavirus HKU9 spike protein: evolution of betacoronavirus receptor binding Motifs. Biochemistry. 2016;55(43):5977–5988. doi: 10.1021/acs.biochem.6b00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tai W., He L., Zhang X., Pu J., Voronin D., Jiang S., Zhou Y., Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cellular & Molecular Immunology. 2020:1–8. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W., Morse J.S., Lalonde T., Xu S. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019‐nCoV. Chembiochem. 2020 doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. BioRxiv. 2020 [Google Scholar]
- 27.Al-Tawfiq J.A. Viral loads of SARS, MERS and SARS-CoV-2 in respiratory specimens: What have we learned? Travel medicine and infectious disease. 2020 doi: 10.1016/j.tmaid.2020.101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang X., Rayner S., Luo M.H. Does SARS‐CoV‐2 has a longer incubation period than SARS and MERS? Journal of medical virology. 2020 doi: 10.1002/jmv.25708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. Journal of virology. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nature Medicine. 2020:1–3. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong M.C., Cregeen S.J.J., Ajami N.J., Petrosino J.F. Evidence of recombination in coronaviruses implicating pangolin origins of nCoV-2019. bioRxiv. 2020 [Google Scholar]
- 33.Sheahan T., Rockx B., Donaldson E., Sims A., Pickles R., Corti D., Baric R. Mechanisms of zoonotic severe acute respiratory syndrome coronavirus host range expansion in human airway epithelium. Journal of virology. 2008;82(5):2274–2285. doi: 10.1128/JVI.02041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the SARS-CoV2 spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walls A.C., Xiong X., Park Y.-J., Tortorici M.A., Snijder J., Quispe J., Cameroni E., Gopal R., Dai M., Lanzavecchia A. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell. 2019;176(5):1026–1039. doi: 10.1016/j.cell.2018.12.028. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Traggiai E., Becker S., Subbarao K., Kolesnikova L., Uematsu Y., Gismondo M.R., Murphy B.R., Rappuoli R., Lanzavecchia A. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nature medicine. 2004;10(8):871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rockx B., Corti D., Donaldson E., Sheahan T., Stadler K., Lanzavecchia A., Baric R. Structural basis for potent cross-neutralizing human monoclonal antibody protection against lethal human and zoonotic severe acute respiratory syndrome coronavirus challenge. Journal of virology. 2008;82(7):3220–3235. doi: 10.1128/JVI.02377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corti D., Zhao J., Pedotti M., Simonelli L., Agnihothram S., Fett C., Fernandez-Rodriguez B., Foglierini M., Agatic G., Vanzetta F. Prophylactic and postexposure efficacy of a potent human monoclonal antibody against MERS coronavirus. Proceedings of the National Academy of Sciences. 2015;112(33):10473–10478. doi: 10.1073/pnas.1510199112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sui J., Li W., Murakami A., Tamin A., Matthews L.J., Wong S.K., Moore M.J., Tallarico A.S.C., Olurinde M., Choe H. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proceedings of the National Academy of Sciences. 2004;101(8):2536–2541. doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sui J., Li W., Roberts A., Matthews L.J., Murakami A., Vogel L., Wong S.K., Subbarao K., Farzan M., Marasco W.A. Evaluation of human monoclonal antibody 80R for immunoprophylaxis of severe acute respiratory syndrome by an animal study, epitope mapping, and analysis of spike variants. Journal of virology. 2005;79(10):5900–5906. doi: 10.1128/JVI.79.10.5900-5906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berry J.D., Hay K., Rini J.M., Yu M., Wang L., Plummer F.A., Corbett C.R., Andonov A. Neutralizing epitopes of the SARS-CoV S-protein cluster independent of repertoire, antigen structure or mAb technology, MAbs. Taylor & Francis. 2010:53–66. doi: 10.4161/mabs.2.1.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ter Meulen J., Bakker A.B., van den Brink E.N., Weverling G.J., Martina B.E., Haagmans B.L., Kuiken T., de Kruif J., Preiser W., Spaan W. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. The Lancet. 2004;363(9427):2139–2141. doi: 10.1016/S0140-6736(04)16506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van den Brink E.N., ter Meulen J., Cox F., Jongeneelen M.A., Thijsse A., Throsby M., Marissen W.E., Rood P.M., Bakker A.B., Gelderblom H.R. Molecular and biological characterization of human monoclonal antibodies binding to the spike and nucleocapsid proteins of severe acute respiratory syndrome coronavirus. Journal of virology. 2005;79(3):1635–1644. doi: 10.1128/JVI.79.3.1635-1644.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ter Meulen J., Van Den Brink E.N., Poon L.L., Marissen W.E., Leung C.S., Cox F., Cheung C.Y., Bakker A.Q., Bogaards J.A., Van Deventer E. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS medicine. 2006;3(7) doi: 10.1371/journal.pmed.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prabakaran P., Gan J., Feng Y., Zhu Z., Choudhry V., Xiao X., Ji X., Dimitrov D.S. Structure of severe acute respiratory syndrome coronavirus receptor-binding domain complexed with neutralizing antibody. Journal of Biological Chemistry. 2006;281(23):15829–15836. doi: 10.1074/jbc.M600697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu Z., Chakraborti S., He Y., Roberts A., Sheahan T., Xiao X., Hensley L.E., Prabakaran P., Rockx B., Sidorov I.A. Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. Proceedings of the National Academy of Sciences. 2007;104(29):12123–12128. doi: 10.1073/pnas.0701000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Y., Lu H., Siddiqui P., Zhou Y., Jiang S. Receptor-binding domain of severe acute respiratory syndrome coronavirus spike protein contains multiple conformation-dependent epitopes that induce highly potent neutralizing antibodies. The Journal of Immunology. 2005;174(8):4908–4915. doi: 10.4049/jimmunol.174.8.4908. [DOI] [PubMed] [Google Scholar]
- 49.Chen W.-H., Hotez P.J., Bottazzi M.E. Potential for Developing the SARS-CoV Receptor Binding Domain Recombinant Protein (RBD) as a Heterologous Human Vaccine for SARS-CoV-2. Hum Vaccin Immunother. 2020 doi: 10.1080/21645515.2020.1740560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coughlin M.M., Prabhakar B.S. Neutralizing human monoclonal antibodies to severe acute respiratory syndrome coronavirus: target, mechanism of action, and therapeutic potential. Reviews in medical virology. 2012;22(1):2–17. doi: 10.1002/rmv.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shanmugaraj B., Siriwattananon K., Wangkanont K., Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19) Asian Pacific Journal of Allergy and Immunology. 2020;38(1):10–18. doi: 10.12932/AP-200220-0773. [DOI] [PubMed] [Google Scholar]
- 52.Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerging microbes & infections. 2020;9(1):382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pak J.E., Sharon C., Satkunarajah M., Auperin T.C., Cameron C.M., Kelvin D.J., Seetharaman J., Cochrane A., Plummer F.A., Berry J.D. Structural insights into immune recognition of the severe acute respiratory syndrome coronavirus S protein receptor binding domain. Journal of molecular biology. 2009;388(4):815–823. doi: 10.1016/j.jmb.2009.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hwang W.C., Lin Y., Santelli E., Sui J., Jaroszewski L., Stec B., Farzan M., Marasco W.A., Liddington R.C. Structural basis of neutralization by a human anti-severe acute respiratory syndrome spike protein antibody, 80R. Journal of Biological Chemistry. 2006;281(45):34610–34616. doi: 10.1074/jbc.M603275200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kirchdoerfer R.N., Wang N., Pallesen J., Wrapp D., Turner H.L., Cottrell C.A., Corbett K.S., Graham B.S., McLellan J.S., Ward A.B. Stabilized coronavirus spikes are resistant to conformational changes induced by receptor recognition or proteolysis. Scientific reports. 2018;8(1):1–11. doi: 10.1038/s41598-018-34171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K., Trilling M., Lu M., Dittmer U., Yang D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS‐CoV, MERS‐CoV, and 2019‐nCoV. Journal of Medical Virology. 2020 doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pang J., Wang M.X., Ang I.Y.H., Tan S.H.X., Lewis R.F., Chen J.I.-P., Gutierrez R.A., Gwee S.X.W., Chua P.E.Y., Yang Q. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel Coronavirus (SARS-CoV2): a systematic review. Journal of Clinical Medicine. 2020;9(3):623. doi: 10.3390/jcm9030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. Jama. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 59.Beigel J.H., Voell J., Kumar P., Raviprakash K., Wu H., Jiao J.-A., Sullivan E., Luke T., Davey R.T., Jr. Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study. The Lancet Infectious Diseases. 2018;18(4):410–418. doi: 10.1016/S1473-3099(18)30002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]