Abstract
Background
The gut microbiome and microbiome-gut-brain (MGB) axis have been receiving increasing attention for their role in the regulation of mental behavior and possible biological basis of psychiatric disorders. With the advance of next-generation sequencing technology, characterization of the gut microbiota in schizophrenia (SZ) patients can provide rich clues for the diagnosis and prevention of SZ.
Methods
In this study, we compared the differences in the fecal microbiota between 82 SZ patients and 80 demographically matched normal controls (NCs) by 16S rRNA sequencing and analyzed the correlations between altered gut microbiota and symptom severity.
Results
The alpha diversity showed no significant differences between the NC and SZ groups, but the beta diversity revealed significant community-level separation in microbiome composition between the two groups (pseudo-F =3.337, p < 0.001, uncorrected). At the phylum level, relatively more Actinobacteria and less Firmicutes (p < 0.05, FDR corrected) were found in the SZ group. At the genus level, the relative abundances of Collinsella, Lactobacillus, Succinivibrio, Mogibacterium, Corynebacterium, undefined Ruminococcus and undefined Eubacterium were significantly increased, whereas the abundances of Adlercreutzia, Anaerostipes, Ruminococcus and Faecalibacterium were decreased in the SZ group compared to the NC group (p < 0.05, FDR corrected). We performed PICRUSt analysis and found that several metabolic pathways differed significantly between the two groups, including the Polyketide sugar unit biosynthesis, Valine, Leucine and Isoleucine biosynthesis, Pantothenate and CoA biosynthesis, C5-Branched dibasic acid metabolism, Phenylpropanoid biosynthesis, Ascorbate and aldarate metabolism, Nucleotide metabolism and Propanoate metabolism pathways (p < 0.05, FDR corrected). Among the SZ group, the abundance of Succinivibrio was positively correlated with the total Positive and Negative Syndrome Scale (PANSS) scores (r = 0.24, p < 0.05, uncorrected) as well as the general PANSS scores (r = 0.22, p < 0.05, uncorrected); Corynebacterium was negatively related to the negative scores of PANSS (r = 0.22, p < 0.05, uncorrected).
Conclusions
Our findings provided evidence of altered gut microbial composition in SZ group. In addition, we found that Succinvibrio and Corynebacterium were associated with the severity of symptoms for the first time, which may provide some new biomarkers for the diagnosis of SZ.
Keywords: Schizophrenia, Gut microbiota, Microbiome-Gut-Brain axis, 16S rRNA sequencing, PANSS
Introduction
Schizophrenia (SZ) is a complex, chronic psychiatric disorder with a heterogeneous genetic and neurobiological background (Bang et al., 2014; Hoekert et al., 2007; McGlashan & Fenton, 1992). Treatments for SZ are available, but their effectiveness is poor for many patients (Higuchi et al., 2019; Kraus et al., 2006). To acquire better therapeutic results, we need to completely understand the pathophysiology of SZ. Previously, researchers have focused on analyzing the human genome (Ripke et al., 2011) and environmental risk factors (Brown et al., 2002; Cannon, Jones & Murray, 2002; Cantor-Graae & Selten, 2005; Mulvany et al., 2001; Sara et al., 2014; Stilo et al., 2017; Van Os, Pedersen & Mortensen, 2004; Varese et al., 2012) to determine the pathogenesis of SZ. However, the identified associations only account for some of the variance in SZ (Wu et al., 2019). Recently, interest in researching the effect of gut microbiota on host physiology and pathology has increased rapidly. The variations in the composition of the gut microbiota influence inflammatory and metabolic pathways across a number of diseases, such as inflammatory bowel disease (Huttenhower, Kostic & Xavier, 2014), obesity and metabolic diseases (Bouter et al., 2017; Hartstra et al., 2015), cancer (Schwabe & Jobin, 2013) and chronic pulmonary diseases (Shukla et al., 2017). Converging evidence also suggests that the gut microbiota communicates with the central nervous system bidirectionally through the microbiome-gut-brain (MGB) axis and thereby influences brain function and behavior (Cryan & Dinan, 2012; Desbonnet et al., 2014; Hsiao et al., 2013; Sampson et al., 2016). A dysregulated MGB axis has been reported in many neuropsychiatric disorders including bipolar disorder (Hu et al., 2019), major depression disorder (Zheng et al., 2016), Alzheimer’s disease (Cattaneo et al., 2017), Parkinson’s disease (Caputi & Giron, 2018) and autism (Tomova et al., 2015).
Recently, a few articles have focused on the role of the MGB axis in SZ. Epidemiological studies have shown that prenatal microbial infections appear to increase the risk of SZ in offspring (Babulas et al., 2006). Additionally, SZ often superinduces gut and digestive disturbances or intestinal inflammation with a high prevalence (Severance et al., 2012, 2015; Sherwin et al., 2016; Yolken et al., 2015). Some clinical studies indicate potential associations between a disturbed gut microbiome and SZ (Lv et al., 2017). Castro-Nallar et al. (2015) sequenced microbes in the oropharynx of patients who have SZ and found a difference between the SZs and normal controls (NCs), which further indicated that the host microbiome have an impact on host’s health. The gut microbiota and its metabolites are critical in promoting neurodevelopment by modulating important agents, such as neurotrophin and neurotransmitters. Fecal microbiota transplantation showed that germ-free mice that received SZ microbiome fecal transplants displayed SZ-relevant behaviors similar to SZ mouse models (Zheng et al., 2019).
Changes in the gut microbiota and its metabolites may cause neuronal damage, apoptosis and abnormal brain development, leading to SZ (Yuan et al., 2019). An increase in intestinal mucosal permeability induced by gut microbiota dysbiosis leads to alterations in intestinal membrane proteins zonulin and mucin and in metabolites indolepropionic acid (IPA), lipopolysaccharides (LPS) and SCFA (Wang, Geier & Howarth, 2016). The metabolite IPA of gut microbiota maintains gut mucosal barrier integrity and Homeostasis of monocytes and T cells (Dodd et al., 2017). The metabolite LPS activates the peripheral immune system, damages the blood brain barrier (BBB), and causes neuroinflammation (Cao et al., 2017); further, LPS and toxic substances are translocated in the gut lumen, aggravating peripheral immune dysfunction to cause neuroimmune activation. The metabolite SCFA protects the brain barrier and intestinal mucosal barrier, and regulates the peripheral immune system and microglia function in the brain and potentially regulates the development and function of meningeal lymphatic vessels in the brain (Louveau et al., 2018), lower levels of SCFA induced by decreased colonic bacteria can damage the intestinal barrier and the BBB, altering microlia vulnerability and morphology and activating immune responses and neuroinflammation. In conclusion, disturbances in the gut microbiota may cause microglia-mediated neuroinflammation and damage to neurons, synapses, and connectivity between brain regions. These disturbances are a possible mechanism for the etiopathology of SZ.
Previous studies discovered that the abundance of some bacteria in the gut of SZs is quite different from that in the gut of NCs. One of these studies found that at the phylum level, Proteobacteria was significantly increased in SZs; the genera Succinivibrio, Megasphaera, Collinsella, Clostridium, Klebsiella and Methanobrevibacter were significantly higher, whereas Blautia, Coprococcus and Roseburia were decreased compared to NCs, and receiver operating characteristic curve analysis demonstrated that 12 microbiota could be used to distinguish SZs from NCs (Shen et al., 2018). Another study based on the metagenomic analysis of gut microbiota showed that the numbers of Lactobacillus group bacteria were elevated in first-episode SZs and significantly correlated with severity (Schwarz et al., 2018). In addition, the investigation of the gut microbiome in US-based patients with chronic SZ revealed that the phylum Proteobacteria was relatively decreased in SZs, and at the genus level, Anaerococcus was relatively increased in SZs, while Haemophilus, Sutterella and Clostridium were decreased (Nguyen et al., 2018) and increased negative symptoms were associated with decreased abundance of family Ruminococcaceae and greater severity of depressive symptoms was correlated with greater abundance of genus Bacteroides. In these studies, the taxonomies of altered bacteria in SZs are inconsistent, and the correlation between altered gut microbiota and symptom severity are not fully understood. The inconsistencies might be due to (1) the small sample size of these studies; (2) various factors, such as region, diet, environment, etc. (Huttenhower et al., 2012); (3) subjects with other mental disorders.
The objective of this study was to characterize the gut microbiome in SZs and preliminary analyze the correlation between the altered gut microbiota and the severity of symptoms. We excluded individuals with any chronic disease that may affect the stability of the gut microbiota, including intestinal inflammation, Constipation, diarrhea and diabetes; expanded the sample size; and controlled the drug use of the NCs to eliminate possible bias. We hypothesized that (1) gut microbial composition might differ between the SZ and NC groups and (2) the altered gut microbiota in SZs might significantly correlate with symptom severity.
Materials and Methods
Participants
A total of 162 subjects were collected in this study from September 2017 to February 2019, including 82 SZs and 80 NCs. The SZs were recruited from Guangzhou Huiai Hospital and were diagnosed by trained and experienced clinical psychiatrists according to the structured clinical interview according to the Diagnostic and Statistical Manual of Mental Disorder-IV-Text Revision (DSM-IV-TR) (SCID) criteria (Wu et al., 2018); The psychiatric symptoms were steady >2 weeks; the Positive and Negative Syndrome Scale (PANSS) evaluated the rate of change ≤20% in 2 weeks and the total score of PANSS ≥30. Seventy-five patients were treated with antipsychotics at the time of the study (Supplemental File 1). The exclusion criteria for patients included (1) any other psychiatric Axis I disorder meeting DSM-IV criteria, including schizoaffective disorder, mental retardation, major depressive disorder, bipolar, delirium, dementia, memory disorder and other cognitive disorders; (2) constipation, diarrhea, diabetes, hypertension, heart disease, thyroid diseases or any somatic diseases; (3) a history of epilepsy, except for febrile convulsions; (4) a history of having received electroconvulsive therapy in the past 6 months; (5) lactating, pregnant, or planning to become pregnant; (6) alcohol dependence or (7) noncompliant drug administration or a lack of legal guardians.
Normal controls were recruited in Guangzhou and surrounding areas through multiple methods, including recruitment flyers in the community, internet ads and word-of-mouth. The age, sex and nationality of all NCs were matched with the SZs. The inclusion criteria of NCs were as follows: (1) the Han nationality, no special religious beliefs; (2) 18–65 years; (3) absence of antibiotic intake for the last 3 months and with no diarrhea at present; (4) absence of any chronic disease that may affect the stability of gut microbiota; (5) BMI 18–30 kg/m2; (6) absence of any major gastrointestinal tract surgery within 5 years; and (7) absence of any head surgery and no mental disorders.
All participants signed the information consent form, indicating their agreement. The sample collection and the protocol of analysis were approved by Guangzhou Brain Hospital. A questionnaire was conducted among all subjects to collect general information, including age, sex, height, weight, years of education, history of taking medicine, and history of smoking and drinking.
Fecal sample collection and 16S ribosome RNA sequencing
Fresh fecal samples were obtained from participants, and all of the samples were stored at −80 °C until DNA extraction. A total of 200 mg of each fecal sample was used for DNA extraction.
Community DNA was extracted under the manual of the MOBIO PowerSoil® DNA Isolation Kit 12,888–100 protocol. Prior to sequencing, the DNA was stored in Tris-EDTA buffer solution at −80 °C. To enable amplification of the V4 region of the 16S rRNA gene and add barcode sequences, unique fusion primers were designed based on the universal primer set 515F (5′-GTGYCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACNVGGGTWTCTAAT-3′) along with barcode sequences. PCR mixtures in 50 μL reaction volumes contained 1 μL of each forward and reverse primer (10 μM), 1 μL of Easy Pfu DNA Polymerase (2.5 U/μL), 4 μL of dNTPs (2.5 mM), 1 μL of template DNA, 1 μL of double distilled water, and 5 μL of 10 × EasyPfu Buffer. Thermal cycling consisted of an initial denaturation step at 95 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 40 s, with a final extension step at 72 °C for 4 min. Amplicons from each sample were run on an agarose gel. The expected band size for 515f–806r is approximately 300–350 bp. Amplicons were quantified with Quant-iT PicoGreen dsDNA Assay Kit (cat. no. P11496; ThermoFisher/Invitrogen, Waltham, MA, USA) following the manufacturer’s instructions. According to the manufacturer’s instructions, the amplicon libraries for high-throughput sequencing on the Illumina MiSeq platform were combined in equal amounts and subsequently quantified (KAPA Library Quantification Kit KK4824).
Bioinformatics and statistical analyses
The raw sequences were processed to concatenate reads into tags according to the overlapping relationship by using QIIME2 (Bolyen et al., 2019). The DADA2 algorithm was performed to demultiplex raw sequences and identify microbial features (Callahan et al., 2016). The output features were rarefied to 1,3581 sequences per sample, which was the lowest value in the dataset. The microbial community structure was characterized using measures of alpha-diversity (within-sample) and beta-diversity (between-samples). The alpha-diversity indices we selected were Evenness, Faith’s Phylogenetic Diversity, Observed Species and Shannon, which represent the evenness and richness of taxa within a single sample, and the differences in diversity between groups were calculated using the nonparametric Kruskal–Wallis H test in QIIME2. The beta-diversity indicates differences in taxa composition between groups, which were calculated using Bray-Curtis dissimilarity. Principal coordinate analysis (PCoA) based on the Bray-Curtis distances matrix was used for visualizing sample relationships, and PERMANOVA with 999 permutations was used to assess the statistical significance of beta-diversity distances between groups. Output matrices were ordinated and visualized using the vegan package from R (Oksanen et al., 2019). We used a pretrained Naïve Bayes classifier for taxonomic analysis. This classifier was trained on the Greengenes database (13.8) (DeSantis et al., 2006), and all differential abundances at different taxonomic levels were tested using the Mann–Whitney U test. Linear discriminant analysis (LDA) effect size (LEfSe) was used to identify different markers, an alpha = 0.01 was used in the factorial Kruskal–Wallis test among groups, and the log value for the LDA score was set to >2. To determine the association between differential abundance at the genus level and clinical characteristics, we further calculated the residuals of relative abundance of those taxa with significant group differences, controlling for age, sex and years of education, by the ‘vglm’ function in the VGAM package (Yee, 2007). Pearson’s correlations were then calculated between the residuals of relative abundance of those taxa from patients and the PANSS scores. Significances of all tests were set as p < 0.05, or FDR corrected p < 0.05 (two side). To obtain insight into the possible functional pathways that differ between SZs and NCs, we used PICRUSt (Langille et al., 2013) to calculate contributions of various features to known biological pathways based on KEGG orthology groups (KOs) using the Kyoto Encyclopedia of Genes and Genomes (KEGG) databases (Ogata et al., 2000).
Results
Clinical data
A total of 82 SZs and 80 NCs were recruited according to the inclusion criteria. Demographic and clinical characteristics of the groups are presented in Table 1. The SZ and NC groups did not differ in age (p = 0.60, uncorrected) or sex (p = 0.35, uncorrected). The years of education (p = 2.04 × 10−6, uncorrected) and BMI (p = 0.01, uncorrected) of the SZ group were lower than those of the NC group. The ratio of tobacco using was higher in the SZ group than in the NC group (p = 0.01, uncorrected), while alcohol intake was lower (p = 3.36 × 10−8, uncorrected). Comparing high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and glucose in serum, the SZ group showed lower values of HDL-C (p = 1.43 × 10−4, uncorrected), LDL-C (p = 7.95 × 10−6, uncorrected) and glucose (p = 1.38 × 10−8, uncorrected) compared to the NC group. In addition, the SZ group showed lower values of total cholesterol (TC) (p = 4.95 × 10−15, uncorrected) and triglyceride (TG) (p = 0.01, uncorrected).
Table 1. Demographic characteristic of schizophrenia and normal controls.
Values are shown as mean ± SD or ratio.
| Characteristic | NC group (n = 80) | SZ group (n = 82) | p-Value |
|---|---|---|---|
| Age | 41.03 ± 14.34 | 42.15 ± 13.13 | 0.60 |
| Sex (M/F) | 39/41 | 46/36 | 0.35 |
| BMI (kg/m2)* | 23.03 ± 3.05 | 24.48 ± 4.33 | 0.01 |
| PANSS | – | 59.12 ± 18.18 | – |
| Education year | 13.95 ± 3.49 | 11.22 ± 3.51 | 2.04 × 10−6 |
| S-HDL-C (mmol/l) | 1.65 ± 0.29 | 1.40 ± 0.50 | 1.43 × 10−4 |
| S-LDL-C (mmol/l) | 3.62 ± 0.95 | 2.97 ± 0.84 | 7.95 × 10−6 |
| S-Glu (mmol/l) | 5.77 ± 1.15 | 4.83 ± 1.04 | 1.38 × 10−8 |
| TC (mmol/l) | 6.24 ± 1.19 | 4.76 ± 0.94 | 4.59 × 10−15 |
| TG (mmol/l) | 1.26 ± 0.69 | 1.56 ± 0.83 | 0.01 |
| Tobacco intake (%) | 5 | 20.7 | 0.01 |
| Alcohol intake (%) | 37.5 | 3.7 | 3.36 × 10−8 |
Notes:
Eight NCs and 10 SZ patients lacked BMI information.
BMI, body mass index; S-HDL-C, serum high-density lipoprotein cholesterol; S-LDL-C, serum low-density lipoprotein cholesterol; S-Glu, serum glucose; TC, total cholesterol; TG, triglyceride.
Sequencing data
We obtained 7,456,515 raw sequences from all subjects (n = 162), ranging from 15,449 to 95,651. After quality filtering and removal of the chimeric sequences, we obtained 6,817,960 high quality reads for further analysis of bacterial composition, ranging from 13,581 to 90,203 and with a mean of 42,086.2 reads. After clustering all the high-quality reads, a total of 2,031 features were obtained, and the frequency per feature ranged from 2 to 533,200, with a mean of 3,356.9.
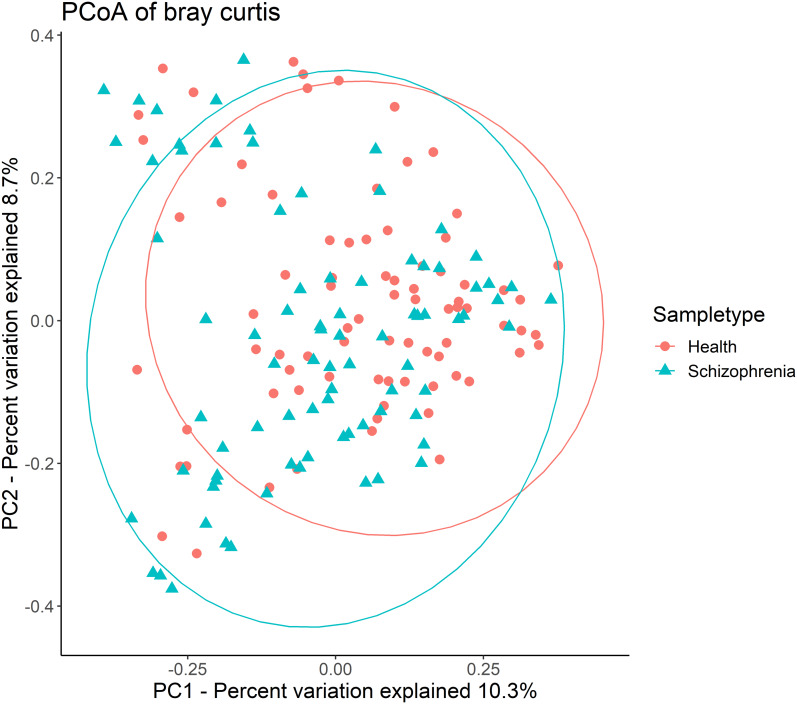
Then, alpha-diversity and beta-diversity calculations were performed. The results showed no significant difference in all alpha-diversity indices between the two groups (Table S1). Analysis of beta-diversity indices using Bray-Curtis dissimilarity revealed significant community-level separation between the SZ and NC groups (pseudo-F =3.337, p = 0.001, uncorrected). PCoA of Bray-Curtis distances showed that the SZ and NC groups formed distinct clusters (Fig. 1). Additionally, the microbiota of the NC group displayed significantly tighter clustering compared to the SZ group, with average Bray-Curtis distances of 0.79 ± 0.05 vs. 0.81 ± 0.06 (p = 0.038, uncorrected).
Figure 1. Principal coordinates analysis (PCoA) plot illustrating beta-diversity distance matrices of Bray-Curtis distance comparing sample distributions between the SZ and NC groups.
Red dots and green triangles represent NCs and SZ patients, respectively.
Bacterial taxonomic compositions and identifications of biomarkers
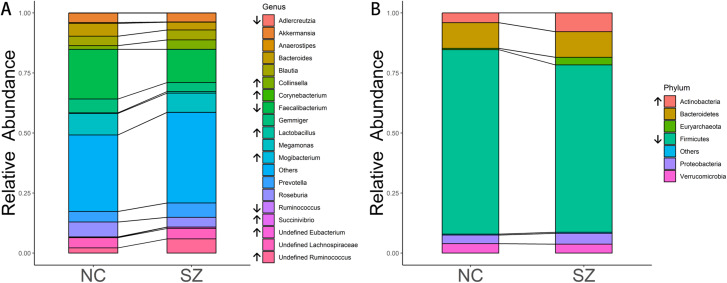
The predominant bacteria at the phylum level were the same between the SZ and NC groups (Fig. 2B), including Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria and Verrucomicrobia. When comparing the relative abundances of the phyla of the two groups, Actinobacteria was significantly higher in the SZ group than in the NC group (p = 0.0046, FDR corrected), whereas Firmicutes was lower (p = 0.026, FDR corrected) (Fig. 2B).
Figure 2. Microbial composition at phylum and genus levels.
(A and B) indicate the most abundant genera and phyla in the NC and SZ groups, respectively. Bacteria that were significantly different between the two groups are shown in (A and B) (p < 0.05, FDR correction, “↑” represent higher in SZs and “↓” represent lower, respectively).
At the genus level, the most abundant genus in the SZs was Faecalibacterium, followed by Megamonas, Prevotella, Ruminococcus and Blautia (Fig. 2A). The bacteria in the NCs were mainly assigned to Faecalibacterium, Megamonas, Gemmiger, Roseburia and Bacteroides. Genera with different relative abundances between the two groups are shown in Fig. 2A. Compared to the NC group, the relative abundance of undefined Ruminococcus (p = 0.0052, FDR corrected), Collinsella (p = 0.00094, FDR corrected), undefined Eubacterium (p = 8.05 × 10−6, FDR corrected), Lactobacillus (p = 0.0148, FDR corrected), Succinivibrio (p = 0.0148, FDR corrected), Mogibacterium (p = 0.0148, FDR corrected) and Corynebacterium (p = 0.0413, FDR corrected) were significantly higher in the SZ group. However, Adlercreutzia (p = 0.0148, FDR corrected), Anaerostipes (p = 0.0025, FDR corrected), Ruminococcus (p = 0.0083, FDR corrected) and Faecalibacterium (p = 0.0223, FDR corrected) were higher in the NC group.
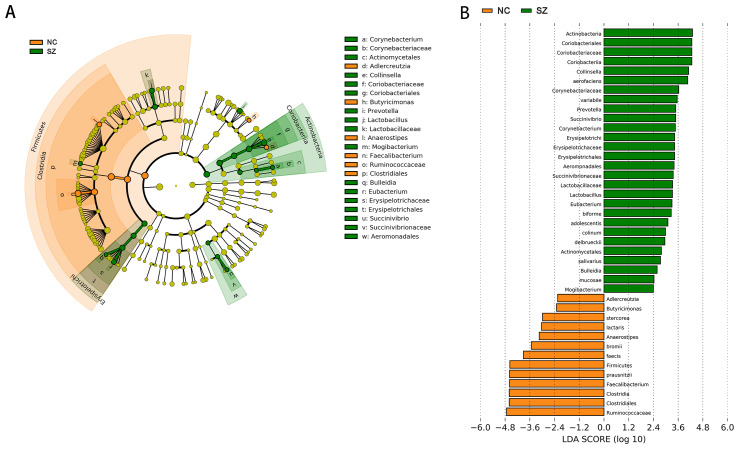
Application of the LefSe method identified a total of 41 features with significantly different abundances between the SZ and NC groups (p < 0.01, uncorrected, LDA score > 2) (Fig. 3B). At the phylum level, the NC group was enriched with Firmicutes, while Actinobacteria was enriched in the SZ group (p < 0.01, uncorrected, LDA score > 2). We also observed that the NC group was differentially enriched with the genera Anaerostipes, Faecalibacterium, Adlercreutzia, Butyricimonas (p < 0.01, uncorrected, LDA score > 2), whereas the SZ group was enriched with Lactobacillus, Mogibacterium, Bulleidia, Eubacterium, Succinivibrio, Corynebacterium, Collinsella and Prevotella (p < 0.01, uncorrected, LDA score > 2) (Fig. 3A).
Figure 3. The differently abundant taxa identified using LEfSe analysis.
(A) LEfSe cladogram showed the most differentially abundant taxa between the two groups. Taxa enriched for NC in red; SZ enriched taxa in green. The size of each dot is proportional to its effect size. (B) Visualization of only taxa meeting an LDA threshold >2. Taxa with enriched levels in SZs are shown in green, red represented taxa with enriched levels in NCs.
Functional properties predicted by PICRUSt
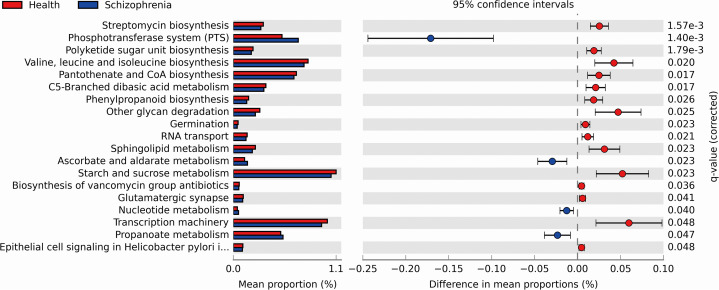
We performed PICRUSt analysis to predict the genetic potentials of the fecal microbiota metagenome based on 16S rRNA sequences. PICRUSt predicted metagenome content to Level 3 KOs and identified 328 functional pathways belonging to different Level 1 KOs, including 19 Cellular Processes, 28 Environmental Information Processing, 28 Genetic Information Processing, 40 Human Diseases, 146 Metabolism, 40 Organismal Systems and 40 Unclassified pathways (Data S1). We identified 19 significantly different functional pathways (Fig. 4, p < 0.05, FDR corrected). We found that varieties of biosynthesis and metabolism pathways were enriched in the NC group, such as Polyketide sugar unit biosynthesis, Valine, Leucine and Isoleucine biosynthesis, Pantothenate and CoA biosynthesis, C5-Branched dibasic acid metabolism and Phenylpropanoid biosynthesis. While Ascorbate and aldarate metabolism, Nucleotide metabolism and Propanoate metabolism pathways were enriched in the SZ group.
Figure 4. Functional prediction analysis of two groups using PICRUSt.
In the figure, the abundance of the biological pathways between the two groups are statistically significant (p < 0.05, FDR corrected). Red and blue represent the NC group and the SZ group, respectively.
Relationship with clinical characteristics
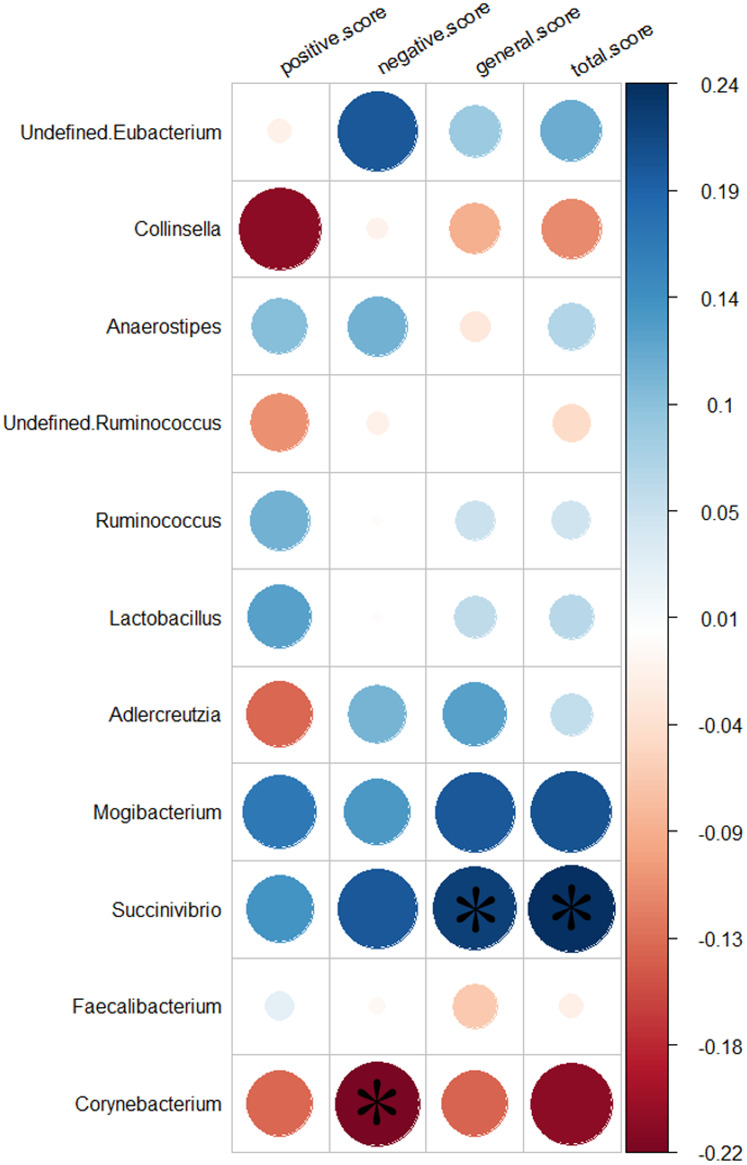
We analyzed the relationship between 11 genera altered in the SZs and the PANSS scores. Greater severity of SZ symptoms was positively correlated with the abundance of the genus Succinivibrio (total score, r = 0.24, p = 0.032, uncorrected; general score, r = 0.22, p = 0.046, uncorrected). While increased negative symptoms were negatively associated with the abundance of the genus Corynebacterium (negative score, r = −0.22, p = 0.044, uncorrected). The results of the relationship between the 11 genera and the severity of symptoms are shown in Fig. 5.
Figure 5. Correlation between the relative abundances of the alter genera and PANSS scores.
The color bar indicates the value of Pearson correlation’s coefficient. The size of circles indicates the degree of significance. “*”: p < 0.05, uncorrected.
Discussion
To the best of our knowledge, this study is the first to indicate that altered gut microbiota is significantly correlated with symptom severity in SZs from South China. Consistent with previous studies, our results demonstrate that the SZs showed altered gut microbiome composition, including two phyla and 11 genera (De Filippo et al., 2010; Huttenhower et al., 2012; Nam et al., 2011; Zhang et al., 2015). Importantly, Succinivibrio was more abundant in SZs and correlated positively with the severity of symptoms. In contrast, Corynebacterium was more highly represented in SZs and negatively associated with the severity of negative symptoms, which may suggest that a greater abundance of Corynebacterium in SZs could remit the symptoms of blunted affect, poverty of speech and loss of drive. Thus, we speculated that an altered gut microbiome profile contributes to the pathogenesis and remission of SZ. Interestingly, Succinivibrio was detected in the SZs but not in the NCs, which may further suggest that Succinivibrio plays an important role in the development of SZ. In addition, we found that Lactobacillus was significantly higher in SZs. Schwarz et al. (2018) found that the abundance of Lactobacillus was significantly increased in first episode SZ and was positively correlated with the severity of symptoms. However, the correlation between Lactobacillus and symptom severity was not significant in this study. One possible reason was the drug use of the subjects included in this study.
Second-generation antipsychotics (SGAs) have been used successfully for the treatment of SZ (Skonieczna-Żydecka et al., 2019), risperidone (RIS) and olanzapine (OLZ) are the most frequently prescribed atypical SGAs (Hálfdánarson et al., 2017). However, long-term SGA treatment can cause health consequences including significant weight gain and hypertriglyceridaemia (Chintoh et al., 2009; De Hert et al., 2011; Galling & Correll, 2015). In this study, 91% SZs was treated with antipsychotics (Data S2). Our results showed that BMI and TG of SZs were significantly higher than that Of NCs, which was consistent with previous studies. Skonieczna-Żydecka et al. (2019) concluded that metabolic disturbances during SGA treatment may be the consequence, at least in part, of gut dysbiosis. In addition, we were surprised to find that the TC in the NC group was significantly higher than that in the SZ group, which we speculated might be due to the higher alcohol intake ratio in the NC group (30 vs. 3, p = 3.36 × 10−8, uncorrected). An expanding body of evidence supports the notion that microbes can metabolise drugs and vice versa drugs can modify the gut microbiota composition. Bahr et al. (2015) identified the Bacteroidetes/Firmicutes ratio was significantly lowered in chronic and short-term RIS users. Morgan et al. (2014) revealed decreased alpha diversity, lower abundance of class Bacteroidia, and increased abundances of Erysipelotrichia, Actinobacteria and Gammaproteobacteria in female mice treated with OLZ. However, Kao et al. (2018) demonstrated no significant effects of OLZ on gut microbiota in female rats. Pełka-Wysiecka et al. (2019) further explored the gut microbiota and OLZ treatment interactions, they classified the included SZs as responders and non-responders, there were no differences in gut microbiota compositions at phyla and genus levels. Hence, the effect of antipsychotics between gut microbiota needs further study.
There are a number of bidirectional signaling pathways by which the gut microbiota, acting via the brain-gut axis, can impact the brain (Kelly et al., 2017), including amino acid metabolism (Saleem et al., 2017), immune system modulation (Erny et al., 2015), hypothalamic-pituitary-adrenal (HPA) axis (Mudd et al., 2017), vagus nerve (Bravo et al., 2011) and the production of bacterial metabolites, such as short-chain fatty acids (SCFA) (Tan et al., 2014). In this study, PICRUSt results showed multiple SCFAs and amino acid metabolic pathways that were significantly enriched between the two groups (Fig. S1). SCFAs are the main metabolites of the gut microbiota (Wong et al., 2006); SCFAs can enter the central nervous system through the blood-brain barrier (De Vadder et al., 2014), stimulating TNF in the body (Morris et al., 2017), activating microglia (Sampson et al., 2016), interfering with membrane metabolism of cells, and thus may induce SZ. He et al. (2018) reported that an increased relative abundance of Lactobacillus in SZs can stimulate TNF production. Based on this, it is speculated that the increased Lactobacillus may induce changes in inflammatory factors and induce SZ. Amino acids and derivatives participate in the biosynthesis and downstream effects of numerous neurotransmitters (Cao et al., 2018). We found that the tryptophan metabolism was significantly enriched in the fecal microbiome of SZs. Zhu et al. (2019) reported that the tryptophan level in mice transplanted with SZ fecal microbiota was significantly lower than that in NC mice, and they also found that tryptophan biosynthesis was significantly enriched in the fecal microbiome of NC mice by shot-gun metagenomic sequencing. Tryptophan is an important source of 5-hydroxytryptamine (5-HT). Tryptophan and kynurenine can cross the blood-brain barrier and have a significant effect on the metabolism of neurotransmitters (Agus, Planchais & Sokol, 2018). Above all, these investigations suggested that gut microbiota may profoundly affect the amino acid metabolism pathway and neurotransmitter levels in SZ patients.
Several methodological issues need to be addressed. First, we did not control the effect of antipsychotic therapy on the gut microbiota due to the lack of data. The form of clinical information will be modified and the data of antipsychotic will be collected. Besides, we plan to recruit patients of first-episode SZ in the future study. Second, in this preliminary study, we adopted the method of 16S rRNA gene sequencing, which has a low phylogenetic power at the species level. According to the findings of this study, we have selected the specific subjects and have applied the metagenomic analysis in the next study. Third, the BMI of the part of subjects and the diet information of all subjects were lacked in this study. Further investigations will include all these data.
Conclusions
In conclusion, our findings provide evidence of altered gut microbial composition in patients who have SZ. In addition, we found that Succinivibrio and Corynebacterium were associated with the severity of symptoms for the first time, which may provide some new biomarkers for the diagnosis of SZ.
Supplemental Information
The abundance of the biological pathways between the two groups are statistically significant (p < 0.05, uncorrected). Red and blue represent NC group and SZ group, respectively.
Acknowledgments
The authors would like to thank the editor as well as the three reviewers for their valuable suggestions and comments that helped to significantly improve the manuscript. We would also like to thank all the volunteers from the Affiliated Brain Hospital of Guangzhou Medical University and South China University of Technology. The authors thank American Journal Experts for English editing and proofreading.
Funding Statement
This work was supported by the National Natural Science Foundation of China (31771074, 81802230), the Guangdong Key Project in “Development of new tools for diagnosis and treatment of Autism” (2018B030335001), and the Science and Technology Program of Guangzhou (201704020168, 201704020113, 201807010064, 201803010100, 201903010032). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Fengchun Wu, Email: 13580380071@163.com.
Kai Wu, Email: kaiwu@scut.edu.cn.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Shijia Li conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, responsible for collecting stool samples and clinical data from healthy controls, and approved the final draft.
Min Zhuo conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Xia Huang performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, responsible for collecting stool samples and clinical data from healthy controls, and approved the final draft.
Yuanyuan Huang performed the experiments, authored or reviewed drafts of the paper, responsible for collecting patients’ stool samples and clinical data, and approved the final draft.
Jing Zhou conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Dongsheng Xiong conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Jiahui Li performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Ya Liu performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Zhilin Pan performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Hehua Li performed the experiments, authored or reviewed drafts of the paper, responsible for collecting patients’ stool samples and clinical data, and approved the final draft.
Jun Chen conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Xiaobo Li conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Zhiming Xiang conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Fengchun Wu conceived and designed the experiments, authored or reviewed drafts of the paper, responsible for collecting and managing patients for this study, and approved the final draft.
Kai Wu conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, the principal investigator of this study, and approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The Guangzhou Brain Hospital approved the sample collection and analysis protocol of this study.
Data Availability
The following information was supplied regarding data availability:
The sequences are available at NCBI GenBank: MT545156–MT547172 and in the Supplemental File.
References
- Agus, Planchais & Sokol (2018).Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host & Microbe. 2018;23(6):716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Babulas et al. (2006).Babulas V, Factor-Litvak P, Goetz R, Schaefer CA, Brown AS. Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. American Journal of Psychiatry. 2006;163(5):927–929. doi: 10.1176/ajp.2006.163.5.927. [DOI] [PubMed] [Google Scholar]
- Bahr et al. (2015).Bahr SM, Tyler BC, Wooldridge N, Butcher BD, Burns TL, Teesch LM, Oltman CL, Azcarate-Peril MA, Kirby JR, Calarge CA. Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Translational Psychiatry. 2015;5(10):e652. doi: 10.1038/tp.2015.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang et al. (2014).Bang M, Kim KR, Song YY, Baek S, Lee E, An SK. Neurocognitive impairments in individuals at ultra-high risk for psychosis: who will really convert? Australian & New Zealand Journal of Psychiatry. 2014;49(5):462–470. doi: 10.1177/0004867414561527. [DOI] [PubMed] [Google Scholar]
- Bolyen et al. (2019).Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez Aés M, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu Y-X, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS, II, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, van der Hooft JJJ, Vargas F, Vázquez-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nature Biotechnology. 2019;37(8):852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouter et al. (2017).Bouter KE, Van Raalte DH, Groen AK, Nieuwdorp M. Role of the gut microbiome in the pathogenesis of obesity and obesity-related metabolic dysfunction. Gastroenterology. 2017;152(7):1671–1678. doi: 10.1053/j.gastro.2016.12.048. [DOI] [PubMed] [Google Scholar]
- Bravo et al. (2011).Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown et al. (2002).Brown AS, Schaefer CA, Wyatt RJ, Begg MD, Goetz R, Bresnahan MA, Harkavy-Friedman J, Gorman JM, Malaspina D, Susser ES. Paternal age and risk of schizophrenia in adult offspring. American Journal of Psychiatry. 2002;159(9):1528–1533. doi: 10.1176/appi.ajp.159.9.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan et al. (2016).Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high-resolution sample inference from illumina amplicon data. Nature Methods. 2016;13(7):581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon, Jones & Murray (2002).Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. American Journal of Psychiatry. 2002;159(7):1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- Cantor-Graae & Selten (2005).Cantor-Graae E, Selten J-P. Schizophrenia and migration: a meta-analysis and review. American Journal of Psychiatry. 2005;162(1):12–24. doi: 10.1176/appi.ajp.162.1.12. [DOI] [PubMed] [Google Scholar]
- Cao et al. (2017).Cao H, Pradhan AK, Karns JS, Wolfgang DR, Hovingh E, Vinyard BT, Van Kessel JAS. 266 Prevalence and risk factors for antimicrobial resistance on U.S. dairy operations. Journal of Animal Science. 2017;95(Suppl. 4):131–132. doi: 10.2527/asasann.2017.266. [DOI] [Google Scholar]
- Cao et al. (2018).Cao B, Wang D, Brietzke E, McIntyre RS, Pan Z, Cha D, Rosenblat JD, Zuckerman H, Liu Y, Xie Q, Wang JJAA. Characterizing amino-acid biosignatures amongst individuals with schizophrenia: a case-control study. Amino Acids. 2018;50:1013–1023. doi: 10.1007/s00726-018-2579-6. [DOI] [PubMed] [Google Scholar]
- Caputi & Giron (2018).Caputi V, Giron MC. Microbiome-gut-brain axis and toll-like receptors in parkinson’s disease. International Journal of Molecular Sciences. 2018;19(6):1689. doi: 10.3390/ijms19061689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Nallar et al. (2015).Castro-Nallar E, Bendall ML, Pérez-Losada M, Sabuncyan S, Severance EG, Dickerson FB, Schroeder JR, Yolken RH, Crandall KA. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ. 2015;3(8):e1140. doi: 10.7717/peerj.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo et al. (2017).Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, Ferrari C, Guerra UP, Paghera B, Muscio C, Bianchetti A, Volta GD, Turla M, Cotelli MS, Gennuso M, Prelle A, Zanetti O, Lussignoli G, Mirabile D, Bellandi D, Gentile S, Belotti G, Villani D, Harach T, Bolmont T, Padovani A, Boccardi M, Frisoni GB. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiology of Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- Chintoh et al. (2009).Chintoh AF, Mann SW, Lam L, Giacca A, Fletcher P, Nobrega J, Remington G. Insulin resistance and secretion in vivo: effects of different antipsychotics in an animal model. Schizophrenia Research. 2009;108(1–3):127–133. doi: 10.1016/j.schres.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Cryan & Dinan (2012).Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- De Filippo et al. (2010).De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert et al. (2011).De Hert M, Vancampfort D, Correll CU, Mercken V, Peuskens J, Sweers K, Van Winkel R, Mitchell AJ. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. British Journal of Psychiatry. 2011;199(2):99–105. doi: 10.1192/bjp.bp.110.084665. [DOI] [PubMed] [Google Scholar]
- De Vadder et al. (2014).De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1–2):84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- DeSantis et al. (2006).DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a cimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied and Environmental Microbiology. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet et al. (2014).Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Molecular Psychiatry. 2014;19(2):146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd et al. (2017).Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, Sonnenburg JL. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551(7682):648–652. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny et al. (2015).Erny D, De Angelis ALH, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermöhlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neuroscience. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galling & Correll (2015).Galling B, Correll CU. Do antipsychotics increase diabetes risk in children and adolescents? Expert Opinion on Drug Safety. 2015;14(2):219–241. doi: 10.1517/14740338.2015.979150. [DOI] [PubMed] [Google Scholar]
- Hartstra et al. (2015).Hartstra AV, Bouter KEC, Bäckhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38(1):159. doi: 10.2337/dc14-0769. [DOI] [PubMed] [Google Scholar]
- He et al. (2018).He Y, Kosciolek T, Tang J, Zhou Y, Li Z, Ma X, Zhu Q, Yuan N, Yuan L, Li CJEP. Gut microbiome and magnetic resonance spectroscopy study of subjects at ultra-high risk for psychosis may support the membrane hypothesis. European Psychiatry. 2018;53:37–45. doi: 10.1016/j.eurpsy.2018.05.011. [DOI] [PubMed] [Google Scholar]
- Higuchi et al. (2019).Higuchi T, Iyo M, Kwon JS, Chou Y-H, Chen H-K, Chen J-Y, Chen T-T, Huang S-Y, Lee J-S, Saeki Y, Tanaka H, Wang T-S, Wu B-J, Katoh T, Ishigouoka J. Randomized, double-blind, placebo, and risperidone-controlled study of lurasidone in the treatment of schizophrenia: Results of an inconclusive 6-week trial. Asia–Pacific Psychiatry. 2019;11(3):e12354. doi: 10.1111/appy.12354. [DOI] [PubMed] [Google Scholar]
- Hoekert et al. (2007).Hoekert M, Kahn RS, Pijnenborg M, Aleman A. Impaired recognition and expression of emotional prosody in schizophrenia: review and meta-analysis. Schizophrenia Research. 2007;96(1–3):135–145. doi: 10.1016/j.schres.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Hsiao et al. (2013).Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al. (2019).Hu S, Li A, Huang T, Lai J, Li J, Sublette ME, Lu H, Lu Q, Du Y, Hu Z, Ng CH, Zhang H, Lu J, Mou T, Lu S, Wang D, Duan J, Hu J, Huang M, Wei N, Zhou W, Ruan L, Li MD, Xu Y. Gut microbiota changes in patients with bipolar depression. Advanced Science. 2019;6:1900752. doi: 10.1002/advs.201900752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenhower et al. (2012).Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT, Creasy HH, Earl AM, FitzGerald MG, Fulton RS, Giglio MG, Hallsworth-Pepin K, Lobos EA, Madupu R, Magrini V, Martin JC, Mitreva M, Muzny DM, Sodergren EJ, Versalovic J, Wollam AM, Worley KC, Wortman JR, Young SK, Zeng Q, Aagaard KM, Abolude OO, Allen-Vercoe E, Alm EJ, Alvarado L, Andersen GL, Anderson S, Appelbaum E, Arachchi HM, Armitage G, Arze CA, Ayvaz T, Baker CC, Begg L, Belachew T, Bhonagiri V, Bihan M, Blaser MJ, Bloom T, Bonazzi V, Paul Brooks J, Buck GA, Buhay CJ, Busam DA, Campbell JL, Canon SR, Cantarel BL, Chain PSG, Chen IMA, Chen L, Chhibba S, Chu K, Ciulla DM, Clemente JC, Clifton SW, Conlan S, Crabtree J, Cutting MA, Davidovics NJ, Davis CC, DeSantis TZ, Deal C, Delehaunty KD, Dewhirst FE, Deych E, Ding Y, Dooling DJ, Dugan SP, Michael Dunne W, Scott Durkin A, Edgar RC, Erlich RL, Farmer CN, Farrell RM, Faust K, Feldgarden M, Felix VM, Fisher S, Fodor AA, Forney LJ, Foster L, Di Francesco V, Friedman J, Friedrich DC, Fronick CC, Fulton LL, Gao H, Garcia N, Giannoukos G, Giblin C, Giovanni MY, Goldberg JM, Goll J, Gonzalez A, Griggs A, Gujja S, Kinder Haake S, Haas BJ, Hamilton HA, Harris EL, Hepburn TA, Herter B, Hoffmann DE, Holder ME, Howarth C, Huang KH, Huse SM, Izard J, Jansson JK, Jiang H, Jordan C, Joshi V, Katancik JA, Keitel WA, Kelley ST, Kells C, King NB, Knights D, Kong HH, Koren O, Koren S, Kota KC, Kovar CL, Kyrpides NC, La Rosa PS, Lee SL, Lemon KP, Lennon N, Lewis CM, Lewis L, Ley RE, Li K, Liolios K, Liu B, Liu Y, Lo C-C, Lozupone CA, Dwayne Lunsford R, Madden T, Mahurkar AA, Mannon PJ, Mardis ER, Markowitz VM, Mavromatis K, McCorrison JM, McDonald D, McEwen J, McGuire AL, McInnes P, Mehta T, Mihindukulasuriya KA, Miller JR, Minx PJ, Newsham I, Nusbaum C, O’Laughlin M, Orvis J, Pagani I, Palaniappan K, Patel SM, Pearson M, Peterson J, Podar M, Pohl C, Pollard KS, Pop M, Priest ME, Proctor LM, Qin X, Raes J, Ravel J, Reid JG, Rho M, Rhodes R, Riehle KP, Rivera MC, Rodriguez-Mueller B, Rogers Y-H, Ross MC, Russ C, Sanka RK, Sankar P, Fah Sathirapongsasuti J, Schloss JA, Schloss PD, Schmidt TM, Scholz M, Schriml L, Schubert AM, Segata N, Segre JA, Shannon WD, Sharp RR, Sharpton TJ, Shenoy N, et al. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenhower, Kostic & Xavier (2014).Huttenhower C, Kostic AD, Xavier RJ. Inflammatory bowel disease as a model for translating the microbiome. Immunity. 2014;40(6):843–854. doi: 10.1016/j.immuni.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hálfdánarson et al. (2017).Hálfdánarson Ó, Zoëga H, Aagaard L, Bernardo M, Brandt L, Fusté AC, Furu K, Garuoliené K, Hoffmann F, Huybrechts KF, Kalverdijk LJ, Kawakami K, Kieler H, Kinoshita T, Litchfield M, López SC, Machado-Alba JE, Machado-Duque ME, Mahesri M, Nishtala PS, Pearson SA, Reutfors J, Saastamoinen LK, Sato I, Schuiling-Veninga CCM, Shyu YC, Skurtveit S, Verdoux H, Wang LJ, Yahni CZ, Bachmann CJ. International trends in antipsychotic use: a study in 16 countries, 2005–2014. European Neuropsychopharmacology. 2017;27(10):1064–1076. doi: 10.1016/j.euroneuro.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Kao et al. (2018).Kao AC, Spitzer S, Anthony DC, Lennox B, Burnet PWJ. Prebiotic attenuation of olanzapine-induced weight gain in rats: analysis of central and peripheral biomarkers and gut microbiota. Translational Psychiatry. 2018;8(1):66. doi: 10.1038/s41398-018-0116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly et al. (2017).Kelly JR, Minuto C, Cryan JF, Clarke G, Dinan TG. Cross talk: the microbiota and neurodevelopmental disorders. Frontiers in Neuroscience. 2017;11:490. doi: 10.3389/fnins.2017.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus et al. (2006).Kraus JE, Sheitman BB, Cook A, Reviere R, Lieberman JA. Olanzapine versus risperidone in newly admitted acutely Ill psychotic patients. Journal of Clinical Psychiatry. 2006;66(12):1564–1568. doi: 10.4088/JCP.v66n1211. [DOI] [PubMed] [Google Scholar]
- Langille et al. (2013).Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature Biotechnology. 2013;31(9):814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau et al. (2018).Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, Da Mesquita S, Frost EL, Gaultier A, Harris TH, Cao R, Hu S, Lukens JR, Smirnov I, Overall CC, Oliver G, Kipnis J. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nature Neuroscience. 2018;21(10):1380–1391. doi: 10.1038/s41593-018-0227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv et al. (2017).Lv F, Chen S, Wang L, Jiang R, Tian H, Li J, Yao Y, Zhuo C. The role of microbiota in the pathogenesis of schizophrenia and major depressive disorder and the possibility of targeting microbiota as a treatment option. Oncotarget. 2017;8(59):100899–100907. doi: 10.18632/oncotarget.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan & Fenton (1992).McGlashan TH, Fenton WS. The positive-negative distinction in schizophrenia—review of natural-history validators. Archives of General Psychiatry. 1992;49(1):63–72. doi: 10.1001/archpsyc.1992.01820010063008. [DOI] [PubMed] [Google Scholar]
- Morgan et al. (2014).Morgan AP, Crowley JJ, Nonneman RJ, Quackenbush CR, Miller CN, Ryan AK, Bogue MA, Paredes SH, Yourstone S, Carroll IM, Kawula TH, Bower MA, Sartor RB, Sullivan PF. The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. PLOS ONE. 2014;9(12):e115225. doi: 10.1371/journal.pone.0115225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris et al. (2017).Morris G, Berk M, Carvalho A, Caso JR, Sanz Y, Walder K, Maes M. The role of the microbial metabolites including tryptophan catabolites and short chain fatty acids in the pathophysiology of immune-inflammatory and neuroimmune disease. Molecular Neurobiology. 2017;54(6):4432–4451. doi: 10.1007/s12035-016-0004-2. [DOI] [PubMed] [Google Scholar]
- Mudd et al. (2017).Mudd AT, Berding K, Wang M, Donovan SM, Dilger RN. Serum cortisol mediates the relationship between fecal Ruminococcus and brain N-acetylaspartate in the young pig. Gut Microbes. 2017;8(6):589–600. doi: 10.1080/19490976.2017.1353849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany et al. (2001).Mulvany F, O’Callaghan E, Takei N, Byrne M, Fearon P, Larkin C. Effect of social class at birth on risk and presentation of schizophrenia: case-control study. BMJ. 2001;323(7326):1398–1401. doi: 10.1136/bmj.323.7326.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam et al. (2011).Nam Y-D, Jung M-J, Roh SW, Kim M-S, Bae J-W. Comparative analysis of Korean human gut microbiota by barcoded pyrosequencing. PLOS ONE. 2011;6(7):e22109. doi: 10.1371/journal.pone.0022109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen et al. (2018).Nguyen TT, Kosciolek T, Eyler LT, Knight R, Jeste DV. Overview and systematic review of studies of microbiome in schizophrenia and bipolar disorder. Journal of Psychiatric Research. 2018;99:50–61. doi: 10.1016/j.jpsychires.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata et al. (2000).Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Research. 2000;27(1):29–34. doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen et al. (2019).Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara R, Simpson GL, Solymos P. Package ‘vegan’. 2019. https://CRAN.R-project.org/package=vegan https://CRAN.R-project.org/package=vegan
- Pełka-Wysiecka et al. (2019).Pełka-Wysiecka J, Kaczmarczyk M, Bąba-Kubiś A, Liśkiewicz P, Wroński M, Skonieczna-Żydecka K, Marlicz W, Misiak B, Starzyńska T, Kucharska-Mazur J, Łoniewski I, Samochowiec J. Analysis of gut microbiota and their metabolic potential in patients with schizophrenia treated with olanzapine: results from a six-week observational prospective cohort study. Journal of Clinical Medicine. 2019;8(10):1605. doi: 10.3390/jcm8101605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke et al. (2011).Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA, Lin D-Y, Duan J, Ophoff RA, Andreassen OA, Scolnick E, Cichon S, St. Clair D, Corvin A, Gurling H, Werge T, Rujescu D, Blackwood DHR, Pato CN, Malhotra AK, Purcell S, Dudbridge F, Neale BM, Rossin L, Visscher PM, Posthuma D, Ruderfer DM, Fanous A, Stefansson H, Steinberg S, Mowry BJ, Golimbet V, De Hert M, Jönsson EG, Bitter I, Pietiläinen OPH, Collier DA, Tosato S, Agartz I, Albus M, Alexander M, Amdur RL, Amin F, Bass N, Bergen SE, Black DW, Børglum AD, Brown MA, Bruggeman R, Buccola NG, Byerley WF, Cahn W, Cantor RM, Carr VJ, Catts SV, Choudhury K, Cloninger CR, Cormican P, Craddock N, Danoy PA, Datta S, de Haan L, Demontis D, Dikeos D, Djurovic S, Donnelly P, Donohoe G, Duong L, Dwyer S, Fink-Jensen A, Freedman R, Freimer NB, Friedl M, Georgieva L, Giegling I, Gill M, Glenthøj B, Godard S, Hamshere M, Hansen M, Hansen T, Hartmann AM, Henskens FA, Hougaard DM, Hultman CM, Ingason A, Jablensky AV, Jakobsen KD, Jay M, Jürgens G, Kahn RS, Keller MC, Kenis G, Kenny E, Kim Y, Kirov GK, Konnerth H, Konte B, Krabbendam L, Krasucki R, Lasseter VK, Laurent C, Lawrence J, Lencz T, Lerer FB, Liang K-Y, Lichtenstein P, Lieberman JA, Linszen DH, Lönnqvist J, Loughland CM, Maclean AW, Maher BS, Maier W, Mallet J, Malloy P, Mattheisen M, Mattingsdal M, McGhee KA, McGrath JJ, McIntosh A, McLean DE, McQuillin A, Melle I, Michie PT, Milanova V, Morris DW, Mors O, Mortensen PB, Moskvina V, Muglia P, Myin-Germeys I, Nertney DA, Nestadt G, Nielsen J, Nikolov I, Nordentoft M, Norton N, Nöthen MM, O’Dushlaine CT, Olincy A, Olsen L, O’Neill FA, Ørntoft TF, Owen MJ, Pantelis C, Papadimitriou G, Pato MT, Peltonen L, Petursson H, Pickard B, Pimm J, Pulver AE, Puri V, Quested D, Quinn EM, Rasmussen HB, Réthelyi JM, Ribble R, Rietschel M, Riley BP, Ruggeri M, Schall U, Schulze TG, Schwab SG, Scott RJ, Shi J, Sigurdsson E, Silverman JM, Spencer CCA, Stefansson K, Strange A, Strengman E, Stroup TS, Suvisaari J, Terenius L, Thirumalai S, Thygesen JH, Timm S, Toncheva D, Van den Oord E, Van Os J, Van Winkel R, Veldink J, Walsh D, Wang AG, Wiersma D, Wildenauer DB, Williams HJ, Williams NM, Wormley B, Zammit S, Sullivan PF, O’Donovan MC, Daly MJ, Gejman PV, The Schizophrenia Psychiatric Genome-Wide Association Study Genome-wide association study identifies five new schizophrenia loci. Nature Genetics. 2011;43(10):969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem et al. (2017).Saleem S, Shaukat F, Gul A, Arooj M, Malik A. Potential role of amino acids in pathogenesis of schizophrenia. International Journal of Health Sciences. 2017;11:63–68. [PMC free article] [PubMed] [Google Scholar]
- Sampson et al. (2016).Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet M-F, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson’s disease. Cell. 2016;167(6):1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara et al. (2014).Sara GE, Large MM, Matheson SL, Burgess PM, Malhi GS, Whiteford HA, Hall WD. Stimulant use disorders in people with psychosis: a meta-analysis of rate and factors affecting variation. Australian & New Zealand Journal of Psychiatry. 2014;49(2):106–117. doi: 10.1177/0004867414561526. [DOI] [PubMed] [Google Scholar]
- Schwabe & Jobin (2013).Schwabe RF, Jobin C. The microbiome and cancer. Nature Reviews Cancer. 2013;13(11):800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz et al. (2018).Schwarz E, Maukonen J, Hyytiäinen T, Kieseppä T, Orešič M, Sabunciyan S, Mantere O, Saarela M, Yolken R, Suvisaari J. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophrenia Research. 2018;192:398–403. doi: 10.1016/j.schres.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Severance et al. (2012).Severance EG, Alaedini A, Yang S, Halling M, Gressitt KL, Stallings CR, Origoni AE, Vaughan C, Khushalani S, Leweke FM, Dickerson FB, Yolken RH. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophrenia Research. 2012;138(1):48–53. doi: 10.1016/j.schres.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance et al. (2015).Severance EG, Prandovszky E, Castiglione J, Yolken RH. Gastroenterology issues in schizophrenia: why the gut matters. Current Psychiatry Reports. 2015;17(5):27. doi: 10.1007/s11920-015-0574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen et al. (2018).Shen Y, Xu J, Li Z, Huang Y, Yuan Y, Wang J, Zhang M, Hu S, Liang Y. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: a cross-sectional study. Schizophrenia Research. 2018;197:470–477. doi: 10.1016/j.schres.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Sherwin et al. (2016).Sherwin E, Sandhu KV, Dinan TG, Cryan JF. May the force be with you: the light and dark sides of the microbiota–gut–brain axis in neuropsychiatry. CNS Drugs. 2016;30(11):1019–1041. doi: 10.1007/s40263-016-0370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla et al. (2017).Shukla SD, Budden KF, Neal R, Hansbro PM. Microbiome effects on immunity, health and disease in the lung. Clinical & Translational Immunology. 2017;6(3):e133. doi: 10.1038/cti.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skonieczna-Żydecka et al. (2019).Skonieczna-Żydecka K, Łoniewski I, Misera A, Stachowska E, Maciejewska D, Marlicz W, Galling B. Second-generation antipsychotics and metabolism alterations: a systematic review of the role of the gut microbiome. Psychopharmacology. 2019;236(5):1491–1512. doi: 10.1007/s00213-018-5102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilo et al. (2017).Stilo SA, Gayer-Anderson C, Beards S, Hubbard K, Onyejiaka A, Keraite A, Borges S, Mondelli V, Dazzan P, Pariante C, Di Forti M, Murray RM, Morgan C. Further evidence of a cumulative effect of social disadvantage on risk of psychosis. Psychological Medicine. 2017;47(5):913–924. doi: 10.1017/S0033291716002993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan et al. (2014).Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. In: Alt FW, editor. Advances in Immunology. Waltham: Academic Press; 2014. pp. 91–119. [DOI] [PubMed] [Google Scholar]
- Tomova et al. (2015).Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, Ostatnikova D. Gastrointestinal microbiota in children with autism in Slovakia. Physiology & Behavior. 2015;138:179–187. doi: 10.1016/j.physbeh.2014.10.033. [DOI] [PubMed] [Google Scholar]
- Van Os, Pedersen & Mortensen (2004).Van Os J, Pedersen CB, Mortensen PB. Confirmation of synergy between urbanicity and familial liability in the causation of psychosis. American Journal of Psychiatry. 2004;161(12):2312–2314. doi: 10.1176/appi.ajp.161.12.2312. [DOI] [PubMed] [Google Scholar]
- Varese et al. (2012).Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, Read J, Van Os J, Bentall RP. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophrenia Bulletin. 2012;38(4):661–671. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Geier & Howarth (2016).Wang H, Geier MS, Howarth GS. Prebiotics: a potential treatment strategy for the chemotherapy-damaged gut? Critical Reviews in Food Science and Nutrition. 2016;56(6):946–956. doi: 10.1080/10408398.2012.741082. [DOI] [PubMed] [Google Scholar]
- Wong et al. (2006).Wong JMW, De Souza R, Kendall CWC, Emam A, Jenkins DJA. Colonic health. Fermentation and Short Chain Fatty Acids. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2019).Wu Y, Bi R, Zeng C, Ma C, Sun C, Li J, Xiao X, Li M, Zhang D-F, Zheng P, Sheng N, Luo X-J, Yao Y-G. Identification of the primate-specific gene BTN3A2 as an additional schizophrenia risk gene in the MHC loci. EBioMedicine. 2019;44:530–541. doi: 10.1016/j.ebiom.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2018).Wu F, Zhang Y, Yang Y, Lu X, Fang Z, Huang J, Kong L, Chen J, Ning Y, Li X, Wu K. Structural and functional brain abnormalities in drug-naive, first-episode, and chronic patients with schizophrenia: a multimodal MRI study. Neuropsychiatric Disease and Treatment. 2018;14:2889–2904. doi: 10.2147/NDT.S174356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee (2007).Yee TW. VGAM: vector generalized linear and additive models. New York: Springer; 2007. [Google Scholar]
- Yolken et al. (2015).Yolken RH, Severance EG, Sabunciyan S, Gressitt KL, Chen O, Stallings C, Origoni A, Katsafanas E, Schweinfurth LAB, Savage CLG, Banis M, Khushalani S, Dickerson FB. Metagenomic sequencing indicates that the oropharyngeal phageome of individuals with schizophrenia differs from that of controls. Schizophrenia Bulletin. 2015;41(5):1153–1161. doi: 10.1093/schbul/sbu197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan et al. (2019).Yuan X, Kang Y, Zhuo C, Huang X-F, Song X. The gut microbiota promotes the pathogenesis of schizophrenia via multiple pathways. Biochemical and Biophysical Research Communications. 2019;512(2):373–380. doi: 10.1016/j.bbrc.2019.02.152. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2015).Zhang J, Guo Z, Xue Z, Sun Z, Zhang M, Wang L, Wang G, Wang F, Xu J, Cao H, Xu H, Lv Q, Zhong Z, Chen Y, Qimuge S, Menghe B, Zheng Y, Zhao L, Chen W, Zhang H. A phylo-functional core of gut microbiota in healthy young Chinese cohorts across lifestyles, geography and ethnicities. ISME Journal. 2015;9(9):1979–1990. doi: 10.1038/ismej.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng et al. (2019).Zheng P, Zeng B, Liu M, Chen J, Pan J, Han Y, Liu Y, Cheng K, Zhou C, Wang H, Zhou X, Gui S, Perry SW, Wong M-L, Licinio J, Wei H, Xie P. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Science Advances. 2019;5(2):eaau8317. doi: 10.1126/sciadv.aau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng et al. (2016).Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Molecular Psychiatry. 2016;21(6):786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- Zhu et al. (2019).Zhu F, Guo R, Wang W, Ju Y, Wang Q, Ma Q, Sun Q, Fan Y, Xie Y, Yang Z, Jie Z, Zhao B, Xiao L, Yang L, Zhang T, Liu B, Guo L, He X, Chen Y, Chen C, Gao C, Xu X, Yang H, Wang J, Dang Y, Madsen L, Brix S, Kristiansen K, Jia H, Ma X. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Molecular Psychiatry. 2019 doi: 10.1038/s41380-019-0475-4. Epub ahead of print 7 August 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The abundance of the biological pathways between the two groups are statistically significant (p < 0.05, uncorrected). Red and blue represent NC group and SZ group, respectively.
Data Availability Statement
The following information was supplied regarding data availability:
The sequences are available at NCBI GenBank: MT545156–MT547172 and in the Supplemental File.