Abstract
Background
Anxiety and depression symptoms in pregnancy typically affect between 10 and 25% of pregnant individuals. Elevated symptoms of depression and anxiety are associated with increased risk of preterm birth, postpartum depression, and behavioural difficulties in children. The current COVID-19 pandemic is a unique stressor with potentially wide-ranging consequences for pregnancy and beyond.
Methods
We assessed symptoms of anxiety and depression among pregnant individuals during the current COVID-19 pandemic and determined factors that were associated with psychological distress. 1987 pregnant participants in Canada were surveyed in April 2020. The assessment included questions about COVID-19-related stress and standardized measures of depression, anxiety, pregnancy-related anxiety, and social support.
Results
We found substantially elevated anxiety and depression symptoms compared to similar pre-pandemic pregnancy cohorts, with 37% reporting clinically relevant symptoms of depression and 57% reporting clinically relevant symptoms of anxiety. Higher symptoms of depression and anxiety were associated with more concern about threats of COVID-19 to the life of the mother and baby, as well as concerns about not getting the necessary prenatal care, relationship strain, and social isolation due to the COVID-19 pandemic. Higher levels of perceived social support and support effectiveness, as well as more physical activity, were associated with lower psychological symptoms.
Conclusion
This study shows concerningly elevated symptoms of anxiety and depression among pregnant individuals during the COVID-19 pandemic, that may have long-term impacts on their children. Potential protective factors include increased social support and exercise, as these were associated with lower symptoms and thus may help mitigate long-term negative outcomes.
Keywords: Anxiety, COVID-19, Depression, Pregnancy, Stress, Physical Activity
1. Introduction
Since it was first recognized in December 2019, the 2019 novel coronavirus (COVID-19) has spread rapidly throughout the world. The health consequences of this virus are distressing: death, strained health care systems, and economic uncertainty. The psychological and social consequences may be equally devastating. People have been physically isolated from family, friends, and community, and schools and daycares around the world have been closed. There is a growing urgency to understand the impact of the COVID-19 pandemic on mental health to best prevent the emergence of serious mental illness as a secondary consequence (Cullen et al., 2020; Geraldo da Silva et al., 2020).
Although limited, previous work shows that infectious disease outbreaks increase symptoms of depression and anxiety. A study of 129 individuals quarantined during the 2003 severe acute respiratory (SARS) outbreak in Toronto, Canada found that 29% of individuals had symptoms of post-traumatic stress disorder and 31% had symptoms of depression approximately one month following their quarantine; longer periods of quarantine were associated with more severe symptoms (Hawryluck et al., 2004). In the early phase of the COVID-19 outbreak, 53.8% of respondents in China's Wuhan region reported moderate or severe psychological impact, with 17% and 29% reporting moderate to severe depression and anxiety symptoms, respectively (Wang et al., 2020). A survey by the Kaiser Family Foundation in late March 2020 found that 53% of women and 37% of men said that stress related to coronavirus had a negative impact on their mental health (Hamel and Salganicoff, 2020).
Pregnancy is a particularly vulnerable time when psychological distress can have negative consequences for both mother and baby. Since women tend to report higher symptoms of anxiety and depression during disease outbreaks than men (Al-Rabiaah et al., 2020; Hamel and Salganicoff, 2020; Wang et al., 2020), women who are pregnant during the COVID-19 pandemic may be especially affected. Sustained, elevated prenatal anxiety and depression symptoms increase the risk of postpartum depression, as well as prenatal infection and illness rates (Bayrampour et al., 2016; Coussons-Read, 2013). Prenatal anxiety and depression symptoms may also cause changes in physical activity, nutrition, and sleep, which in turn affect maternal mood and fetal development (Coussons-Read, 2013). Prenatal anxiety and depression also increases the risk of miscarriage, preterm birth, lower birthweight, and lower Apgar scores at birth (Accortt et al., 2015; Grigoriadis et al., 2018; Qu et al., 2017; Rondo et al., 2003; Stein et al., 2014). Children of mothers who experienced high stress during pregnancy are more likely to have cognitive and behavioural problems, and are at higher risk for later mental health problems themselves (Glover, 2014; MacKinnon et al., 2018; Stein et al., 2014; Van den Bergh, Dahnke, and Mennes, 2018; Van den Bergh et al., 2017). Prenatal anxiety and depression are also associated with changes to brain structure and function in infants and children (Adamson et al., 2018; Lebel et al., 2016; Qiu et al., 2013; Sandman et al., 2015). These long-lasting psychological and neurological effects highlight the importance of mitigating prenatal distress now, to support both pregnant individuals and their babies.
It is also important to look for potential resilience factors that may help protect against high prenatal stress. Social support can buffer the effects of prenatal stress, and has been shown to mitigate the impacts of prenatal anxiety and depression symptoms on maternal and infant stress response systems (Thomas, et al., 2018). Physical activity is also associated with reduced depressive and anxiety symptoms in pregnant individuals (Demissie et al., 2011), and thus may provide another resilience factor.
Given the potential negative psychological sequelae of psychological, health and financial uncertainty coupled with social isolation, there is an urgent need to determine the prevalence of psychological distress among pregnant individuals during this pandemic and identify protective factors so that targeted interventions can be quickly implemented. The aims of the current study were to determine the prevalence of anxiety and depression symptoms in pregnant people during the COVID-19 pandemic and identify potential resilience factors associated with lower symptoms. The social distancing universally recommended by governments around the world may be especially problematic during pregnancy because social support has a well-recognized role in buffering the negative effects of stress (Reid and Taylor, 2015).
2. Methods
2.1. Participants
The current study reports data collected from an ongoing study: Pregnancy during the COVID-19 Pandemic. This study recruited pregnant individuals across Canada via social media to complete an online survey. Study advertisements and the study website were shared via Twitter, Facebook, and Instagram. Ads were distributed to groups for expecting mothers, young parents, and midwifery and obstetric groups, and participants were encouraged to share the study with their friends and family. Inclusion criteria were: living in Canada, able to read and write English, and having a confirmed pregnancy <35 weeks gestation. The data reported here were collected between April 5–20, 2020. This study was approved by the Conjoint Health Research Ethics Board (CHREB) at the University of Calgary, REB20–0500.
2.2. Demographics
Participants provided comprehensive demographic information including their birth month and year, postal code, education level, household income range, their baby's due date, and number of other children.
2.3. COVID-19
Participants completed a questionnaire about COVID-19 infections and isolations, as well as COVID-19-related life changes such job loss. This questionnaire was developed specifically for this study, based on previous work assessing stress during natural disasters (King and Laplante, 2015). Participants were asked specifically about concerns due to COVID-19 with the following statements/questions: “During the COVID-19 pandemic, I have felt more alone than usual”, “How much do you think your life is in danger during the COVID-19 pandemic?”, “How much are you worried that exposure to the COVID-19 virus will harm your unborn baby?”, and “Are you concerned that you or your baby are not receiving the care that you need?”. Participants answered on a scale of 0 (not at all) to 100 (very much so). Participants were also asked “How has the COVID-19 pandemic affected your relationship with your partner?” on a scale of 0–100, with 0 (it has strained our relationship), 50 (not much has changed) and 100 (it has brought us closer together).
2.4. Anxiety and depression symptoms
Maternal depressive symptoms were assessed using the Edinburgh Depression Scale (EPDS) (Cox et al., 1987; Kozinszky and Dudas, 2015), a self-report questionnaire with possible scores ranging from 0 to 30. Scores ≥13 are used to identify women with clinically concerning depression symptoms and have been shown to have maximal consistency with a diagnosis of major depressive disorder (Cox et al., 1987). For a cut-off of 13 on the EPDS, sensitivity ranges from 38 to 43% (depending on trimester) and specificity is 98–99% (Bergink et al., 2011). The PROMIS Anxiety Adult 7-item short form was used to assess general anxiety symptoms; T-scores 60–69.9 are considered moderately elevated anxiety symptoms and scores at or above 70 are considered severely elevated (Cella et al., 2010); possible scores range from 36.3–82.7. Pregnancy-related anxiety symptoms were assessed with a 10-item questionnaire about feelings surrounding the health of the baby and circumstances of the birth (Rini et al., 1999); possible scores on this questionnaire range from 10 to 40 . On all measures, higher scores indicate worse symptoms. There is no cut score for the pregnancy-related anxiety scale, but previous treatment studies used a median split to define groups with higher versus lower pregnancy anxiety symptoms (Urizar et al., 2019). In our sample the median was 19, which we used to divide the sample into groups with higher and lower pregnancy-related anxiety symptoms.
2.5. Social support
Participants completed the social support effectiveness questionnaire (SSEQ) (Rini et al., 2006), which evaluates the type and self-perceived effectiveness of the support they receive from their partner or another support person, and the interpersonal support evaluation list (ISEL) (Cohen and Hoberman, 1983), which measures broader perceived social support from friends, family, and others.
2.6. Physical activity
We asked questions about physical activity from the Godin-Shephard Leisure-Time Exercise Questionnaire (Godin, 2011), which is a validated self-report measure of exercise frequency in which participants report the number of times per week they engaged in mild, moderate, and strenuous exercise of more than 15 min. A total score was calculated, per standard procedure, by multiplying episodes of mild exercise by 3, moderate by 5, and strenuous exercise by 9. Individuals with scores below 14 are considered sedentary, 14–23 are moderately active, and 24 or more are considered active.
2.7. Data analysis
Survey data were manually checked for accuracy and consistency before analysis. From an original 2225 respondents, we identified and removed 238 invalid records because either participants had not provided consent or they provided invalid due dates (i.e., their gestation fell outside the range of 1–35 weeks).
All analyses were conducted using SPSS 26.0. Descriptive statistics were computed for demographics and main study variables. An analysis of covariance (ANCOVA) was used to compare nulliparous to primiparous and multiparous pregnant individuals on measures of mental health symptoms (EPDS, PROMIS anxiety, pregnancy-related anxiety). Mental health symptoms were included as continuous variables. Age and gestation were included as covariates. The significance was set at p<0.017 using Bonferroni correction for 3 multiple comparisons.
Bivariate correlations were used to determine relationships between mental health symptoms measures and social support measures. Multivariate binomial logistic regression was used to identify how COVID-19 related stressors (loss of employment, social isolation, relationship strain) and worries (concern about threat to own life, harm to baby, and not receiving the care needed) were associated with clinically elevated mental health symptoms (EPDS, PROMIS anxiety, pregnancy-related anxiety). Clinically elevated mental health symptoms were defined using cutoffs from previous literature: ≥13 on the EPDS (Cox et al., 1987), and T-scores ≥ 60 for the PROMIS anxiety scale (Cella et al., 2010). The loss of employment variable was binomial (yes/no); all other variables were measured from 0 to 100. The significance threshold was set at p<0.0028 using Bonferroni correction for 18 multiple comparisons. The multivariate model was used to determine unique associations between COVID-19 factors and anxiety and depression symptoms. Parity was included as a covariate in the pregnancy-related anxiety model because of its significant association with pregnancy-related anxiety symptoms. No covariates were included in the other models. Univariate models were conducted as supplementary analysis, also with Bonferroni correction at p<0.00028.
A logistic regression was used to identify resilience factors (physical activity, perceived partner support, perceived general social support) that were associated with lower odds of clinically elevated symptoms of anxiety and depression. Partner support was operationalized as the Total Support score from the SSEQ and general social support was operationalized as the Total Support from ISEL; both were continuous variables. The total score from the Godin was our measure of physical activity. The significance was set at p<0.0056 using Bonferroni correction for 9 multiple comparisons.
3. Results
3.1. Participants
A total of 1987 individuals provided data for at least one measure on the survey between April 5–20, 2020 and were included in the current analysis; specific numbers of individuals providing data for each measure are listed in Table 1 . Not all participants provided data for each question, so numbers included in each analysis vary between 1581 and 1987. Missing data were handled with listwise deletion for each separate analysis; n is provided in the tables for each analysis.
Table 1.
Sample characteristics. Mean, standard deviation, and range are provided for key demographic characteristics and depression and anxiety symptoms in the sample. The number of datapoints available for each comparison is also given. .
| Measure | n | Mean | Standard deviation | Range | Cronbach's Alpha |
|---|---|---|---|---|---|
| Gestation (weeks) | 1987 | 22.5 | 8.4 | 4–35.9 | – |
| Age (years) | 1900 | 32.4 | 4.2 | 18.6–47.6 | – |
| Anxiety and Depression Symptoms | |||||
| Pregnancy-related anxiety questionnaire | 1757 | 19.1 | 5.1 | 8–38 | 0.82 |
| Edinburgh postnatal depression scale (EPDS) | 1764 | 10.7 | 5.3 | 0–30 | 0.88 |
| PROMIS anxiety T-scores | 1757 | 60.1 | 8.1 | 36–83 | 0.94 |
| COVID-19 Stressors | |||||
| Job loss due to COVID-19 | 1581 | 254 yes / 1327 no | – | ||
| Threat to own life from COVID-19 | 1795 | 46.4 | 24.3 | 0–100 | – |
| Threat to baby's life from COVID-19 | 1793 | 51.7 | 25.1 | 0–100 | – |
| Strained relationship with partner during COVID-19* | 1735 | 56.3 | 21.3 | 0–100 | – |
| Social isolation due to COVID-19 | 1785 | 64.1 | 26.1 | 0–100 | – |
| Concerned not receiving necessary care due to COVID-19 | 1585 | 35.7 | 27.6 | 0–100 | – |
| Resilience Factors | |||||
| Physical activity (total score from Godin) | 1947 | 33.1 | 21.2 | 0–119 | – |
| Partner social support (SSEQ Total support) | 1685 | 55.8 | 14.9 | 4–80 | 0.94 |
| General social support (ISEL) | 1674 | 34.1 | 6.3 | 6–42 | 0.88 |
*Relationship strain was measured on a scale of 0–100, with 0 being It has strained our relationship and 100 being it has brought us closer together; 50 was not much has changed; so values <50 indicate strain and values >50 indicate closeness. All other COVID-19 stressors were measured from 0 [not at all] to 100 [very much so] with higher values indicating more strain.
Participants were aged 32.4 +/- 4.2 years (range 18.6–47.6 years). 51% of participants had other children (37% had one child, 11% had two children, and 3.5% had three or more other children). Participants lived across Canada (12% British Columbia, 41% Alberta, 4% Saskatchewan, 3% Manitoba, 29% Ontario, 3% Quebec, 1% New Brunswick, 4% Nova Scotia, 1% Prince Edward Island, 2% Newfoundland and Labrador, and 1% from the Territories). The majority were married (77.9%) or cohabitating (19.4%) with a partner. Most participants self-identified as Caucasian (87.1%), with others identifying as First Nations (0.7%), Metis (1.2%), Inuit (0.1%), Black (0.7%), Chinese (1.6%), Filipino (0.9%), Korean (0.2%), West Asian (e.g., Afghan, Iranian; 0.4%), South Asian (e.g., East Indian, Pakistani, Sri Lankan; 2.6%), Southeast Asian (e.g., Cambodian, Indonesian; 0.3%), Hispanic/Latinx (1.1%), and Mixed Race or Other (3.3%). Most participants reporting having completed a trade or community college diploma (23%), bachelor's degree (41%), or higher (28%). Participants had a median household income range of $100,000-$124,999 CDN/year [$70,000–88,000 USD].
While Alberta residents are over-represented in the data compared to Alberta's population within Canada (11.6% of Canadian residents live in Alberta), there were no significant differences in weeks gestation or maternal age between Alberta respondents and the rest of the sample, Alberta respondents were equally likely to be born in Canada, and had a similar breakdown by ethnicity (p>0.05). Alberta residents had higher incomes (p<0.001; median=$125,000–149,000/year vs $100,000–125,000/year), different education profiles (p = 0.002; higher proportion of high school diplomas and bachelor degrees), and were more likely to be married than respondents from elsewhere (p<0.001; 83% vs 74%), which is consistent with population demographics in Alberta and Canada (Statistics Canada, 2013, 2016, 2019).
3.2. COVID-19 stressors
One participant had a confirmed case of COVID-19; 25 others reported suspected but unconfirmed cases. At the time of this initial survey, most provinces in Canada were only testing serious cases (i.e., in hospital) or healthcare workers. None of these individuals were hospitalized. 11 individuals reported other people with COVID-19 infections within their household (6 partners; 1 child, 1 housemate; 3 unspecified).
At the time of the survey, 18.3% of participants reported job loss due to COVID-19 (16.1% laid off, 2.2% indicated their employment was terminated).
Participants rated their social isolation as 64 +/- 26, their worries that their own life was in danger due to COVID-19 as 46 +/- 24, and worries that the virus would cause harm to their unborn baby as 52 +/- 25, all on a scale of 0–100 (not at all to very much so). Average score on relationship strain (where scores <50 indicate more strain and scores >50 indicate the pandemic brought them closer with their partner) were 56 +/- 21.
Most participants (89%) reported changes in prenatal care due to the pandemic, including canceled appointments (36%), or not being allowed to bring a support person (90%). Average scores on the question of whether participants believed that the quality of their prenatal care had decreased were 36 +/- 28, on a scale of 0–100. 35% of respondents made changes to their birth plan because of the pandemic, including the location (11%), support people (25%), and childcare arrangements (11%). 74% had trouble accessing other healthcare during their pregnancy, most commonly reporting they could not access massage therapy services (58%), followed by chiropractic (26%); 9% reported that they were unable to access psychological counselling services.
3.3. Anxiety and depression symptoms
Mean scores are shown in Table 1. 37.0% of participants had clinically elevated symptoms of depression (EPDS scores ≥13). 46.3% of participants had moderately elevated anxiety symptoms (T-scores 60–69), and 10.3% severely elevated anxiety symptoms (T-scores>70). 56.6% total had clinically elevated anxiety symptoms. As expected, measures of anxiety and depression symptoms were moderately to strongly associated with each other, and negatively associated with perceived social support (Table 2 ).
Table 2.
Bivariate correlations among mental health and social support measures. All p-values <0.001.
| PRAQ | Anxiety | EPDS | ISEL | |
|---|---|---|---|---|
| Pregnancy-related anxiety questionnaire (PRAQ) | ||||
| PROMIS Anxiety | 0.50 | |||
| Edinburgh postnatal depression scale (EPDS) | 0.46 | 0.80 | ||
| Interpersonal support evaluation list (ISEL) | −0.24 | −0.26 | −0.35 | |
| Social support effectiveness questionnaire (SSEQ) | −0.20 | −0.31 | −0.37 | 0.42 |
3.4. Parity
Nulliparous individuals were younger than primiparous and multiparous individuals (31.3 years vs 33.3, 33.6 years, respectively; F = 67.4, p<0.001) and were further along in gestation (23.2 weeks vs 21.8, 21.6 weeks, respectively; F = 7.9, p<0.001). Comparison of anxiety and depression symptoms by parity revealed no differences for EPDS (F = 2.6, p = 0.078) or PROMIS Anxiety (F = 0.34, p = 0.71), controlling for age and gestation. However, nulliparous individuals had higher pregnancy-related anxiety symptoms (F = 35.7, p < 0.0001) compared to primiparous and multiparous individuals (Table 3 ).
Table 3.
Comparison of mental health symptoms by parity.
| Nulliparous (n = 971) | Primiparous (n = 735) | Multiparous (n = 277) | F-value | p-value | |
|---|---|---|---|---|---|
| Edinburgh postnatal depression scale | 10.6 | 10.6 | 10.8 | 2.6 | 0.078 |
| PROMIS Anxiety T-scores | 60.4 | 60.0 | 59.3 | 0.34 | 0.71 |
| Pregnancy-related anxiety | 20.2 | 18.3 | 17.6 | 35.7 | <0.001 |
Significant results (p<0.017) are indicated in bold.
3.5. COVID-19 worries and stressors in association with anxiety and depression symptoms
We used binomial logistic regression to determine which COVID-19 related worries (threat to own life, harm to baby, not getting needed care) and stressors (loss of employment, changes to relationship with partner, feelings of isolation) were associated with and clinically elevated anxiety and depression symptoms. The odds for clinically elevated depression symptoms were increased by COVID-19-related worries and by partner relationship strain, but not by loss of employment. Odds for clinically elevated depression symptoms increased by 1% for each unit increase in perceived threat to own life, harm to baby and not getting care needed, 5% for each unit increase in feelings of isolation, and 2% for each unit increase in relationship strain (all measures on 0–100 scale). Loss of employment did not increase the odds of clinically elevated depression symptoms. Similar findings were observed for general anxiety and pregnancy-related anxiety symptoms (Table 4 ). For both depression and general anxiety symptoms, the largest effects were for social isolation. Results of univariate binomial logistic regression models showed significant associations between most COVID-19 factors and depression, anxiety, and pregnancy-related anxiety symptoms (Supplementary Table 1). Only loss of employment (for all 3 symptoms) and relationship strain (for pregnancy-related anxiety symptoms) were not significant in the univariate analysis.
Table 4.
Multivariate models of COVID-19 specific factors predicting elevated anxiety and depression symptoms.
| B | SE | Wald | df | p | Odds Ratio | 95% CI for Odds Ratio | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Depression Symptoms | ||||||||
| Constant | −4.42 | 0.36 | 152.52 | 1 | <0.001 | .012 | ||
| Threat to life | 0.011 | 0.003 | 9.93 | 1 | 0.002 | 1.01 | 1.01 | 1.02 |
| Harm to baby | 0.010 | 0.003 | 9.95 | 1 | 0.002 | 1.01 | 1.01 | 1.02 |
| Not getting needed care | 0.013 | 0.003 | 26.26 | 1 | <0.001 | 1.01 | 1.01 | 1.02 |
| Relationship strain | −0.016 | 0.003 | 25.13 | 1 | <0.001 | 0.98 | 0.98 | 0.99 |
| Social isolation | 0.045 | 0.004 | 163.92 | 1 | <0.001 | 1.05 | 1.04 | 1.05 |
| Loss of employment | 0.21 | 0.18 | 1.42 | 1 | 0.23 | 1.24 | 0.87 | 1.76 |
| General Anxiety Symptoms | ||||||||
| Constant | −3.38 | 0.32 | 114.62 | 1 | <0.001 | 0.03 | ||
| Threat to life | 0.021 | 0.003 | 39.09 | 1 | <0.001 | 1.02 | 1.01 | 1.03 |
| Harm to baby | 0.011 | 0.003 | 12.87 | 1 | 0.001 | 1.01 | 1.01 | 1.02 |
| Not getting needed care | 0.012 | 0.003 | 19.83 | 1 | <0.001 | 1.01 | 1.01 | 1.02 |
| Relationship strain | −0.006 | 0.003 | 3.47 | 1 | 0.063 | 0.99 | 0.99 | 1.00 |
| Social isolation | 0.032 | 0.003 | 119.46 | 1 | <0.001 | 1.03 | 1.03 | 1.04 |
| Loss of employment | 0.180 | 0.18 | 1.02 | 1 | 0.31 | 1.20 | 0.85 | 1.70 |
| Pregnancy-Related Anxiety Symptoms# | ||||||||
| Constant | −0.0.991 | 0.31 | 10.22 | 1 | <0.001 | 0.37 | ||
| Threat to life | 0.014 | 0.003 | 18.05 | 1 | <0.001 | 1.01 | 1.01 | 1.02 |
| Harm to baby | 0.014 | 0.003 | 20.22 | 1 | <0.001 | 1.01 | 1.01 | 1.02 |
| Not getting needed care | 0.020 | 0.003 | 50.18 | 1 | <0.001 | 1.02 | 1.02 | 1.03 |
| Relationship strain | −0.007 | 0.003 | 4.16 | 1 | 0.04 | 0.99 | 0.99 | 1.00 |
| Social isolation | 0.007 | 0.003 | 6.68 | 1 | 0.01 | 1.01 | 1.00 | 1.01 |
| Loss of employment | −0.12 | 0.18 | 0.46 | 1 | 0.50 | 0.89 | 0.63 | 1.26 |
Loss of employment was binary (yes/no); all other variables are 0–100 scale. Significant results (p<0.0028) are shown in bold. #Model includes parity as a covariate.
3.6. Resilience factors
The mean physical activity score on the Godin was 33, indicating that the sample could be considered ‘active’, using the classifications established by the Godin. Average scores on the SSEQ Total Support (partner social support) were 55.8 +/- 14.9. Average scores on the ISEL Total Support (general social support) were 34.1 +/- 6.3, which are consistent with previous reports in pregnant women, M = 35.4–38.7 (Chou et al., 2008; Christian et al., 2009; Messer et al., 2013).
The odds of clinically elevated depression and anxiety symptoms were lower if participants and had better perceived social support (independent effects for partner and general support) (Table 5 ). The odds of clinically elevated anxiety symptoms (both general anxiety and pregnancy-related anxiety) were lower if participants reported more physical activity.
Table 5.
Resilience factors predicting reduced anxiety and depression symptoms.
| B | SE | Wald | df | p | Odds Ratio | 95% CI for Odds Ratio | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Depression Symptoms | ||||||||
| Constant | 3.42 | .33 | 107.97 | 1 | <0.001 | 30.47 | ||
| Physical Activity | −0.01 | 0.003 | 6.66 | 1 | 0.01 | 0.99 | 0.988 | 0.99 |
| Partner support | −0.03 | 0.004 | 68.91 | 1 | <0.001 | 0.97 | 0.96 | 0.98 |
| General support | −0.06 | 0.01 | 36.63 | 1 | <0.001 | 0.95 | 0.93 | 0.96 |
| General Anxiety Symptoms | ||||||||
| Constant | 3.49 | 0.34 | 108.07 | 1 | <0.001 | 32.84 | ||
| Physical activity | −0.01 | 0.002 | 8.28 | 1 | 0.004 | 0.99 | 0.988 | 0.998 |
| Partner support | −0.03 | .004 | 57.38 | 1 | <0.001 | 0.97 | 0.96 | 0.98 |
| General support | −0.04 | 0.01 | 13.45 | 1 | <0.001 | 0.97 | 0.95 | 0.98 |
| Pregnancy-Related Anxiety Symptoms# | ||||||||
| Constant | 3.39 | 0.36 | 91.44 | 1 | <0.001 | 29.70 | ||
| Physical activity | −0.01 | 0.003 | 7.39 | 1 | 0.007 | 0.99 | 0.988 | 0.998 |
| Partner support | −0.02 | 0.004 | 24.32 | 1 | <0.001 | 0.98 | 0.97 | 0.99 |
| General support | −0.05 | 0.01 | 29.54 | 1 | <0.001 | 0.95 | 0.93 | 0.97 |
Physical activity is the total score form the Godin. Partner support is the Total Support measure from the SSEQ; general support is the Total Support from the ISEL. Significant results (p<0.0056) are indicated in bold. #Model includes parity as a covariate.
4. Discussion
Pregnant participants reported high levels of depression, general anxiety, and pregnancy-specific anxiety symptoms. Higher symptoms were associated with more concern about threats of COVID-19 to the life of the mother and baby, as well as concerns about not getting the necessary prenatal care, relationship strain, and social isolation due to the COVID-19 pandemic. These findings suggest that the COVID-19 pandemic presents serious psychological challenges for pregnant individuals, with the potential for both short term (e.g., preterm birth, postpartum depression) and long-lasting impacts on the developing fetus. These findings highlight the urgent need to reduce psychological distress during pregnancy. Increased perceived social support and increased physical activity were associated with reduced symptoms, and thus may be possible targets for intervention.
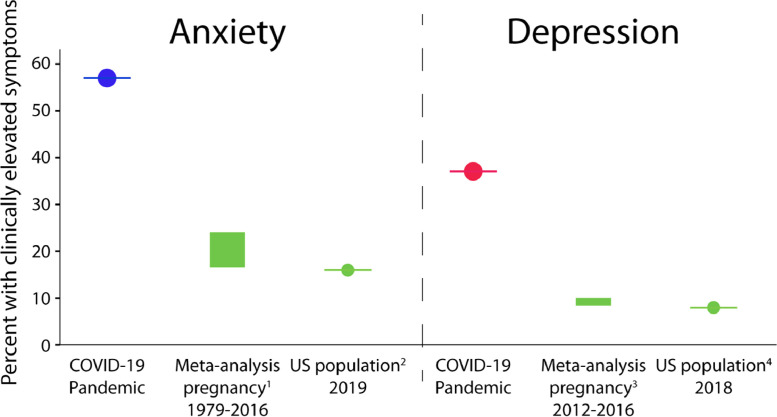
Elevated symptoms (above cut-off scores) of depression (37%), anxiety (59%) were higher than expected based on previous pre-COVID-19 cohort studies assessing symptoms in pregnant women with similar demographic profiles. Prenatal depression is estimated to affect 9–11% of individuals at any given time, with 18% of individuals experiencing a depressive episode at some point during pregnancy (Gavin et al., 2005; Woody et al., 2017). The England-based Avon Longitudinal Study of Parents and Children (ALSPAC) found that 17% of 2390 pregnant women reported clinically elevated depressive symptoms (≥13 on the EPDS) in the first wave of the study (1990–1992), while 25% of 180 women in the second generation (2012–2016) reported clinically elevated depressive symptoms (Pearson et al., 2018). In the Canadian Alberta Pregnancy Outcomes and Nutrition (APrON) study (Kaplan et al., 2014), a study with similar demographic profiles to those seen here, 11% of women had clinically elevated depression symptoms on the EPDS (Leung et al., 2017). Normative data for the United States indicates prevalence of clinically elevated depression symptoms in 8% of adults (Brody et al., 2018). These comparisons suggest that symptoms of depression have increased substantially during the COVID-19 pandemic (Fig. 1 ).
Fig. 1.
To understand depression and anxiety symptoms in context, we compared results to published meta-analyses and normative scores on our measures of depression and anxiety. The prevalence of clinically elevated anxiety (blue) and depression (red) symptoms in the current study was substantially higher compared to meta-analyses (green boxes indicate full range of estimates) and the US population norms (green circles). References 1: (Dennis et al., 2017); 2: (Cella et al., 2019) 3: (Gavin et al., 2005) 4: (Brody et al., 2018).
In a survey of Chinese residents early in the COVID-19 outbreak (Jan 31-Feb 2, 2020), 17% of respondents reported moderate or severe depression, and 29% reporting moderate to severe anxiety (C. Wang et al., 2020). The rates of elevated depressive and anxiety symptoms in our pregnancy cohort are even higher, suggesting that the psychological impact of the outbreak may be of particular concern for pregnant individuals.
Pregnancy-related anxiety symptoms were similarly elevated in our cohort (mean=19.1) compared to recent studies with similar demographics, which reported mean scores of 7.3 (Tomfohr-Madsen et al., 2019) and 7.5 (Thomas et al., 2017). General anxiety was elevated compared to a meta-analysis of pregnancy which reports 18–25% prevalence (Dennis et al., 2017) and the general US population prevalence of 16% (Cella et al., 2019); see Fig. 1. High levels of prenatal distress, particularly pregnancy anxiety, are concerning due to unique associations with elevated risk of preterm birth (Bussieres et al., 2015). The elevated anxiety and depression symptoms appear to be, at least in part, a consequence of the COVID-19 pandemic given that COVID-19-related worries were associated with higher symptoms. The odds of depression increased by 1–5% for each unit (on a 0–100 scale) increase in COVID-19 worries, results that were mirrored in the anxiety outcomes. Importantly, participants’ worries that they were not getting adequate prenatal care due to COVID-19 were associated with higher symptoms in all categories, with the largest effect for pregnancy-related anxiety symptoms. This suggests that maintaining high quality prenatal care is a priority for pregnant individuals, and changes to care may lead to increased anxiety symptoms.
Consistent with the broader literature, better social support was associated with lower symptoms of depression and anxiety. The finding that higher perceived support and support effectiveness are associated with decreased depression and anxiety symptoms is consistent with the notion that social support buffers the effects of stress on anxiety and depression symptoms (Cohen, 2004) and previous research showing decreased prenatal and postnatal anxiety and depression among women with higher levels of social support (Akiki et al., 2016; Friedman et al., 2020). Social support is an important determinant of physical and psychological well-being, especially during pregnancy when individuals take on new responsibilities and roles (Dunkel Schetter, 2011). Supportive social relationships directly affect mental health by encouraging positive health behaviors, increasing positive feelings, and enhancing emotion regulation (Cohen and Wills, 1985) and indirectly by reducing the physiological stress response (Giesbrecht et al., 2013). Social support also reduces the effects of prenatal maternal stress on infant stress responses, suggesting that positive social relationships buffer the biological cascade of stress from mother to infant (Thomas et al., 2018).
Previous studies in multiple populations (Cotman and Berchtold, 2002; Erickson et al., 2011; Vankim and Nelson, 2013), including pregnant individuals (Demissie et al., 2011), indicate that physical activity is associated with reduced depression and anxiety symptoms. Our results were highly consistent, although the effect did not reach significance for depression after Bonferroni correction, but was at trend-level (p = 0.01). These associations have implications for pandemic control measures that limit opportunities for physical activity (e.g., closure of parks, beaches, and gyms) and suggests that encouraging physical activity among pregnant individuals may help reduce feelings of anxiety and depression.
The high anxiety and depression symptoms reported by participants are concerning for both maternal and child health. Children whose mothers experienced high prenatal stress are at higher risk of cognitive and behavioural problems, as well as mental illness in their own lives (Brouwers et al., 2001; DiPietro et al., 2006; Glover, 2014; Huizink et al., 2004; Kinsella and Monk, 2009; Mennes et al., 2006; O'Connor et al., 2003; Stein et al., 2014; Van den Bergh et al., 2005; Weinstock, 2008). Prenatal anxiety and depression symptoms are also associated with changes to brain structure and function in children (Adamson et al., 2018; Lebel et al., 2016; Sandman et al., 2015). Such changes in offspring development are known to occur via multiple mechanisms, including epigenetic, hormonal (e.g., cortisol), behavioral (e.g., lifestyle factors), and social (e.g., lack of adequate support) factors (Beijers et al., 2014), all of which are modifiable and therefore represent potential intervention/prevention targets.
Given the potentially serious consequences of untreated anxiety and depression symptoms in pregnancy on physical and psychological outcomes, interventions are urgently needed to reduce symptoms and build resilience. Psychological interventions for preventing and treating depression and anxiety in pregnancy are effective, with cognitive behavior therapy (CBT) emerging as a front-line treatment and interpersonal therapy (IPT) potentially offering additional benefits to reduce depression and increase social support (Field, 2017; Manber et al., 2019; O'Connor et al., 2019). Preliminary evidence also provides support for e-health interventions; however, trials to date are relatively small and scaling for widespread dissemination is urgently needed (Felder et al., 2020; Heller et al., 2020; Loughnan et al., 2019). Evaluation of e-health treatments should be a priority, given that in-person psychological treatments are currently not available or severely limited. Treatment of pregnancy anxiety is also effective with brief midwife or obstetric lead interventions; however, the ability to deliver these via telehealth has not been tested (Stoll et al., 2018). There is also suggestion that online programs improve partner social support and satisfaction, but to our knowledge these have not been tested in pregnancy (Doss et al., 2020). Psychological treatments may require specific investments from government to ensure wide access but could have large future returns.
One of the factors that may be closely associated with pregnancy-specific anxiety symptoms is parity, as first-time mothers tend to report greater pregnancy-related anxiety than parous women (Huizink et al., 2016). Indeed, in our sample, nulliparous individuals had significantly higher pregnancy-related anxiety symptoms than primiparous and multiparous individuals. Parity was not significantly related to EPDS or PROMIS Anxiety scores.
Our sample was slightly older and more likely to be married or cohabitating than the Canadian averages for pregnancy (Chalmers et al., 2008; Provencher et al., 2018). While this suggests that our data may not be entirely representative of Canadian pregnant individuals, samples with higher education, older age, and where more individuals are partnered tend to have fewer prenatal anxiety and depression symptoms. Thus, the elevated anxiety and depression symptoms seen here would be highly unexpected under normal circumstances. Given the low sociodemographic risks in our sample and the fact that Canada has had a relatively contained outbreak and universal health care, the results may be worse in populations with higher sociodemographic risks (e.g., low education, low income) or living in countries with larger outbreaks and/or worse containment measures. Symptoms of anxiety and depression can vary across pregnancy (Bayrampour et al., 2016; Bennett et al., 2004; Gavin et al., 2005), and the rapid changes in government policies and outbreak risks during the current pandemic could add further confounding. Therefore, longitudinal studies with multiple assessment points will be necessary to better understand the nature of anxiety and depression symptoms in pregnant women during the current pandemic. Future studies should consider other factors that may additionally contribute to anxiety and depression symptoms such as history of mental health problems.
5. Conclusions
Pregnant individuals are experiencing substantially elevated anxiety and depression symptoms during the COVID-19 pandemic that are significantly related to COVID-19 specific worries about threats to their own lives, their baby's health, not getting enough prenatal care, and social isolation. These levels far exceed those normally expected during pregnancy and those experienced by other groups of people during the current pandemic. Social support and physical activity appear to be protective resilience factors. Given the known effects of stress on pregnancy, infant, and child outcomes, there is an urgent need to support pregnant individuals during this critical time to mitigate long-term negative outcomes.
Declaration of Competing Interest
The authors report no conflicts of interest.
Acknowledgments
Funding
This research was supported by the Alberta Children's Hospital Research Institute and the Owerko Centre; the funder had no role in the research.
Acknowledgements
We thank Mary Kate Dichoso, Ashley Dhillon, Diego Santillo, and Pooja Sohal for their assistance with survey set up.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jad.2020.07.126.
Appendix. Supplementary materials
References
- Accortt E.E., Cheadle A.C., Schetter C.D. Prenatal depression and adverse birth outcomes: an updated systematic review. Matern. Child Health J. 2015;19(6):1306–1337. doi: 10.1007/s10995-014-1637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson B., Letourneau N., Lebel C. Prenatal maternal anxiety and children's brain structure and function: a systematic review of neuroimaging studies. J. Affect. Disord. 2018;241:117–126. doi: 10.1016/j.jad.2018.08.029. [DOI] [PubMed] [Google Scholar]
- Akiki S., Avison W.R., Speechley K.N., Campbell M.K. Determinants of maternal antenatal state-anxiety in mid-pregnancy: role of maternal feelings about the pregnancy. J. Affect. Disord. 2016;196:260–267. doi: 10.1016/j.jad.2016.02.016. [DOI] [PubMed] [Google Scholar]
- Al-Rabiaah A., Temsah M.H., Al-Eyadhy A.A., Hasan G.M., Al-Zamil F., Al-Subaie S., Somily A.M. Middle east respiratory syndrome-corona virus (MERS-CoV) associated stress among medical students at a university teaching hospital in Saudi Arabia. J. Infect. Public Health. 2020 doi: 10.1016/j.jiph.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayrampour H., Tomfohr L., Tough S. Trajectories of perinatal depressive and anxiety symptoms in a community cohort. J. Clin. Psychiatry. 2016;77(11):e1467–e1473. doi: 10.4088/JCP.15m10176. [DOI] [PubMed] [Google Scholar]
- Beijers R., Buitelaar J.K., de Weerth C. Mechanisms underlying the effects of prenatal psychosocial stress on child outcomes: beyond the HPA axis. Eur. Child. Adolesc. Psychiatry. 2014;23(10):943–956. doi: 10.1007/s00787-014-0566-3. [DOI] [PubMed] [Google Scholar]
- Bennett H.A., Einarson A., Taddio A., Koren G., Einarson T.R. Prevalence of depression during pregnancy: systematic review. Obstet. Gynecol. 2004;103(4):698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- Bergink V., Kooistra L., Lambregtse-van den Berg M.P., Wijnen H., Bunevicius R., van Baar A., Pop V. Validation of the Edinburgh depression scale during pregnancy. J. Psychosom. Res. 2011;70(4):385–389. doi: 10.1016/j.jpsychores.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Brody D., Pratt L., J. H. Prevalence of depression among adults aged 20 and over: united States. NCHS Data Brief. 2018;(303):2013–2016. [PubMed] [Google Scholar]
- Brouwers E.P., van Baar A.L., Pop V.J. Does the Edinburgh postnatal depression scale measure anxiety? J. Psychosom. Res. 2001;51(5):659–663. doi: 10.1016/s0022-3999(01)00245-8. https://www.ncbi.nlm.nih.gov/pubmed/11728506 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Bussieres E., Tarabulsy G., Pearson J., Tessier R., Forest J.C., Giguere Y. Maternal prenatal stress and infant birth weight and gestational age: a meta-analysis of prospective studies. Dev. Rev. 2015;36:179–199. [Google Scholar]
- Cella D., Choi S.W., Condon D.M., Schalet B., Hays R.D., Rothrock N.E., Reeve B.B. PROMIS((R)) Adult Health Profiles: efficient Short-Form Measures of Seven Health Domains. Value Health. 2019;22(5):537–544. doi: 10.1016/j.jval.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D., Riley W., Stone A., Rothrock N., Reeve B., Yount S., Group P.C. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J. Clin. Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers B., Dzakpasu S., Heaman M., Kaczorowski J. The Canadian maternity experiences survey: an overview of findings. J. Obstet. Gynaecol. Can. 2008;30(3):217–228. doi: 10.1016/S1701-2163(16)32758-X. [DOI] [PubMed] [Google Scholar]
- Chou F.H., Avant K.C., Kuo S.H., Fetzer S.J. Relationships between nausea and vomiting, perceived stress, social support, pregnancy planning, and psychosocial adaptation in a sample of mothers: a questionnaire survey. Int. J. Nurs. Stud. 2008;45(8):1185–1191. doi: 10.1016/j.ijnurstu.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Christian L.M., Franco A., Glaser R., Iams J.D. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav. Immun. 2009;23(6):750–754. doi: 10.1016/j.bbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. Social relationships and health. Am. Psychol. 2004;59(8):676–684. doi: 10.1037/0003-066X.59.8.676. [DOI] [PubMed] [Google Scholar]
- Cohen S., Hoberman H. Positive events and social supports as buffers of life change stress. J. Appl. Soc. Psychol. 1983;13:99–125. [Google Scholar]
- Cohen S., Wills T.A. Stress, social support and the buffering hypothesis. Psychol. Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- Cotman C.W., Berchtold N.C. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/S0166-2236(02)02143-4%3C. [DOI] [PubMed] [Google Scholar]
- Coussons-Read M.E. Effects of prenatal stress on pregnancy and human development: mechanisms and pathways. Obstet. Med. 2013;6(2):52–57. doi: 10.1177/1753495X12473751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J.L., Holden J.M., Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. http://www.ncbi.nlm.nih.gov/pubmed/3651732 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Cullen W., Gulati G., Kelly B.D. Mental health in the Covid-19 pandemic. QJM. 2020 doi: 10.1093/qjmed/hcaa110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demissie Z., Siega-Riz A.M., Evenson K.R., Herring A.H., Dole N., Gaynes B.N. Physical activity and depressive symptoms among pregnant women: the PIN3 study. Arch. Womens Ment. Health. 2011;14(2):145–157. doi: 10.1007/s00737-010-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis C.L., Falah-Hassani K., Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br. J. Psychiatry. 2017;210(5):315–323. doi: 10.1192/bjp.bp.116.187179. [DOI] [PubMed] [Google Scholar]
- DiPietro J.A., Novak M.F., Costigan K.A., Atella L.D., Reusing S.P. Maternal psychological distress during pregnancy in relation to child development at age two. Child Dev. 2006;77(3):573–587. doi: 10.1111/j.1467-8624.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- Doss B.D., Knopp K., Roddy M.K., Rothman K., Hatch S.G., Rhoades G.K. Online programs improve relationship functioning for distressed low-income couples: results from a nationwide randomized controlled trial. J. Consult. Clin. Psychol. 2020;88(4):283–294. doi: 10.1037/ccp0000479. [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu. Rev. Psychol. 2011;62:531–558. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- Erickson K.I., Voss M.W., Prakash R.S., Basak C., Szabo A., Chaddock L., White S.M.. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder J.N., Epel E.S., Neuhaus J., Krystal A.D., Prather A.A. Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: a randomized clinical trial. JAMA Psychiatry. 2020 doi: 10.1001/jamapsychiatry.2019.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T. Prenatal depression risk factors, developmental effects and interventions: a review. J. Pregnancy Child Health. 2017;4(1) doi: 10.4172/2376-127X.1000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L.E., Gelaye B., Sanchez S.E., Williams M.A. Association of social support and antepartum depression among pregnant women. J. Affect. Disord. 2020;264:201–205. doi: 10.1016/j.jad.2019.12.017. [DOI] [PubMed] [Google Scholar]
- Gavin N.I., Gaynes B.N., Lohr K.N., Meltzer-Brody S., Gartlehner G., Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet. Gynecol. 2005;106(5 Pt 1):1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Geraldo da Silva, A., Marques Miranda, D., Paim Diaz, A., Silva Teles, A., Fernandes Malloy-Diniz, L., & Pacheco Palha, A. (2020). Mental health: why it still matters in the midst of a pandemic. Braz. J. Psychiatry, in press. [DOI] [PMC free article] [PubMed]
- Giesbrecht G.F., Poole J.C., Letourneau N., Campbell T., Kaplan B.J. The buffering effect of social support on hypothalamic-pituitary-adrenal axis function during pregnancy. Psychosom. Med. 2013;75(9):856–862. doi: 10.1097/PSY.0000000000000004. [DOI] [PubMed] [Google Scholar]
- Glover V. Maternal depression, anxiety and stress during pregnancy and child outcome; what needs to be done. Best Pract. Res. Clin. Obstet. Gynaecol. 2014;28(1):25–35. doi: 10.1016/j.bpobgyn.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Godin G. The Godin-Shephard leisure-time physical activity questionnaire. Health Fitness J. Can. 2011;4(1):18–22. [Google Scholar]
- Grigoriadis S., Graves L., Peer M., Mamisashvili L., Tomlinson G., Vigod S.N., Richter M. Maternal anxiety during pregnancy and the association with adverse perinatal outcomes: systematic review and meta-analysis. J. Clin. Psychiatry. 2018;79(5) doi: 10.4088/JCP.17r12011. [DOI] [PubMed] [Google Scholar]
- Hamel, L., & Salganicoff, A. (2020). Is there a widening gender gap in coronavirus stress?Retrieved from.
- Hawryluck L., Gold W.L., Robinson S., Pogorski S., Galea S., Styra R. SARS control and psychological effects of quarantine, Toronto, Canada. Emerg. Infect. Dis. 2004;10(7):1206–1212. doi: 10.3201/eid1007.030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller H.M., Hoogendoorn A.W., Honig A., Broekman B.F.P., van Straten A. The effectiveness of a guided internet-based tool for the treatment of depression and anxiety in pregnancy (MamaKits Online): randomized controlled trial. J. Med. Internet Res. 2020;22(3):e15172. doi: 10.2196/15172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink A.C., Delforterie M.J., Scheinin N.M., Tolvanen M., Karlsson L., Karlsson H. Adaption of pregnancy anxiety questionnaire-revised for all pregnant women regardless of parity: PRAQ-R2. Arch. Womens Ment. Health. 2016;19(1):125–132. doi: 10.1007/s00737-015-0531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink A.C., Mulder E.J., Buitelaar J.K. Prenatal stress and risk for psychopathology: specific effects or induction of general susceptibility? Psychol. Bull. 2004;130(1):115–142. doi: 10.1037/0033-2909.130.1.115. [DOI] [PubMed] [Google Scholar]
- Kaplan B.J., Giesbrecht G.F., Leung B.M., Field C.J., Dewey D., Bell R.C., Martin J.W.. The Alberta Pregnancy Outcomes and Nutrition (APrON) cohort study: rationale and methods. Matern. Child Nutr. 2014;10(1):44–60. doi: 10.1111/j.1740-8709.2012.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S., Laplante D.P. Using natural disasters to study prenatal maternal stress in humans. Adv. Neurobiol. 2015;10:285–313. doi: 10.1007/978-1-4939-1372-5_14. [DOI] [PubMed] [Google Scholar]
- Kinsella M.T., Monk C. Impact of maternal stress, depression and anxiety on fetal neurobehavioral development. Clin. Obstet. Gynecol. 2009;52(3):425–440. doi: 10.1097/GRF.0b013e3181b52df1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinszky Z., Dudas R.B. Validation studies of the Edinburgh Postnatal Depression Scale for the antenatal period. J. Affect. Disord. 2015;176:95–105. doi: 10.1016/j.jad.2015.01.044. [DOI] [PubMed] [Google Scholar]
- Lebel C., Walton M., Letourneau N., Giesbrecht G.F., Kaplan B.J., Dewey D. Prepartum and postpartum maternal depressive symptoms are related to children's brain structure in preschool. Biol. Psychiatry. 2016;80(11):859–868. doi: 10.1016/j.biopsych.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Leung B., Letourneau N., Bright K., Giesbrecht G.F., Ntanda H., Gagnon L., Team A.P. Appraisal of the psychiatric diagnostic screening questionnaire in a perinatal cohort: the APrON study. Scand. J. Public Health. 2017;45(6):658–665. doi: 10.1177/1403494817717835. [DOI] [PubMed] [Google Scholar]
- Loughnan S.A., Sie A., Hobbs M.J., Joubert A.E., Smith J., Haskelberg H., Newby J.M. A randomized controlled trial of 'MUMentum Pregnancy': internet-delivered cognitive behavioral therapy program for antenatal anxiety and depression. J. Affect. Disord. 2019;243:381–390. doi: 10.1016/j.jad.2018.09.057. [DOI] [PubMed] [Google Scholar]
- MacKinnon N., Kingsbury M., Mahedy L., Evans J., Colman I. The association between prenatal stress and externalizing symptoms in childhood: evidence from the avon longitudinal study of parents and children. Biol. Psychiatry. 2018;83(2):100–108. doi: 10.1016/j.biopsych.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Manber R., Bei B., Simpson N., Asarnow L., Rangel E., Sit A., Lyell D. Cognitive behavioral therapy for prenatal insomnia: a randomized controlled trial. Obstet. Gynecol. 2019;133(5):911–919. doi: 10.1097/AOG.0000000000003216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M., Stiers P., Lagae L., Van den Bergh B. Long-term cognitive sequelae of antenatal maternal anxiety: involvement of the orbitofrontal cortex. Neurosci. Biobehav. Rev. 2006;30(8):1078–1086. doi: 10.1016/j.neubiorev.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Messer L., Maxson P., Miranda M.L. The urban built environment and associations with women's psychosocial health. J. Urban Health: Bull. New York Acad. Med. 2013;90(5):857–871. doi: 10.1007/s11524-012-9743-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor E., Senger C.A., Henninger M.L., Coppola E., Gaynes B.N. Interventions to prevent perinatal depression: evidence report and systematic review for the US preventive services task force. JAMA. 2019;321(6):588–601. doi: 10.1001/jama.2018.20865. [DOI] [PubMed] [Google Scholar]
- O'Connor T.G., Heron J., Golding J., Glover V., Team A.S. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J. Child Psychol. Psychiatry. 2003;44(7):1025–1036. doi: 10.1111/1469-7610.00187. https://www.ncbi.nlm.nih.gov/pubmed/14531585 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Pearson R.M., Carnegie R.E., Cree C., Rollings C., Rena-Jones L., Evans J., Lawlor D.A. Prevalence of prenatal depression symptoms among 2 generations of pregnant mothers: the avon longitudinal study of parents and children. JAMA Netw. Open. 2018;1(3) doi: 10.1001/jamanetworkopen.2018.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher, C., Milan, A., Hallman, S., & D'Aoust, C. (2018). Fertility: overview, 2012-2016. Retrieved from.
- Qiu A., Rifkin-Graboi A., Chen H., Chong Y.S., Kwek K., Gluckman P.D, Meaney M.J. Maternal anxiety and infants' hippocampal development: timing matters. Transl. Psychiatry. 2013;3:e306. doi: 10.1038/tp.2013.79. . . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu F., Wu Y., Zhu Y.H., Barry J., Ding T., Baio G., Hardiman P.J. The association between psychological stress and miscarriage: a systematic review and meta-analysis. Sci. Rep. 2017;7(1):1731. doi: 10.1038/s41598-017-01792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K.M., Taylor M.G. Social support, stress, and maternal postpartum depression: a comparison of supportive relationships. Soc. Sci. Res. 2015;54:246–262. doi: 10.1016/j.ssresearch.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Rini C.K., Dunkel Schetter C., Hobel C., Glynn L., Sandman C.A. Effective social support: antecedents and consequences of partner support during pregnancy. Pers. Relatsh. 2006;13(2):207–229. [Google Scholar]
- Rini C.K., Dunkel-Schetter C., Wadhwa P.D., Sandman C.A. Psychological adaptation and birth outcomes: the role of personal resources, stress, and sociocultural context in pregnancy. Health Psychol. 1999;18(4):333–345. doi: 10.1037//0278-6133.18.4.333. https://www.ncbi.nlm.nih.gov/pubmed/10431934 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Rondo P.H., Ferreira R.F., Nogueira F., Ribeiro M.C., Lobert H., Artes R. Maternal psychological stress and distress as predictors of low birth weight, prematurity and intrauterine growth retardation. Eur. J. Clin. Nutr. 2003;57(2):266–272. doi: 10.1038/sj.ejcn.1601526. [DOI] [PubMed] [Google Scholar]
- Sandman C.A., Buss C., Head K., Davis E.P. Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biol. Psychiatry. 2015;77(4):324–334. doi: 10.1016/j.biopsych.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Canada. (2013). Canadian vital statistics, marriages database, 1981-2008. Retrieved fromhttps://www150.statcan.gc.ca/n1/pub/91-209-x/2013001/article/11788/tbl/tbl1-eng.htm.
- Statistics Canada. (2016). Highest level of educational attainment (detailed). Retrieved fromhttps://www12.statcan.gc.ca/census-recensement/2016/dp-pd/hlt-fst/edu-sco/Table.cfm?Lang=E&T=21&Geo=00&SP=1&view=2&age=1&sex=1.
- Statistics Canada. (2019). The income of Canadians, 2017. Retrieved fromhttps://www150.statcan.gc.ca/n1/pub/11-627-m/11-627-m2019013-eng.pdf.
- Stein A., Pearson R.M., Goodman S.H., Rapa E., Rahman A., McCallum M., Pariante C.M. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384(9956):1800–1819. doi: 10.1016/S0140-6736(14)61277-0. [DOI] [PubMed] [Google Scholar]
- Stoll K., Swift E.M., Fairbrother N., Nethery E., Janssen P. A systematic review of nonpharmacological prenatal interventions for pregnancy-specific anxiety and fear of childbirth. Birth. 2018;45(1):7–18. doi: 10.1111/birt.12316. [DOI] [PubMed] [Google Scholar]
- Thomas J.C., Letourneau N., Bryce C.I., Campbell T.S., Giesbrecht G.F., APrON Study Team Biological embedding of perinatal social relationships in infant stress reactivity. Dev. Psychobiol. 2017;59(4):425–435. doi: 10.1002/dev.21505. [DOI] [PubMed] [Google Scholar]
- Thomas J.C., Letourneau N., Campbell T.S., Giesbrecht G.F., APrON Study Team Social buffering of the maternal and infant HPA axes: mediation and moderation in the intergenerational transmission of adverse childhood experiences. Dev. Psychopathol. 2018;30(3):921–939. doi: 10.1017/S0954579418000512. [DOI] [PubMed] [Google Scholar]
- Tomfohr-Madsen L., Cameron E.E., Dunkel Schetter C., Campbell T., O'Beirne M., Letourneau N., Giesbrecht G.F. Pregnancy anxiety and preterm birth: the moderating role of sleep. Health Psychol. 2019;38(11):1025–1035. doi: 10.1037/hea0000792. [DOI] [PubMed] [Google Scholar]
- Urizar G.G., Jr., Yim I.S., Rodriguez A., Schetter C.D. The SMART Moms Program: a Randomized Trial of the Impact of Stress Management on Perceived Stress and Cortisol in Low-Income Pregnant Women. Psychoneuroendocrinology. 2019;104:174–184. doi: 10.1016/j.psyneuen.2019.02.022. [DOI] [PubMed] [Google Scholar]
- Van den Bergh B.R., Dahnke R., Mennes M. Prenatal stress and the developing brain: risks for neurodevelopmental disorders. Dev. Psychopathol. 2018;30(3):743–762. doi: 10.1017/S0954579418000342. [DOI] [PubMed] [Google Scholar]
- Van den Bergh B.R., Mulder E.J., Mennes M., Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci. Biobehav. Rev. 2005;29(2):237–258. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Van den Bergh B.R., van den Heuvel M.I., Lahti M., Braeken M., de Rooij S.R., Entringer S., King S. Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy. Neurosci. Biobehav. Rev. 2017 doi: 10.1016/j.neubiorev.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Vankim N.A., Nelson T.F. Vigorous physical activity, mental health, perceived stress, and socializing among college students. Am. J. Health Promot. 2013;28(1):7–15. doi: 10.4278/ajhp.111101-QUAN-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Pan R., Wan X., Tan Y., Xu L., Ho C.S., Ho R.C. Immediate psychological responses and associated factors during the initial stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int. J. Environ. Res. Public Health. 2020;17(5) doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 2008;32(6):1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Woody C.A., Ferrari A.J., Siskind D.J., Whiteford H.A., Harris M.G. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J. Affect. Disord. 2017;219:86–92. doi: 10.1016/j.jad.2017.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.