Abstract
A novel Gram-positive, catalase negative, rod-shaped strain, FI11369T, was isolated from gari, a traditional West African fermented food derived from cassava. Based on 16S rRNA gene sequence similarity, the closest type strains were Lactobacillus xiangfangensis LMG 26013T (99.4 % similarity), Lactobacillus plajomi NBRC 107333T (99.1 %), Lactobacillus paraplantarum DSM 10667T (99.1 %), Lactobacillus pentosus DSM 20314T (99.0 %), Lactobacillus plantarum subsp. plantarum ATCC 14917T (99.0 %), Lactobacillus modestisalitolerans NBRC 107235T (98.9 %), Lactobacillus plantarum subsp. argentoratensis DSM 16365T (98.9 %) and Lactobacillus daowaiensis NCIMB 15183T (98.8 %). The genome of strain FI11369T was sequenced and the average nucleotide identity (ANI) was compared with its closest relatives. ANI analysis showed that the closest relative, L. xiangfangensis DSM 27103T, had only a 82.4 % similarity. The main fatty acids of FI11369T were saturated C16 : 0 (18.2 %), unsaturated C18 : 1 ω9c (43.8 %) and cyclopropane C19 : 0 cyclo (ω10c and/or ω6; 22.5 %). Based on the genotypic and phenotypic data obtained in this study, a novel Lactobacillus species, Lactobacillus garii sp. nov., with the type strain FI11369T (=NCIMB 15148=DSM 108249), is proposed.
Keywords: Lactobacillus garii, gari, fermented food, cassava, Africa
The genus Lactobacillus , which includes more than 200 species, is a taxonomically complex group due to the high level of phenotypic and genotypic diversity [1]. Lactobacilli are Gram-positive, mostly non-motile, catalase-negative, non-spore forming and rod-shaped bacteria. Their habitats are nutrient-rich environments such as food, soil, plants, animals and humans [2]. Lactobacilli dominate the microbiota of the vast majority of fermented foods and most studies have focused on their role in food fermentation and prevention of food spoilage [3], as well as their importance in the gut and their applications as probiotics [4].
Gari, a fermented food derived from cassava (Manihot esculenta), is widely consumed in West and Central Africa. The steps to obtain gari include washing and grating fresh cassava roots, followed by fermenting and dewatering at ambient temperature (ca. 30 °C) for up to 72 h. The fermented pressed cake is disintegrated and roasted into gari [5, 6]. In a previous study of the diversity of lactic acid bacteria in gari, Lactobacillus plantarum was the most frequently isolated species, followed by Leuconostoc fallax and Lactobacillus fermentum [6].
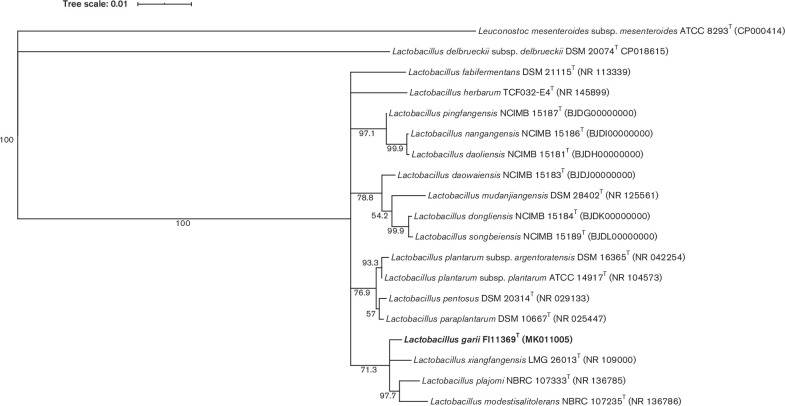
Strain FI11369T was isolated as part of a study to sequence the whole genomes of micro-organisms present in African fermented foods. In this study, a sample of gari, produced in the suburb of Pokuase, Accra (Ghana) was collected and preserved at 4 °C until further processing in the UK. The sample of gari was homogenized in PBS and 100 µl of a 10−5 dilution of the homogenate were plated on MRS (Oxoid) agar medium [7]. Plates were incubated for 48 h at 37 °C and 10 out of 194 colonies were picked based on different morphology and sub-cultured for three rounds until pure cultures were obtained. A pure culture of strain FI11369T, the only selected colony with irregular edges, was obtained after three rounds of sub-culturing. DNA from strain FI11369T was extracted using the cetyltrimethylammonium bromide-based extraction protocol [8] and the genome was sequenced. Libraries were obtained using the Nextera XT DNA library Prep kit (Illumina) according to manufacturer instructions and sequenced for 150 cycles using the Illumina NextSeq platform at the Quadram Institute Bioscience (QIB; Norwich, UK). The 887 369 reads generated were quality trimmed with BBDuk (version 38.68) to at least a quality of Q21. Cleaned reads were assembled with SPAdes (version 3.11.1) [9] and annotated using patric [10]. The genome was assembled into 158 contigs, with an N50 of 57 125 bp. The 16S rRNA gene sequence (accession no. MK011005) was extracted from the genome assembly (accession no. QWZQ00000000; size 2 972 171 bp). The sequence was compared with all the type strains present in the nucleotide collection database from the National Center for Biotechnology Information (NCBI). Sequence similarity with the closest type strains was calculated using the pairwise nucleotide sequence alignment for taxonomy tool from EzBioCloud [11]. The highest similarity was found with the type strains of Lactobacillus xiangfangensis (99.4 % similarity), Lactobacillus plajomi (99.1 %), Lactobacillus paraplantarum (99.1 %), Lactobacillus pentosus (99.0 %), Lactobacillus plantarum subsp. plantarum (99.0 %), Lactobacillus modestisalitolerans (98.9 %), Lactobacillus plantarum subsp. argentoratensis (98.9 %), Lactobacillus daowaiensis (98.8%), Lactobacillus fabifermentans (98.6%), Lactobacillus nangangensis (98.6 %), Lactobacillus daoliensis (98.5 %), Lactobacillus pingfangensis (98.5 %), Lactobacillus herbarum (98.5 %), Lactobacillus mudanjiangensis (98.0%), Lactobacillus dongliensis (98.1 %) and Lactobacillus songbeiensis (98.1 %). All sequences were aligned using clustal_w version 2.1 [12]. A 16S rRNA gene phylogeny was reconstructed using the maximum-likelihood method with the Jukes–Cantor model [13] incorporated into Geneious version 11.1.3 (Biomatters) (Fig. 1) . Branching support was estimated with 1000 bootstrap replicates. In addition, the 16S rRNA gene was amplified by PCR using primers AMP_F (5′-GAG AGT TTG ATY CTG GCT CAG-3′) and AMP_R (5′-AAG GAG GTG ATC CAR CCG CA-3′) and sequenced by a Sanger sequencing service (Eurofins Genomics Germany GmbH, Germany). The partial 16S rRNA gene sequence (accession no. MN817919) obtained by Sanger sequencing was identical to the 16S rRNA gene sequence extracted from the genome.
Fig. 1.
Phylogenetic tree based on 16S rRNA gene sequences showing the relationship of strain FI11369T and the related Lactobacillus species. Tree reconstructed by the maximum-likelihood method. Leuconostoc mesenteroides subsp. mesenteroides ATCC 8293T was used as an outgroup. Bootstrap values are indicated at branch points based on 1000 replications. Bootstrap values below 62 % are not shown. Bar, 0.01 substitutions per nucleotide position.
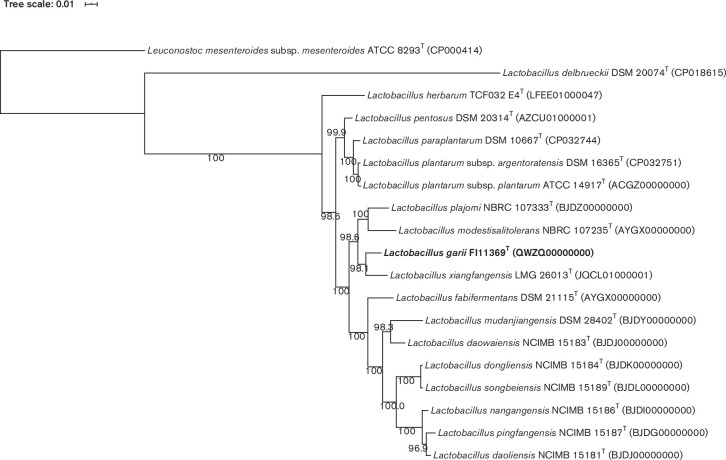
To reconstruct a well-supported phylogenomic tree, we extracted the following single-copy marker genes from 18 type strains using amphora2 [14]: frr, infC, nusA, pgk, pyrG, rplA, rplC, rplD, rplE, rplF, rplK, rplL, rplM, rplN, rplP, rplS, rplT, rpmA, rpoB, rpsB, rpsC, rpsE, rpsI, rpsJ, rpsK, rpsM, rpsS, smpB and tsf. Sequences were aligned using RAxML version 8.2.12 [15]. Single protein alignments were concatenated with the script from phylogenomics-tools (github.com/kbseah/phylogenomics-tools). Positions of the alignment that had more than 75 % gaps were removed using Geneious version 11.1.3 (www.geneious.com). The resulting alignment was then used for maximum-likelihood phylogenomic reconstruction with FastTree version 2.1.11 [16] (Fig. 2) .
Fig. 2.
Phylogenomic tree based on the concatenated frr, infC, nusA, pgk, pyrG, rplA, rplC, rplD, rplE, rplF, rplK, rplL, rplM, rplN, rplP, rplS, rplT, rpmA, rpoB, rpsB, rpsC, rpsE, rpsI, rpsJ, rpsK, rpsM, rpsS, smpB and tsf gene sequences showing the relationship of strain FI11369T and the related Lactobacillus species. Tree reconstructed with an alignment spanning 6923 aa using the approximately-maximum-likelihood method implemented in FastTree 2.1.11. Leuconostoc mesenteroides subsp. mesenteroides ATCC 8293T was used as an outgroup. The node labels represent SH-like branching support values. Bar, 0.01 substitutions per aa position.
The average nucleotide identity (ANI) of strain FI11369T was compared to the type strains that were closest based on our phylogenomic reconstruction, which included: L. xiangfangensis , L. plajomi , L. paraplantarum , L. pentosus , L. plantarum subsp. plantarum , L. modestisalitolerans , L. plantarum subsp. argentoratensis , L. daowaiensis , L. fabifermentans , L. nangangensis , L. daoliensis , L. pingfangensis , L. herbarum , L. mudanjiangensis , L. dongliensis and L. songbeiensis . The ANI and digital DNA–DNA hybridization (dDDH) parameters were calculated using FastANI [17] and formula 2 from the Genome-to-Genome Distance Calculator 2.1 (GGDC 2.1) software [18] respectively using the default settings. The ANI values ranged from 82.4 to 79.7% and the dDDH values ranged from 38.8 to 19.6 % (Table 1), which are well below the generally accepted threshold to identify the same species (95–96 % for ANI and 70 % for dDDH [18–20]). Thus, we confirmed that strain FI11369T represents a novel species. The DNA G+C content of FI11369T is 48.3 mol%, similar to the 46.6 mol% G+C of its closest relative L. xiangfangensis DSM 27103T [21].
Table 1.
ANI values (%) and dDDH prediction values (%) between Lactobacillus garii sp. nov. and its closely related species
|
Species |
Strain |
Accession No. |
ANI |
dDDH |
|---|---|---|---|---|
|
LMG 26013 |
82.4 |
23.8 |
||
|
NBRC 107333 |
81.6 |
22.1 |
||
|
L. plantarum subsp. argentoratensis |
DSM 16365 |
81.1 |
21.2 |
|
|
DSM 10667 |
80.8 |
20.9 |
||
|
NBRC 107235 |
80.6 |
21.2 |
||
|
DSM 20314 |
80.6 |
25.1 |
||
|
L. plantarum subsp. plantarum |
ATCC 14917 |
80.4 |
20.6 |
|
|
TCF032-E4 |
80.1 |
38.8 |
||
|
DSM 21115 |
80.1 |
21.2 |
||
|
NCIMB 15181 |
80.0 |
20.4 |
||
|
NCIMB 15184 |
80.0 |
20.4 |
||
|
NCIMB 15186 |
79.9 |
20.0 |
||
|
NCIMB 15183 |
79.9 |
20.4 |
||
|
NCIMB 15189 |
79.7 |
20.4 |
||
|
DSM 28402 |
79.7 |
19.6 |
||
|
NCIMB 15187 |
79.7 |
20.2 |
Morphological and biochemical tests were performed after growth of FI11369T at 30 °C in aerobic conditions, unless otherwise stated. Colony morphology was observed after incubation on MRS agar plates. Cell morphology was investigated using scanning electron microscopy (Zeiss Supra 55 VP) as previously described [22]. The cells of FI11369T were rod-shaped, 1–2 µm in length and formed short chains or pairs (Fig. 3). Motility and spore formation were determined by phase-contrast microscopy using Olympus microscope CX41 using cells grown on MRS agar and broth for 2 days. Gram-staining was performed using a commercial Gram-staining kit (Remel, Thermo Fisher Scientific). Catalase activity was determined by treating the cells with 3 % H2O2. Gas production from glucose was analysed in MRS broth using inverted Durham tubes. Relationship with oxygen was determined on MRS broth or MRS plates under aerobic and anaerobic conditions. The latter were achieved by providing anaerobic gas mix (85 % N2, 5 % CO2 and 10 % H2) in an anaerobic cabinet (Whitley A95 Workstation). Growth at different temperatures (6, 12, 20, 25, 28, 30, 37, 40 and 42 and 55 °C), pH (9, 8.8, 8.4, 8, 7.8, 7.4, 7, 6, 5, 4.0, 3.8, 3.4, 3.2 or 3.0) and salt levels (commercial MRS with 2, 4, 6, 8, 10, 14 or 16 % additional NaCl) was measured using OD600 after 3 days of incubation. Production of d- and l-lactic acid was analysed using the K-DLATE assay kit (Megazyme). Fermentation products from glucose were analysed by HPLC as previously described [23]. Nitrate and nitrite reduction, indole production, Voges–Proskauer reaction, H2S production, deamination of arginine, hydrolysis of gelatin and urease production were determined using the API 20E system. Hydrolysis of hippurate was tested using hippurate discs (Sigma-Aldrich). Pyrrolidonylarylamidase production was tested using pyrase strips (Sigma-Aldrich). Bile–aesculin tolerance was tested by growing the isolates on bile aesculin agar (Sigma-Aldrich). Haemolytic activity was checked on Columbia blood agar with 5 % sheep blood. Pyruvate utilization was tested in broth medium as previously described [24]. Tellurite tolerance was tested on MRS agar supplemented with 0.02 % of potassium tellurite. Carbon source utilization by strain FI11369T and the closest relative L. xiangfangensis DSM 27103T (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH) was examined using the API 50 CH system (bioMérieux) following the manufacturer’s instructions. Strips were incubated at 30 °C and readings were made after 48 h (Table 2). Whole-cell fatty acids were analysed by gas chromatography of fatty acid methyl esters (GC-FAME) using the Sherlock microbial identification method [25, 26] after growth of the strain in trypticase soy medium for 2 days at 28 °C. Peptidoglycan was isolated from cells grown in MRS broth for 10 h at 30 °C and its structure and cell-wall sugar composition was studied after total hydrolysis (100 °C, 4 N HCl, 16 h) and partial hydrolysis (4 N HCl, 45 min, 100 °C) as previously described [27]. Both analyses were performed by the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures identification service. The main fatty acids (>10 %) of FI11369T were saturated C16 : 0 (18.2 %), unsaturated C18 : 1 ω9c (43.8 %) and cyclopropane C19 : 0 cyclo (ω10c and/or ω6; 22.5 %) (Table 3). The total hydrolysate of the peptidoglycan of strain FI11369 contained muramic acid (Mur) and the amino acids diaminopimelic acid (Dpm), alanine (Ala) and glutamic acid (Glu) in a molar ration of 1.6 : 0.9 : 1.6 : 1. Enantiomeric analysis of the peptidoglycan amino acids revealed the presence of meso-Dpm. The partial hydrolysate contained peptides M-Glu, Ala-Glu, Glu-Dpm, Dpm-Ala, Glu-Dpm-Ala and Glu-Dpm-Ala-Dpm. These data strongly suggested that the peptidoglycan type of the strain was A1γ meso-Dpm direct.
Fig. 3.
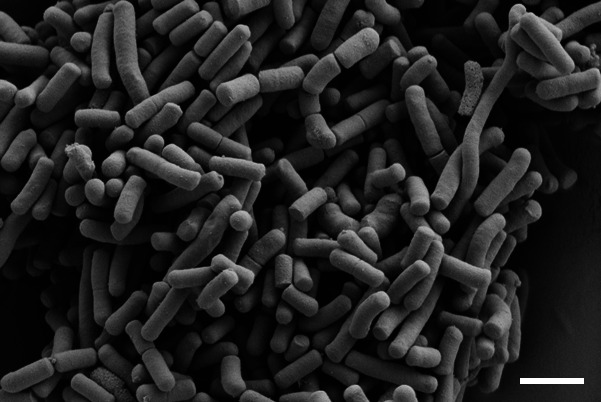
Scanning electron microscope image of strain FI11369T cells after 48 h incubation at 30 °C in MRS broth. Bar, 1 µm.
Table 2.
Distinctive features of the carbohydrate fermentation profiles of strain FI11369T and closest phylogenetically related species
Strains:1, FI11369T(data from this study); 2, Lactobacillus xiangfangensis DSM 27103T(data from this study); 3, Lactobacillus plajomi NBRC107333T [22]; 4, Lactobacillus modestisalitolerans NBRC 107235T [22]. +, Positive; −, negative; w, weak reaction; d, delayed (>72h).
|
Carbon source |
Strain |
|||
|---|---|---|---|---|
|
1 |
2 |
3 |
4 |
|
|
d-Xylose |
w |
w |
− |
− |
|
d-Adonitol |
− |
w |
− |
− |
|
d-Galactose |
w |
− |
+ |
+ |
|
Amygdalin |
+ |
− |
− |
− |
|
Arbutin |
+ |
− |
− |
− |
|
Aesculin |
+ |
d |
w |
w |
|
Salicin |
+ |
− |
+ |
+ |
|
Lactose |
− |
− |
− |
+ |
|
Melibiose |
− |
− |
− |
+ |
|
Raffinose |
− |
− |
− |
+ |
|
l-Arabitol |
− |
− |
+ |
− |
|
Gluconate |
− |
− |
+ |
+ |
Table 3.
Comparative fatty acid compositions of strains FI11369T and the closely related Lactobacillus species
Strains: 1, FI11369T (data from this study); 2, Lactobacillus xiangfangensis DSM 27103T (data from this study); 3, Lactobacillus plajomi NBRC107333T [24]; 4, Lactobacillus modestisalitolerans NBRC 107235T [22]. Values are percentages of total fatty acids. Fatty acids amounting to less than 0.5% of the total fatty acids are not shown. nd, not detected. The major components of cellular fatty acid are highlighted in bold.
|
Fatty acid |
Strain |
|||
|---|---|---|---|---|
|
1 |
2 |
3 |
4 |
|
|
Saturated: |
||||
|
C14 : 0 |
0.7 |
0.8 |
6.6 |
nd |
|
C16 : 0 |
18.2 |
20.0 |
11.4 |
13.0 |
|
C18 : 0 |
2.9 |
2.5 |
nd |
nd |
|
C19 : 0 iso |
2.0 |
nd |
nd |
nd |
|
Unsaturated: |
||||
|
C16 : 1 ω7c and/or ω6c |
1.1 |
0.9 |
2.6 |
nd |
|
C18 : 1 ω9c |
43.8 |
28.1 |
36.1 |
51.5 |
|
C18 : 1 ω6c and/or ω7c |
8.8 |
6.9 |
9.7 |
nd |
|
Cyclopropane: |
||||
|
C19 : 0 cyclo ω10c and/or ω6 |
22.5 |
40.8 |
33.7 |
35.6 |
Description of Lactobacillus garii sp. nov.
Lactobacillus garii (ga’ri.i. N.L. gen. n. garii of gari).
Cells of strain FI11369T are Gram-positive, non-motile, non-spore-forming, catalase-negative, straight rod-shaped, 1–2 µm long, and usually occur in pairs or in short chains. They are facultative anaerobes. Colonies grown aerobically on MRS agar at 30 °C for 48 h are irregular and umbonate. Strain FI11369T grows at 12–40 °C (weakly at 6 and 42 °C, optimum at 30–37 °C), at pH range 4.0–8.8 (optimum at pH 6) and with 0–8 % NaCl (delayed growth at 10 %, optimum in the absence of NaCl supplementation). Gas is not produced from glucose. Only d-lactate is synthesized from glucose. Acid is produced from d-ribose, d-xylose, d-galactose, d-glucose, d-fructose, d-mannose, d-mannitol, d-sorbitol, N-acetyl glucosamine, amygdalin, arbutin, aesculin, salicin, cellobiose, maltose, sucrose, trehalose and gentiobiose. Acid is not produced from glycerol, erythritol, d-arabinose, l-arabinose, l-xylose, d-adonitol, ß-d-xylopyranoside, l-sorbose, l-rhamnose, dulcitol, inositol, α-d-mannopyranoside, α-d-glucopyranoside, lactose, melibiose, inulin, melezitose, raffinose, starch, glycogen, xylitol, turanose, d-lyxose, d-tagatose, d-fucose, l-fucose, d-arabitol, l-arabitol, gluconate and 2- or 5-keto-gluconate. Strain FI11369T is positive for α-haemolytic activity, bile–aesculin test and Voges–Proskauer test and negative for pyruvate utilization, tellurite tolerance, hippurate hydrolysis, pyrrolidonylarylamidase production, deamination of arginine, H2S production, nitrate and nitrite reduction, urease production and gelatin hydrolysis. Cellular fatty acids mainly comprised saturated C16 : 0, unsaturated C18 : 1 ω9c and cyclopropane C19 : 0 cyclo (ω10c and/or ω6). Cells contain meso-diaminopimelic acid in their cell-wall peptidoglycan. The peptidoglycan type is A1γ meso-Dpm direct. The genome size of the type strain is 2 972 171 bp and the DNA G+C content is 48.3 mol%.
The type strain, FI11369T (=NCIMB 15148=DSM 108249), was isolated in the UK from gari produced in Ghana. The GenBank accession numbers of the 16S rRNA gene and the genome sequence of FI11369T are MN817919 and QWZQ00000000, respectively.
Funding information
This work was supported by the UK Biotechnology and Biological Sciences Research Council (BBSRC) via a Global Challenge Research Fund Data and Resources award and Institute Strategic Programmes for Food Innovation and Health (BB/R012512/1, and its constituent projects Theme 1 BBS/E/F/000PR10343 and Theme 3 BBS/E/F/000PR10346) and Gut Microbes and Health (BB/R012490/1, Theme 3 BBS/E/F/000PR10356). M.D. was the beneficiary of a Clarin COFUND outgoing grant (ACA17-16) co-funded by the 7th WP of the European Union, Marie Curie Actions and the FICyT Foundation.
Acknowledgements
The authors would like to thank Catherine Booth and Kathryn Cross in the Quadram Institute Bioscience, Norwich, UK, for scanning electron microscopy.
Author contributions
Maria Diaz: Conceptualization, Investigation, Visualization, Writing - original draft. Lizbeth Sayavedra: Writing – review and editing. Amy Atter: Resources. Shikha Saha: Investigation. Melinda J Mayer: Funding acquisition, Writing - review and editing. Wisdom Amoa-Awa: Resources, Writing - review and editing. Arjan Narbad: Funding acquisition, Writing - review and editing.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Abbreviations: aa, amino acid; ANI, average nucleotide identity; dDDH, digital DNA-DNA hybridization.
The 16S rRNA gene sequence accession number is MN817919. The genome sequence accession number is QWZQ00000000.
References
- 1.Salvetti E, Harris HMB, Felis GE, O'Toole PW. Comparative genomics of the genus Lactobacillus reveals robust phylogroups that provide the basis for reclassification. Appl Environ Microbiol. 2018;84:e00993–00918. doi: 10.1128/AEM.00993-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein EJC, Tyrrell KL, Citron DM. Lactobacillus species: taxonomic complexity and controversial susceptibilities. Clin Infect Dis. 2015;60 Suppl 2:S98–S107. doi: 10.1093/cid/civ072. [DOI] [PubMed] [Google Scholar]
- 3.Duar RM, Lin XB, Zheng J, Martino ME, Grenier T, et al. Lifestyles in transition: evolution and natural history of the genus Lactobacillus . FEMS Microbiol Rev. 2017;41:S27–S48. doi: 10.1093/femsre/fux030. [DOI] [PubMed] [Google Scholar]
- 4.Heeney DD, Gareau MG, Marco ML. Intestinal Lactobacillus in health and disease, a driver or just along for the ride? Curr Opin Biotechnol. 2018;49:140–147. doi: 10.1016/j.copbio.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franz CMAP, Huch M, Mathara JM, Abriouel H, Benomar N, et al. African fermented foods and probiotics. Int J Food Microbiol. 2014;190:84–96. doi: 10.1016/j.ijfoodmicro.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Kostinek M, Specht I, Edward VA, Schillinger U, Hertel C, et al. Diversity and technological properties of predominant lactic acid bacteria from fermented cassava used for the preparation of Gari, a traditional African food. Syst Appl Microbiol. 2005;28:527–540. doi: 10.1016/j.syapm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 7.De man JC, Rogosa M, Sharpe ME. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. doi: 10.1111/j.1365-2672.1960.tb00188.x. [DOI] [Google Scholar]
- 8.Wilson K. Preparation of genomic DNA from bacteria. Curr Protoc Mol Biol. 2001;Chapter 2:2.4.1–2.4.2. doi: 10.1002/0471142727.mb0204s56. [DOI] [PubMed] [Google Scholar]
- 9.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wattam AR, Davis JJ, Assaf R, Boisvert S, Brettin T, et al. Improvements to PATRIC, the all-bacterial bioinformatics database and analysis resource center. Nucleic Acids Res. 2017;45:D535–D542. doi: 10.1093/nar/gkw1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon S-H, Ha S-M, Kwon S, Lim J, Kim Y, et al. Introducing EzBioCloud: a taxonomically United database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol. 2017;67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 13.Chor B, Hendy MD, Snir S. Maximum likelihood Jukes-Cantor triplets: analytic solutions. Mol Biol Evol. 2006;23:626–632. doi: 10.1093/molbev/msj069. [DOI] [PubMed] [Google Scholar]
- 14.Wu M, Scott AJ. Phylogenomic analysis of bacterial and archaeal sequences with AMPHORA2. Bioinformatics. 2012;28:1033–1034. doi: 10.1093/bioinformatics/bts079. [DOI] [PubMed] [Google Scholar]
- 15.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price MN, Dehal PS, Arkin AP. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain C, Rodriguez-R LM, Phillippy AM, Konstantinidis KT, Aluru S. High throughput ani analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat Commun. 2018;9:5114. doi: 10.1038/s41467-018-07641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier-Kolthoff JP, Auch AF, Klenk H-P, Göker M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics. 2013;14:60. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, et al. DNA–DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol. 2007;57:81–91. doi: 10.1099/ijs.0.64483-0. [DOI] [PubMed] [Google Scholar]
- 20.Richter M, Rosselló-Móra R. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci U S A. 2009;106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu CT, Wang F, Li CY, Liu F, Huo GC. Lactobacillus xiangfangensis sp. nov., isolated from Chinese pickle. Int J Syst Evol Microbiol. 2012;62:860–863. doi: 10.1099/ijs.0.031468-0. [DOI] [PubMed] [Google Scholar]
- 22.Pitino I, Randazzo CL, Cross KL, Parker ML, Bisignano C, et al. Survival of Lactobacillus rhamnosus strains inoculated in cheese matrix during simulated human digestion. Food Microbiol. 2012;31:57–63. doi: 10.1016/j.fm.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Niu H, Chen Y, Xie J, Chen X, Bai J, et al. Ion-Exclusion chromatography determination of organic acid in uridine 5'-monophosphate fermentation broth. J Chromatogr Sci. 2012;50:709–713. doi: 10.1093/chromsci/bms046. [DOI] [PubMed] [Google Scholar]
- 24.Miyashita M, Yukphan P, Chaipitakchonlatarn W, Malimas T, Sugimoto M, et al. Lactobacillus plajomi sp. nov. and Lactobacillus modestisalitolerans sp. nov., isolated from traditional fermented foods. Int J Syst Evol Microbiol. 2015;65:2485–2490. doi: 10.1099/ijs.0.000290. [DOI] [PubMed] [Google Scholar]
- 25.Miller LT. Single derivatization method for routine analysis of bacterial whole-cell fatty acid methyl esters, including hydroxy acids. J Clin Microbiol. 1982;16:584–586. doi: 10.1128/JCM.16.3.584-586.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuykendall L, Roy M, O'neill J, Devine T. Fatty acids, antibiotic resistance, and deoxyribonucleic acid homology groups of Bradyrhizobium japonicum. International Journal of Systematic and Evolutionary Microbiology. 1988;38:358–361. [Google Scholar]
- 27.Schumann P. Methods in Microbiology. Elsevier; 2011. Peptidoglycan structure; pp. 101–129. [Google Scholar]