Graphical abstract
Abbreviation: Nt, nucleotide; miRNA, MicroRNA; EBoV, Ebola; GO, gene ontology
Keywords: COVID19, SARS-CoV2, In-silico, miRNA, Hairpin, Gene-ontology
Highlights
-
•
The rising epidemics of SARS-CoV2 has globally made a serious concern.
-
•
Thus, understanding the virus-host relationship remains a serious concern.
-
•
Here, we adopted the in-silico approach to picturized the similarities between the miRNAs of SARS-CoV2 genomes and human.
-
•
Further, the assessments and prediction of miRNAs helped us to analyze and understand the mechanism of pathogenesis.
Abstract
Background
The progressive SARS-CoV2 outbreaks worldwide have evoked global investigation. Despite the numerousin-silico approaches, the virus-host relationship remains a serious concern. MicroRNAs are the small non-coding RNAs that help in regulating gene profiling. The current study utilized miRNA prediction tools along with the PANTHER classification system to demonstrate association and sequence similarities shared between miRNAs of SARS-CoV2 and human host.
Method
An in-silico approach was carried out using Vmir analyzer to predict miRNAs from SARS-CoV2 viral genomes. Predicted miRNAs from SARS-CoV2 viral genomes were used for effective hybridization sequence identification along the nucleotide similarities with human miRNAs from miRbase database. Further, it was proceeded to analyze the gene ontology using miRDB with PANTHER classification.
Result
Based on the prediction and analysis, we have identified 22 potential miRNAs from five genomes of SARS-CoV2 linked with 12 human miRNAs. Analysis of human miRNAs hsa-mir-1267, hsa-mir-1-3p, hsa-mir-5683 were found shared between all the five viral SARS-CoV2 miRNAs. Further, PANTHER classification analyzed the gene-ontology being carried by these associations showed that 44 genes were involved in biological functions that includes genes specific for signaling pathway, immune complex generation, enzyme binding with effective role in the virus-host relationship.
Conclusion
Our analysis concludes that the genes identified in this study can be effective in analyzing the virus-host interaction. It also provides a new direction to understand viral pathogenesis with a probable new way to link, that can be used to understand and relate the miRNAs of the virus to the host conditions.
1. Introduction
The ongoing pandemic of COVID-19 which originated at Wuhan and its progressing rate of transmission into 188 countries and territories has created a havoc in the society declaring it as a global health emergency by WHO on 30 January 2020 (Rasmussen et al., 2020). Phylogenetically, SARS-CoV2 carries a positive-sense single-stranded RNA genome; included in the family Coronaviridae, order Nidovirales, and is a β Coronavirus of 2B group which approximately 30 kb size and shares 79.5 % with SARS-CoV and 96 % genome similarity with Bat Coronavirus, respectively (Wang et al., 2020). The clinical manifestations concerned with SARS-CoV2 are fever, dry cough, low or normal peripheral white blood cell count, and low lymphocyte commonly termed as novel coronavirus-infected pneumonia (NCIP) (Qin, 2020). Till date, the total confirmed cases were recorded as approximately 13.2 million including 5,75,540 deaths worldwide. Since then, the situation has been deteriorating in the European provinces and American regions, where the South Asian countries have also been carrying a worse COVID-19 burden.
Currently more than 400 genome sequences SARS-CoV2 are available at NCBI databases and that gives a plethora of information to aid the development of the drug and vaccine. Immuno-informatics combined with molecular approaches carried out so far to study the genome characteristics include comparison within various CoV genomes, structural behavior of different proteins in SARS-CoV2 and mutational variations (30) that have been identified along the SARS-CoV2 genomes (Ahmed et al., 2020; Silipo et al., 2015; ul Qamar et al., 2020). Further, researchers are specifically targeting the spike protein using various predicted B-cell and T-cell epitopes (Baruah and Bose, 2020; Bhattacharya et al., 2020; Feng et al., 2020; Kalita et al., 2020; Program and Sciences, 2018; Shanmugaraj et al., 2020). These studies were not only useful to understand host-pathogen interaction but also to design valuable antiviral therapeutics. Eventually, we tried to focus on a different way to understand the mechanism of pathogenesis in SARS-CoV2 and found that the studies on miRNAs and RNAi is useful in developing as an alternative approach, where the investigators reported many miRNAs mediating gene silencing activity (Saxena and Dwivedi, 2013). Even, these miRNAs helps in regulating diverse biological functions like development, apoptosis, tumorigenesis, proliferation, stress response, and fat metabolism (Chen et al., 2013; Mishra et al., 2020).
The miRNAs are short generally 19–26 bp in length, ∼22-nt non-coding RNA species that control the post-transcriptional genes level expressions. The RNA polymerase II helps in transcribing the gene profiling and to from primary miRNA in the nucleus (Kincaid et al., 2011; Kumar et al., 2018; Praianantathavorn et al., 2016; Sannigrahi et al., 2017; Yousef et al., 2009). After the miRNA is formed, the hairpin complex is transcribed and recognized by Drosha (RNase III enzyme) and DGCR8 (dsRNA-binding protein). After recognition, the pre-miRNA is exported from the nucleus to cytoplasm with the help of enzyme exportin-5 and Ran (Ras-related Nuclear protein). Soon in the cytoplasm, the pre-miRNA is converted to mature RNA i.e. ds RNA by RNase III ribonuclease Dicer into duplex mature RNA (Girardi et al., 2018; Mishra et al., 2020; Yadav et al., 2014). This duplex RNA with the help of RNA-induced silencing complex (RISC) targets the messenger RNA and degrades the translational activity. This complexity emerged between the 3′ untranslated regions (UTR) of miRNA and the seed region of miRNA (2−7bp) helps in cleaving and blocking the translations (Duygu et al., 2020; Tahi, 2012). The mechanism adopted by the virus to generate miRNA helps to altercate the relevant host. These miRNAs generated can be used to find pertinent targets. Subsequently, several studies have conferred and explored the miRNAs as antiviral therapies against HIV 1, HSV, Dengue, Influenza, and Hepatitis C (HCV) (Mallick et al., 2009; Yousef et al., 2009). The efficacy of miRNA based treatment is demonstrated and found promising in the case of HCV treatment (Mishra et al., 2020).
Currently, the prevention and treatment approaches to SARS-CoV2 are very limited due to complexity in their genome sequences. However, approaches like convalescent plasma therapy (CPT) has provided a promising result in this aspect (Duan et al., 2020). Further, to determine the viral pathogenesis and the outcome of the infection, it is necessary to understand the host-pathogen interactions. Although the studies between the host innate immune system and the role of miRNA-mediated RNA silencing in SARS-CoV2 infection has not been enlightened yet. So, our current approach of using Vmir analyzer in identifying the maximum possible miRNAs among the genome sequences in SARS-Cov2 will help to identify the potent and effective target that triggers the host-pathogen interactions. Further, the in-silico based techniques used here will be helpful to scrutinize the role of the human miRNA on SARS-CoV2. Thus, we collected SARS-CoV2 genomes from different geographical locations mainly from China, India, USA, Italy, South America (Jamaica) to identify the notable host miRNA targets identifications. Our analysis revealed various miRNAs accompanying the host-pathogen relationship which is necessary for its survival and replication. Further, the genes targeted by these miRNAs can provide a definitive insight into the pathways involved in infection. This approach of targeting the genomes will provide an innovative platform as an antiviral strategy and to understand the interactions network.
2. Materials and methods
2.1. Prediction of precursor miRNA
The SARS-CoV-2 miRNA prediction was carried out using the complete genome sequence of SARS-CoV2 carrying accession number (NC_045512.2, MT435086, MT339041, MT066156 and MT507794.1) for China, India, USA, Italy and Jamaica respectively; obtained from the National Centre for Biotechnology Information (NCBI). Briefly, the viral genomes were scanned for hairpin-structured miRNA precursors using the VMir Analyzer program. Vmir, an ab-intio prediction program that was designed specifically to identify pre-miRNA in the viral genome. The scanned hairpin was visualized in Vmir visualizer (Duygu et al., 2020; Sardar et al., 2020). The potential hairpin-like structures were extracted as a candidate’s miRNA precursor.
2.2. Prediction of Human miRNA from precursor miRNA hairpin
Human miRNA sequences are available in the miRBase database (http://www.mirbase.org). This is dependent on the average length of microRNA (∼22bp). Each of the Candidate precursor miRNAs was searched for the nucleotide similarity with all human miRNAs by using the SSEARCH (it is useful for finding a short sequence within the library of miRNA) menu of the miRbase database. According to the principal, each of the input viral hairpin segments was aligned with all of the microRNAs in the miRBase then the highly similar were identified as target miRNA. The mature duplex microRNA consisted of two strands of microRNA that are complementary to each other. The complementary strand of the target miRNA can be complementary to the input viral sequences and along with the hybridization between the viral gene fragments and complementary template of the potential miRNA, which will analyzed and sorted out using RNA hybrid (Hasan et al., 2014).
2.3. Hybridization between viral precursor miRNAs and human miRNAs
Energetically most favorable hybridization between target microRNA and viral miRNA was predicted by the RNA hybrid tool (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid). The results of RNA hybrid were categorized in terms of pairing energy (minimum free energy) and hybridization pattern. Four types of hybridization patterns were obtained from RNA hybrid analysis including 5′ canonical, 3′ compensatory, 5′ seed, and ineffective hybridization (Hasan et al., 2014; Yadav et al., 2014).
2.4. Identification of mature miRNAs from pre-miRNAs
Mature miRNAs were identified from pre-miRNAs sequences using Mature Bayes (http://mirna.imbb.forth.gr/MatureBayes.html), an online tool that uses Naive Bayes Classifier (NBC) taking into account of the sequences as well as structural information of experimental predicted miRNA precursors. All the potential pre-miRNAs identified by ViralmiR was used for analysis (Gkirtzou et al., 2010).
2.5. Criteria for selection of potent miRNA
According to the microRNA target prediction principle which requires the sufficient base pairing between the miRNA and target miRNAs that can be classified into 5′ canonical, 3′ compensatory, 5′ seed, and ineffective hybridization. The 5′ dominant classes of target sites can be divided into 2 subtypes: 5′ seed and 5′ canonical. Both indicate the effective base pairing within 2nd to 8th position from the 5′ end of miRNA. For 3′ compensatory pattern, the candidate miRNA should show half sequence from middle to 3′ end of miRNA that will perfectly match with miRNA. Pairing energy or minimum free energy (mef) indicating the stability of the hybridization. For the selection of potential miRNA, the pairing energy at -10 kcal/mol was utilized as a cut-off score. The miRNA targeting SARS-CoV2 genes with effective hybridization patterns (5′ canonical, 3′ compensatory, 5′ seed) and minimum free energy of -10 kcal/mol were selected as potential miRNA (Hasan et al., 2014; Sardar et al., 2020; Tahi, 2012; Yousef et al., 2009).
2.6. Prediction of the secondary structure of miRNA precursor
The RNAfold web server (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi) was used to predict the secondary structure of pre-miRNA. This program is used to predict the most stable secondary structure of SARS-CoV2 hairpin sequences. The sequence applied for prediction analysis included pre-miRNA about 200bp upstream and about 100bp downstream flanking sequences at each end of the precursor. In all the cases, folding structures with minimal free energy were depicted (Lorenz et al., 2016; Wang et al., 2019).
2.7. (Gene ontology) GO analysis
Gene ontology analysis of the retrieved target genes was performed using PANTHER (Protein Analysis through Evolutionary Relationships) classification system (http://www.pantherdb.org) to gain insight into the molecular function, biological process and cellular components of the target genes products. Gene ID’s of target genes were used for this analysis to find GO terms related to gene products (Chen and Glover, 2016; Dalmer and Clugston, 2019; Hu et al., 2019).
3. Results
3.1. Prediction of precursor miRNA (Pre-miRNA) hairpins with VMir analyser
SARS-CoV-2 viral genome was screened with VMir analyzer program and the result of VMir analyzer was visualized by VMir Viewer program which shows complete output graphically with sequence length and score Fig. 1 . Under default parameters (with a window size of 500 nt and step size of 10 nt), this ab-intio program scans the sequences and the analysis were performed across the sequences. The VMir analyzed and predicted all the possible miRNAs from the five genomes of SARS-CoV2 and it was depicted in Fig. 2 . The software predicted 633 (China), 625 (India), 630 (US), 632 (Italy) and 631 (Jamaica) candidate hairpins respectively. Further filtration after implementing the parameters such as minimum hairpin size 60 nt, maximum hairpin size 120 nt, minimum hairpin scores 115 and minimum window count 25 on the genome sequences NC_045512.2 (Chinese), MT435086 (Indian), MT339041 (US), MT066156 (Italian) and MT507794.1 (Jamaican) obtained a customized hairpin miRNAs structure which was found to be 52 (China, INDIA, US), 53 (Italy) and 51 (Jamaica) respectively (Hasan et al., 2014). Additionally, the Vmir analyzer predicted all possible hairpins either in direct orientation or in reverse orientation according to the location (x-axis) and Vmir score (y-axis).
Fig. 1.
Graphical view of VMir analysis of the SARS-CoV2 genome. A) The scatter plot of Vmir predictions of all possible hairpin pre-miRNAs. Simultaneously, the hairpin is plotted according to the positions of the viral genome under default parameters with a window size of 500 nt and step size of 10 nt. B) Customized view of predicted pre-miRNAs after filtering (minimum hairpin size: 60 nt, maximum hairpin size: 120 nt, minimum hairpin score: 115, and minimum window count: 25) were shown.
Fig. 2.
Prediction of possible miRNAs from SARS-CoV2 viral genomes and its association with human miRNAs. The picture shows the work flow of the steps taken to filter the miRNAs and the steps carried out to obtain the possible number of miRNAs. Here the (Red) depicts the filtered miRNAs from human (12) found to have association with SARS-CoV2. The miRNAs obtained from the viral genomes were further analyzed to observed their secondary structure and the possible gene ontologies.
3.2. Prediction of Human miRNA from precursor miRNA hairpin
The sequences of all the five viral SARS-CoV2 miRNA candidate precursors obtained was searched using SSEARCH menu the miRbase database for finding the nucleotide similarity with all human microRNAs using the default parameters (E-cut off value: 10, Maximum no. of hits: 100, Specific organism: human). This identified 22 sequences as a candidate miRNA precursor among the five viral miRNAs, based on the sequence similarity with human miRNAs. Subsequently, human miRNAs carrying a minimum of 19bp sequence similarity with candidate miRNA precursors were selected as prime target miRNAs as depicted in Table 1 . Similarly, the possible interaction shared between the human miRNAs and SARS-CoV2 viral miRNAs was shown with the help of diverging radial interaction plot Fig. 3 . Additionally, miRNAs seed region (2–7) were selected based on 3′ untranslated region of candidate miRNA precursor which was used to find the potential miRNA targets. In general; to depict a picture of gene silencing activity, the perfect complementarity between region 3′ untranslated (UTR) of the miRNA and the (2–7 bp) seed region of miRNA is significant. Additionally, the precursor miRNA hairpins were also classified accordingly based on directions respectively as MD (forward direction) and MR (the reverse direction).
Table 1.
Alignments between the precursor SARS-CoV2 miRNAs hairpin sequences with human miRNAs where effective hybridization pairing energy is also indicated.
| S No. | Origin | Hairpin | Hybridization paring energy mfe(kcal/mol) | Score | Alignment between human miRNA & SARS-CoV2 |
|---|---|---|---|---|---|
| 1 | China | MD 51 | −14.2 | 112.2 | |
| 2 | China | MD 306 | −33.5 | 118.8 | |
| 3 | China | MR231 | −16.0 | 111.2 | |
| 4 | China | MR304 | −19.8 | 64 | |
| 5 | India | MD 254 | −25.8 | 135.3 | |
| 6 | India | MR 248 | −20.8 | 118.8 | |
| 7 | India | MD 87 | −21.9 | 129.2 | |
| 8 | India | MD 157 | −23.6 | 117.0 | |
| 9 | India | MR 190 | −10.8 | 114.8 | |
| 10 | India | MR 137 | −17.6 | 112.4 | |
| 11 | USA | MD 52 | −14.2 | 112.2 | |
| 12 | USA | MD 83 | −20.8 | 129.2 | |
| 13 | USA | MD 143 | −20.2 | 123.7 | |
| 14 | USA | MD 154 | −23.6 | 117.0 | |
| 15 | USA | MR 143 | −10.8 | 112.4 | |
| 16 | Italy | MD 52 | −14.2 | 112.2 | |
| 17 | Italy | MD 154 | −23.6 | 117.0 | |
| 18 | Italy | MR 193 | −17.6 | 114.8 | |
| 19 | Italy | MR 140 | −10.8 | 112.4 | |
| 20 | Jamaica | MD 52 | −14.2 | 112.2 | |
| 21 | Jamaica | MD 302 | −33.5 | 118.8 | |
| 22 | Jamaica | MR 233 | −16.0 | 111.2 |
Fig. 3.
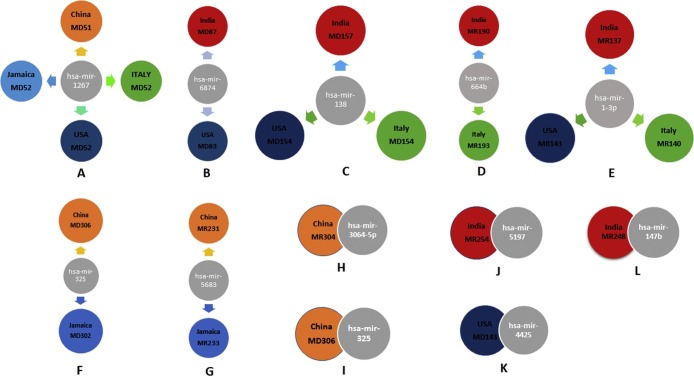
The diverging radial plot shows the common and unique miRNAs with sequence similarities found across the five viral SARS-CoV2 genomes and human. A-L) shows the miRNAs shared between the five SARS-CoV2 genomes from China, India, US, Italy and Jamaica which was found to be linked with the human miRNAs.
3.3. Hybridization between viral precursor miRNAs and human miRNAs
For effective hybridization between target human miRNAs and precursor miRNAs of SARS-CoV2, we implemented the RNAhybrid (http://bibiserv2.cebitec.uni-bielefeld.de/rnahybrid/) tool. It is a widely used miRNAs target prediction tool based on parameters depicted as the minimum free energy hybridization shared between the long and short RNAs. The minimum free energy (MFE) or pairing energy specifies the stability of hybridization. Along with, the potential miRNA carrying pairing energy of -10 kcal/mol is essential for an effective hybridization. According to the criteria obtained for selection, we found 4 effective pairings hsa-mir-1267, hsa-mir-325, hsa-mir-5683, hsa-mir-3064-5p among the China; 6 effective pairings hsa-mir-5197, hsa-mir-147b, hsa-mir-6874, hsa-mir-138-1, hsa-mir-664b, hsa-mir-1-3p among India, 5 including hsa-mir-1267, hsa-mir-6874, hsa-mir-4425, hsa-mir-138-1, hsa-mir-1-3p among the US; 4 interactions hsa-mir-1267, has-mir-138-1, has-mir-664b, hsa-mir-1-3p among Italy and finally 3 collective pairing hsa-mir-1267, hsa-mir-325, hsa-mir-5683 among Jamaica particularly the human miRNAs involved with viral SARS-CoV2 miRNAs Fig. 5.
Fig. 5.
Schematic diagram illustrating the mechanism of SARS-COV2 miRNAs driven pathogenesis. Briefly, after entering into the host/ human cell, the SARS-COV2 releases its genomic RNA, which is translated into proteins necessary for its RNA synthesis. Simultaneously, some of the RNAs are converted to miRNAs that targets specific host mRNAs, thereby altering the expression level of the genes as well as the pathways associated with them, thereby resulting in viral pathogenesis.
3.4. Identification of mature miRNA from pre-miRNA
After initial identification and validation, pre-miRNAs sequences were subjected to Mature Bayes for retrieving the small mature miRNAs. Large pre-miRNAs sequences were cleaved to short mature miRNAs of 22 nucleotide length. Mature miRNAs obtained were based on 5′ and 3′ stem location where we retrieved 10 mature miRNA from 5 pre miRNA from China; 12 mature miRNAs from 6 pre-miRNAs sequences of India, 10 mature miRNAs from 5 pre-miRNAs sequences of the US, 8 mature miRNAs from 4 pre-miRNAs of Italy and 6 mature miRNA from 3 pre-miRNA of Jamaica Table 2 . Depending on the assembly of RISC complex, one or both strands can serve as mature miRNA molecules, which was kept for further analysis.
Table 2.
Selected mature miRNAs of SARS-CoV2 predicted by MATUREBAYES database.
| Mature miRNA | Length | Location | Position | Sequence |
|---|---|---|---|---|
| COV2_MD3 | 22 | 5' | 38 | AAAAGGCGTTTTGCCTCAACTT |
| COV2_MD3 | 22 | 3' | 56 | ACTTGAACAGCCCTATGTGTGTTC |
| COV2_MD51 | 22 | 5' | 41 | CTTATTACAGAGCAAGGGCTGG |
| COV2_MD51 | 22 | 3' | 53 | CAAGGGCTGGTGAAGCTGCTAA |
| COV2_MD306 | 22 | 5' | 35 | TGATCCTTCGTGGACATCTTCG |
| COV2_MD306 | 22 | 3' | 52 | CTTCGTATTGCTGGACACCATC |
| COV2_MR231 | 22 | 5' | 26 | TCAGCAACACAGTTGCTGATTC |
| COV2_MR231 | 22 | 3' | 52 | TGATTCTCTTCCTGTTCCAAGC |
| COV2_MR304 | 22 | 5' | 15 | AAGAGTAGACTATATATCGTAA |
| COV2_MR304 | 22 | 3' | 54 | TTTATATAGCCCATCTGCCTTG |
| COV2_MD 254 | 22 | 5' | 24 | ACTACTTTAGATTCGAAGACCC |
| COV2_MD 254 | 22 | 3' | 37 | CGAAGACCCAGTCCCTACTTAT |
| COV2_MR 248 | 22 | 5' | 14 | CATTTCATCTGTGAGCAAAGGT |
| COV2_MR248 | 22 | 3' | 56 | AAACTTTTGTGCACAAATGAGG |
| COV2_MD 87 | 22 | 5' | 23 | TCAGGCATTAGTGTCTGATGTT |
| COV2_MD 87 | 22 | 3' | 40 | ATGTTGGTGATAGTGCGGAAGT |
| COV2_MD 157 | 22 | 5' | 39 | TATGGATCAAGAATCCTTTGGT |
| COV2_MD 157 | 22 | 3' | 54 | CTTTGGTGGTGCATCGTGTTGT |
| COV2_MR 190 | 22 | 5' | 16 | AATGTGTGGCATAAGAATAGAA |
| COV2_MR 190 | 22 | 3' | 64 | CACTACAAGGCTGTGCATCATA |
| COV2_MR137 | 22 | 5' | 22 | TTAGAGAAAGTGTGTCTCTTAA |
| COV2_MR137 | 22 | 3' | 39 | CTTAACTACAAAGTAAGAATCA |
| COV2_MD52 | 22 | 5' | 41 | CTTATTACAGAGCAAGGGCTGG |
| COV2_MD52 | 22 | 3' | 53 | CAAGGGCTGGTGAAGCTGCTAA |
| COV2_MD83 | 22 | 5' | 23 | TCAGGCATTAGTGTCTGATGTT |
| COV2_MD83 | 22 | 3' | 40 | ATGTTGGTGATAGTGCGGAAGT |
| COV2_MD143 | 22 | 5' | 7 | TTTTGCTTTCCATGCAGGGTGC |
| COV2_MD143 | 22 | 3' | 43 | AGCTTTGTGAAGAAATGCTGGA |
| COV2_MD154 | 22 | 5' | 39 | TATGGATCAAGAATCCTTTGGT |
| COV2_MD154 | 22 | 3' | 54 | CTTTGGTGGTGCATCGTGTTGT |
| COV2_MR143 | 22 | 5' | 22 | TTAGAGAAAGTGTGTCTCTTAA |
| COV2_MR143 | 22 | 3' | 39 | CTTAACTACAAAGTAAGAATCA |
| CoV2_MD52 | 22 | 5' | 41 | CTTATTACAGAGCAAGGGCTGG |
| CoV2_MD52 | 22 | 3' | 53 | CAAGGGCTGGTGAAGCTGCTAA |
| CoV2_MD154 | 22 | 5' | 39 | TATGGATCAAGAATCCTTTGGT |
| CoV2_MD154 | 22 | 3' | 54 | CTTTGGTGGTGCATCGTGTTGT |
| CoV2_MR193 | 22 | 5' | 16 | AATGTGTGGCATAAGAATAGAA |
| CoV2_MR193 | 22 | 3' | 64 | CACTACAAGGCTGTGCATCATA |
| COV2_MR140 | 22 | 5' | 22 | TTAGAGAAAGTGTGTCTCTTAA |
| COV2_MR140 | 22 | 3' | 39 | CTTAACTACAAAGTAAGAATCA |
| COV2_MD52 | 22 | 5' | 41 | CTTATTACAGAGCAAGGGCTGG |
| COV2_MD52 | 22 | 3' | 53 | CAAGGGCTGGTGAAGCTGCTAA |
| COV2_MD302 | 22 | 5' | 35 | TGATCCTTCGTGGACATCTTCG |
| COV2_MD302 | 22 | 3' | 52 | CTTCGTATTGCTGGACACCATC |
| COV2_MR233 | 22 | 5' | 26 | TCAGCAACACAGTTGCTGATTC |
| COV2_MR233 | 22 | 3' | 42 | TGATTCTCTTCCTGTTCCAAGC |
3.5. Prediction of secondary structure of miRNA precursor
The RNAfold web server was used to predict the secondary structure of pre-miRNA. Expanded sequence dependence thermodynamic parameters were used to observe the hairpin structure of SARS-CoV2 (Mathews et al., 1999). Further, the minimum free energy (mfe) and partition function along with avoid isolated base pairs (isolated base pairs predicted structures contains isolated base pairs helices of length 1 which may be undesirable) were selected as a fold algorithm. Subsequently, the RNAfold program was able to predict the most stable and standardized secondary structure. The pre-miRNA about 200bp upstream and 100bp downstream flanking sequences at each end of the precursor were used for analyzing. Further, based on the prediction and hybridization between the viral miRNA and human miRNA scenario, we analyzed the folding structures along with the centroid regions of the viral miRNA; which was found to be relevant for interactions and targeting. The following depicted centroid structures MD 51, MD 306, MR 231, MR304 (China); MD 254, MR 248, MD 87, MD 157, MR 190, MR137 (India), MD 52, MD 83, MD 143, MD 154 (US); MD 143, MD 52, MD 154, MR 193, MR140 (Italy) and MD 52, MD 302, MR233 (Jamaica) were depicted in Fig. 4 respectively.
Fig. 4.
Predicated secondary structure of potential hairpins candidate of SARS-CoV2 from India, US, Italy, China and Jamaica. Only centroid structures were depicted from A) India a) MD 254 b) MR248 c) MD87 d) MD157 e) MR190 f) MR137; B) US g) MD52 h) MD83 i) MD143 j) MD154; C) Italy k) MD143 l) MD52 m) MD154 n) MR193 o) MR140; D) China p) MD51, q) MD306, r) MR231, s) MR304 and E) Jamaica t) MD52 u) MD302 v) MR233.
3.6. Depiction of molecular, biological and cellular characterization
Target prediction by miRDB for all the viral SARS-CoV2 miRNAs gave detailed and precise data of all the collection of genes that can be targeted in the human genome. Basically, the server uses the MirTarget algorithm, which is based on a 7-mer seeding approach and further predicts the miRNAs targets among 3′ UTRs of the human genes. Thus, we selected target genes with miRDB score >80 because a predicted target with a prediction score >80 is most likely to be real and not required any other supporting shreds of evidence. Finally, we proceeded through the PANTHER classification system to analyze the gene ontology and their involvement in different clusters of molecular functions, cellular components, and biological processes. This clustering was helpful to prove the significant determinant target gene involved in molecular functions, cellular components, and biological processes supplementary (file 1 & 2).
4. Discussion
The SARS-CoV2 has become a serious public threat globally and has drawn much attention because of its associations with mortality and the development of vaccines (Ahmed et al., 2020; Shanmugaraj et al., 2020). Despite this, much effort has been carried out in studying the detailed mechanism of the SARS-CoV2 pathogenesis. Many in-silico works were reported regarding this aspect but a similar bioinformatics approach was carried out where it involved in identifying the potential miRNAs for MERS-CoV. This study ruled out the possibility of the MERS- CoV’s miRNAs in locating significant importance to human miRNAs but the study does not determine the gene involvement with the pathogenic conditions (Hasan et al., 2014). Further, to gain empathy and understand more about the nature of pathogenesis; identification of the novel miRNAs along with the gene-ontology encoded by SARS-CoV2 became one of the preliminary aims. In recent years, these miRNAs have emerged as an important intervention in biomedical research because of its involvement in several biological phenomena. So, studying virus-encoded miRNAs can therefore become an important platform to develop and understand the viral-host relationship which could be better aimed for therapeutics (Biology et al., 2016; Duygu et al., 2020; Ghosh et al., 2007; Girardi et al., 2018; Grundhoff, n.d.; Tahi, 2012; Yadav et al., 2014; Yousef et al., 2009).
It was already reported from the studies that the human miRNAs target viral genes and functions as antiviral mediators to suppress the viral pathogenesis. By silencing those genes, human miRNAs ensure the prevention of complicated events. A study was carried out in ZIKA virus infection where it suggested that the miRNAs in astrocytes are dysregulated followed by the dysregulation of host miRNAs during the infection; which in turn halts the host gene functions (Karim, 2019). On the contrary, one of the most exciting aspects comes from the virus-encoded miRNAs, providing their role in the survival and targeting the host genes where it successfully activates the host immune system (Bernier and Sagan, 2018). In this regard, the host has developed various defenses against viruses such as the miRNA mediated host gene silencing is one of the strategies. Silencing the host genes provides the virus to invade the defenses adopted by the host, to replicate within the host and to avoid antiviral strategies (Russo and Potenza, 2011). Further, to investigate whether the SARS-CoV2 can mediate and target the host genes we adopted this approach to find the potential role of the host genes in virus survival and replications.
Initially, 1600 pre-miRNAs were obtained by Vmir (Grundhoff et al., 2006). Previously, the miRNAs encoded in Epstein Barr Virus (EBV) were reported to be 24, which was obtained by Vmir (Griffiths-Jones et al., 2008). Even, some ZIKA miRNAs targeted genes were identified which was found to be associated with the cell cycle process, cell communication, immune system regulation, etc. Halting these processes can have an immediate response in the viral transport, immune evasion which was found to be essential in ZIKA virus replications (Cristina et al., 2016; Karim, 2019). Takin into account, we utilized a series of bioinformatics tools, which predicted 22 viral miRNAs against the human genome and based on our computational investigations, we hypothesize those miRNAs having a significant role in understanding the host-pathogen relationship. The human miRNAs hsa-mir-5197, hsa-mir-147b, hsa-mir-6874, hsa-mir-138-1, hsa-mir-664b, hsa-mir-1267, hsa-mir-4425, hsa-mir-1-3p, hsa-mir-5197, hsa-mir-325, hsa-mir-5683 and hsa-mir-3064-5p were found to show perfect complementarity and identity with the virus SARS-CoV2 miRNAs.
To gain more insights into the associate functions with their gene’s involvement; PANTHER classification was adopted to understand the relationship between the predicted miRNAs shared between the SARS-CoV2 and human. With respect to molecular functions, cluster target genes products were depicted to play a role in protein binding (GO:0005515), enzyme binding (GO:0019899), protein domain specific binding (GO:0019904), protein kinase activity (GO:0004672) and Rho-GTPase binding (GO: 0017048). These specific functions are required to lead to an abnormal state in the body. Further, the cellular response implicated by the gene (GO:0009987) implies the pathological damage. This has been reported earlier with Hepatitis C virus infection along with fatty liver conditions. However, in SARS-CoV2; only 2–11 % infected patients carry liver morbidities but remains indistinct whether the liver damage in COVID-19 patients was caused by a viral infection or drug toxicity (Liu et al., n.d.). The analysis can be based on the phenomenon that liver dysfunction can be impaired by SARS-CoV2 viral infection through the release of viral miRNA. Subsequently, biological process cluster classifications also predicted involvement of the genes linked to immune system (GO:0002520), response to cytokine (GO:0034097), biological adhesion (GO:0065007) and the regulation of signaling pathways (GO:0009966). These combined biological functions are necessary to implement a significant defense against viral infection. Finally, the cellular component classification suggested the target gene products i.e. immunoglobulin generation (GO:0019814), bounding to organelle (GO:0098588). This PANTHER classification system predicted the gene functions on a wide-scale but there occurs always a phenomena where some viruses might downregulate host genes expression to increase their gene expression either in the nucleus or in the cytoplasm (Chen et al., 2013; Dombkowski et al., n.d.; Mishra et al., 2020). Another phenomenon associated is the basal transcription machinery of the SARS-CoV2 (TAF’s) of TFIID complex which could prevent the RNA polymerase II to assemble on the promoters of the host genes at the initiation step that can specifically block the transcription. Therefore, the suppression of such genes could lead to a turnover and provide opportunities for viral miRNA to escape degradation. Similarly, the genes predicted in our study also bear some similar features with the genes associated with basal cell carcinoma which was primarily involved in biological adhesions and differentiation (Dombkowski et al., 2020). Additionally, a similar study was carried out to predict the interactions along the miRNA of MERS-CoV and human miRNAs hsa-miR-628-5p, hsa-miR-18a-3p, and hsa-miR-332-3p (Hasan et al., 2014). Even, the hsa-mir-1-3p found in our study was also prominent in EboV ensuring the cellular signaling pathways and immune response dysregulations (Mishra et al., 2020). Further, the functional annotation also carries some of the genes involved in signaling pathway (GO:0048014) where it has been reported that the signaling gene coding from the region CXCL16 for salmonella has been found to elicit cell-mediated immunity via IFN-ϒ production, further ARRB2 coding for signal gene plays an important role in inflammation evoking T-lymphocytes and cytokine production in lungs (Khan et al., 2020; Liu et al., n.d.). Simultaneously, genes of highest enrichment (GO:0002520, GO: 0015031) were found to be similar to MERS-CoV and SARS-CoV, which was found to be associated as virus-encoded miRNAs in the metabolic process (Khan et al., 2020). The miRNA’s also act as a mediator against the viral evasion, thereby controlling the apoptosis (Cullen, 2013; Riley et al., 2012; Yang et al., 2013). Particularly, GO:0042981 a gene predicted from our study was found to be involved in the regulation of apoptosis, where it was originated to be in close association with the infected cell. Additionally, the SARS-CoV2 also initiates various pathways; among them is the Rho-GTPase pathways which have been found to increase the vulnerability of infection among the infected host (Cullen, 2013; Heidbreder et al., 2010; Yang et al., 2013). Similar gene GO:0017048 predicted and analysed in our study were found to be involved in the Rho-GTPase binding. Finally, the gene’s (GO:0048014, GO:0032879, GO:0007186, GO:0034097) were predicted to be associated with various functions like regulation of Tie signaling pathways, regulation of localization, G-protein-couples receptor signaling and response to cytokine respectively. All these associated functions were found to be mostly involved in the regulation and survival of viral infection in the host. Apart from that these miRNAs encoded genes have been found to be linked in the prognosis assessment of the SARS-CoV2 infections (Lukassen et al., 2020; Schneider et al., 2020; Vigorito et al., 2007; Zhou et al., 2014). Additionally, the human miRNAs hsa-mir-1267, hsa-mir-1-3p, hsa-mir-5683 were found prominent in all the five genomes of SARS-CoV2 predicted miRNAs; where the human miRNA hsa-mir-1267 shares the similarities with China, Italy, US and Jamaica; human miRNA hsa-mir-1-3p with India, US, Italy and hsa-mir-5683 with China and Jamaica respectively based on nucleotide similarity and gene ontology. These also define and predict the sequence similarities shared between the five SARS-CoV2 genomes being identified. So, our analysis resulted in the prediction of 44 essential genes involved with the biological functions, 13 with the molecular functions most prominently in protein binding and 13 genes with cellular components where one recognizing the immunoglobulin complex. Basically, the study carried out gave outline information about the dependence and involvement in survival and replication of SARS-CoV2 inside the human host. Further the insight analysis of miRNAs shared between the SARS-CoV2 and human also came into limelight. In conclusion, we propose the virus-host relationship in terms of miRNAs and its associated genes which may involve the upregulation or downregulations accompanying the dependent mechanisms in survival and replication of viruses.
Based on data obtained in our computational approach and its analysis, we designed a mechanism of SARS-CoV2 pathogenesis through miRNA associated pathways. After the entry into the host cell cytoplasm, the SARS-CoV2 releases its genomic RNA, which utilizes the host machinery for its survival and replications Fig. 5. Probably the possible interactions which were predicted in our study may be helpful in mediating the association pathways in the host which accelerates the pathogenic conditions. Simultaneously, a phylogenetic tree was constructed to show the sequence similarities among the data obtained from the miRNA sequences of the SARS-CoV2 Fig. 6 .
Fig. 6.
The tree suggests the neighbourhood relationship shared among the miRNA sequences of SARS-CoV2 viral genomes. Briefly, the miRNA sequences in SARS-CoV2 genomes become clusters i.e. (US MD154, Italy MD154, India MR190, India MD157); (India MD87, US MD83); (China MD306, Jamaica MD302); (China MR231, Jamaica MR233); (US MR143, Italy MR140, India MR137) and (China MD51, US MD52, Italy MD52, Jamaica MD52). The evolutionary history was inferred using the UPGMA method. The optimal tree with the sum of branch length = 3.41800150 is shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the p-distance method and are in the units of the number of base differences per site. This analysis involved 22 nucleotide sequences. Codon positions included were 1st + 2nd+3rd + Noncoding. All ambiguous positions were removed for each sequence pair (pairwise deletion option). There was a total of 105 positions in the final dataset. Evolutionary analyses were conducted in MEGA X.
5. Conclusion
In this study, we portrayed the pathogenesis of SARS-CoV2 through miRNAs thereby mediating a generalized picture of the putative miRNAs shared between the human genome and SARS-CoV2. The analysis was further carried out to identify the role of the essential genes involved in the survival and replication of the virus inside the host cytoplasm. Thereby our analysis predicted several novel miRNAs of SARS-CoV2 and as expected, the genes targeted by the miRNAs were found to be mostly involved in various biological and molecular functions. Additionally, the predicted 22 SARS-CoV2 miRNAs linked or shared with 12 human miRNAs can be utilized to understand the relationship with the virus. A much-needed work has to be carried out in this area but still, our findings provided a new insight to understand viral pathology. Last but not the least, a detailed experimental evidence is required to provide an assessment of these identified miRNAs to understand the viral replications more in-depth.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.compbiolchem.2020.107352.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Ahmed S.F., Quadeer A.A., McKay M.R. Preliminary identification of potential vaccine targets for the COVID-19 Coronavirus (SARS-CoV-2) based on SARS-CoV Immunological Studies. Viruses. 2020:12. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruah V., Bose S. Immunoinformatics-aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019-nCoV. J. Med. Virol. 2020;92:495–500. doi: 10.1002/jmv.25698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier A., Sagan S.M. The diverse roles of microRNAs at the host–virus interface. Viruses. 2018;10:1–26. doi: 10.3390/v10080440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M., Sharma A.R., Patra P., Ghosh P., Sharma G., Patra B.C., Lee S.S., Chakraborty C. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): immunoinformatics approach. J. Med. Virol. 2020;92:618–631. doi: 10.1002/jmv.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biology C., Hu Y., Song J., Liu L., Li J., Tang B., Wang J., Zhang X., Zhang Y., Wang L., Liao Y., He Z., Li Q. The International Journal of Biochemistry Different microRNA alterations contribute to diverse outcomes following EV71 and CA16 infections : insights from high-throughput sequencing in rhesus monkey peripheral blood mononuclear cells. Int. J. Biochem. Cell Biol. 2016;81:20–31. doi: 10.1016/j.biocel.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Chen C.J., Cox J.E., Kincaid R.P., Martinez A., Sullivan S. Divergent MicroRNA Targetomes of Closely Related Circulating Strains of a Polyomavirus. Journal of Virology) Americam society of Microbiology. 2013;87:11135–11147. doi: 10.1128/JVI.01711-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.E., Glover G.H. Large-scale gene function analysis with PANTHER Classification System. Nature protocols. 2016;25:289–313. doi: 10.1007/s11065-015-9294-9.Functional. [DOI] [Google Scholar]
- Cristina J., Echeverría N., Gambaro F., Fajardo A. 2016. Genome-wide Prediction of microRNAs in Zika Virus Genomes Reveals Possible Interactions With Human Genes Involved in the Nervous System Development. [Google Scholar]
- Cullen B.R. review MicroRNAs as mediators of viral evasion of the immune system. Nature Immunology. 2013;14:205–210. doi: 10.1038/ni.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmer T.R.A., Clugston R.D. Gene ontology enrichment analysis of congenital diaphragmatic hernia-associated genes. Pediatr. Res. 2019;85:13–19. doi: 10.1038/s41390-018-0192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombkowski, A.A., Sultana, Z., Craig, D.B., Jamil, H., n.d. Cancer Informatics In silico Analysis of Combinatorial microRNA Activity Reveals Target Genes and Pathways Associated with Breast Cancer Metastasis 13–29. https://doi.org/10.4137/CIN.S6631. [DOI] [PMC free article] [PubMed]
- Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Yong, Peng C., Yuan M., Huang J., Wang Z., Yu J., Gao X., Wang D., Yu X., Li L., Zhang J., Wu X., Li B., Xu Y., Chen W., Peng Y., Hu Yeqin, Lin L., Liu X., Huang S., Zhou Z., Zhang L., Wang Y., Zhang Z., Deng K., Xia Z., Gong Q., Zhang W., Zheng X., Liu Y., Yang H., Zhou D., Yu D., Hou J., Shi Z., Chen S., Chen Z., Zhang X., Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. 2020;117 doi: 10.1073/pnas.2004168117. 202004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duygu M., Demİrcİ S., Adan A. 2020. Computational Analysis of microRNA-mediated Interactions in SARS- CoV-2 Infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Qiu M., Zou S., Li Y., Luo K., Chen R., Sun Y., Wang K., Zhuang X., Zhang S., Chen S., Mo F. Multi-epitope vaccine design using an immunoinformatics approach for 2019 novel coronavirus in China (SARS-CoV-2) bioRxiv. 2020 doi: 10.1101/2020.03.03.962332. 2020.03.03.962332. [DOI] [Google Scholar]
- Ghosh Z., Chakrabarti J., Mallick B. miRNomics — The bioinformatics of microRNA genes. Biochemical and Biophysical Research Communications. 2007;363:6–11. doi: 10.1016/j.bbrc.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Girardi E., López P., Pfeffer S., Sullivan C. On the Importance of Host MicroRNAs During Viral Infection. Frontiers in genetics. 2018;9:1–17. doi: 10.3389/fgene.2018.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkirtzou K., Tsamardinos I., Tsakalides P., Poirazi P. MatureBayes: a probabilistic algorithm for identifying the mature miRNA within novel precursors. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S., Saini H.K., Van Dongen S., Enright A.J. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:154–158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundhoff, A., n.d. Chapter 8 Computational Prediction of Viral miRNAs 721, 143–152. https://doi.org/10.1007/978-1-61779-037-9. [DOI] [PubMed]
- Grundhoff A., Sullivan C.S., Ganem D. A combined computational and microarray-based approach identifies novel microRNAs encoded by human gamma-herpesviruses. RNA. 2006;12:733–750. doi: 10.1261/rna.2326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M., Akter R., Ullah S., Abedin J., Ullah G.M.A., Hossain Z. 2014. A Computational Approach for Predicting Role of Human MicroRNAs in MERS-CoV Genome 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder C.E., Shi F., Zhou X. Downregulation of the Rho GTPase signaling pathway is involved in the microRNA-138-mediated inhibition of cell migration and invasion in tongue squamous cell carcinoma. International Journal of Cancer. 2010;512:505–512. doi: 10.1002/ijc.25320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Liao Y., Zheng J., Gou L., Regmi A., Zafar M.I., Chen L. In silico integration approach reveals key MicroRNAs and their target genes in follicular thyroid carcinoma. Biomed Res. Int. 2019;2019 doi: 10.1155/2019/2725192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalita P., Padhi A.K., Zhang K.Y.J., Tripathi T. 2020. Design of a Peptide-based Subunit Vaccine Against Novel Coronavirus SARS-CoV-2 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim M. 2019. In Silico Analysis Revealed Zika Virus miRNAs Associated With Viral Pathogenesis Through Alteration of Host Genes Involved in Immune Response and Neurological Functions; pp. 1–11. [DOI] [PubMed] [Google Scholar]
- Khan A., Sany R.U., Islam S. 2020. Epigenetic Regulator miRNA Pattern Differences Among SARS- CoV, SARS-CoV-2 and SARS-CoV-2 World-wide Isolates Delineated the Mystery Behind the Epic Pathogenicity and Distinct Clinical Characteristics of Pandemic COVID-19 * Correspondence : Dr. Abul Bas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid R.P., Burke J.M., Sullivan C.S. 2011. RNA Virus microRNA That Mimics a B-cell oncomiR 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Ashish, Kumar Akhilesh, Ingle H., Kumar S., Mishra R., Verma K., Biswas D. 2018. Crossm MicroRNA hsa-miR-324-5p Suppresses H5N1 Virus Replication; pp. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z., Wang, J., Xu, Y., Guo, M., Mi, K., Xu, R., Pei, Y., Zhang, Q., n.d. Implications of the virus-encoded miRNA and host miRNA in the pathogenicity of SARS-CoV-2.
- Lorenz R., Wolfinger M.T., Tanzer A., Hofacker I.L. Predicting RNA secondary structures from sequence and probing data. Methods. 2016;103:86–98. doi: 10.1016/j.ymeth.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Lukassen S., Chua R.L., Trefzer T., Kahn N.C., Schneider M.A., Muley T., Winter H., Meister M., Veith C., Boots A.W., Hennig B.P., Kreuter M., Conrad C. 2020. SARS-CoV- 2 Receptor ACE 2 and TMPRSS 2 Are Primarily Expressed in Bronchial Transient Secretory Cells 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick B., Ghosh Z., Chakrabarti J. 2009. MicroRNome Analysis Unravels the Molecular Basis of SARS Infection in Bronchoalveolar Stem Cells; p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews D.H., Sabina J., Zuker M., Turner D.H. 1999. Expanded Sequence Dependence of Thermodynamic Parameters Improves Prediction of RNA Secondary Structure. [DOI] [PubMed] [Google Scholar]
- Mishra R., Kumar A., Ingle H., Kumar H. 2020. The Interplay Between Viral-Derived miRNAs and Host Immunity During Infection 10; pp. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praianantathavorn K., Jinato T., Poovorawan Y. 2016. Human microRNAs Profiling in Response to Influenza a Viruses; pp. 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Program B., Sciences N. 2018. In Silico B-cell and T-cell Epitope-based Vaccine Designing Against Chikungunya Virus. [Google Scholar]
- Qin Q. 2020. Unique Epidemiological and Clinical Features of the Emerging 2019 Novel Coronavirus Pneumonia (COVID-19) Implicate Special Control Measures; pp. 0–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S.A., Smulian J.C., Lednicky J.A., Wen T.S., Jamieson D.J. Coronavirus Disease 2019 (COVID-19) and Pregnancy: what obstetricians need to know. Am. J. Obstet. Gynecol. 2020:2019. doi: 10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley K.J., Rabinowitz G.S., Yario T.A., Luna J.M., Darnell R.B., Steitz J.A. latency. EMBO J. 2012;31:2207–2221. doi: 10.1038/emboj.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A., Potenza N. Antiviral effects of human microRNAs and conservation of their target sites. FEBS Lett. 2011;585:2551–2555. doi: 10.1016/j.febslet.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Sannigrahi M.K., Sharma R., Singh V., Panda N.K. 2017. Role of Host miRNA Hsa-miR-139-3p in HPV-16 – Induced Carcinomas; p. 23. [DOI] [PubMed] [Google Scholar]
- Sardar A.R., Satish D., Birla S., Gupta D. 2020. Comparative Analyses of SAR-CoV2 Genomes From Different Geographical Locations and Other Coronavirus Family Genomes Reveals Unique Features Potentially Consequential to Host-virus Interaction and Pathogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena V.L., Dwivedi A. In silico identification of miRNAs and their target prediction from Japanese encephalitis. Journal of Bioinformatics and Sequence Analysis. 2013;5:25–33. doi: 10.5897/JBSA2012.0056. [DOI] [Google Scholar]
- Schneider M.A., Muley T., Winter H., Meister M., Boots A.W., Hennig B.P., Kreuter M., Eils R. 2020. SARS-CoV-2 Receptor ACE2 and TMPRSS2 Are Predominantly Expressed in a Transient Secretory Cell Type in Subsegmental Bronchial Branches AUTHORS. [Google Scholar]
- Shanmugaraj B., Siriwattananon K., Wangkanont K., Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19) Asian Pacific J. allergy Immunol. 2020;38:10–18. doi: 10.12932/AP-200220-0773. [DOI] [PubMed] [Google Scholar]
- Silipo A.T., Planinsic R.M., Wittwer E.D., Sprung J., Nicholson W.T. 1 + 1 + 1 + 1 =? A Case Approach to Perioper. Drug-Drug Interact. 2015;000:123–128. doi: 10.1007/978-1-4614-7495-1_23. [DOI] [Google Scholar]
- Tahi F. A fast ab-initio method for predicting miRNA precursors in genomes. Nucleic Acids Research. 2012;40:1–9. doi: 10.1093/nar/gks146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ul Qamar M.T., Alqahtani S.M., Alamri M.A., Chen L.-L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J. Pharm. Anal. 2020 doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito E., Perks K.L., Abreu-goodger C., Bunting S., Xiang Z., Kohlhaas S., Das P.P., Miska E.A., Rodriguez A., Bradley A., Smith K.G.C., Rada C., Enright A.J., Toellner K., Maclennan I.C.M., Turner M. 2007. Article microRNA-155 Regulates the Generation of Immunoglobulin Class-Switched Plasma Cells; pp. 847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Liu Y., Zhong X., Liu H., Lu C., Li C., Zhang H. DMFold: a novel method to predict RNA secondary structure with pseudoknots based on deep learning and improved base pair maximization principle. Front. Genet. 2019;10:1–12. doi: 10.3389/fgene.2019.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang Y., Ye D., Liu Q. A review of the 2019 Novel Coronavirus (COVID-19) based on current evidence. Int. J. Antimicrob. Agents. 2020:105948. doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S., Singh A., Verma S.K. 2014. Computational Identification of MicroRNAs for Targeting Long and Short Segments of Lassa Virus. [Google Scholar]
- Yang G., Huang T., Peng L., Yang C., Liu R., Huang H. Epstein-Barr Virus _ Encoded LMP1 Upregulates MicroRNA-21 to Promote the Resistance of Nasopharyngeal Carcinoma Cells to Cisplatin-Induced Apoptosis by Suppressing PDCD4 and Fas-L. Plos one. 2013;8:1–15. doi: 10.1371/journal.pone.0078355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef M., Showe L., Showe M. MINIREVIEW A study of microRNAs in silico and in vivo : bioinformatics approaches to microRNA discovery and target identification. The FEBS Journal. 2009;276:2150–2156. doi: 10.1111/j.1742-4658.2009.06933.x. [DOI] [PubMed] [Google Scholar]
- Zhou A., Li S., Wu J., Ahmed F., Zhang S. Interplay between microRNAs and host pathogen recognition receptors (PRRs) signaling pathways in response to viral infection. Virus Res. 2014;184:1–6. doi: 10.1016/j.virusres.2014.01.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.