Abstract
Background & Aims
The incidence and outcomes of coronavirus disease 2019 (COVID-19) in immunocompromised patients are a matter of debate.
Methods
We performed a prospective nationwide study including a consecutive cohort of liver transplant patients with COVID-19 recruited during the Spanish outbreak from 28 February to 7 April, 2020. The primary outcome was severe COVID-19, defined as the need for mechanical ventilation, intensive care, and/or death. Age- and gender-standardised incidence and mortality ratios (SIR and SMR) were calculated using data from the Ministry of Health and the Spanish liver transplant registry. Independent predictors of severe COVID-19 among hospitalised patients were analysed using multivariate Cox regression.
Results
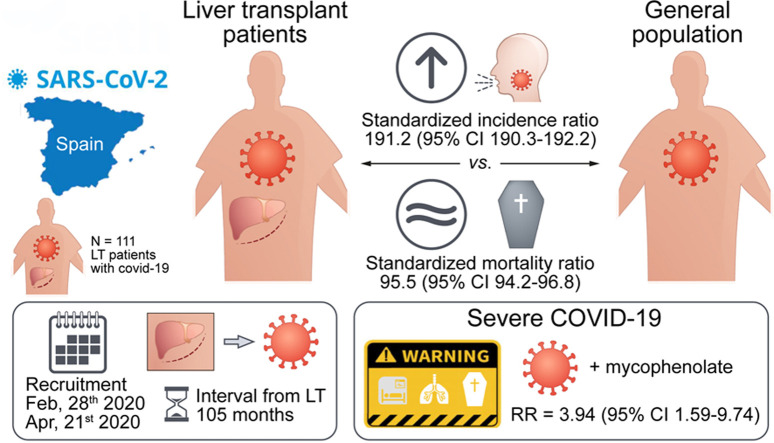
A total of 111 liver transplant patients were diagnosed with COVID-19 (SIR = 191.2 [95% CI 190.3–192.2]). The epidemiological curve and geographic distribution overlapped widely between the liver transplant and general populations. After a median follow-up of 23 days, 96 patients (86.5%) were admitted to hospital and 22 patients (19.8%) required respiratory support. A total of 12 patients were admitted to the ICU (10.8%). The mortality rate was 18%, which was lower than in the matched general population (SMR = 95.5 [95% CI 94.2–96.8]). Overall, 35 patients (31.5%) met criteria of severe COVID-19. Baseline immunosuppression containing mycophenolate was an independent predictor of severe COVID-19 (relative risk = 3.94; 95% CI 1.59–9.74; p = 0.003), particularly at doses higher than 1,000 mg/day (p = 0.003). This deleterious effect was not observed with calcineurin inhibitors or everolimus and complete immunosuppression withdrawal showed no benefit.
Conclusions
Being chronically immunosuppressed, liver transplant patients have an increased risk of acquiring COVID-19 but their mortality rates are lower than the matched general population. Upon hospital admission, mycophenolate dose reduction or withdrawal could help in preventing severe COVID-19. However, complete immunosuppression withdrawal should be discouraged.
Lay summary
In liver transplant patients, chronic immunosuppression increases the risk of acquiring COVID-19 but it could reduce disease severity. Complete immunosuppression withdrawal may not be justified. However, mycophenolate withdrawal or temporary conversion to calcineurin inhibitors or everolimus until disease resolution could be beneficial in hospitalised patients.
Keywords: COVID-19, SARS-CoV-2, Pneumonia, Epidemiology, Transplantation, Immunosuppression, Mycophenolate, Calcineurin inhibitors, Tacrolimus, Everolimus, Standardised incidence, Standardised mortality
Graphical abstract
Highlights
-
•
The incidence of coronavirus disease 2019 (COVID-19) is higher in liver transplant patients.
-
•
Mortality rates are lower than those observed in the matched general population.
-
•
Immunosuppression withdrawal may not be justified.
-
•
Mycophenolate may increase the risk of severe COVID-19 in a dose-dependent manner.
-
•
Calcineurin inhibitors and everolimus are not deleterious for COVID-19.
Introduction
Spain has registered the fastest spread of coronavirus disease 2019 (COVID-19) in Europe with 210,773 cases detected by 28 April 2020, and a case-fatality rate of 11.3%,1 which was much higher than those observed in China, the origin of the pandemic.2 The liver transplantation (LT) network in Spain comprises 25 institutions in which more than 13,000 living LT patients are monitored. Transplant recipients are considered by some authors as a high-risk sub-population for COVID-19 because they receive lifelong immunosuppressive therapy.
According to data on previous coronaviruses (MERS-CoV and SARS-CoV), it has been hypothesised that COVID-19 has an earlier phase of viral replication and a later phase characterised by viral clearance as a result of the immune response. This second phase may eventually trigger a deregulation of CD4+ T cells, activation of CD8+ T cells and macrophages, and a cytokine storm,3 , 4 ultimately producing the most severe forms of COVID-19. Immunomodulatory agents could ameliorate this harmful immune response4 but this could lead to an increase in viral load and delayed disease recovery. Interestingly, the most frequently used immunosuppressive agents in LT recipients, namely calcineurin inhibitors,5 have shown intriguing capacities to inhibit the replication of coronaviruses.6 Therefore, baseline immunomodulation could protect LT patients against the most severe clinical forms of COVID-19.7
In this prospective nationwide study, we aimed to analyse the epidemiological pattern, clinical features, and outcomes of LT patients diagnosed with COVID-19 as compared with those observed in the general Spanish population.
Patients and methods
This is a prospective nationwide study promoted by the Spanish Society of Liver Transplantation (SETH), which was launched after the outbreak of COVID-19 in Spain on 28 February 2020. The study was performed according to the principles of the declaration of Helsinki and the European Union regulation 2016/679. The study was approved by the research ethics committee of the Hospital Clinic Barcelona (HCB 2020/0384) and the research protocol was registered at ClinicalTrials.gov (NCT04361591). Oral informed consent to participate in the study was obtained from all patients/relatives, and written informed consent was retrieved after viral clearance.
Identification and characterisation of LT patients with COVID-19
Patients who receive a LT in Spain undergo lifelong surveillance by the transplant team and they are instructed to make contact for any health-related issue. All LT recipients with known COVID-19 were prospectively enrolled in the registry up to 7 April 2020, when the national authorities declared that the epidemiological curve had reached a plateau. There were no exclusion criteria. LT patients with COVID-19 were declared to the SETH registry using an anonymised online platform. The transplant institution was responsible for declaring cases as soon as they became aware of them. Nurse transplant coordinators actively searched for COVID-19 patients by telephone contact.
COVID-19 was confirmed by a real-time reverse transcriptase PCR assay of nasal and pharyngeal swab specimens.8 In Spain, patients admitted to the hospital were tested onsite within the first 24 h, whereas outpatients with mild symptoms were tested at home or at ‘drive-through’ testing sites within the next 72 h after contacting a dedicated phone number. All clinical information was extracted by experienced transplant physicians from reliable electronic medical data sources. Demographic data, comorbidities (Charlson comorbidity index9), clinical features, laboratory parameters, and transplant-related information including baseline immunosuppression (drugs and trough concentrations) were recorded. Chest X-rays were reported by a single member of the radiology department at each centre. Modifications of immunosuppression therapy were registered as well as specific drugs prescribed for COVID-19.
Management protocols for COVID-19 were broadly similar within the national territory according to the SETH and the Ministry of Health recommendations. Patients were admitted to hospital if they had hypoxaemia (arterial oxygen partial pressure <70 mmHg) and/or radiological chest X-ray abnormalities. Patients with significant comorbidities or who were over the age of 60 years were also admitted at the discretion of the responsible clinician even if they did not fulfil the above-mentioned criteria. Patients could be treated with oral hydroxychloroquine and/or azithromycin for 5–7 days. In patients with moderate-severe COVID-19, antiviral therapy with lopinavir/ritonavir or remdesivir was allowed as compassionate use. Patients with acute distress respiratory syndrome could receive boluses of steroids and/or tocilizumab. Regarding immunosuppression, COVID-19 protocols encouraged clinicians to reduce, but not to withdraw, immunosuppression in LT patients. Patients were followed until 21 April 2020, thereby ensuring a minimum of 2 weeks of follow-up for clinical outcomes.
Study definitions and outcomes
The main outcome evaluated was severe COVID-19, which was a composite endpoint including need for mechanical ventilation, admission to the intensive care unit (ICU), and/or death. This endpoint was used in a previous study describing the clinical features of COVID-19 in China.2 Secondary outcomes were: respiratory insufficiency (arterial oxygen partial pressure <60 mmHg), need for mechanical ventilation, admission to the ICU and liver allograft dysfunction (conventionally defined as raised bilirubin >4 times from baseline values or international normalised ratio >1.4). Patients were considered cured of COVID-19 when discharged from the hospital or after 2 consecutive PCR-negative results separated by more than 48 h, whichever occurred first.
Data sources, calculation of standardised ratios, and statistical analysis
Data regarding populations at risk were obtained from the National Statistics Institute (general population) and from the Spanish Liver Transplant Registry (LT population), both updated in 2019. The incidence and outcomes of the COVID-19 epidemic in the general Spanish population were retrieved from the official publication of the Ministry of Health, which is updated on a daily basis (https://www.mscbs.gob.es/). To obtain the cumulative incidence of COVID-19, data was accessed on 7 April 2020, whereas mortality data were obtained on 21 April 2020, aligning with the periods of recruitment and surveillance, respectively, of LT patients. Relative risks of COVID-19 (incidence and mortality) in LT patients as compared with the general population were expressed as standardised incidence and mortality ratios, which result from the ratio of the observed to the expected events. Expected incidence and outcomes in LT patients were obtained from the numbers observed in the general population, after balancing for age and gender. Ninety-five percent confidence intervals (95% CI) were calculated under the assumption that the number of cases observed followed a Poisson distribution.
Continuous variables were reported as mean and standard deviations or as median and inter-quartile range (IQR), as appropriate. Categorical variables were summarised as counts and percentages. Among hospitalised patients, independent predictors of severe COVID-19 were identified using Cox regression analysis. Patients were censored when discharged or on 21 April 2020. Variables available at COVID-19 onset showing a p ≤0.30 in the univariate analysis entered the initial multivariate model. Non-significant co-variates were removed from the model in a backward stepwise process, commencing with those with the highest p value. All possible interactions were tested. A confounding factor was confirmed when its removal from the model resulted in >15% variation in the remaining β coefficients. The statistical analysis was performed using SPSS version 22.0 (IBM Corp, Armonk, NY, USA). Every hypothesis tested was 2-tailed and considered significant at p <0.05.
Data availability
A raw data set underpinning the present research publication may be found in the following repository: https://doi.org/10.17632/5ssscvfmxn.1.
Results
Standardised incidence of COVID-19 in LT patients
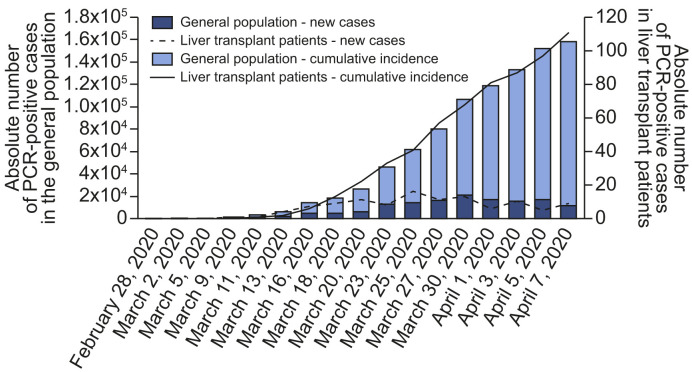
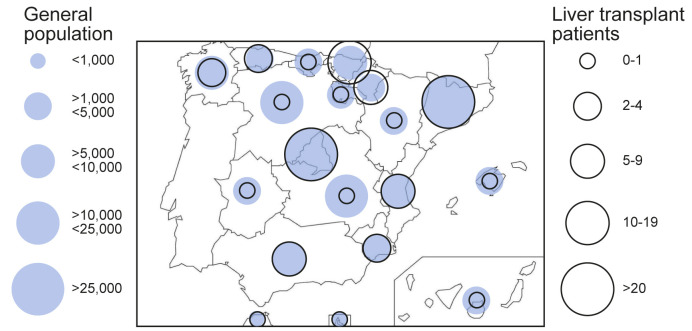
Epidemiological curves in the general and LT populations were broadly similar (Fig. 1 ). Peaks of new cases occurred between 25 March and 1 April, 2020 with subsequent stabilisation, and incipient decline at database closure. The geographical distribution of COVID-19 in the general population and in LT recipients overlapped widely (Fig. 2 ). The autonomous regions with the highest absolute number of confirmed cases were Madrid and Catalonia, both in the general population and in the LT population. Within the study period, 146,690 persons were diagnosed with COVID-19 in Spain (cumulative incidence 311.93 cases/105 habitants). Cumulative incidence was increased in older people, particularly beyond 60 years old (Table S1). However, the alive LT population in Spain comprises 13,255 patients, among whom there were 111 confirmed cases of COVID-19 (cumulative incidence 837.41 cases/105 patients). After adjusting for age and gender, the number of observed cases almost doubled the expected number of cases (Table S1) thus resulting in a standardised incidence ratio of 191.22 (95% CI 190.28–192.16). In the subset of patients older than 60 years, the standardised incidence ratio was even higher (206.48; 95% CI 205.45–207.52).
Fig. 1.
Epidemiological curve of COVID-19 in Spain from February 28, 2020 until the registry closure on 7 April 2020.
Absolute number of cases are shown for the whole Spanish population and for the liver transplant population.
Fig. 2.
Geographical distribution of COVID-19 among autonomous regions in Spain.
Absolute number of cases are shown for the whole Spanish population and for the liver transplant population.
Clinical characteristics and outcomes of LT patients with COVID-19
A total of 111 LT patients with COVID-19 were included (Table 1 ). The mean age was 65.34 ± 10.96 years and there was a male predominance (71.2%). The median interval from transplantation was 105 months (IQR 35–168). None of the patients had been infected by the liver donor. The most frequent comorbidity was hypertension (57.7%). The vast majority of patients (n = 109; 98.2%) were receiving chronic immunosuppression. Calcineurin inhibitors were the predominant immunosuppressants, either alone (n = 34; 30.6%), or in combination with mycophenolate (n = 29; 26.1%) or everolimus (n = 9; 8.1%). The remaining 37 patients (33.3%) received calcineurin inhibitor-free immunosuppression, based on mycophenolate and/or everolimus (Table 1). COVID-19 was symptomatic in 93% of patients. The most frequent symptoms were fever (74.8%) and cough (70.3%). Asymptomatic patients (n = 7) were tested for COVID-19 because of incidental radiological findings (n = 2) or after a high-risk contact (n = 5). Regarding laboratory parameters, the absolute lymphocyte count was 670/μl (IQR 430–1,040) and D dimer was 600 ng/ml (IQR 345–1,630). The chest X-ray showed abnormalities in 78.4% of patients, with a bilateral diffuse pattern in 58.6% of patients.
Table 1.
Clinical characteristics of 111 liver transplant recipients with COVID-19 included in the Spanish Society of Liver Transplantation (SETH) registry.
| Variable | COVID-19 SETH registry n = 111 |
Non-severe COVID-19 n = 76 |
Severe COVID-19 n = 35 |
p |
|---|---|---|---|---|
| Age | 65.34 ± 10.96 | 63.88 ± 11.94 | 68.51 ± 7.70 | 0.038 |
| Gender (women); % (n) | 28.8 (32) | 25 (19) | 37.1 (13) | 0.189 |
| Previous medical history | ||||
| Diabetes, % (n) | 47.7 (53) | 43.4 (33) | 57.1 (20) | 0.179 |
| Hypertension, % (n) | 57.7 (64) | 59.2 (45) | 54.3 (19) | 0.626 |
| ACE inhibitors, % (n) | 29.7 (33) | 31.6 (24) | 25.7 (9) | 0.530 |
| Cardiomyopathy, % (n) | 19.8 (22) | 17.1 (13) | 25.7 (9) | 0.290 |
| Bronchopulmonary, % (n) | 11.7 (13) | 11.8 (9) | 11.4 (4) | 0.850 |
| Charlson comorbidity index; median (IQR) | 4 (2–6) | 3 (2–4.7) | 5 (4–7) | 0.002 |
| Interval since transplantation | ||||
| Months, median (IQR) | 105 (35–168) | 97.5 (30–160) | 127 (57–208) | 0.197 |
| <12 months, % (n) | 13.5 (15) | 11.8 (9) | 17.1 (6) | 0.448 |
| Aetiology of liver disease | ||||
| Alcohol, % (n) | 30.6 (34) | 28.9 (22) | 35.3 (12) | 0.571 |
| Hepatitis C, % (n) | 28.8 (32) | 30.3 (23) | 25.7 (9) | 0.623 |
| Hepatitis B, % (n) | 10.8 (12) | 11.8 (9) | 8.6 (3) | 0.750 |
| Autoimmune, % (n) | 8.1 (9) | 6.6 (5) | 11.4 (4) | 0.459 |
| Clinical presentation of COVID-19 | ||||
| Fever, % (n) | 74.8 (83) | 75 (57) | 74.3 (26) | 0.936 |
| Dyspnoea, % (n) | 41.4 (46) | 25 (19) | 77.1 (27) | <0.001 |
| Cough, % (n) | 70.3 (78) | 67.1 (51) | 77.1 (27) | 0.282 |
| Gastrointestinal, % (n) | 34.2 (38) | 36.8 (28) | 28.6 (10) | 0.394 |
| Asymptomatic, % (n) | 6.3 (7) | 9.2 (7) | 0 (0) | 0.095 |
| Laboratory parameters in hospitalised patients (n = 96); median (IQR) | ||||
| PaO2/FiO2 ratio (onset)∗ | 400 (309–436) | 403 (333–444) | 328 (279–420) | 0.044 |
| Lymphocyte (onset)-count/μl | 670 (430–1,040) | 715 (415–1,000) | 600 (430–1,200) | 0.449 |
| Lymphocyte count (min)-count/μl | 455 (275–755) | 500 (380–840) | 310 (200–500) | 0.013 |
| D dimer (onset)-ng/ml | 600 (345–1,630) | 540 (340–1,010) | 1,100 (450–2,796) | 0.127 |
| D dimer (max)-ng/ml | 1,050 (517–3,299) | 800 (390–2,543) | 2,229 (809–4,290) | 0.032 |
| Ferritin (max)-ng/ml | 847 (376–1975) | 511 (256–1,663) | 1,459 (770–2,264) | 0.004 |
| Estimated glomerular filtration rate, ml/min† | 62.5 (41.3–79.7) | 63.2 (43.5–82.5) | 53.3 (39–74) | 0.396 |
| Chest X-ray abnormalities | ||||
| Normal, % (n) | 21.6 (24) | 31.6 (24) | 0 (0) | 0.002 |
| Unilateral, % (n) | 19.8 (22) | 18.4 (14) | 22.8 (8) | |
| Bilateral, % (n) | 58.6 (65) | 50 (38) | 77.1 (27) | |
| COVID-19 specific therapy in hospitalised patients (n = 96) | ||||
| Azithromycin, % (n) | 62.5 (60) | 63.9 (39) | 60 (21) | 0.702 |
| Hydroxychloroquine, % (n) | 91.7 (88) | 90.2 (55) | 94.3 (33) | 0.482 |
| Lopinavir/ritonavir, % (n) | 41.7 (40) | 36.1 (22) | 51.4 (18) | 0.142 |
| Remdesivir, % (n) | 1 (1) | 0 (0) | 2.9 (1) | 0.365 |
| Interferon beta, % (n) | 3.1 (3) | 1.6 (1) | 5.7 (2) | 0.552 |
| Tocilizumab, % (n) | 15.6 (15) | 4.9 (3) | 34.3 (12) | <0.001 |
| Corticosteroids (boluses), % (n) | 12.5 (12) | 4.9 (3) | 25.7 (9) | 0.007 |
| Immunosuppression at baseline (drugs) | ||||
| Tacrolimus, % (n) | 59.5 (66) | 64.5 (49) | 48.6 (17) | 0.113 |
| Cyclosporine, % (n) | 5.4 (6) | 5.3 (4) | 5.7 (2) | 0.922 |
| Mycophenolate, % (n) | 51.4 (57) | 43.4 (33) | 68.6 (24) | 0.014 |
| Everolimus, % (n) | 20.7 (23) | 22.4 (17) | 17.1 (6) | 0.528 |
| Corticosteroids (maintenance), % (n) | 21.8 (24) | 18.7 (14) | 28.6 (15) | 0.241 |
| Immunosuppression (combinations) | ||||
| CNI, % (n) | 30.6 (34) | 32.9 (25) | 25.7 (9) | 0.374 |
| CNI + mycophenolate, % (n) | 26.1 (29) | 27.6 (21) | 22.9 (8) | |
| CNI + everolimus, % (n) | 8.1 (9) | 9.2 (7) | 5.7 (2) | |
| Mycophenolate +/− everolimus, % (n) | 33.3 (37) | 27.6 (21) | 45.7 (16) | |
| None, % (n) | 1.8 (2) | 2.6 (2) | 0 (0) | |
| Immunosuppression baseline (dose- mg) | ||||
| Tacrolimus (n = 66); median (IQR) | 2.5 (1.5–5) | 2.5 (1.7–5) | 2 (1–4.25) | 0.986 |
| Cyclosporine (n = 6); median (IQR) | 75 (50–150) | 125 (62.5–150) | 125 (75–150) | 0.400 |
| Mycophenolate (n = 57); median (IQR) | 1,000 (1,000–2,000) | 1,000 (1000–1,500) | 1,500 (1,000–2,000) | 0.056 |
| Everolimus (n = 23); median (IQR) | 2 (1–2.1) | 2 (1.1–2.4) | 2 (0.87–2.25) | 1 |
| Corticosteroids (maintenance) (n = 24); median (IQR) | 10 (5–20) | 5 (4.7–16.2) | 10 (8.7–30) | 0.673 |
| Immunosuppression (trough concentrations onset – ng/mL) | ||||
| Tacrolimus (n = 66); median (IQR) | 4.95 (3.32–6.92) | 5 (4–6.7) | 4.2 (2.7–6.9) | 0.772 |
| Cyclosporine (n = 6); median (IQR) | 99 (51.5–148.6) | 99 (88.5–125) | 85.1 (25–145.2) | 1 |
| Everolimus (n = 23); median (IQR) | 3.5 (2.9–5.5) | 3.5 (2.9–5.4) | 3.5 (1.6–6.4) | 1 |
Severe COVID-19 was defined as need for mechanical ventilation, admission to intensive care unit, and/or death.
CNI, calcineurin inhibitor.
Pao2/FiO2 was not available in 22 patients.
Estimated glomerular filtration rate calculated by the Modification of Diet in Renal Disease-4 (MDRD-4).
The median follow-up after the diagnosis of COVID-19 was 23 days, with a minimum of 15 days for the last included patient. Ninety-six patients (86.5%) were admitted to hospital according to the following criteria: 18 patients (18.8%) had hypoxaemia, 81 patients (84.4%) had radiological chest abnormalities, 77 patients (80.2%) were older than 60 years, and 51 patients (53.1%) had significant comorbidities (Charlson comorbidity index >3). Most of admitted patients met more than one criterion: 11 patients (11.5%) met 2 criteria, 32 patients (33.3%) met 3 criteria, and 36 patients (37.5%) met 4 criteria. Only 6 patients did not meet any of the recommendations described above and the reason for hospital admission was the presence of severe clinical symptoms. There was a significant elevation of transaminases (doubled nadir) in 16 patients (14.7%). Three patients developed liver graft dysfunction (2.7%) but none of them had graft loss. Respiratory insufficiency occurred in 44 patients (39.6%). Twenty-two patients (19.8%) required respiratory support after a median of 5 days (IQR 1.75–9.5) after hospital admission, being non-invasive in 13 patients and invasive in 9 patients. Twelve patients were admitted to the ICU (10.8%). The mortality rate was 18% with a median interval from hospital admission of 7 days (IQR 5–9.75). At database closure, 81 patients (73%) had been discharged or remained asymptomatic at home, and 10 patients (9%) were still in hospital. After a median of 17 days (IQR 13–27.5), a PCR-negative result for COVID-19 was confirmed in 18 patients.
Thirty-five patients (31.5%) met the criteria of severe COVID-19 (Table 1). These patients were characterised by older age (p = 0.038), more frequent dyspnoea (p <0.001) and a reduced Pao 2/FiO2 ratio (p = 0.044) on admission. During the hospital stay, severe COVID-19 patients presented reduced lymphocyte counts (p = 0.013), increased D dimer (p = 0.032), and increased serum ferritin (p = 0.004). Regarding management, severe COVID-19 patients received more frequently tocilizumab (p <0.001) and boluses of corticosteroids (p = 0.007). Patients receiving mycophenolate or attempting a complete withdrawal of immunosuppression were more prevalent in the severe COVID-19 group (p = 0.014, and p = 0.016, respectively). Conversely, tacrolimus-containing immunosuppression was more frequent in the non-severe COVID-19 group, although without statistical significance (p = 0.113).
Risk factors of severe COVID-19 among hospitalised patients
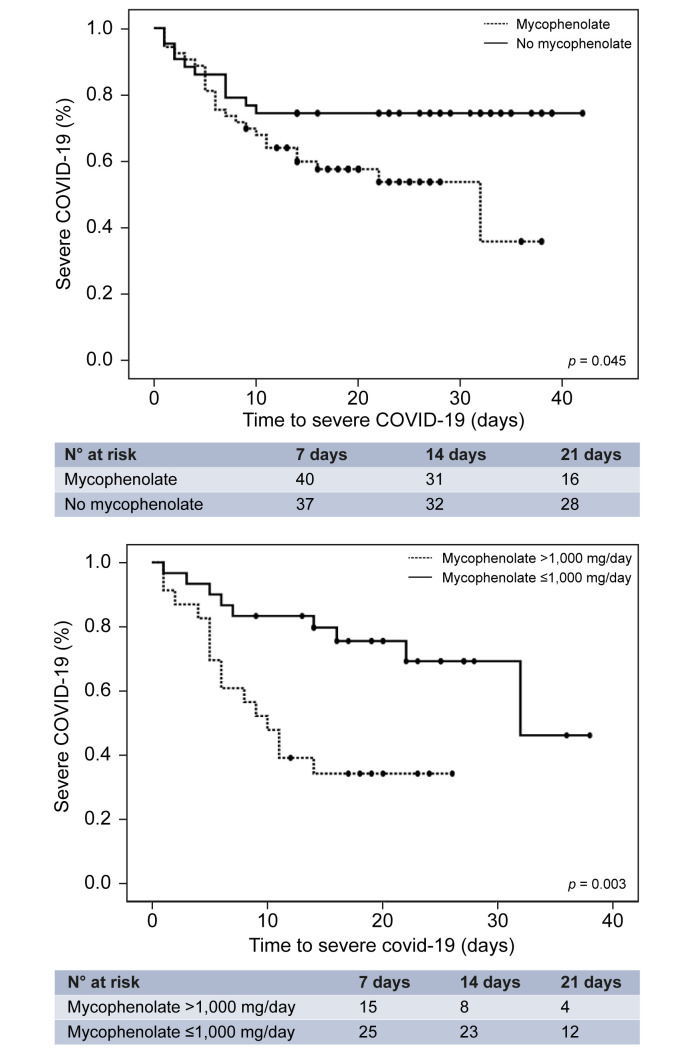
Baseline risk factors of severe COVID-19 among hospitalised patients (n = 96) were screened using Cox regression analysis (Table 2 ). The multivariate analysis identified the following independent predictors: Charlson comorbidity index (relative risk [RR] = 1.28; 95% CI 1.05–1.56), male gender (RR = 2.49; 95% CI 1.14–5.41), dyspnoea at diagnosis (RR = 7.25; 95% CI 2.95–17.82), and baseline immunosuppression containing mycophenolate (RR = 3.94; 95% CI 1.59–9.74). Age, estimated glomerular filtration rate, and complete withdrawal of immunosuppression upon hospital admission were not significant, but they were kept in the analysis to be controlled as potential confounding factors. Kaplan-Meier curves illustrate the negative prognostic impact of mycophenolate, particularly at doses higher than 1,000 mg/day (Fig. 3 ). Among patients receiving full-dose of mycophenolate at baseline (i.e. 2,000 mg/day), complete drug withdrawal had a trend towards reduced severe COVID-19 (41.7% vs. 69.2%; p = 0.16). Interestingly, baseline therapy with calcineurin inhibitors, mTOR inhibitors (everolimus), and maintenance corticosteroids, independently of their trough concentrations/doses, were not associated with increased risk of severe COVID-19. Therapy with hydroxychloroquine and/or azithromycin at hospital admission did not decrease the risk of severe COVID-19 (p = 0.64 and p = 0.45, respectively).
Table 2.
Clinical predictors of severe COVID-19 among patients admitted into the hospital (n = 96).
| Variables | Univariate analysis |
Multivariate analysis (initial model) |
Multivariate analysis (final model) |
|||
|---|---|---|---|---|---|---|
| RR (95% CI) | p | RR (95% CI) | p | RR (95% CI) | p | |
| Age | 1.05 (1.01–1.10) | 0.017 | 1.05 (0.98–1.13) | 0.166 | 1.03 (0.98–1.09) | 0.182 |
| Gender (male) | 1.56 (0.77–3.17) | 0.212 | 7.66 (2.20–26.69) | 0.001 | 2.49 (1.14–5.41) | 0.021 |
| Diabetes | 1.59 (0.80–3.17) | 0.181 | 0.26 (0.10–0.68) | 0.006 | ||
| Hypertension | 0.93 (0.47–1.84) | 0.838 | ||||
| ACE inhibitors | 0.23 (0.34–1.56) | 0.414 | ||||
| Charlson comorbidity index | 1.25 (1.11–1.40) | <0.001 | 1.34 (1.02–1.76) | 0.031 | 1.28 (1.05–1.56) | 0.015 |
| Interval since liver transplantation (>12 mo) | 1 (0.99–1) | 0.466 | ||||
| Dyspnoea | 4.19 (1.89–9.30) | <0.001 | 15.91 (4.17–60.73) | <0.001 | 7.25 (2.95–17.82) | <0.001 |
| Cough | 0.96 (0.43–2.15) | 0.934 | ||||
| Fever | 0.73 (0.34–1.58) | 0.429 | ||||
| Gastrointestinal symptoms | 0.72 (0.34–1.52) | 0.392 | ||||
| PaFiO2 | 0.99 (0.99–0.99) | 0.038 | 0.99 (0.99–0.99) | 0.012 | ||
| Lymphocyte count | 1 (0.99–1) | 0.418 | ||||
| D Dimer | 1 (1–1) | 0.409 | ||||
| Estimated glomerular filtration rate | 0.99 (0.98–1.01) | 0.249 | 1.01 (0.99–1.04) | 0.171 | 1.01 (0.98–1.03) | 0.077 |
| Tacrolimus∗ | 0.54 (0.29–1.07) | 0.079 | 0.19 (0.05–0.68) | 0.011 | ||
| Cyclosporine∗ | 0.76 (0.18–3.22) | 0.708 | ||||
| Trough concentrations (tacrolimus)∗ | 1.01 (0.93–1.10) | 0.762 | ||||
| Trough concentrations (cyclosporine)∗ | 1.04 (0.92–1.19) | 0.485 | ||||
| Mycophenolate∗ | 2.62 (1.25–5.49) | 0.011 | 5.32 (1.60–17.69) | 0.006 | 3.94 (1.59–9.74) | 0.003 |
| Everolimus∗ | 0.77 (0.32–1.88) | 0.575 | ||||
| Trough concentrations (everolimus)∗ | 0.86 (0.59–1.32) | 0.490 | ||||
| Corticosteroids∗ | 1.53 (0.72–3.22) | 0.262 | 1.77 (0.59–5.27) | 0.303 | ||
| Withdrawal of immunosuppression† | 2.03 (0.91–4.51) | 0.083 | 0.34 (0.08–1.51) | 0.155 | 0.58 (0.24–1.40) | 0.229 |
| Hydroxychloroquine† | 0.64 (0.33–5.88) | 0.640 | ||||
| Azithromycin† | 0.77 (0.38–1.53) | 0.454 | ||||
Severe COVID-19 was defined as requirement of respiratory support, admission in intensive care unit and/or death. Univariate and multivariate Cox's regression analyses were used. RR, relative risk.
These variables pertain to active immunosuppression therapy at COVID-19 diagnosis.
These therapies were started at COVID-19 diagnosis. Other therapies against COVID-19 were not included as they were initiated selectively in unresponsive cases and would confound the analysis.
Fig. 3.
Kaplan-Meier curves showing the impact of mycophenolate-containing immunosuppression (upper panel) and increased doses (lower panel) on the development of severe COVID-19.
The p values were determined using the log rank test.
Standardised mortality ratio of COVID-19 in LT patients
In the general population, a total of 21,717 deaths were registered by 21 April 2020. Overall mortality rates were 14.8%, but were as high as 27.9% in patients older than 60 years (Table S2). Among the LT population, 20 patients with COVID-19 died during the study period (mortality rate 18%). After adjusting for age and gender, the number of observed deaths among LT patients was slightly lower than the expected number of deaths (Table S2), thus resulting in a standardised mortality ratio of 95.55 (95% CI 94.25–96.85). When evaluating subgroups according to age strata, no death was registered among LT patients younger than 60. Adjusted mortality rates in patients older than 60 were similar in LT recipients and in the general population (standardised mortality ratio = 100.29; 95% CI 98.93–101.66).
Discussion
This is the first nationwide study evaluating the incidence and outcomes of COVID-19 in immunosuppressed patients as compared with age and gender-matched counterparts from the general population. Despite a similar epidemiological pattern, LT patients had almost double the standardised incidence rates of COVID-19. Chronic exposure to immunosuppressive agents did not increase standardised mortality rates but our findings suggest that mycophenolate, particularly at doses higher than 1,000 mg/day, could increase the risk of severe COVID-19 among hospitalised LT patients. As other immunosuppressants such as calcineurin inhibitors (tacrolimus and cyclosporine) and mTOR inhibitors (everolimus) did not show this association, the present study provides key evidence to modulate immunosuppression in order to improve outcomes in LT patients with COVID-19.
Similar epidemiological curves and geographical distributions of COVID-19 were observed between LT recipients and the general population. Despite routine recommendations given to LT patients regarding social distancing, they had almost double the risk of developing COVID-19 as compared with the age- and gender-matched general population. The most plausible explanations are chronic immunosuppression and increased comorbidities, which would make them more vulnerable. Precautions should be intensified within a context of an epidemic to prevent transmission. Regarding outcomes, patients had a minimum of 2 weeks of surveillance to ensure that the need for intensive care and mortality were adequately captured, as previous studies reported that these outcomes may occur earlier.2 The current evidence of COVID-19 in solid-organ transplant recipients is based on short and uncontrolled case-series with contradictory conclusions. Whereas some studies report aggressive COVID-19 with fatal outcomes,10 , 11 other authors suggest that severe respiratory disease may be anecdotal.12 Because transplant patients have more comorbidities than the general population,13 the expected severity of COVID-19 would be increased. Although standardised rates of severe COVID-19 could not be calculated (owing to unreliable data from the general population), we demonstrated that standardised mortality rates were actually lower in LT patients as compared with the general population. The clinical features associated with severe COVID-19 were similar to those previously reported:2 older age, male gender, increased comorbidities, raised D dimer, serum ferritin, and decreased absolute lymphocyte count. PaFiO2 and dyspnoea at admission were also associated with an increased risk of severe COVID-19, but only the latter variable was included in the final multivariate model because of missing PaFiO2 information in 22 patients. Noteworthy, the interval from LT until SARS-CoV-2 infection had no impact on the risk of evolving to severe COVID-19. However, the number of patients diagnosed of COVID-19 within the first year after LT was limited (n = 15) and further studies are required to confirm this finding.
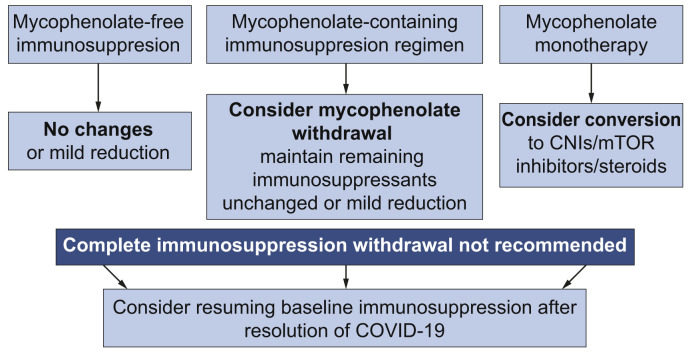
The relationship of immunosuppression and COVID-19 outcomes is frequently referred to as a double-edged sword.14 Too much immunosuppression could result in increased viral load and delayed recovery whereas a competent immune system could be responsible for the most severe forms of the disease.15 In the transplant setting, many authors advocate the potential benefit of immunosuppression in COVID-19 based on personal experiences or on uncontrolled clinical observations.12 , [16], [17], [18], [19] In the present study, the vast majority of patients were receiving calcineurin inhibitors (tacrolimus or cyclosporine), mTOR inhibitors (everolimus), and/or mycophenolate. Whereas calcineurin and mTOR inhibitors were not associated with worse outcomes, at least not with their usual trough concentrations, mycophenolate therapy at baseline was an independent predictor of severe COVID-19 in a dose-dependent manner. This interesting observation may be explained by the different mechanisms of action. Mycophenolate produces a cytostatic effect on activated lymphocytes.20 In COVID-19, the virus SARS-CoV-2 has a direct cytotoxic effect on lymphocytes, particularly CD8+,3 , 21 thereby explaining the association between lymphopenia and worse outcomes.2 Therefore, mycophenolate and SARS-CoV-2 may exert a synergic and deleterious effect on depleting peripheral lymphocytes, which would be responsible for an aberrant immune reconstitution as demonstrated with other viruses.22 In contrast, mTOR inhibitors increase the quality and functionality of memory T cells and reduce the replication of a myriad of viruses including cytomegalovirus, herpes virus-8, Epstein-Barr, and human immunodeficiency virus.23 Regarding calcineurin inhibitors, some studies have shown antiviral effects in vitro against coronaviruses6 and they could also ameliorate the cytokine storm.15 A randomised controlled trial with calcineurin inhibitors and/or mTOR inhibitors in immunocompetent individuals could be of great interest. An algorithm proposal to modify baseline immunosuppression in LT patients diagnosed with COVID-19 according to the study findings is presented in Fig. 4 .
Fig. 4.
Proposed algorithm to modify immunosuppression in liver transplant patients with COVID-19 according to the findings of the present study.
The recommendations should be adapted to each patient taking into account the interval from liver transplantation and the individualised risk of rejection. CNI, calcineurin inhibitors; mTOR, mammalian target of rapamycin.
Given the lack of solid scientific evidence, the therapeutic approach against COVID-19 varied among participating institutions and usually included a combination of hydroxychloroquine, azithromyzin, antivirals, corticosteroids, and/or monoclonal antibodies in a stepwise process. In the only randomised trial published hitherto, antiviral therapy with lopinavir/ritonavir failed to reduce mortality in patients with severe COVID-19.24 In our cohort, hydroxychorloquine and/or azithromyzin were started early after diagnosis but we did not observe a clear benefit. Antivirals (lopinavir/ritonavir, remdesivir, and interferon), boluses of corticosteroids, and monoclonal antibodies (tocilizumab) were prescribed mainly in unresponsive cases in the present cohort and it is hard to extract valid conclusions. Caution should be taken in the absence of well-designed randomised trials.25
Some limitations of the present study should be highlighted. Underdiagnosing of COVID-19 could be an issue given the suboptimal sensitivity of PCR of nasal and pharyngeal swab specimens and the limited access to diagnostic tests at some timepoints during the COVID-19 epidemic in Spain. The true cumulative incidence of COVID-19 may have been underestimated, although in a similar proportion in LT patients and in the general population, thus making standardised incidence ratio equally reliable. Under-reporting is another potential limitation in prospective registries but we believe it had a limited impact in the present study because LT patients/relatives are tightly connected by phone with the nurse transplant coordinator at each centre to report any intercurrent health issue. The limited sample size may have weakened some analyses and results marginally but not significantly and should be carefully interpreted as some could be clinically meaningful. Finally, the present study was neither designed nor powered to evaluate the effect of specific therapies against COVID-19 and no solid conclusion may be derived in this regard.
In conclusion, being chronically immunosuppressed and with increased comorbidities, LT patients are more likely to develop COVID-19 within an outbreak scenario and therefore social distancing and other preventative measures should be enhanced in these patients. Mortality rates were lower than those observed in the age- and gender-matched general population, thereby suggesting that chronic immunosuppression could exert a certain protective effect against the most severe forms of COVID-19. A complete immunosuppression withdrawal after the diagnosis of COVID-19 may not be justified. However, in patients receiving mycophenolate, dose reduction, or temporary conversion to calcineurin inhibitors or everolimus may be considered until complete recovery from COVID-19.
Abbreviations
COVID-19, coronavirus disease 2019; ICU, intensive care unit; IQR, inter-quartile range; LT, liver transplantation; RR, relative risk; SETH, Spanish liver transplant society (Sociedad Española de Trasplante Hepático).
Financial support
The Spanish Society of Liver Transplantation (SETH) was the only funding agent and covered the fee of an expert English language reviewer.
Authors' contributions
JC, MLR-P: Study design, statistical analysis and manuscript preparation.
MS: Literature review, data recording and manuscript draft review for important intellectual content.
AA-M, A.M-S, JG, JN, MG, JB-S, AC, LL, AC, AF-Y, CL, IF, CF, MN, MI, LC, SP, PR, CV, MLG-D, RG-G, LH, FN, AO, JMÁ, GB-F, EF, FG-P, JLM, and ST: Identification and inclusion of patients, data recording and manuscript draft review for important intellectual content.
GDR: Provided and analysed data from the Spanish Liver Transplant Registry.
JAP: Original concept, study design, literature review, and manuscript draft review for important intellectual content.
Conflicts of interest
JC has received lecture fees from Chiesi, Astellas, and Novartis and is an advisory to Chiesi. MR-P has received lecture fees from Astellas, Novartis, and Intercept Pharma. MS has received lecture fees from Astellas, Novartis, and Chiesi and is an advisory to Jazz and Novartis. MG has received speaker fees from and/or acted as advisor for Astellas, Novartis, Chiesi, Baxter, and Medtronic. MN has received lecture fees from Astellas Pharma. MI has received lecture fees from BMS. CV has received lecture fees from Novartis, Abbie, and Gilead. AC has received lecture fees from Astellas Pharma. GB-F has received lecture fees from Bayer and Astellas. JLM has received lecture fees from Bayer and Gilead. JP has received lecture fees by Astellas, Chiesi, and Gilead. AA-M, AM-S, JG, JN, JB-S, AC, LL, AC, AF-Y, CL, IF, CF, LC, SP, PR, MG-D, RG-G, LH, FN, AO, JA, EF, FG-P, ST and GDR have no conflict of interest to disclose regarding this manuscript.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We greatly appreciate the endeavours of the nurse transplant coordinators in attending transplant recipients and for providing continuous support to their families. We thank Ms Paloma Bellés from AOPC for her contribution in secretariat and logistic aspects. We also acknowledge Ms Donna Pringle for professional English language polishing.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jhep.2020.07.040.
Supplementary data
References
- 1.Ministerio de Sanidad, Gobierno de España https://www.mscbs.gob.es/en/profesionales/saludPublica/ccayes/alertasActual/nCov-China/situacionActual.htm Available at.
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Peralvarez M., Guerrero-Misas M., Thorburn D., Davidson B.R., Tsochatzis E., Gurusamy K.S. Maintenance immunosuppression for adults undergoing liver transplantation: a network meta-analysis. Cochrane Database Syst Rev. 2017;3:CD011639. doi: 10.1002/14651858.CD011639.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka Y., Sato Y., Sasaki T. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses. 2013;5:1250–1260. doi: 10.3390/v5051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romanelli A., Mascolo S. Immunosuppression drug-related and clinical manifestation of coronavirus disease 2019: a therapeutical hypothesis. Am J Transplant. 2020;20:1947–1948. doi: 10.1111/ajt.15905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Zhu L., Gong N., Liu B., Lu X., Chen D., Chen S. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020;77:748–754. doi: 10.1016/j.eururo.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akalin E., Azzi Y., Bartash R., Seethamraju H., Parides M., Hemmige V. Covid-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl. 2020;26:832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 13.Tovikkai C., Charman S.C., Praseedom R.K., Gimson A.E., van der Meulen J. Time-varying impact of comorbidities on mortality after liver transplantation: a national cohort study using linked clinical and administrative data. BMJ Open. 2015;5:e006971. doi: 10.1136/bmjopen-2014-006971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritchie A.I., Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? Lancet. 2020;395:1111. doi: 10.1016/S0140-6736(20)30691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willicombe M., Thomas D., McAdoo S. COVID-19 and calcineurin inhibitors: should they get left out in the storm? J Am Soc Nephrol. 2020;31:1145–1146. doi: 10.1681/ASN.2020030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minotti C., Tirelli F., Barbieri E., Giaquinto C., Dona D. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? A systematic review. J Infect. 2020;81:e61–e66. doi: 10.1016/j.jinf.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Columbia University Kidney Transplant Program Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31:1150–1156. doi: 10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb G.J., Moon A.M., Barnes E., Barritt A.S., Marjot T. Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol. 2020;5:P643–P644. doi: 10.1016/S2468-1253(20)30125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhoori S., Rossi R.E., Citterio D., Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:532–533. doi: 10.1016/S2468-1253(20)30116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allison A.C., Eugui E.M. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 21.Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221:1732–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brennan D.C., Legendre C., Patel D., Mange K., Wiland A., McCague K. Cytomegalovirus incidence between everolimus versus mycophenolate in de novo renal transplants: pooled analysis of three clinical trials. Am J Transplant. 2011;11:2453–2462. doi: 10.1111/j.1600-6143.2011.03674.x. [DOI] [PubMed] [Google Scholar]
- 23.Bowman L.J., Brueckner A.J., Doligalski C.T. The role of mTOR inhibitors in the management of viral infections: a review of current literature. Transplantation. 2018;102:S50–S59. doi: 10.1097/TP.0000000000001777. [DOI] [PubMed] [Google Scholar]
- 24.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferner R.E., Aronson J.K. Chloroquine and hydroxychloroquine in Covid-19. BMJ. 2020;369:m1432. doi: 10.1136/bmj.m1432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A raw data set underpinning the present research publication may be found in the following repository: https://doi.org/10.17632/5ssscvfmxn.1.