Abstract
Background
Behavioral interventions produce clinically significant weight reduction, with many participants regaining weight subsequently. Most interventions focus on an individual, but dietary and physical activity behaviors occur with, or are influenced by, domestic partners. According to interdependence theory, couples who approach behavior change as a problem to be tackled together versus independently are more likely to utilize communal coping processes to promote behavior change. We utilized interdependence theory to develop a partner-assisted intervention to increase long-term weight loss.
Methods
Community-dwelling individuals (index participants) cohabitating with a partner with 1) overweight and at least one obesity-related comorbidity or 2) obesity are randomized to participate in a standard weight management program alone or with their partner. The weight management program involves biweekly, in-person, group sessions focusing on weight loss for six months, followed by three group sessions and nine telephone calls focusing on weight loss maintenance for twelve months. In the partner-assisted arm, partners participate in half of the group sessions and telephone calls. Couples receive training in principles of cognitive behavioral therapy for couples, including sharing thoughts and feelings and joint problem solving, to increase communal coping. The primary outcome is participant weight loss at 24 months, with caloric intake and moderate-intensity physical activity as secondary outcomes. Partner weight and caloric intake will also be analyzed. Mediation analyses will examine the role of interdependence variables and social support.
Discussion
This trial will provide knowledge about effective ways to promote long-term weight loss and the role of interdependence constructs in weight loss.
Clinical trials identifier: NCT 03801174.
Keywords: Couples, Dyads, Weight loss, Weight loss maintenance, Randomized controlled trial
Abbreviations: BMI, body mass index; CBCT, cognitive-behavioral couples therapy; IOS, inclusion of other in self; RCT, randomized controlled trial; RD, registered dietitian; REDCap, Research Electronic Data Capture; URCS, Unidimensional Relationship Closeness Scale; UW, University of Wisconsin
1. Introduction
Two-thirds of United States adults have excess body weight, resulting in increased disease risk [1,2], decreased health-related quality of life [3,4], and high health care costs to individuals, employers, insurers, and health systems [[5], [6], [7]]. Behavioral interventions involving dietary modification and physical activity, combined with cognitive and behavioral strategies, are efficacious for short-term weight loss [[8], [9], [10], [11]]. Yet, most people regain weight following significant weight loss [11]. Accordingly, efforts have focused on identifying effective strategies that promote weight loss maintenance, with modest success [[12], [13], [14], [15], [16], [17], [18], [19]].
One novel approach to promote initial weight loss and subsequent maintenance is to leverage the domestic partnership. Domestic partners share eating habits, physical activity patterns, and other health behaviors, offering frequent opportunities to provide informational, emotional, and instrumental support [20]. Furthermore, domestic partners are the preferred source of support for most adults [21,22]. Given that 53% of the US population is married and another 7% cohabit with a partner, the potential impact of intervening on domestic partners considerable [23].
Despite the potential for partners to increase health behavior change, trials of weight loss interventions involving partners have yielded inconsistent effects [24]. Meta-analyses comparing couples-oriented to participant-only interventions, most of which were conducted in the 1970s–80s, indicated superiority of partner-assisted interventions immediately following weight loss programs, but not thereafter [25,26]. Many of these studies had design limitations, including failure to specify a primary outcome, endpoint, effect size, or include a power analysis [[27], [28], [29], [30], [31], [32], [33]]. Additionally, most trials included interventions focused on initial weight loss, not weight loss maintenance [27,28,30,31,33,34]. Finally, the interventions tested were not rooted in contemporary knowledge regarding communal coping.
According to Lewis' interdependence model of communal coping and behavior change [35], couples who approach behavior change as a problem to be tackled together versus independently are more likely to utilize effective communal coping strategies. Effective methods to promote communal coping in the context of weight management have not been identified [24].
To promote communal coping among couples, we are taking the novel approach of applying principles of cognitive-behavioral couples therapy (CBCT) [36]. CBCT is an empirically-supported, efficacious intervention for preventing and treating relationship distress that trains couples in communication skills for sharing their thoughts and feelings, listening supportively, and making decisions together. Components of evidence-based couples interventions have been applied to medical problems by systematically training individuals and their partners in communication skills to provide each other with effective informational, emotional, and instrumental support [37,38]. A pilot study of a couple-based intervention for physical activity among cancer survivors led to increases in activity in both individuals and their partners and increases in partner support for physical activity [39].
Previous studies have primarily applied principles of CBCT via individual sessions between a trained therapist and the couple. However, weight loss programs are often offered in groups to reduce cost, increase reach, and provide emotional support from similar others. This study evaluates a novel application of CBCT principles in a group-based intervention for weight loss initiation followed by individual maintenance intervention. We present the study design, intervention, outcomes, and analysis plan for Partner2Lose, a randomized trial comparing partner-assisted and participant-only weight management interventions.
2. Methods
2.1. Study design
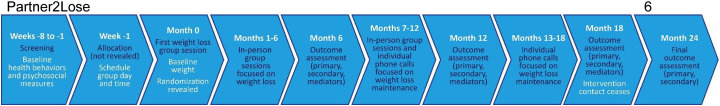
Partner2Lose is a parallel, two-arm randomized controlled trial in which couples are randomized to participant-only or partner-assisted intervention. The study flow is shown in Fig. 1 . In both arms, index participants receive intervention contact for 18 months, including 6 months of weight-loss initiation and 12 months of maintenance, followed by no intervention contact for 6 months. In the partner-assisted arm, partners co-participate in the intervention, and couples receive training in communication skills. The primary endpoint is participant weight measured at 24 months. Our aims are: 1) Test the hypothesis that average participant weight loss is at least 2.5 kg greater in the partner-assisted arm than the participant-only arm at 24 months; 2) Test the hypotheses that average participant daily caloric is significantly lower, and minutes spent doing moderate-intensity physical activity is significantly greater, in the partner-assisted arm than the participant-only arm at 24 months; and 3) Assess the extent to which interdependence constructs mediate the effect of the partner-assisted weight management intervention on participant weight loss.
Fig. 1.
Study flow.
2.2. Setting and eligibility criteria
We are conducting this study in the greater Madison, WI area (Dane County). This geographic area has 536,000 residents, of whom approximately 31% had obesity in 2015 [40]. Approval was obtained from the University of Wisconsin Health Sciences Institutional Review Board. Participant and partner eligibility criteria are summarized in Table 1 .
Table 1.
Participant and partner eligibility criteria.
| Criteria | Participant | Partner |
|---|---|---|
| Inclusion |
|
|
| Exclusion |
|
|
2.3. Recruitment and enrollment
This study is being conducted in five cohorts of 45–50 couples each. Each new cohort begins as the previous cohort transitions from weight loss to maintenance intervention in month 7. As in our previous studies [15,41], we recruit each cohort over eight weeks and do not begin group sessions until we have achieved our recruitment target for a cohort.
We recruit in three ways. First, we place advertisements on bulletin boards in public meeting places, in clinics, and in local print and online publications. Second, we send recruitment electronic mail messages to faculty and staff of the University of Wisconsin (UW)-Madison. Third, we mail recruitment letters to patients in UW Health Internal Medicine and Family Medicine clinics. After one week, we may call people who received a recruitment letter to ascertain interest in the study. Regardless of how they learn about the study, people are referred to the study website for initial eligibility screening. Given population rates of overweight/obesity and that weight and health behaviors are highly correlated within couples [[42], [43], [44], [45]], both members of a couple may be eligible to be index participants (hereafter, participants). When an individual goes to the screening website, they are required to designate one member of the couple as the participant to complete screening, after which they enter their partner's contact information.
The screening website provides a study description followed by questions to determine initial eligibility of participants. People who screen as eligible are asked to provide their name and contact information as well as that of their partner. An e-mail is then sent to the partner with a website link to complete partner eligibility screening. Partners who have not responded within three days are sent a reminder email that is copied to the participant. Couples who pass initial eligibility screening are scheduled for an in-person visit where they provide written consent and complete baseline study measures. Final eligibility is determined via participant and partner weights and participant blood pressure.
2.4. Randomization and blinding
Eligible couples are categorized into one of sixteen strata representing their specific combination of our four stratification variables: participant birth sex, participant baseline BMI (<35 kg/m2 and ≥35 kg/m2), partner baseline BMI (<27 kg/m2 and ≥27 kg/m2), and site (A or B). We stratify by participant sex because the literature has shown sex differences in participant perceptions of partner support in heterosexual couples (the expected majority) and greater weight loss for men than for women [30,47]. We stratify by participant BMI of 35 because it is a cut-point for eligibility for intensive treatments such as bariatric surgery. Stratification by partner BMI of 27 balances groups with partners of the same eligibility for the study, which could affect the support they provide [34]. Lastly, we offer participation at two different community locations (sites) per cohort to increase convenience. We stratify by site to balance any possible effects related to site such as day and time of class meetings.
We randomize couples within each strata 1:1 to the partner-assisted or participant-only arm using a block randomization methodology with random block sizes (known only to the statisticians) within each strata. This methodology will allow for sample sizes of each group to remain near equal throughout the study duration. In each cohort, the intervention is delivered to four groups of ~11–13 participants, with two groups corresponding to the participant-only arm and two corresponding to the partner-assisted arm.
Couples learn of the day of the week and time of their group meetings at the end of the screening visit, when study personnel open a sealed envelope. At this point, the couple is considered as allocated but not randomized; neither study personnel nor couples know which group times correspond to which treatment assignment. Participants attend the first group session alone. After they provide a baseline weight at that session, the principal investigator reveals assignment to the participant-only versus partner-assisted intervention, at which point the couple is considered as randomized. If allocated participants do not attend the first visit, the couple is not considered as randomized: They need not be included in intent-to-treat analyses, and their place in the block randomization scheme can be replaced by a future couple. To address possible dissatisfaction with randomization in the participant-alone arm, we emphasize the importance of the participant-only comparator arm during consent and the first group session; emphasize that partners in both arms participate in assessments; and offer two group sessions including partners to couples in the participant-only arm after 24-month assessments. After the first group session, all outcomes are collected by study personnel blinded to randomization assignment.
2.5. Intervention
2.5.1. Participant intervention (both arms)
Participants in both arms receive identical standard weight management intervention. Couples in the partner-assisted arm receive additional content related to couples communication skills, increasing the total amount of intervention contact. The schedule and content of intervention contacts for both arms is shown in Table 2 .
Table 2.
Intervention mode and content for participant and partner interventions.
| Class | Participant only arm |
Partner-assisted arm |
|||
|---|---|---|---|---|---|
| Modea | Content | Modea | Content | ||
| Week | Initiation | ||||
| 0 | 1 | G,IP |
|
G,IP |
|
| 2 | 2 | G,IP |
|
G,IPb, a |
|
| 4 | 3 | G,IP |
|
GIPb |
|
| 6 | 4 | G,IP |
|
G,IP |
|
| 8 | 5 | G,IP |
|
G,IPb |
|
| 10 | 6 | G,IP |
|
G,IP |
|
| 12 | 7 | G,IP |
|
G,IPb |
|
| 14 | 8 | G,IP |
|
G,IP |
|
| 16 | 9 | G,IP |
|
G,IPb |
|
| 18 | 10 | G,IP |
|
G,IP |
|
| 20 | 11 | G,IP |
|
G,IPb |
|
| 22 | 12 | G,IP |
|
G,IP |
|
| 24 | 13 | G,IP |
|
G,IP |
|
| Month | Maintenance | ||||
| 7 | 14 | G,IP |
|
G,IPb |
|
| 7 | I,T |
|
I,Tb |
|
|
| 8 | 15 | G,IP |
|
G,IPb |
|
| 8 | I,T |
|
I,Tb |
|
|
| 9 | G,IP |
|
G,IPb |
|
|
| 9 | I,T |
|
I,Tb |
|
|
| 10 | I,T |
|
I,T |
|
|
| 11 | I,T |
|
I,T |
|
|
| 12 | I,T |
|
I,Tb |
|
|
| 14 | I,T |
|
I,T |
|
|
| 16 | I,T |
|
I,T |
|
|
| 18 | I,T |
|
I,Tb |
|
|
G = group, IP = in person, T = telephone, I = individual.
Partner participates.
2.5.1.1. Participant weight loss initiation intervention (months 1–6, both arms)
We impart a standard reduced-calorie dietary approach based on previous studies [48]. Groups meet every two weeks and are co-led by a registered dietitian (RD) and an exercise physiologist. At the first group session, participants receive a personalized daily calorie budget involving a 500-cal deficit based on their maintenance caloric requirement, which is calculated based on their weight at the screening visit [15,49]. They are guided to create a personalized, realistic, 6-month weight loss goal with a target of one to two pounds per week. Participants are provided with a physical activity tracker and are encouraged to download a dietary mobile application to their cell phones to promote self-monitoring.
Participants are weighed upon arrival at each group session. The RD then offers 60 min of dietary education and behavioral goal setting. Participants are asked to create a goal related to a menu of three to four topics tailored to each session (e.g., for the class on dining out, the four options are: restaurant menus, modifications, planning, or meals for home). Participants indicate their goal selections on a form collected by study staff. Classes conclude with 15 min of exercise education and demonstration led by the exercise physiologist. Across sessions, a range of exercises are demonstrated, including cardiovascular, strength training, and stretching. Modifications are demonstrated to accommodate different fitness levels. Participants are encouraged to achieve or work up to current recommendations for physical activity, including at least 150 min per week of moderate-intensity aerobic physical activity or 75 min per week of vigorous activity [50].
2.5.1.2. Participant weight loss maintenance intervention (months 7–18, both arms)
Participants transition to the behavioral maintenance intervention in month 7 regardless of whether they achieved their 6-month weight loss goal for three reasons: 1) Based on previous studies, most participants achieve maximum weight loss at 6 months [51]; 2) It is not feasible for some participants to continue the group-based initial protocol while others transition to the individual maintenance protocol; and 3) Our maintenance protocol allows for participants to continue pursuing weight loss during the maintenance phase if desired. Additionally, the protocol stipulates that participants who relapse (defined as gain >3 pounds) should revert to creating dietary and physical activity goals and monitoring these behaviors, thus re-focusing on weight loss.
We are using an evidence-based maintenance intervention involving a shift to maintenance-oriented skills training, from in-person to telephone delivery, and decreased contact frequency [15]. The three group sessions address reasons the body might regain weight, skills for maintaining weight loss, habits of successful losers, and the role of physical activity in maintenance. In the first group session (month 7), participants are provided with a personalized maintenance-level calorie budget. Consistent with recommendations [52], participants are advised that >250 min per week of moderate-to-vigorous intensity activity is suggested during maintenance. The nine telephone calls address four skills for weight loss maintenance [15]: making salient satisfaction with outcomes, self-monitoring, relapse prevention, and social support. An RD or health educator places all telephone calls using a semi-structured script programmed into REDCap (Research Electronic Data Capture [53]), which records participant responses for reference in subsequent calls. The first call is designed to take 20–25 min, with subsequent calls taking 15 min. There is a two-week window around each target call date. If the interventionist is unable to contact a participant in the call window, that telephone call is skipped. Continuity is maintained because each call involves a review of data from the previous call and a review of the same behavioral skills.
In months 19–24, participants do not receive intervention but are encouraged to continue self-monitoring. This transition mimics real-world practice in which participants would be expected to apply the maintenance skills they have learned once intervention contacts cease. The 6-month period without intervention contact will allow us to examine sustainability of intervention effects.
2.5.2. Partner intervention (partner-assisted arm)
Table 3 shows the application of interdependence theory to the partner intervention. We train couples in communication and support skills and help them apply these to 1) increase their perception of weight loss as “our problem” (transformation of motivation) and 2) enhance partners' efficacy and abilities to engage in communal coping and support participants' weight loss efforts in a way that is mutually acceptable and satisfying, described as part of communal coping [54]. During the study, if partners no longer wish to participate in the intervention, we retain participants in the intervention. If participants no longer wish to receive the intervention, their partners no longer receive the intervention. In both cases, we ask both members of the couple to return for outcome assessments.
Table 3.
Application of interdependence theory to partner intervention.
| Interdependence construct | Operationalization |
|---|---|
| Transformation of motivation |
|
Communal coping process
|
|
As this is a partner-assisted intervention, partners are not required to have excess weight or pursue weight loss. Because obesity and health behaviors are highly correlated within couples, however, we expect that some partners will attempt weight loss themselves. Partners receive a handbook with the same handouts as participants and, at joint meetings, receive the same dietary and physical education as participants. We encourage partners to set their own SMART (specific, measurable, attainable, relevant, and timebound) goals, but we do not address them during intervention contacts. As the focus is on participant weight loss, all text messages include participant (not partner) goals and partner (not participant) support plans.
2.5.2.1. Partner involvement in weight loss initiation intervention (months 1–6)
Partners attend half of the group meetings, once monthly. One advantage of this reduced dose for partners compared to participants is that it provides opportunities for participants to interact with one another, which is believed to be an important component of group-based weight loss interventions. Another advantage is that it provides participants a safe space to discuss issues they may not wish to share with their partner, such as specific eating habits. Finally, the smaller dose may improve participant and partner adherence by reducing partner burden.
In joint meetings involving participants and partners, all attendees receive the same dietary and physical activity education, which can provide the basis for instrumental support and may benefit partners who are attempting weight loss themselves. In the first joint meeting, the RD provides ground rules and discusses the role of the partner in weight management. She notes that couples vary in how they like to give and receive support and states that this study focuses on two communication skills: shared decision making and sharing thoughts and feelings. Thus, in the first joint session, the RD reviews guidelines for shared decision making; in the second, she reviews guidelines for speaker and listener roles when sharing thoughts and feelings.
In subsequent joint sessions, the RD provides time for couples to break out and engage in guided exercises to identify individual challenges and preferred supportive behaviors and practice the communication skills. To enhance support commitment, couples work together to generate a partner support plan in relation to the participant's goal. Couples are encouraged to share successes and challenges if comfortable with the large group. Additionally, in several sessions, study staff role-play situations in which a participant and partner have not utilized the communication skills and invite participants to provide feedback about how the conversation could have been different. Couples are advised that the interventionist and study clinical psychologist are available to meet with couples who are having communication challenges. At the end of each group session, participants are asked to select a partner support plan from a menu of seven options fixed across the study (i.e., do it together, provide gentle reminders, praise your partner, remember the long game, check in with your partner, be mindful of how your choices affect your partners' goals, and talk with your partner to develop a support plan at home) and provide it to the study team.
2.5.2.2. Partner involvement in weight loss maintenance intervention (months 7–18)
Partners attend all three in-person group sessions. In each one, couples break out and practice applying the communication skills to participants' maintenance plans. Partners also participate in five of the nine maintenance telephone calls. During all joint telephone calls, partners are asked to reflect on participants' satisfaction with outcomes and to add other outcomes participants may not have mentioned, including any benefits that they have experienced as a couple. Couples work together to develop a plan to support participants' relapse prevention and self-monitoring plans. They are encouraged to communicate regularly about the identified plans, assess progress, and work together to overcome barriers between calls. Calls to couples are 10–15 min longer than participant-only calls.
2.5.3. Text messaging to participants and partners (both arms)
We use text messaging to communicate with participants in both arms over 24 months in two ways. The first is as automated reminders. At baseline, we program REDCap to send 80 automated messages over a 24-month time period using Twilio. Twilio is a secure, third-party web application integrated with REDCap to send survey invites/messages to participants via text message. The text messages remind participants to attend the next upcoming class, complete survey measures, wear their physical activity trackers for outcome assessments, and to attend in-person assessments. These messages are tailored to participant role (participant vs. partner) and arm assignment.
Secondly, text messaging is used to reinforce didactic content and behavioral principles during the intervention (Table 4 ). During the weight loss initiation phase (months 1–6), participants (both arms) and partners (partner-assisted arm) receive three text messages per week. The messages are designed in two-week cycles corresponding to the biweekly group visits. In each two-week cycle, participants receive text messages reminding them of the goal topic (both arms) and support plan (partner-assisted arm) indicated on forms turned in during the group session. They also receive behavioral or didactic content tailored to the topic covered in the corresponding group session. Participants who miss a group session receive a telephone call from study staff requesting them to select a goal and, if appropriate, support plan. If participants do not respond to the telephone call, a study team member selects a goal/support plan for them.
Table 4.
Text messages to reinforce intervention content.
| Months | Frequency | Recipient | Text Message Content |
|---|---|---|---|
| 1–6 | 3/week | Participants in participant-only arm | Participant goal |
| Behavioral or didactic content | |||
| Behavioral or didactic content | |||
| Participants in partner-assisted arm | Participant goal and partner support plan | ||
| Social support tip | |||
| Behavioral or didactic content | |||
| Partners in partner-assisted arm | Participant goal and partner support plan | ||
| Social support tip | |||
| Behavioral or didactic content | |||
| 7–9 | 2/week | Participants in both arms | Maintenance principles |
| Partners in partner-assisted arm | |||
| 10–12 | 1/week | Participants in both arms | Maintenance principles |
| Partners in partner-assisted arm | |||
| 13–18 | Every 2 weeks | Participants in both arms | Maintenance principles |
| Partners in partner-assisted arm |
During the weight loss maintenance phase, the frequency of text messages decreases to twice per week in months 7–9, once per week in months 10–12, and once every two weeks in months 13–18. Text messages to participants (both arms) and partners (partner-assisted arm) address the four behavioral maintenance principles (i.e., satisfaction with outcomes, self-monitoring, relapse prevention, and social support). In contrast to the text messages sent in months 1–6, which are tailored to participants' goals and desired support plans and are scheduled every week, these maintenance messages are scheduled at baseline and are automatically sent at scheduled times in months 7–18.
2.6. Protocol changes due to Covid-19 pandemic
In March of 2020, we made several changes to recruitment, intervention delivery, and outcome assessment processes to allow study continunuation when in-person research visits are not permitted or desirable. First, we changed two eligibility criteria to enable screening visits to be conducted virtually: 1) Removed the blood pressure criterion (study physicians determined that this was not a safety risk) and 2) Added possession of harrdware to permit video conferencing and Internet connection. Second, we expanded our recruitment strategy to include postings on local websites (e.g., Madison365, La Comunidad) and a press release by the UW Health media office. Third, we shifted in-person group sessions to WebEx, a UW-Madison-approved videoconferencing platform. Fourth, we shipped calibrated scales to each couple's home. We e-mail each member of the couple a link to a REDCap survey along with instructions to type in their weight and upload a photograph of their weight displayed on the scale. This is done for participants at each bi-weekly group session during months 1–6 and for participants and partners at each outcome assessment at 6-month intervals. Fifth, we added several questions to each outcome assessment survey assessing the impact of the pandemic on dietary and activity habits. The duration of these protocol changes, and any further changes, will be reported in the context of trial results, and sensitivity analyses will be performed as appropriate (e.g., comparing weight loss among participants who received the intervention completely virtually versus a hybrid of in-person and virtual visits).
2.7. Interventionist training and fidelity
Interventionists read key articles selected by the study team to learn the behavioral concepts addressed in the study (e.g., cognitive behavioral therapy for couples). They also listened to recorded intervention telephone calls from a previous weight loss maintenance study [15]. Prior to each group session for the first cohort, the RD practiced the lesson for the study investigators and colleagues. Prior to beginning maintenance calls for the first cohort, the RD practiced calls with several team members and colleagues.
Each group session and maintenance call is audio recorded. Three investigators are responsible for fidelity monitoring (CV, LP, KG). These investigators meet with the interventionists for 1.5 h every two weeks to review audio recorded sessions. The investigators review additional audio recordings individually using fidelity checklists created for this study. The calls reviewed are both chosen at random by the investigators and flagged by the interventionists for review.
Fidelity to the text messages is maintained by automated delivery and by time and date stamps. In the initiation phase, the system automatically delivers the selected bundled messages. Study staff can review in REDCap that messages were delivered and that the appropriate bundle was selected. The maintenance and reminder messages are all scheduled at baseline. The software system maintains fidelity over the 24-month period by delivering messages at pre-determined dates and time.
2.8. Safety monitoring
Adverse events are assessed each time study staff interact with participants (every group session, maintenance phone call, and outcome assessment visit) using a yes/no question (“Have you had any health events since the last time we talked to you?”) with the opportunity to elaborate. Responses are categorized according to CTCAE version 5.0. Adverse events are summarized for annual meetings with the UW Institute for Clinical and Translational Research Data Monitoring Committee. Severe adverse events are reported according to local institutional review board requirements.
2.9. Outcome assessments
Demographic characteristics are assessed during the screening visit. Whether participants and partners engaged in any other weight loss program is assessed at 6, 12, 18, and 24 months. Attendance at group sessions and telephone calls is recorded in the study database to allow calculation of intervention adherence for possible dose-response analyses. Primary (participant body weight) and secondary (participant caloric intake and physical activity) outcomes are assessed at baseline and every six months. Baseline weight is obtained at the first group session, and physical activity is recorded for one week prior to that session; baseline for all other measures is obtained during the eligibility visit so could vary by up to 8 weeks. Participant and partner mediators are measured at baseline (eligibility visit) and every three months. There is a 10-day window on each side of target assessment dates. All measures except the dietary recall are obtained via REDCap survey. Participants and partners each receive $40 for every completed interim assessment and $60 for the month-24 assessment, for a total possible incentive of $180 each ($360 per couple).
We take several steps to minimize missing data. The RD schedules participants for an outcome assessment visit during the group session or telephone call that precedes an assessment time point. When this is not possible, study personnel send personalized email reminders, followed by a telephone call, to attempt to schedule the participant for the outcome assessment appointment. For survey data collected via REDCap survey, automated reminder emails are sent at specified intervals until the end of the data collection window. Links to the REDCap survey and dietary recall program are e-mailed to participants at the beginning of the assessment window, with reminders sent every five days until the data collection window is closed. If participants have not completed the surveys upon arrival at the in-person weight measurement, they are asked to complete them on a study-provided tablet.
2.9.1. Primary and secondary outcome
Participant body weight is obtained in person using a calibrated digital Tanita WB-800S plus Digital Scale in light clothing with pockets emptied and shoes removed.
Participant caloric intake is assessed using the Automated Self-Administered 24-h dietary recall (ASA24) [55,56]. Participants receive a link to the website by e-mail and may call study staff to ask for assistance. Electronic messages are sent to remind participants to complete recalls on one weekday and one weekend day during the data collection window.
Participant physical activity is assessed with a Fitbit Inspire Flex® or similar model, a valid and reliable triaxial accelerometer [57] that is well-accepted by consumers and compatible with iOS and Android devices. Participants receive the device at the in-person screening visit, along with instructions to wear it 24 h a day for seven days during outcome assessment windows. They have the option of wearing it all the time but are prompted during outcome assessment windows. Fitbit data will be collapsed and summarized for each participant as total and average minutes of moderate activity (as emphasized in the intervention) and total number of steps over each 6-month outcome assessment window. In accordance with current physical activity guidelines [50], Fitbit quantifies moderate activity as spending at least 10 min in an activity that burns three times as many calories as at rest. Fitbit trackers calculate active minutes using metabolic equivalents (METs). When at rest, the metabolic MET equals 1. Fitbit uses a level of 3 MET or higher to indicate moderate-intensity exercise.Participants keep the Fitbits after study completion.
2.9.2. Couple interdependence and support constructs for mediation analyses
Consistent with recommendations of Dibble [58], transformation of motivation is assessed with both Aron's Inclusion of Other in Self (IOS) scale [59] and the Unidimensional Relationship Closeness Scale (URCS) [58]. The IOS comprises seven Venn-like diagrams representing different degrees of overlap between the partner and self. IOS scores range from 1 (completely separate, non-overlapping circles) to 7 (completely overlapping circles). Participants complete four versions of the IOS: relationship with their partner, how they manage weight, how they manage healthy eating, and how they manage being physically active. The IOS has produced test-retest correlations >0.80 and has been associated with relationship longevity. The theoretical basis supports the transformation of motivation concept in that self-expansion occurs when the interests and experiences of one's partner are integrated into one's self definition. The URCS has 12 items (e.g., “My relationship with my partner is close”) rated on a 7-point scale (strongly disagree to strongly agree). The URCS has produced highly reliable scores (>0.90) across different relationship types, demonstrates measurement invariance across relationship types, and is associated with relational satisfaction.
We assess communal coping in three domains (use of communal coping, couple efficacy, and outcome efficacy) related to dietary and physical activity changes for both participant and partner. We focus on these three domains because of evidence showing: a) the interdependence of coping behaviors in chronic illness within couples (use of communal coping) [60]; b) the importance of efficacy perceptions in predicting a wide range of health outcomes (couple efficacy); and c) the relevance of behavioral expectations (outcome efficacy) [61]. There is no standardized measure of these concepts across behaviors; instead, the measurement approach must be tailored to each health behavior. Items have been developed to measure these constructs in the context of smoking cessation [62], HIV [63,64], vasculitis [65], and colon cancer prevention [66]. Use of communal coping for diet and physical activity is measured with 10 items (e.g., “How often do your partner and you talk about ways to eat healthier?” 1 never to 5 very often). Couple efficacy in communal coping for diet and physical activity is measured with 10 items (e.g., “How confident are you that, as a couple, you two could talk about ways to eat healthier?” 0 = not at all confident to 10 = very confident). Outcome efficacy of communal coping for diet and physical activity is measured with 10 items (e.g., “How effective would it be to talk, as a couple, about ways to eat healthier?” 0 = not at all effective to 10 = very effective). We also measure social support from their partner for diet and physical activity using measures from Ball and Crawford [67]. These measures have produced reliable scores and demonstrated validity in women with overweight/obesity [68]. Respondents are asked to indicate how often their partner has said or done a variety of actions in the past 6 months (e.g., complimented me on my eating habits) on a scale ranging from 1 almost never to 5 almost always.
2.9.3. Partner outcomes
At the screening visit and every 6 months, we obtain weight, dietary intake, interdependence constructs, and social support using the previously described methods from partners in both arms. Fitbits are not provided to partners due to budgetary constraints.
2.10. Analyses
Descriptive statistics, including graphical displays, will be used to summarize all study variables. Distributional assumptions of the continuous outcome variables will be carefully examined and transformations made, if necessary. We will construct individual and mean trajectory plots of the longitudinal outcome variables to understand their general trends over the study period. In addition, we will explore the variability and correlation structure of the longitudinal outcome variables.
Aim 1
: Test the hypothesis that average participant weight loss is at least 2.5 kg greater in the partner-assisted arm than the participant-only arm at 24 months.
The unit of time will be months relative to randomization. The outcome is participant weight measured at each 6-month, in-person assessment visit. Therefore, month0 represents participant weight at the point of randomization (i.e., the first group visit), and month24 represents the primary study endpoint. The intent-to-treat analysis of between-arm comparison in participant net weight loss from month0 to month24 will be examined under a multilevel longitudinal mixed-effects model [69]. Predictors in the model will include linear time and its interaction with the intervention indicator (without the intervention effect to reflect the equality in baseline means constrained by randomization). The 16 strata will also be included as fixed effects. Participant- and group-level random intercepts and time slope will be included to account for the correlation between longitudinal measurements within participants and between participants within the small group. We will estimate the parameters in the model using the SAS procedure MIXED (Cary, NC). Contrasts will be written in the context of this model to test the difference of mean weight loss between the two treatment arms at month24.
Our plans for preventing and dealing with missing data follow the guidelines set forth by the National Research Council's Panel on Handling Missing Data in Clinical Trials. We employ numerous strategies to achieve <20% participant attrition, noted previously. In case data are not missing at random, multiple imputations using auxiliary variables (e.g. physical activity measurements) will be conducted as sensitivity analyses.
Aim 2
: Test the hypotheses that average participant daily caloric intake is significantly lower, and time spent doing moderate-intensity physical activity is significantly greater, in the partner-assisted arm than the participant-only arm at 24 months.
Estimated participant caloric intake and physical activity are measured at months 0, 6, 12, 18, and 24. Changes in estimated participant caloric intake and physical activity will be analyzed using the same methods and models presented for the Aim 1 analyses of weight. Previous experience with these outcomes has shown they are often non-normally distributed; therefore, outcomes may be analyzed as generalized linear mixed models, estimated with adaptive Gaussian quadrature, as needed. As exploratory analyses, we will use the models described for Aim 1, Aim 2 to examine effects of the partner-assisted intervention on partner weight and caloric intake measured every six months.
Aim 3
: Assess the extent to which interdependence constructs mediate the effect of the partner-assisted weight management intervention on participant weight loss.
The potential mediators will be operationalized at the couple level—separately for diet and physical activity—as standardized sums of the items for 1) transformation of motivation, 2) couple efficacy, 3) outcome efficacy, 4) communal coping, 5) social support, averaged across participant and partner. First, we will investigate the effect of the intervention on each mediator, using models analogous to those in Aim 1, Aim 2 with controls for the baseline values of all mediators. As the intervention is randomized, these estimates can be interpreted causally. However, since the mediators were not randomized, all subsequent models are exploratory and warrant causal interpretation only under additional assumptions [70]. Second, we will estimate the direct effect of the intervention on final (24-month) weight with an analogous model, including all mediators at 6, 12, and 18 months. Third, we will estimate the indirect effect of the intervention jointly via all mediators as the difference between the total effect of the intervention estimated from an analogous model without intermediate measures of the mediators minus the direct effect estimated in step 2 [71]. Fourth, we will estimate stage-specific mediation by first including only all mediators measured at 6 months, then additionally all mediators measured at 12 months, and finally all mediators measured at 18 months. Then we will compute the contribution of each stage to the mediation process as the difference between the direct and indirect effects estimated in the previous stage [71]. Finally, we will explore mediation by individual mediator constructs analogously, albeit noting that these individual-mediator models will lack a causal interpretation if the various mediator constructs influence each other within a given stage [71]. Standard errors for estimates requiring the combination of coefficients from multiple models will be constructed using the bootstrap. We will penalize standard errors for multiple testing [72].
2.11. Sample size and power considerations
The sample size estimate is based on the primary hypothesis that participant weight loss will be at least 2.5 kg lower at 24 months in the partner-assisted than participant-only arm. This effect size was chosen because smaller differences are unlikely to be considered meaningful to researchers, providers, or participants [73]. Additionally, it is similar in magnitude to proposed effect sizes for other weight loss maintenance trials [17,19,51]. To enable comparisons with other trials, we will also calculate percent weight loss. Sample size was determined by multiplying the number of participants required for a t-test of post scores by 2(1-ρ) and adding one extra participant per arm [74]. Based on a previous study by members of our team [15], we anticipate a common standard deviation of 19.9 kg and a correlation (ρ) between the month0 and month24 time points of 0.96. Thus, we need 160 participants total (80 in each arm) to detect a 2.5 kg difference with 80% power and a type-I error rate of 5%. We further inflate the sample size to 1) incorporate the intra-class correlation of small group members and 2) compensate for attrition using procedures for group-randomized designs [75]. Assuming a maximum of 15 participants in each small group and intra-class correlation among group members of 0.01, the updated sample size is 180. Inflating the sample size by an attrition rate of 20% through month 24, our target sample size is 230 participants (115 per arm).
3. Discussion
This study is the first, to our knowledge, to apply principles of cognitive behavioral therapy for couples to target communal coping to support long-term participant weight loss. We are systematically involving partners and training couples in communal coping skills. This approach contrasts with previous trials in which partners have been taught to monitor participant progress [27,32] or provide a particular type of social support [30,31,33,76]. By intervening on longstanding communication patterns among couples, we hope to improve transformation of motivation, use of communal coping, couple efficacy, outcome efficacy, and perceived social support for dietary and physical activity change.
Our study is also among the first to use both interdependence and support constructs to frame an intervention that supports behaviors conducive to weight loss. A recent randomized controlled trial evaluated the effect of increasing autonomy support in couples enrolled in a six-month weight loss program [76]. Autonomy support included more general areas of support: empathy, listening skills, and giving feedback. Our work builds on this by recognizing variability in individuals' preferences for the amount and types of support for weight loss as well as ways in which couples problem-solve and make behavior changes together. Furthermore, we will evaluate both shorter- (i.e., 6 months) and longer-term (i.e., 24 months) effects of increasing communal coping.
Our trial is novel in that our measurement approach includes both transformation of motivation and other interpersonal processes that are expected to account for behavior change and weight loss. [76] This theoretical and measurement approach is fully aligned in our use of mediation analyses that will provide important information regarding mechanisms by which a partner-assisted intervention can assist in weight loss and weight loss maintenance. Using this approach, our study will help advance theory and measurement in this research area. Our study also illustrates how these concepts inform intervention components that may account for behavioral change and weight loss.
Our study is also novel in its use of text messaging. One advantage to using text messages is that it balances efficacy with burden. Recruitment and retention of couples is inherently more difficult than of a single individual due to the need for both partners to meet eligibility criteria and be retained in the intervention. To maintain engagement of partners between contacts, we send text messages to inform partners of participant goals and desired support plans. Our intent is that these text messages will stimulate a conversation outside the intervention setting wherein couples can utilize the communication skills we teach to promote participant adherence to dietary and physical activity changes. Another advantage of using text messaging is that it enforces intervention fidelity. Messages are scheduled at pre-determined times, and a log is created when they are sent. This method not only allows for cost-efficient scalability, but can help reduce staff burden, too. Text messaging is not only an affordable communication method, but remains the most popular communication method to reach diverse populations [77].
This study has some limitations. Generalizability is affected by several study design features. First, we are likely to recruit people who prefer a reduced-calorie diet as opposed to other diets. This would be a limitation no matter which dietary approach we used. Second, we require that couples have separate mobile phones with data plans. According to Pew Research, over 81% of US adults own a smartphone and over 96% own a cell phone. Low-income racial/ethnic minorities are more likely than low-income Whites to own mobile devices and to use features such as text messaging or smartphone applications [77], so we do not expect this intervention to exacerbate disparities. Third, there may be a self-selection bias such that couples with less closeness are less likely to enroll. Fourth, the study is conducted in a single, largely Caucasian city in the upper Midwest, whose residents may not be fully representative of the national population. Fifth, this intervention approach may not generalize to relationships other than a participant-partner dyad. In addition to limits on generalizability, another limitation is that requiring partners to attend half of group sessions may change group dynamics and/or affect efficacy. This disadvantage might be offset by providing participants an opportunity to converse with one another during group sessions and an opportunity to discuss potentially sensitive topics with the interventionist during intervention telephone calls.
In conclusion, Partner2Lose is among the first RCTs to test the efficacy of an intervention to fundamentally change the way couples think and communicate about weight loss. By teaching couples speaker and listener roles for joint problem solving and sharing thoughts and feelings, we aim to have partners consider weight loss as a couple's issue rather than a participant's issue. By encouraging couples to co-develop goals and support plans, we aim to have participants adopt and adhere to healthier dietary and physical activity behaviors. Our intervention represents a practical, sustainable approach to move the needle on the intractable problem of obesity.
Funding
This study was supported by a grant to the first author from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK111491-01). Dr. Voils is supported by a Research Career Scientist award (RCS 14-443) from the Health Services Research & Development service of the Department of Veterans Affairs. The views contained in this article are those of the authors and not those of the U.S. Government.
Acknowledgments
The authors would like to thank Maren Olsen, PhD, for feedback on the analytic plan and Jenna Quinto, PhD, for editing.
References
- 1.Serdula M.K. Prevalence of attempting weight loss and strategies for controlling weight. J. Am. Med. Assoc. 1999;282(14):1353–1358. doi: 10.1001/jama.282.14.1353. [DOI] [PubMed] [Google Scholar]
- 2.Kenchaiah S. Obesity and the risk of heart failure. N. Engl. J. Med. 2002;347(5):305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 3.Wilson P.W. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch. Intern. Med. 2002;162(16):1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 4.Fontaine K.R., Barofsky I. Obesity and health-related quality of life. Obes. Res. 2001;2(3):173–182. doi: 10.1046/j.1467-789x.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 5.Cawley K., Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J. Health Econ. 2012;31(1):219–230. doi: 10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Tsai A.G., Williamson D.F., Glick H.A. University of Pennsylvania; Philadelphia, PA: 2006. A Quantitative Assessment of Cost of Obesity Studies. in Working Paper. [Google Scholar]
- 7.Finkelstein E.A. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Affairs. 2009;28(5):w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 8.Foster G.D. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann. Intern. Med. 2010;153(3):147–157. doi: 10.1059/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wadden T.A. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N. Engl. J. Med. 2005;353(20):2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 10.Johns D.J. Diet or exercise interventions vs combined behavioral weight management programs: a systematic review and meta-analysis of direct comparisons. J. Acad. Nutr. Diet. 2014;114(10):1557–1568. doi: 10.1016/j.jand.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacLean P.S. NIH working group report: innovative research to improve maintenance of weight loss. Obesity. 2015;23(1):7–15. doi: 10.1002/oby.20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leahey T.M. A randomized controlled trial testing an internet delivered cost-benefit approach to weight loss maintenance. Prev. Med. 2016;92:51–57. doi: 10.1016/j.ypmed.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yancy W.S., Jr. Effect of escalating financial incentive rewards on maintenance of weight loss: a randomized clinical trial. JAMA Netw. Open. 2019;2(11) doi: 10.1001/jamanetworkopen.2019.14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yancy W.S., Jr. Financial incentive strategies for maintenance of weight loss: results from an internet-based randomized controlled trial. Nutr. Diabetes. 2018;8(1) doi: 10.1038/s41387-018-0036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voils C.I. Maintenance of weight loss after initiation of nutrition training: a randomized trial. Ann. Intern. Med. 2017;166(7):463–471. doi: 10.7326/M16-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherwood N.E. Enhancing long-term weight loss maintenance: 2 year results from the keep it off randomized controlled trial. Prev. Med. 2013;56(3–4):171–177. doi: 10.1016/j.ypmed.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svetkey L.P. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. J. Am. Med. Assoc. 2008;299(10):1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 18.Wing R.R. A self-regulation program for maintenance of weight loss. N. Engl. J. Med. 2006;355(15):1563–1571. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 19.Kiernan M. Promoting healthy weight with "stability skills first": a randomized trial. J. Consult. Clin. Psychol. 2012;81(2):336–346. doi: 10.1037/a0030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyler D., Stimpson J.P., Peek M.K. Health concordance within couples: a systematic review. Soc. Sci. Med. 2007;64(11):2297–2310. doi: 10.1016/j.socscimed.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Keicolt-Glaser J., Newton T. Marriage and health: his and hers. Psychol. Bull. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- 22.Umberson D. Gender, marital status and the social control of health behavior. Soc. Sci. Med. 1992;34(8):907–917. doi: 10.1016/0277-9536(92)90259-s. [DOI] [PubMed] [Google Scholar]
- 23.Center P.R. 2019. Marriage and Cohabitation in the U.S. [Google Scholar]
- 24.Martire L.M. Review and meta-analysis of couple-oriented interventions for chronic illness. Ann. Behav. Med. 2010;40(3):325–342. doi: 10.1007/s12160-010-9216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Black D.R., Gleser L.J., Kooyers K.J. A meta-analytic evaluation of couples weight-loss programs. Health Psychol. 1990;9(3):330–347. doi: 10.1037//0278-6133.9.3.330. [DOI] [PubMed] [Google Scholar]
- 26.McLean N. Family involvement in weight control, weight maintenance and weight-loss interventions: a systematic review of randomised trials. Int. J. Obes. Relat. Metab. Disord. 2003;27(9):987–1005. doi: 10.1038/sj.ijo.0802383. [DOI] [PubMed] [Google Scholar]
- 27.Murphy J. The long-term effects of spouse involvement upon weight loss and maintenance. Behav. Ther. 1982;13:681–693. [Google Scholar]
- 28.Black D., Lantz C. Spouse involvement and a possible long-term follow-up trap in weight loss. Behav. Res. Ther. 1984;22(5):557–562. doi: 10.1016/0005-7967(84)90059-7. [DOI] [PubMed] [Google Scholar]
- 29.Pearce J.W., LeBow M.D., Orchard J. Role of spouse involvement in the behavioral treatment of overweight women. J. Consult. Clin. Psychol. 1981;49(2):236–244. doi: 10.1037//0022-006x.49.2.236. [DOI] [PubMed] [Google Scholar]
- 30.Wing R.R. A “family-based” approach to the treatment of obese type II diabetic patients. J. Consult. Clin. Psychol. 1991;59(1):156–162. doi: 10.1037//0022-006x.59.1.156. [DOI] [PubMed] [Google Scholar]
- 31.Rosenthal B., Allen G.J., Winter C. Husband involvement in the behavioral treatment of overweight women: initial effects and long-term follow-up. Int. J. Obes. 1980;4(2):165–173. [PubMed] [Google Scholar]
- 32.Saccone A.J., Israel A.C. Effects of experimenter versus significant other-controlled reinforcement and choice of target behavior on weight loss. Behav. Ther. 1978;9:271–278. [Google Scholar]
- 33.Dubbert P.M., Wilson G.T. Goal-setting and spouse involvement in the treatment of obesity. Behav. Res. Ther. 1984;22(3):227–242. doi: 10.1016/0005-7967(84)90003-2. [DOI] [PubMed] [Google Scholar]
- 34.Brownell K.D., Stunkard A.J. Couples training, pharmacotherapy, and behavior therapy in the treatment of obesity. Arch. Gen. Psychiatry. 1981;38(11):1224–1229. doi: 10.1001/archpsyc.1981.01780360040003. [DOI] [PubMed] [Google Scholar]
- 35.Lewis M.A. Understanding health behavior change among couples: an interdependence and communal coping approach. Soc. Sci. Med. 2006;62(6):1369–1380. doi: 10.1016/j.socscimed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Epstein N., Baucom D. American Psychological Association; Washington, DC: 2002. Enhanced Cognitive-Behavioral Therapy for Couples: A Contextual Approach. [Google Scholar]
- 37.Baucom D.H. A couple-based intervention for female breast cancer. Psycho-Oncology. 2009;18(3):276–283. doi: 10.1002/pon.1395. [DOI] [PubMed] [Google Scholar]
- 38.Fischer M.S., Baucom D.H., Cohen M.J. Cognitive-Behavioral couple therapies: review of the evidence for the treatment of relationship distress, psychopathology, and chronic health conditions. Fam. Process. 2016;55(3):423–442. doi: 10.1111/famp.12227. [DOI] [PubMed] [Google Scholar]
- 39.Porter L.S. Pilot randomized trial of a couple-based physical activity videoconference intervention for sedentary cancer survivors. Health Psychol. 2018;37(9):861–865. doi: 10.1037/hea0000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Institute for Health Metrics and Evaluation County Profile for Dane County Wisconsin. 2015. http://www.healthdata.org/sites/default/files/files/county_profiles/US/2015/County_Report_Dane_County_Wisconsin.pdf [cited 2020 March 12]; Available from:
- 41.Yancy W.S., Jr. A randomized trial of a low-carbohydrate, ketogenic diet versus orlistat plus a low-fat diet for weight loss. Arch. Intern. Med. 2010;170(2):136–145. doi: 10.1001/archinternmed.2009.492. [DOI] [PubMed] [Google Scholar]
- 42.Cobb L.K. Changes in body mass index and obesity risk in married couples over 25 years: the ARIC cohort study. Am. J. Epidemiol. 2016;183(5):435–443. doi: 10.1093/aje/kwv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macario E., Sorensen G. Spousal similarities in fruit and vegetable consumption. Am. J. Health Promot. 1998;12(6):369–377. doi: 10.4278/0890-1171-12.6.369. [DOI] [PubMed] [Google Scholar]
- 44.Jacobson P. Spouse resemblance in body mass index: effects on adult obesity prevalence in the offspring generation. Am. J. Epidemiol. 2007;165(1):101–108. doi: 10.1093/aje/kwj342. [DOI] [PubMed] [Google Scholar]
- 45.Di Castelnuovo A. Spousal concordance for major coronary risk factors: a systematic review and meta-analysis. Am. J. Epidemiol. 2009;169(1):1–8. doi: 10.1093/aje/kwn234. [DOI] [PubMed] [Google Scholar]
- 46.Callahan C.M. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med. Care. 2002;40(9):771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Schierberl Scherr A.E., McClure Brenchley K.J., Gorin A.A. Examining a ripple effect: do spouses' behavior changes predict each other's weight loss? J. Obes. 2013;2013 doi: 10.1155/2013/297268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yancy W.S., Jr. Effect of allowing choice of diet on weight loss: a randomized trial. Ann. Intern. Med. 2015;162(12):805–814. doi: 10.7326/M14-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Institute of Medicine . The National Academies Press; Washington, DC: 2002. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. [DOI] [PubMed] [Google Scholar]
- 50.US Department of Health and Human Services . US Department of Health and Human Services; Washington, DC: 2018. 2018 Physical Activity Guidelines for Americans. [Google Scholar]
- 51.Dombrowski S.U. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. Br. Med. J. 2014;348:g2646. doi: 10.1136/bmj.g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donnelly J.E. American College of Sports Medicine position stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc. 2009;41(2):459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 53.Harris P.A. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Helgeson V.S. Communal coping and adjustment to chronic illness: theory update and evidence. Personal. Soc. Psychol. Rev. 2018;22(2):170–195. doi: 10.1177/1088868317735767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Subar A.F. Formative research of a quick list for an automated self-administered 24-hour dietary recall. J. Am. Diet. Assoc. 2007;107(6):1002–1007. doi: 10.1016/j.jada.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Blanton C. The USDA Automated Multiple-Pass Method accurately estimates group total energy and nutrition intake. J. Nutr. 2006;136:2594–2599. doi: 10.1093/jn/136.10.2594. [DOI] [PubMed] [Google Scholar]
- 57.Reid R.E.R. Validity and reliability of Fitbit activity monitors compared to ActiGraph GT3X+ with female adults in a free-living environment. J. Sci. Med. Sport. 2017;20(6):578–582. doi: 10.1016/j.jsams.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 58.Dibble J.L., Levine T.R., Park H.S. The Unidimensional Relationship Closeness Scale (URCS): reliability and validity evidence for a new measure of relationship closeness. Psychol. Assess. 2012;24(3):565–572. doi: 10.1037/a0026265. [DOI] [PubMed] [Google Scholar]
- 59.Aron A., Aron E., Smollan D. Inclusion of other in the self scale and the structure of interpersonal closeness. J. Pers. Soc. Psychol. 1992;63:596–612. [Google Scholar]
- 60.Cutrona C. Sage Publications; Thousand Oaks: 1996. Social Support in Couples. [Google Scholar]
- 61.Bandura A. Freeman; New York: 1997. Self-Efficacy: The Exercise of Control. [Google Scholar]
- 62.Grinberg A. American Psychological Association; Chicago, IL: 2012. Measurement of Communal Coping in Dual-Smoker Couples. [Google Scholar]
- 63.Salazar L.F. Development and validation of HIV-related dyadic measures for men who have sex with men. J. Sex Res. 2013;50(2):164–177. doi: 10.1080/00224499.2011.636845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lewis M.A. Health-related social control and relationship interdependence among gay couples. Health Educ. Res. 2006;21(4):488–500. doi: 10.1093/her/cyh075. [DOI] [PubMed] [Google Scholar]
- 65.Lewis M. Interpersonal processes and vasculitis management. Arthritis Care Res. 2006;55:670–675. doi: 10.1002/art.22092. [DOI] [PubMed] [Google Scholar]
- 66.Lewis M. American Society of Preventive Oncology; 2003. Couples' preferences for beahvior change following polypectomy. [Google Scholar]
- 67.Ball K., Crawford D. An investigation of psychological, social, and environmental correlates of obesity and weight gain in young women. Int. J. Obes. 2006;30:1240–1249. doi: 10.1038/sj.ijo.0803267. [DOI] [PubMed] [Google Scholar]
- 68.Kiernan M. Social support for healthy behaviors: scale psychometrics and prediction of weight loss among women in a behavioral program. Obesity. 2012;20(4):756–764. doi: 10.1038/oby.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fitzmaurice G., Laird N., Ware J. Wiley; Hoboken, NJ: 2004. Applied Longitudinal Analysis. [Google Scholar]
- 70.VanderWeele T.J. Oxford University Press; New York, NY: 2015. Explanation in Causal Inference: Methods for Mediation and Interaction. [Google Scholar]
- 71.VanderWeele T.J., Vansteelandt S. Mediation analysis with multiple mediators. Epidemiol. Methods. 2014;2(1):95–115. doi: 10.1515/em-2012-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57(1):289–300. [Google Scholar]
- 73.Ludwig D., Ebbeling C. Weight-loss maintenance-mind over matter? N. Engl. J. Med. 2011;363(22):2159–2161. doi: 10.1056/NEJMe1011361. [DOI] [PubMed] [Google Scholar]
- 74.Borm G., Fransen J., Lemmens W. A simple sample size formula for analysis of covariance in randomized clinical trials. J. Clin. Epidemiol. 2007;60(12):1234–1238. doi: 10.1016/j.jclinepi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 75.Donner A., Klar N. Oxford University Press; New York: 2000. Design and Analysis of Cluster Randomization Trials in Health Research. [Google Scholar]
- 76.Gorin A.A. A randomized controlled trial of a theory-based weight-loss program for couples. Health Psychol. 2020;39(2):137–146. doi: 10.1037/hea0000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pew Research Center . 2018. Mobile Fact Sheet. [Google Scholar]