Abstract
Background
Coronavirus disease 2019 (COVID-19) has become a pandemic. Despite the growing number of patients with COVID-19 infection, data on the clinical characteristics of pregnant patients are still limited.
Methods
We retrospectively included childbearing-age female patients with laboratory-confirmed COVID-19 at Renmin Hospital of Wuhan University from January 15 to February 23, 2020. Demographic, clinical, radiological, laboratory, and treatment data were reviewed. Clinical characteristics of pregnant and nonpregnant patients were compared.
Results
One hundred eleven childbearing-age women with COVID-19 were included, including 16 patients (14.4%) with severe or critical disease. Compared with nonpregnant patients (n = 80), pregnant patients (n = 31) were less likely to have dyspnea (16.1% vs 37.5%), asthenia (3.2% vs 33.8%), and ≥3 symptoms (22.6% vs 45.0%); had a significantly higher neutrophil count (5.2 vs 2.5 ×109/L) and a higher percentage of CD3+ cells (76.7% vs 73.7%) and CD8+ cells (32.3% vs 28.4%); and had a dramatically lower percentage of lymphocytes (18.2% vs 31.8%), a lower CD4+/CD8+ ratio (1.2 vs 1.4), and a lower level of IgG (9.8 vs 11.9 g/L). Of note, pregnant patients had a significantly lower percentage of severe disease (3.2% vs 18.8%) and a substantially higher level of inflammation markers including neutrophil-to-lymphocyte ratio (4.4 vs 1.9) and systematic inflammatory index (812.8 vs 354.7) than nonpregnant patients. Seventeen live births were recorded, and all of these showed negative results of postnatal COVID-19 detection together with a normal Apgar score.
Conclusions
Pregnant patients with COVID-19 had a lower level of severity and an enhanced inflammatory response and cell immunity when compared with nonpregnant patients.
Keywords: COVID-2019, pregnant women, clinical characteristics, severity
Coronavirus disease 2019 (COVID-19) occurred in Wuhan, the capital city of Hubei Province, China, beginning in December 2019, and rapidly spread throughout China [1–4]. The World Health Organization (WHO) has declared COVID-19 a public health emergency of international concern. Now, COVID-19 has become a global outbreak. Most of the published studies on COVID-19 have collected and analyzed clinical data from nonpregnant adults [1–4]. To date, only a limited number of pregnant women with COVID-19 infection have been studied to investigate the possibility of intrauterine vertical transmission, and no evidence for intrauterine infection was found [5, 6].
Current knowledge and clinical management of pregnant women with COVID-19 is mainly based on information from the general population [7]. In spite of the growing number of pregnant women with COVID-19, data on the clinical characteristics and disease severity of pregnant patients are still limited. Considering the particularity of immune status and physiological features in pregnant women, there is an urgent need to investigate the differences in the clinical characteristics and severity of COVID-19 between pregnant and nonpregnant women and the potential impact of COVID-19 infection on the clinical outcomes of the fetus and neonate. Answering these questions will be useful to the development of effective preventive and therapeutic strategies in clinical settings. Herein, we retrospectively and simultaneously identified clinical data from pregnant and childbearing-age nonpregnant women with laboratory-confirmed COVID-19 infection at Renmin Hospital in Wuhan University, Wuhan, China. In this study, we compare the detailed clinical characteristics of pregnant patients with nonpregnant patients, and we present the neonatal outcomes in pregnant patients.
METHODS
Study Design and Included Patients
First, we retrospectively reviewed the electronic medical records of patients with laboratory-confirmed COVID-19 admitted to Renmin Hospital of Wuhan University from January 15 to February 23, 2020. As previous studies have reported [1], diagnosis of COVID-19 was based on the result of real-time reverse-transcriptase polymerase chain reaction (RT-PCR) of routine nasal and pharyngeal swab specimens or serum IgM and IgG antibody detection (≥10 AU/mL was defined as a positive result) using fully automatic chemical luminescence immunoanalysis technology per the manufacturer’s instruction, according to the New Coronavirus Pneumonia Prevention and Control Program guidelines published by the National Health Commission of China [8]. Female patients aged 22–41 years were included for further analysis. This study was approved by the Research Ethics Committee of Renmin Hospital of Wuhan University (approval number: WDRY2020-K076). Considering the urgent need for public health outbreak investigation, written informed consent was waived. All data were anonymously collected and analyzed. All studies and treatments administered were given as part of routine standard of care.
Data Collection
Demographic, clinical, laboratory, and radiological parameters and treatment data including age, gestation, exposure history, coexisting disorders, signs, symptoms, chest computed tomography (CT) scans, and laboratory findings and treatments (eg, antiviral therapy, antibiotics/antifungal medication, systemic corticosteroid therapy, oxygen therapy, mechanical ventilation, kidney replacement therapy, extracorporeal membrane oxygenation) were identified from electronic medical records. Laboratory analyses included complete blood count, liver and renal function, electrolyte testing, coagulation function, C-reactive protein, procalcitonin, lactate dehydrogenase, myocardial enzymes, and cell and humoral immunity index. Inflammation indexes including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systematic inflammatory index (SII) were calculated using specific parameters of blood tests. NLR was defined as the absolute neutrophil count divided by the lymphocyte count. PLR was calculated by dividing the absolute platelet count by the lymphocyte count. SII was defined as platelet count × neutrophil count/lymphocyte count (/μL). Radiological analyses included x-ray and CT scans. Pregnant women who received CT scans signed written informed consent. For pregnant women, we collected neonatal outcomes, including gestational age at delivery, birthweight, Apgar score (1 minute, 5 minute), record of premature delivery, severe neonatal asphyxia, and neonatal death. A team of experienced obstetrician and gynecologists and respiratory physicians reviewed and extracted the data. Last follow-up was March 10, 2020.
Statistical Analysis
Categorical variables were expressed as the counts and percentages. Continuous variables were described as medians and interquartile range (IQR) values or simple ranges. Category variables were adopted using the chi-square test or Fisher exact test. Continuous variables were compared using independent group t tests or the Mann-Whitney test. All analyses were performed with the use of SPSS, version 20.0, and GraphPad Prism, version 6.0. For unadjusted comparisons, a 2-sided P <.05 was considered statistically significant. Considering the possibility of type I error and analyses not adjusted for multiple comparisons, the results should be descriptively interpreted.
RESULTS
In total, 111 hospitalized childbearing-age women with laboratory-confirmed COVID-19 were included (Table 1). The median age (range) was 31.0 (22.0–41.0) years. Fifteen (13.5%) had coexisting disorders, including cardiovascular disease (5 [4.5%]), diabetes (4 [3.6%]), renal disease (2 [1.8%]), respiratory disease (1 [0.9%]), gastric ulcer (1 [0.9%]), mental sickness (1 [0.9%]), and malignancy (1 [0.9%]). Common symptoms included fever (64 [57.7%]), cough (62 [55.9%]), dyspnea (35 [31.5%]), asthenia (28 [25.2%]), and digestive tract symptoms (26 [23.4%]). Chest CT scans showed unilateral or bilateral abnormalities in the lungs of 103 (92.8%) patients, 5 patients with mild disease showed no abnormalities in both lungs (1 in the pregnant group and 4 in the nonpregnant group), and 3 pregnant patients refused the CT scan. Laboratory analyses (Table 2) showed that lymphopenia (lymphocyte count [IQR], 1.3 [1.0–1.7] ×109/L) occurred in 36 patients (32.4%), neutropenia (neutrophil count [IQR], 2.9 [2.0–4.8] ×109/L) in 24 patients (21.6%), hypoalbuminemia (41 [37–43] g/L) in 48 patients (43.2%), hypokalemia (3.9 [3.6–4.2] mmol/L) in 12 patients (10.8%), prolonged prothrombin time (11.5 [11.0–12.0] seconds) in 9 patients (8.1%), and elevated lactate dehydrogenase (189 [160–223] U/L) in 22 patients (19.8%). Mild disease emerged in 5 patients (4.5%), moderate disease in 90 patients (81.1%), severe disease in 12 patients (10.8%), and critical disease in 4 patients (3.6%) according to the New Coronavirus Pneumonia Prevention and Control Program guidelines (5th edition) published by the National Health Commission of China. Uncomplicated illness occurred in 5 patients (4.5%), mild pneumonia in 89 patients (80.2%), severe pneumonia in 12 patients (10.8%), and acute respiratory distress syndrome (ARDS) in 5 patients (4.5%) following the WHO guidelines for COVID-19. Most patients received antiviral therapy (104 [93.7%]), antibacterial therapy (89 [80.2%]), glucocorticoid therapy (41 [36.9%]), intravenous immune globulin (41 [36.9%]), and oxygen therapy (37 [33.3%]). One patient was transferred to the intensive care unit (ICU), and 1 received continuous renal replacement therapy (Table 1).
Table 1.
Clinical Characteristics of the Study Patients
| Total (n = 111) | Pregnant (n = 31) | Nonpregnant (n = 80) | P Value | |
|---|---|---|---|---|
| Age | ||||
| Median (range), y | 32.0 (22.0–41.0) | 29.0 (24.0–41.0) | 33.0 (22.0–41.0) | .001 |
| Distribution, No. (%) | ||||
| 22~29 y | 39 (35.1) | 17 (54.8) | 22 (27.5) | .007 |
| 30~39 y | 63 (56.8) | 13 (41.9) | 50 (62.5) | .050 |
| 40~41 y | 9 (8.1) | 1 (3.2) | 8 (10.0) | .432 |
| Gestation, No. (%) | ||||
| 1~13 wk (+6 d) | 5 (4.5) | 5 (16.1) | / | / |
| 14~27 wk (+6 d) | 6 (5.4) | 6 (19.4) | / | / |
| 28~40 wk | 20 (18.0) | 20 (64.5) | / | / |
| Coexisting disorders, No. (%) | ||||
| Cardiovascular diseases | 5 (4.5) | 1 (3.2) | 4 (5.0) | .916 |
| Respiratory diseases | 1 (0.9) | 0 (0.0) | 1 (1.3) | .621 |
| Diabetes | 4 (3.6) | 3 (9.7) | 1 (1.3) | .117 |
| Malignancy | 1 (0.9) | 0 (0.0) | 1 (1.3) | .621 |
| Renal diseases | 2 (1.8) | 1 (3.2) | 1 (1.3) | .926 |
| Gastric ulcer | 1 (0.9) | 0 (0.0) | 1 (1.3) | .621 |
| Mental sickness | 1 (0.9) | 1 (3.2) | 0 (0.0) | .621 |
| Total | 15 (13.5) | 6 (19.4) | 9 (11.3) | .263 |
| Signs and symptoms, No. (%) | ||||
| Fever on admission | 64 (57.7) | 15 (48.4) | 49 (61.3) | .219 |
| Cough | 62 (55.9) | 14 (45.2) | 48 (60.0) | .158 |
| Nasal congestion | 2 (1.8) | 0 (0.0) | 2 (2.5) | .926 |
| Rhinorrhea | 1 (0.9) | 1 (3.2) | 0 (0.0) | .621 |
| Sore throat | 14 (12.6) | 1 (3.2) | 13 (16.3) | .125 |
| Myalgia or arthralgia | 9 (8.1) | 1 (3.2) | 8 (10.0) | .432 |
| Headache | 2 (1.8) | 0 (0.0) | 2 (2.5) | .926 |
| Dizziness | 3 (2.7) | 0 (0.0) | 3 (3.8) | .659 |
| Dyspnea | 35 (31.5) | 5 (16.1) | 30 (37.5) | .030 |
| Asthenia | 28 (25.2) | 1 (3.2) | 27 (33.8) | .002 |
| Digestive tract symptoms | 26 (23.4) | 3 (9.7) | 23 (28.8) | .060 |
| No symptoms | 14 (12.6) | 9 (29.0) | 5 (6.3) | .001 |
| ≥3 symptoms | 43 (38.7) | 7 (22.6) | 36 (45.0) | .030 |
| Abnormalities on chest CT, No. (%) | ||||
| Normal | 5 (4.5) | 1 (3.2) | 4 (5.0) | .067 |
| Unilateral | 32 (28.8) | 11 (35.5) | 21 (26.3) | .335 |
| Bilateral | 71 (64.0) | 16 (51.6) | 55 (68.8) | .092 |
| Not applicable | 3 (2.7) | 3 (9.7) | 0 (0.0) | .030 |
| Treatments | ||||
| Antiviral medication, No. (%) | 104 (93.7) | 29 (93.5) | 75 (93.8) | .692 |
| Oseltamivir, No. (%) | 40 (36.0) | 16 (51.6) | 24 (30.0) | .033 |
| Arbidol, No. (%) | 92 (82.9) | 25 (80.6) | 67 (83.8) | .697 |
| Ribavirin, No. (%) | 22 (19.8) | 8 (25.8) | 14 (17.5) | .325 |
| Intravenous antibiotics, No. (%) | 89 (80.2) | 29 (93.5) | 60 (75.0) | .053 |
| Antifungal medication, No. (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | / |
| Systemic glucocorticoids, No. (%) | 41 (36.9) | 20 (64.5) | 21 (26.3) | <.001 |
| Oxygen therapy, No. (%) | 37 (33.3) | 2 (6.5) | 35 (43.8) | <.001 |
| Mechanical ventilation, No. (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | / |
| Invasive, No. (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | / |
| Noninvasive, No. (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | / |
| Use of ECMO, No. (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | / |
| Use of intravenous immune globulin, No. (%) | 41 (36.9) | 7 (22.6) | 34 (42.5) | .051 |
| Use of CRRT, No. (%) | 1 (0.9) | 0 (0.0) | 1 (1.3) | .621 |
| Admission to intensive care unit, No. (%) | 1 (0.9) | 0 (0.0) | 1 (1.3) | .621 |
Abbreviations: CRRT, continuous renal replacement therapy; CT, computed tomography; ECMO, extracorporeal membrane oxygenation.
Table 2.
Laboratory Findings of the Study Patients
| Median (IQR) | |||||
|---|---|---|---|---|---|
| Normal Range | Total (n = 111) | Pregnant (n = 31) | Nonpregnant (n = 80) | P Value | |
| Blood cell count | |||||
| White blood cell count, *109/L | 3.5–9.5 | 5.2 (3.8–7.2) | 6.9 (5.6–9.1) | 4.6 (3.5–6.1) | <.001 |
| Lymphocyte count, *109/L | 1.1–3.2 | 1.3 (1.0–1.7) | 1.1 (0.9–1.5) | 1.3 (1.1–1.8) | .113 |
| Lymphocyte, % | 20.0–50.0 | 28.4 (19.2–36.7) | 18.2 (12.4–23.9) | 31.8 (24.9–38.7) | <.001 |
| White blood cell count—lymphocyte count, *109/L | 0.3–8.4 | 3.6 (2.6–5.6) | 5.6 (4.1–8.0) | 3.2 (2.2–4.3) | <.001 |
| Neutrophil count, *109/L | 1.8–6.3 | 2.9 (2.0–4.8) | 5.2 (3.6–7.4) | 2.5 (1.7–3.3) | <.001 |
| Neutrophil, % | 50.0–70.0 | 61.8 (52.4–72.4) | 73.6 (68.5–81.9) | 56.6 (50.1–65.2) | <.001 |
| Platelet count, *109/L | 125–350 | 205 (158–255) | 180 (165–233) | 213 (157–257) | .414 |
| Hemoglobin, g/L | 115–150 | 125 (115–133) | 120 (112–130) | 127 (117–133) | .779 |
| Blood biochemical analysis | |||||
| C-reactive protein, mg/L | <10.0 | 2.5 (2.5–15.0) | 8.8 (2.5–33.4) | 2.5 (2.5–10.3) | .480 |
| Procalcitonin, ng/mL | <0.10 | 0.034 (0.023–0.056) | 0.068 (0.043–0.090) | 0.03 (0.01–0.04) | .715 |
| Sodium, mmol/L | 137–147 | 140 (139–144) | 140 (137–144) | 140 (139–143) | .610 |
| Potassium, mmol/L | 3.5–5.3 | 3.9 (3.6–4.2) | 3.9 (3.6–4.0) | 4.0 (93.6–4.3) | .086 |
| Chloride, mmol/L | 99–110 | 108 (105–109) | 107 (105–109) | 107 (105–109) | .324 |
| Albumin, g/L | 40–55 | 41 (37–43) | 37 (33–39) | 41 (39–43) | <.001 |
| Total bilirubin, μmol/L | 0–23 | 7.8 (6.0–10.3) | 8.4 (7.3–11.9) | 7.3 (5.6–9.4) | .624 |
| Alanine aminotransferase, U/L | 7–40 | 15.5 (10.8–22.0) | 15.5 (11.0–24.3) | 15.5 (10.0–20.8) | .411 |
| Aspartate aminotransferase, U/L | 13–35 | 19.5 (16.0–24.0) | 21.0 (16.0–25.8) | 19.0 (16.0–23.0) | .313 |
| Lactate dehydrogenase, U/L | 10–250 | 189 (160–223) | 200 (181–254) | 182 (152–218) | .311 |
| Blood urea nitrogen, mmol/L | 2.6–7.5 | 3.40 (2.76–4.03) | 3.02 (2.41–3.40) | 3.58 (2.94–4.28) | .138 |
| Creatinine, μmol/L | 41–73 | 47.5 (42.0–53.0) | 43.0 (37.3–49.8) | 50.0 (44.0–53.0) | .380 |
| Creatine kinase-MB, ng/mL | <5.00 | 0.58 (0.43–0.73) | 0.61 (0.35–1.12) | 0.54 (0.45–0.67) | .531 |
| Myohemoglobin, μg/L | 0–110 | 20 (14–28) | 16 (11–29) | 22 (16–28) | .356 |
| NT-pro B-type natriuretic peptide, pg/mL | 0–450 | 30 (17–62) | 45 (18–91) | 22 (12–47) | .268 |
| Prothrombin time, sec | 9.0–13.0 | 11.5 (11.0–12.0) | 11.0 (10.7–11.3) | 11.7 (11.2–12.4) | <.001 |
| Activated partial thromboplastin time, sec | 25.0–31.3 | 28.2 (26.2–30.3) | 27.8 (25.0–29.7) | 28.2 (26.5–30.4) | .332 |
| Fibrinogen, g/L | 2.00–4.00 | 3.64 (2.84–4.43) | 4.43 (3.99–5.12) | 3.10 (2.51–3.81) | <.001 |
| D-dimer, mg/L | 0.0–0.6 | 0.5 (0.2–1.4) | 1.8 (0.8–3.3) | 0.3 (0.2–0.5) | .015 |
| Fibrinogen degradation products, mg/L | 0.00–5.00 | 1.49 (0.56–4.57) | 5.15 (2.19–9.66) | 0.70 (0.37–1.56) | .211 |
| Antithrombin-3, % | 80.0–120.0 | 92.4 (82.6–99.9) | 92.9 (82.3–102.9) | 91.9 (83.1–97.6) | .329 |
| Cell immunity, *109/L | |||||
| CD3+ cell % | 56.0–86.0 | 74.9 (69.8–78.6) | 76.7 (73.5–80.0) | 73.7 (68.4–77.4) | .014 |
| CD3+ cell count | 723.0–2737.0 | 858.0 (704.8–1131.0) | 938.5 (741.5–1061.8) | 845.0 (635.8–1138.5) | .717 |
| CD4+ cell % | 33.0–58.0 | 40.3 (35.9–45.8) | 39.7 (35.3–41.5) | 41.1 (36.0–46.1) | .313 |
| CD4+ cell count | 404.0–1612.0 | 477.5 (341.8–640.8) | 463.0 (360.5–597.25) | 481.0 (325.3–653.3) | .606 |
| CD8+ cell % | 13.0–39.0 | 29.5 (23.8–34.2) | 32.3 (26.6–38.0) | 28.4 (22.3–32.4) | .003 |
| CD8+ cell count | 220.0–1129.0 | 357.5 (233.0–458.8) | 426.0 (344.3–465.3) | 329.0 (228.3–452.5) | .079 |
| CD4+/CD8+ ratio | 0.9–2.0 | 1.4 (1.1–1.8) | 1.2 (0.9–1.6) | 1.4 (1.1–2.0) | .023 |
| CD19+ cell % | 5.0–22.0 | 12.2 (9.5–15.0) | 10.3 (8.7–13.7) | 12.7 (9.9–16.5) | .091 |
| CD19+ cell count | 80.0–616.0 | 129.0 (96.3–185.5) | 127.5 (94.0–163.3) | 132.0 (96.5–209.0) | .324 |
| CD16+ CD56+ cell % | 5.0–26.0 | 11.1 (7.9–14.9) | 9.7 (7.8–14.8) | 11.2 (8.2–14.8) | .244 |
| CD16+ CD56+ cell count | 84.0–724.0 | 123.0 (81.3–171.8) | 123.0 (74.0–163.8) | 123.0 (85.8–171.8) | .361 |
| Humoral immunity, g/L | |||||
| IgG | 7.0–16.0 | 11.35 (10.10–12.88) | 9.76 (8.19–11.13) | 11.90 (10.90–13.50) | <.001 |
| IgM | 0.4–2.3 | 1.25 (0.91–1.56) | 1.08 (0.90–1.44) | 1.27 (0.92–1.71) | .170 |
| IgA | 0.7–4.0 | 2.01 (1.52–2.33) | 1.83 (1.45–2.18) | 2.05 (1.56–2.44) | .179 |
| IgE, IU/mL | <100.0 | 34.7 (9.15–118.5) | 29.9 (9.15–59.65) | 37.7 (99.2–125.8) | .174 |
| C3 | 0.9–1.8 | 1.0 (0.8–1.1) | 1.1 (1.0–1.2) | 0.9 (0.8–1.0) | <.001 |
| C4 | 0.1–0.4 | 0.2 (0.2–0.3) | 0.2 (0.2–0.4) | 0.2 (0.2–0.3) | .255 |
Abbreviation: IQR, interquartile range.
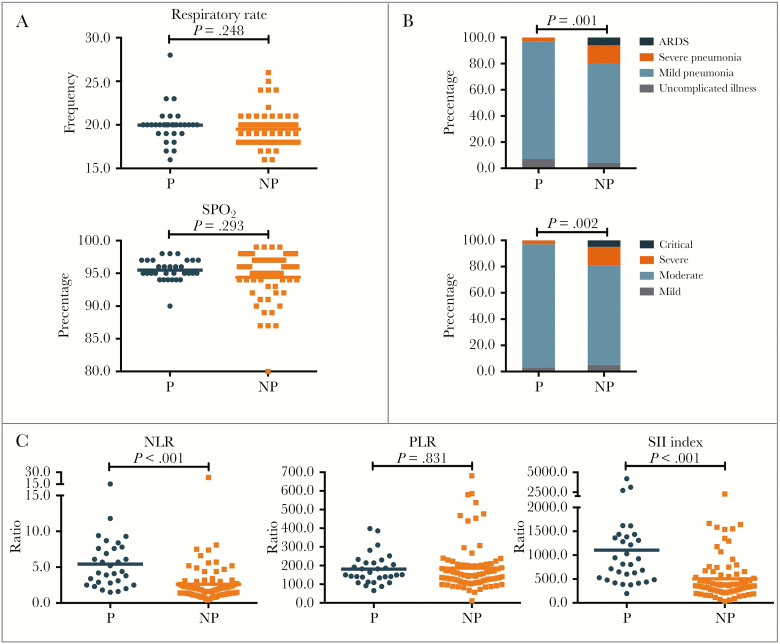
Thirty-one pregnant patients and 80 nonpregnant patients were included (Table 1). Compared with nonpregnant patients, pregnant patients were younger (median age, 29.0 vs 33.0 years; P < .001), less likely to have dyspnea (5 [16.1%] vs 30 [37.5%]; P = .030), less likely to have asthenia (1 [3.2%] vs 27 [33.8%]; P = .002), and less symptomatic (≥3 symptoms: 7 [22.6%] vs 36 [45.0%]; P = .030; no symptoms: 9 [29.0%] vs 5 [6.3%]; P = .001). Respiratory rate (20/minute vs 20/minute; P = .248) (Figure 1A) and oxygen saturation (95% vs 96%; P = .293) (Figure 1A) at initial diagnosis were analogous between the 2 groups. Notably, pregnant patients had a significantly lower percentage of severe pneumonia and ARDS according to the WHO guidelines for COVID-19 (1 [3.2%] vs 16 [14.4%]; P = .001) (Figure 1B) and severe or critical disease according to the Chinese COVID-19 guidelines (1 [3.2%] vs 15 [18.8%]; P = .002) (Figure 1B), indicating a lower level of severity of COVID-19 in pregnant patients.
Figure 1.
Comparison of disease severity between pregnant and nonpregnant women with COVID-2019. A, Comparison of respiratory rate and oxygen saturation between P and NP women with COVID-2019. B, Comparison of disease severity classification between P and NP women with COVID-2019 according to World Health Organization guidelines for COVID-19 and Chinese guidelines for COVID-19. C, Comparison of NLR ratio, PLR ratio, and SII index at initial diagnosis between P and NP women with COVID-2019. Abbreviations: NLR, neutrophil-to-lymphocyte ratio; NP, nonpregnant; P, pregnant; PLR, platelet-to-lymphocyte ratio; SII, systematic inflammatory index.
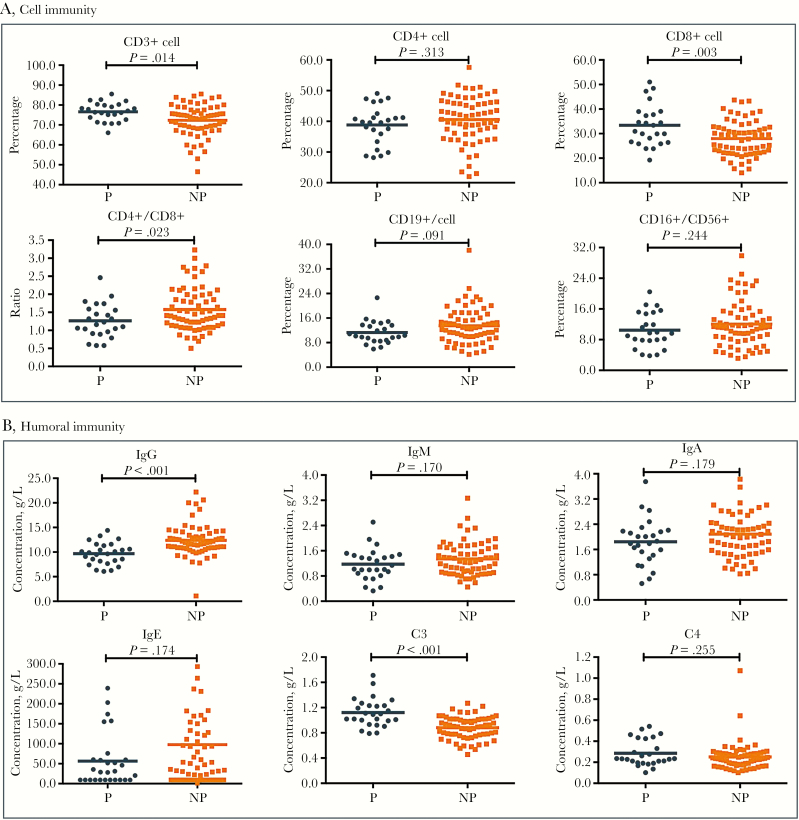
Laboratory analyses (Table 2) showed that pregnant patients had significantly higher white blood cell counts (6.9 vs 4.6 ×109/L; P < .001), neutrophil counts (5.2 vs 2.5 ×109/L; P < .001), higher levels of fibrinogen (4.43 vs 3.10 g/L; P < .001), dramatically lower percentages of lymphocytes (18.2% vs 31.8%; P < .001), lower levels of albumin (37 vs 41 g/L; P < .001), and shorter prothrombin times (11.0 vs 11.7 seconds; P < .001). Intriguingly, pregnant patients had substantially higher levels of inflammation markers including NLR ratio (4.4 vs 1.9; P < .001) (Figure 1C) and SII (812.8 vs 354.7; P < .001) (Figure 1C) but similar PLR ratios (150.9 vs 146.6; P = .831) (Figure 1C) when compared with nonpregnant patients. In addition, cluster analysis of peripheral immune cells suggested that, in comparison with nonpregnant patients, pregnant patients had enhanced cell immunity with increased CD3+ cells (76.7% vs 73.7%; P = .014) (Figure 2A), CD8+ cells (32.3% vs 28.4%; P = .003) (Figure 2A), and C3 levels (1.1 vs 0.9 g/L; P < .001) (Figure 2B), but insufficient humoral immunity, with reduced CD4+/CD8+ ratios (1.2 vs 1.4; P = .023) (Figure 2A) and IgG levels (9.76 vs 11.90 g/L; P < .001) (Figure 2B).
Figure 2.
Comparison of cell and humoral immunity between pregnant and nonpregnant women with COVID-2019. A, Comparison of the percentage of CD3+ cells, CD4+ cells and CD8+ cells, CD19+ cells, CD16+ CD56+ cells, and CD4+/CD8+ ratio at initial diagnosis between P and NP women with COVID-2019. B, Comparison of the level of IgG, IgM, IgA, IgE, C3, and C4 at initial diagnosis between P and NP women with COVID-2019. Abbreviations: NP, nonpregnant; P, pregnant.
Treatment options are summarized in Table 1. The percentages of pregnant patients who received oseltamivir (51.6% vs 30.0%; P = .033) and glucocorticoid (64.5% vs 26.3%; P < .001) were significantly higher than in nonpregnant patients. The percentage of oxygen therapy was significantly lower in the pregnant group than the nonpregnant group (6.5% vs 43.8%; P < .001). More nonpregnant patients received intravenous immune globulin than pregnant patients (42.5% vs 22.6%; P = .051), but the difference did not reach statistical significance. One patient was transferred to the ICU, and 1 received renal replacement therapy in the nonpregnant group. Only 1 patient had died in the nonpregnant group as of March 10, 2020.
Seventeen live births were recorded (Table 3). The median age of these puerperae (range) was 29 (24–34) years. The median body length (range) was 49 (45–52) cm, and the median birthweight (range) was 3120 (2300–3750) g. Only 1 premature neonate at 35 gestational weeks plus 6 days had a birthweight <2500 g (Table 3). Seventeen live births had a median 1-minute Apgar score of 9 and a median 5-minute Apgar score of 10. One live birth had a 1-minute Apgar score of 7 and a 5-minute Apgar score of 9. All of live births had negative results of immediately postnatal COVID-19 detection. Two had positive results for COVID-19 2 days after birth mainly due to the contact transmission. Among these 2 live births, 1 had neonatal fever, and a CT scan showed viral pneumonia. After active treatment, she has totally recovered. No neonatal hypoglycemia, neonatal congenital malformation, severe neonatal asphyxia, or neonatal death was observed in these newborns (Table 3).
Table 3.
Neonatal Outcomes
| Patient ID | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P13 | P14 | P15 | P16 | P17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Puerpera age, y | 29 | 32 | 34 | 29 | 26 | 24 | 33 | 31 | 28 | 27 | 26 | 30 | 28 | 31 | 29 | 33 | 29 |
| Gestational age at delivery | 37 wk, 1 d | 39 wk, 1 d | 37 wk, 6 d | 36 wk | 35 wk, 6 d | 40 wk, 1 d | 40 wk | 41 wk | 36 wk, 2 d | 39 wk, 4 d | 40 wk, 3 d | 38 wk, 1 d | 37 wk, 1 d | 39 wk, 1 d | 38 wk, 4 d | 39 wk, 3 d | 38 wk |
| Premature delivery | No | No | No | Yes | Yes | No | No | No | Yes | No | No | No | No | No | No | No | No |
| Neonatal sex | Female | Female | Female | Female | Female | Female | Male | Female | Female | Female | Male | Male | Male | Female | Male | Male | Female |
| Apgar score (1 min) | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 7 | 9 | 9 |
| Apgar score (5 min) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 9 | 10 | 10 |
| Birthweight, g | 2890 | 3750 | 3400 | 2830 | 2300 | 3360 | 3450 | 3140 | 2900 | 2650 | 3680 | 3720 | 2940 | 3570 | 2650 | 3000 | 3120 |
| Neonatal body length | 49 | 51 | 50 | 46 | 50 | 50 | 52 | 48 | 48 | 49 | 52 | 51 | 49 | 50 | 47 | 49 | 45 |
| Neonatal congenital malformation | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Neonatal fever | No | No | No | No | No | No | No | No | No | No | No | No | No | Yes | No | No | No |
| Neonatal hypoglycemia | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Severe neonatal asphyxia | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Neonatal death | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Postnatal admission to intensive care unit | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Postnatal mechanical ventilation | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
DISCUSSION
To our knowledge, this study is 1 of few case series of hospitalized childbearing-age female patients with laboratory-confirmed COVID-19. In comparison with nonpregnant patients, pregnant patients were less likely to have symptoms; had significantly higher white blood cell counts, neutrophil counts, fibrinogen and C3 levels, and percentages of CD3+ and CD8+ cells; had dramatically lower percentages of lymphocyte, albumin levels, CD4+/CD8+ ratios, and IgG levels; and had shorter prothrombin time. Of note, pregnant patients had a significantly lower percentage of severe disease according to both the WHO and Chinese COVID-19 guidelines and had a substantially higher level of inflammation markers including NLR ratio and SII than nonpregnant patients. In addition, 17 live births were recorded, all of which showed negative results for COVID-19 detection immediately postnatally, and none experienced severe comorbidities.
It is well known that the morbidity and mortality of viral pneumonia are higher in pregnant women compared with the general population when there is no effective antiviral therapy [9, 10]. The influenza epidemic of 1918 and the Asian flu epidemic of 1957 had a maternal mortality rate of 30%~50% [11]. For severe acute respiratory syndrome (SARS) due to SARS-coronavirus (CoV) infection in 2003, the case fatality rate of the pregnant cases was 25%. Fifty percent needed ICU admission, and 33% required endotracheal intubation, while in the present study the ICU admission rate was 17.5% (P = .012) and the intubation rate was 12.5% (P = .065) in the nonpregnant group [10]. Pregnant women infected by Middle East respiratory syndrome coronavirus (MERS-CoV) had a case mortality as high as 40% [9, 12]. However, the current study showed that pregnant patients were less likely to have a severe or critical type of COVID-19 (3.2%) according to both the WHO and Chinese COVID-19 guidelines, which is significantly lower than the rate of 18.8% in nonpregnant women and also significantly lower than 15.7% in the whole population from a large-scale national analysis [4]. Moreover, this national analysis reported lower rates of severe disease among women and younger patients than among men and older patients [4]. Similarly, Chen et al. collected 118 pregnant women with COVID-19 and reported that the risk of severe disease compared favorably with the risk in general populations of patients with COVID-19, indicating no increased risk of severe disease among pregnant patients [13]. Unlike influenza, SARS, and MERS-CoV, pregnant patients with COVID-19 were also less likely to have symptoms such as dyspnea, asthenia, and so on, suggesting that COVID-19 has distinct clinical features for pregnant women. Even though more pregnant patients received oseltamivir and glucocorticoid than the nonpregnant group, these is still no evidence that these 2 drugs could effectively inhibit SARS-CoV-2.
We also surveyed the distinct immunological features between pregnant and nonpregnant patients. In spite of a lower percentage of lymphocyte, pregnant patients had a substantially higher percentage of CD3+ and CD8+ cells, as well as inflammation markers including NLR, SII, and C3 level, when compared with nonpregnant patients. Previous studies together with the pathological examination found that cytokine release storm was the main cause of severe disease [14–16]. Therefore, the different immunological features found in this study might contribute to the mild effect of COVID-19 in pregnant women. Furthermore, 14.4% of the females and 18.8% of nonpregnant women in our study were found to have severe disease, which is lower than the rates of 22.0%–31.6% of the total population in Wuhan city during the period of January to February 2020 [1–3]. Consistently, previous studies have found that female patients with COVID-19 have a significantly lower rate of death and severe disease than male patients [2]. COVID-19 infects the human body through binding angiotensin-converting enzyme II (ACE2), and ACE2 expression is significantly higher in men than women [17–19]. Meanwhile, it has been reported that estrogen was a protective factor from severe pneumonia in animal models [20, 21]. Collectively, the unique immune and pathophysiological features found in this study might contribute to the finding that pregnant women are less likely to develop severe COVID-19 infection. Clarification of the related mechanisms might provide clues for the development of novel preventive or therapeutic strategies, as effective methods are still undetermined to overcome COVID-19 infection.
Among the 31 pregnant women, 17 live births were recorded, and all of these showed results negative for postnatal COVID-19 detection at the first testing, and 2 became positive thereafter, indicating that vertical transmission is rare. A case of a newborn infant who tested positive for COVID-19 at the Wuhan Children’s Hospital in Hubei Province was reported on February 5, 2020, 30 hours following the infant’s birth [22], suggesting that strict quarantine is needed to prevent mother-to-child coronavirus transmission during delivery [23]. As for the newborn infants in our study, all of them were live births with a normal Apgar score, and no severe neonatal asphyxia was observed. In contrast, a high incidence of preterm delivery, admission to the ICU, spontaneous abortion, and perinatal death have been reported in pregnant women with SARS [22, 24]. The discrepancy in obstetrical outcomes might be due to the severe hypoxia caused by SARS disease, while this was less likely to happen in pregnant women with COVID-19 in this study.
There are several limitations of this study that should be acknowledged. First, the sample size was relatively small, and the retrospective nature of this study will inevitably entail selection bias. Hence, we should cautiously interpret these findings, and large-scale, multicenter studies are still needed. Second, all of the included cases were from Wuhan; it would be better to collect patients in other cities of China, and even in other countries, to obtain a more comprehensive understanding of the clinical characteristics in pregnant and nonpregnant childbearing-age women with COVID-19. Third, because of the short follow-up period, a small portion of patients remained in the hospital. The potential impact of disease severity in pregnant and nonpregnant patients on clinical outcomes was not evaluated. Forth, data collection was clinically driven and was not systematic, so the findings should be descriptively interpreted. Given that COVID-19 is a novel infection, no systematic management protocols were in place, and the decision to perform certain laboratories or to administer certain treatments was the clinician’s, and some therapies were not based on known efficacy/recommendations. Last but not least, clinical interpretation of laboratory comparisons between the pregnant and nonpregnant groups was limited by the inherent changes that occur in a normal pregnancy. The optimal comparisons would be conducted between mild/moderate and severe/critical disease groups in future investigations.
In conclusion, this single-center investigation involving 111 childbearing-age women with COVID-19 revealed that pregnant patients had a lower level of severity of COVID-19 together with an enhanced inflammatory response and cell immunity when compared with nonpregnant patients. These findings should provide useful information for understanding the pathogenesis and clinical course of pregnant patients with COVID-19 and will be helpful in the forumation of the principles of obstetric treatment for pregnant women with COVID-19 infection.
Acknowledgments
We thank the family members who were involved in this study, and we thank the patient for granting permission to publish this information.
Financial support. This work was supported by Scientific Research Projects from the Health and Family Planning Commission in Hubei Province (grant WJ2017M026 to Dr. B. Cheng), Wuhan University School of Medicine (grant TFZZ2018018 to Dr. B. Cheng), Wuhan University post-doctoral research project (grant 169557 to Dr. B. Cheng), Shanghai Pujiang Talent Plan (grant 2019PJD048 to Dr. S. Ren), Shanghai Key disciplines of Respiratory (grant 2017ZZ02012 to Dr. S. Ren), and the Shanghai Major Diseases Multidisciplinary Cooperation Diagnosis and Treatment Construction Project.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. J.Y., S.R., and G.W. made the same contributions to the study concept and design and are co-senior authors. B.C. and T.J. were in charge of the manuscript drafting. L.Z., R.H., J.T., Y.J., B.H., J.L., and M.W. took responsibility for obtaining written consent from patients, obtaining ethical approval, collecting samples, and confirming data accuracy. T.J. and S.R. made contributions to data acquisition, analysis, and interpretation. B.C. and L.Z. were the pediatricians in charge of treatment of the newborn babies. R.H., J.T., and Y.J. were the obstetricians of the pregnant women and were responsible for data collection and confirmation. J.L. and M.W. were in charge of the laboratory tasks, including sample processing and detection. J.Y. and G.W. made substantial revisions to the manuscript.
References
- 1. Wang D, Hu B, Hu C, et al. . Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen N, Zhou M, Dong X, et al. . Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan WJ, Zhong NS. Clinical characteristics of Covid-19 in China. Reply. N Engl J Med 2020; 382:1861–2. [DOI] [PubMed] [Google Scholar]
- 5. Schwartz DA. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020. [DOI] [PubMed] [Google Scholar]
- 6. Chen H, Guo J, Wang C, et al. . Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020; 395:809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qiao J. What are the risks of COVID-19 infection in pregnant women? Lancet 2020; 395:760–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Health Commission of the People’s Republic of China. The notice of launching guideline on diagnosis and treatment of the novel coronavirus pneumonia (NCP). Revised version of the 5th edition Available at: http://www.nhc.gov.cn/xcs/zhengcwj/202002/d4b895337e19445f8d728fcaf1e3e13a.shtml. Accessed 8 February 2020.
- 9. Alserehi H, Wali G, Alshukairi A, Alraddadi B. Impact of Middle East respiratory syndrome coronavirus (MERS-CoV) on pregnancy and perinatal outcome. BMC Infect Dis 2016; 16:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong SF, Chow KM, Leung TN, et al. . Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol 2004; 191:292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gottfredsson M. The Spanish flu in Iceland 1918. Lessons in medicine and history [in Icelandic]. Laeknabladid 2008; 94:737–45. [PubMed] [Google Scholar]
- 12. Alfaraj SH, Al-Tawfiq JA, Memish ZA. Middle east respiratory syndrome coronavirus (MERS-CoV) infection during pregnancy: report of two cases & review of the literature. J Microbiol Immunol Infect 2019; 52:501–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen L, Li Q, Zheng D, et al. . Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med 2020; 382:e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017; 39:529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Channappanavar R, Fehr AR, Vijay R, et al. . Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 2016; 19:181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu Z, Shi L, Wang Y, et al. . Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020; 8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamming I, Timens W, Bulthuis ML, et al. . Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004; 203:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Z, Xiao X, Wei X, et al. . Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol. 2020; 92:595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou P, Yang XL, Wang XG, et al. . A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vermillion MS, Ursin RL, Attreed SE, Klein SL. Estriol reduces pulmonary immune cell recruitment and inflammation to protect female mice from severe influenza. Endocrinology 2018; 159:3306–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vom Steeg LG, Klein SL. Sex and sex steroids impact influenza pathogenesis across the life course. Semin Immunopathol 2019; 41:189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses 2020; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wei M, Yuan J, Liu Y, et al. . Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020; 323:1313–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ng PC, Leung CW, Chiu WK, et al. . SARS in newborns and children. Biol Neonate 2004; 85:293–8. [DOI] [PubMed] [Google Scholar]