Abstract
Prenatal ethanol exposure (PE) causes many cognitive and behavioral deficits including increased drug addiction risk, demonstrated by enhanced ethanol intake and behavioral phenotypes associated with addiction risk. Additionally, preclinical studies show that PE persistently changes the function of dopaminergic neurons in the ventral tegmental area, a major neural substrate for addiction, and alters these neurons’ responses to psychostimulants. Accordingly, PE could also lead to increased risk of addiction to drugs of abuse, other than ethanol. In the present study, addiction risk was examined utilizing paradigms of amphetamine conditioned place preference (CPP) and intravenous self-administration. Ethanol was administered to pregnant dams via intragastric gavage (6 g/kg, during gestational days 8-20). Behavioral tests were conducted in adult male offspring. Amphetamine at a low dose (0.3 mg/kg, i.p.) induced CPP in PE but not control rats, whereas at a higher dose (0.6 mg/kg, i.p.) both groups acquired CPP. There was no group difference in amphetamine-induced CPP reinstatement. Furthermore, PE rats self-administered more amphetamine at a low dose (0.02 mg/kg/infusion) than controls, while no group differences were observed at a higher dose (0.1 mg/kg/infusion). Rats with PE also exhibited greater reactivity to contextual drug cues after extended abstinence and amphetamine-induced reinstatement of drug seeking. These results support that PE persistently leads to increased psychostimulant addiction risk later in life, manifested in many elements of addictive behavior following limited psychostimulant exposure. The observations provide insights into prevention strategies for drug addiction in individuals with fetal alcohol spectrum disorders.
Keywords: fetal alcohol spectrum disorders, addiction risk, conditioned place preference, intravenous self-administration, contextual cue, drug-induced reinstatement
1. Introduction
Only a small proportion of the people who repeatedly use drugs of abuse develop drug dependence in the long run [1]. Thus, we need to identify genetic and environmental risk factors that determine addictive behaviors [2]. Prenatal exposure to toxicants or stress has been shown to increase the incidence of multiple mental disorders, including addiction [3–5]. Prenatal ethanol exposure (PE) leads to fetal alcohol spectrum disorders (FASD), which consist of various physical, behavioral, and cognitive abnormalities/dysfunctions [6, 7]. It is well documented that PE causes increased addiction risk. Clinical studies have shown that alcohol dependence in human adolescence is highly correlated with PE [8–10]. Animal studies have also demonstrated that PE gives rise to a variety of behavioral phenotypes associated with increased addiction risk, such as enhanced locomotor activity in a novel environment [11–14], anxiety- and depression-like behavior [15, 16], behavioral sensitization to psychostimulants [17, 18], and augmented HPA axis responsivity [15, 19]. Moreover, it has been reported that PE directly facilitates addictive behaviors, including enhanced ethanol preference or intake and learning of drug-associated cues in the conditioned place preference (CPP) paradigm [18, 20, 21]. Interestingly, the above findings are in line with evidence showing that prenatal exposure to other drugs of abuse, e.g., psychostimulants, opioids, marijuana/cannabinoids, and nicotine, could also increase addiction risk [22–24].
It is well accepted that dysfunction of the mesolimbic/cortical dopamine (DA) systems (the brain reward pathway), the targets of drugs of abuse including ethanol [25], is responsible for increased drug addiction risk [25, 26]. Particularly, DA neurons located in the ventral tegmental area (VTA), the origin of the mesolimbic/cortical DA systems, play an important role in mediating addictive behaviors. For example, intra-VTA psychostimulant administration enhances cocaine self-administration [27] and reinstates drug-seeking behavior [28]. Prenatal ethanol exposure causes increased activity in VTA DA neurons in response to psychostimulants [29, 30]. Moreover, adult rats subjected to moderate PE show structural changes (in dendritic length, branching, and spine density) in the nucleus accumbens and dorsal striatum, the major subcortical target areas of the mesolimbic DA system [31,32]. These findings support that PE could lead to increased risk of addiction to drugs of abuse, other than ethanol. However, most of the clinical and animal studies so far have been focused on the effects of PE on alcohol abuse. In the present study, we investigated if PE could lead to increased risk of psychostimulant addiction.
First, a locomotor test was performed to assess whether locomotion was augmented by PE, because increased locomotor response to novelty is a well-documented behavioral phenotype predicting high addiction risk [31, 32]. In addition, two of the most widely accepted paradigms in studying drug addiction were utilized: CPP [33, 34] and intravenous drug self-administration [27, 35]. The CPP paradigm has several advantages (Bardo and Bevins, 2000), including limited drug exposure and behavioral tests being conducted in a drug-free state. Most drugs of abuse have been shown to induce CPP in animals [34]. Intravenous self-administration, on the other hand, uses operant procedures resembling human drug taking behavior and therefore has high validity [36, 37] in examining many facets of addictive behaviors [38, 39]. The CPP and self-administration paradigms can both be applied to examine major elements of drug addiction, including acquisition, extinction, and reinstatement (relapse) of drug seeking [40]. In the present study, both high and low doses of amphetamine were used to investigate the different elements of addictive behavior in PE rats. Together, they can provide comprehensive evidence regarding drug addiction risk after PE [41, 42]. The results can have important implications for the FASD research field. First, they can validate the rat model of PE for studying increased risk for drug addiction in FASD. Second, they can permit more thorough investigations of possible neural mechanisms underlying PE-induced behavioral deficits. Third, the model could be employed to study intervention strategies. For example, we may explore whether readily-translatable intervention approaches, such as neonatal handling and environmental enrichment [43, 44], can reduce increased addiction risk after PE. Lastly, although clinical studies have shown that PE may increase the risk of addiction to different drugs of abuse, preclinical studies mainly focus on PE effects on alcohol dependence alone. The results of the present preclinical study will assess whether PE increases the risk of addiction to substances of abuse beyond alcohol.
2. Methods
2.1. Animals and prenatal ethanol exposure
The methods of breeding, prenatal treatment, and cross-fostering were reported previously [45]. Briefly, adult male (300-450 g) and virgin female (225-250 g) Sprague-Dawley rats (Envigo, Indianapolis, IN) were housed together under a 12 h/12 h light/dark cycle until vaginal plugs were found (gestational day 0). Pregnant dams were then randomly assigned to PE or control group and singly housed. During gestational day (GD) 8 through GD 20, rats were treated by intragastric intubation twice (6 h apart) during the light phase every weekday, with 3 g/kg ethanol (15% w/v) or vehicle (22.5% w/v sucrose water, isocaloric to ethanol) per treatment. A single daily treatment with 4 g/kg solution was given on weekends. Our previous study employing a similar PE treatment paradigm has shown that the blood ethanol concentration (BAC) 1 h after the 2nd gavage was 116.8 ± 10.5 mg/dl [46]. The PE treatment corresponded to heavy prenatal alcohol exposure in humans [46, 47]. To be nutritionally equivalent, controls were pair-fed with the PE rats on GDs 8-20. In addition, dams received thiamine injections (8 mg/kg; i.m.; twice a week) to avoid vitamin B1 deficiency induced by ethanol exposure or the pair-feeding procedure.
To mitigate the potential impact of PE dams’ alcohol withdrawal on their maternal behavior, a cross-fostering procedure was performed. On postnatal day (PD) 1, litters were randomly culled to 10 pups with the maximal number of males (up to 8). Offspring of PE dams were transferred to foster dams, which received no prenatal treatment and gave birth 2 days earlier than their PE counterparts. Control litters were cross-fostered among themselves. Weaning was conducted on PD 21, after which time same-sex rats were housed at 2-3 per cage. Experiments started when animals reached the age of 7-8 weeks, and the behavioral tests were carried out during the 12 h dark phase. Experiments were conducted only in males, to limit the scope of the study. No more than 3 rats out of each litter were used in an experiment to minimize potential litter effects. All of the procedures were approved by the Institutional Animal Care and Use Committee of University at Buffalo, The State University of New York.
2.2. Apparatuses and drugs
Locomotor activity was assessed in a standard rat cage (42.5×22.5×19.25 cm) without bedding, as previously described [32]. Cages were located in a modified file cabinet, which represented a novel environment for animals. Rats’ activity was monitored by an infrared motion-sensor system (Kinder Scientific, Poway, CA). Photo beam breaks were recorded at a temporal resolution of 0.01 s.
The CPP apparatus was made of two large Plexiglas side chambers (24×35×35 cm) connected by a smaller center chamber (15.5×19.5×19.5 cm) [48]. One side chamber had 4 dark gray walls and a wire mesh (0.65×0.65 cm) floor; the other chamber had 3 dark gray walls and a white wall on the far side, as well as a different wire mesh (1.3×1.3 cm) floor. The center chamber had punched aluminum floor (0.4 cm diameter holes). The entire CPP box was covered with a transparent Plexiglas lid. During the conditioning phase, two gray Plexiglas partitions were inserted between the center and side chambers to restrict the animal’s movement within a specific side chamber. The partitions were removed during the testing phase. The room was kept in semi-darkness with only a dim light projecting to the ceiling. Network video cameras (Panasonic BL-C1A, Panasonic US, Newark, NJ) were mounted above the CPP apparatuses, recording the animals’ activity. The videos were manually coded by individuals blind to the experimental design.
Self-administration experiments were conducted in standard operant conditioning chambers (interior dimensions: 30.5×24.1×29.2 cm, Model #ENV-007, Med Associates, St. Albans, VT) individually situated in sound-attenuating cubicles. House light, tone, cue light, and a retractable lever were equipped in each chamber. Lever press led to contingent drug infusions delivered through a syringe pump (Model #PHM-100, Med Associates) connected to a watertight swivel (Instech Laboratories, Plymouth Meeting, PA). In the pre-training phase, lever press triggered water delivery through a dipper cup (0.02 ml, Model #ENV-202M, Med Associates). Rats’ responding was recorded with the MED-PC IV software (Med Associates).
D-amphetamine hemisulfate salt (Sigma-Aldrich, St. Louis, MO), dissolved in 0.9% sterile saline, was made once a week. Dosage was based on salt weight. All the other drugs were supplied by Patterson Veterinary Supply (Greeley, CO).
2.3. Locomotor test
Seven-week-old male rats (control: n = 38 from 14 litters; PE: n = 36/13 litters) were used in the locomotor test. Rats were placed in dark locomotor chambers individually for 60 min (divided into 5 min epochs in data analysis, see Fig. 1 left panels). The distance travelled and the movement time were recorded.
Fig. 1.
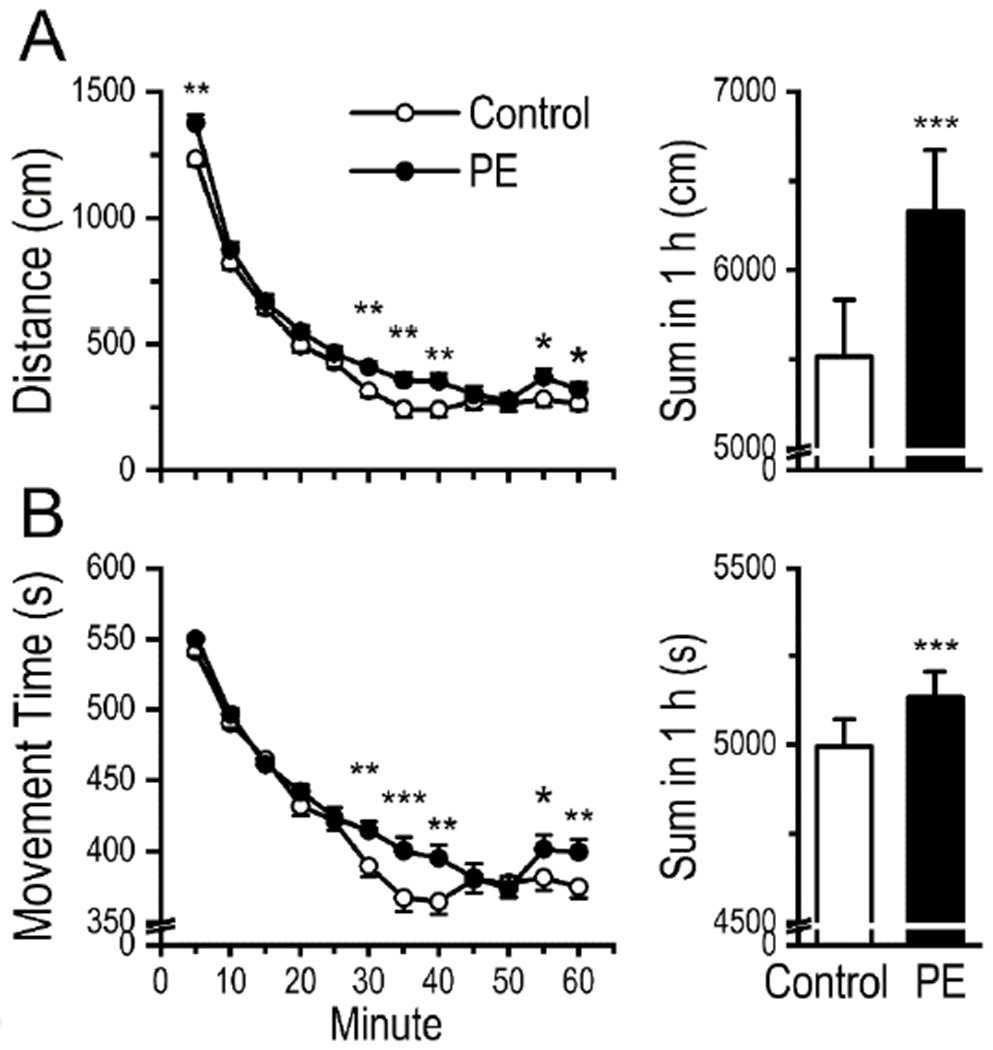
Rats with prenatal ethanol exposure (PE) exhibited higher locomotor activity in a novel environment. (A) The distance travelled was greater in PE rats than that in controls. Left panel depicts distance traveled within 5 min epochs. Right panel is a summary plot for total distance traveled in 1 h. (B) The time spent on movement was also greater in PE rats than in controls. Left panel depicts movement time within 5 min epochs. Right panel is a summary plot for total movement time in 1 h. Data are presented as Mean ± SEM. *: p < 0.05, **: p < 0.01, ***: p < 0.001, control vs. PE rats, planned comparisons after ANOVA.
2.4. Conditioned place preference test
Eight-week-old male rats (control: n = 37 from 13 litters; PE: n = 42/12 litters) underwent the CPP test. Fig. 2A presents the timeline for the experimental procedures. A pre-test was conducted to determine the initial unconditioned place preference. Animals were transported to the testing room and allowed to habituate for 15 min. Each animal was then placed in the center chamber and allowed to explore the entire apparatus freely for 15 min. Animals spending more than 70% of the time in a particular side chamber were removed from the study. Only 1 control rat and 1 PE rat were removed.
Fig. 2.
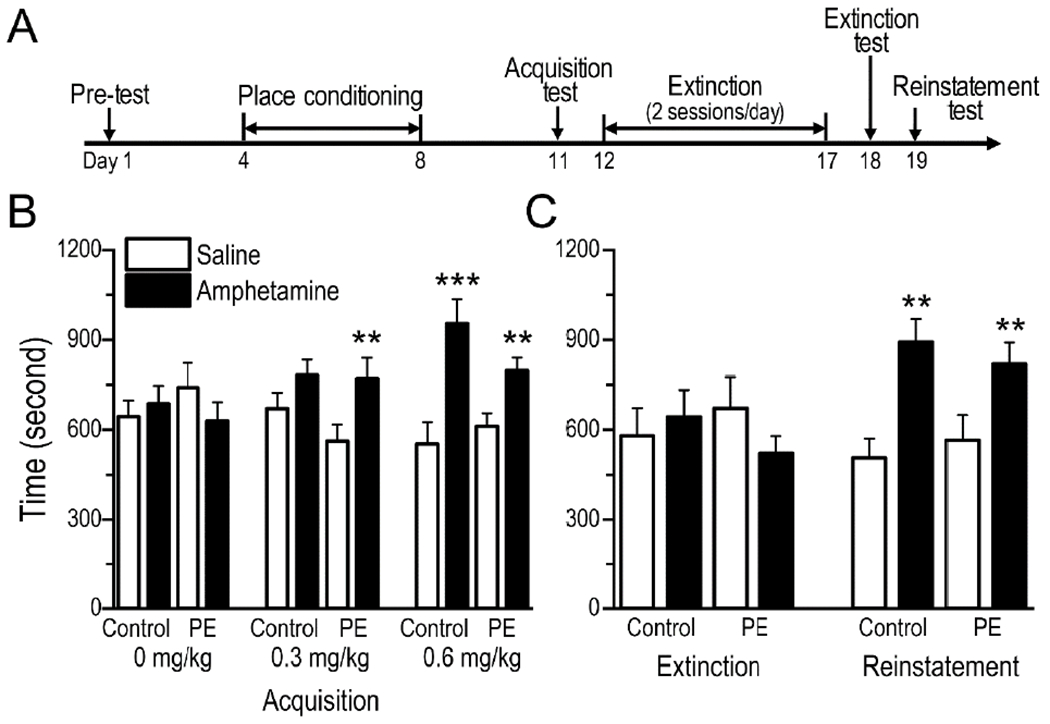
Prenatal ethanol exposure (PE) facilitated acquisition of amphetamine-induced conditioned place preference (CPP). (A) Timeline of the amphetamine CPP experiments. (B) Amphetamine CPP was observed in PE but not control rats at the 0.3 mg/kg (i.p.) dose. In contrast, CPP was observed in both groups at the 0.6 mg/kg (i.p.) dose. The uneven pairings of saline and amphetamine did not influence the results, because 0 mg/kg amphetamine (i.p.) did not induce CPP in either group. (C) Extinction training eliminated CPP in both PE and control rats conditioned with amphetamine at 0.6 mg/kg. In addition, 0.3 mg/kg amphetamine (i.p.) reinstated CPP in both control and PE rats. No group differences were observed in either extinction or reinstatement of CPP. Data are presented as Mean ± SEM, **: p < 0.01, ***: p < 0.001, time spent in amphetamine- vs. saline-paired compartment in control or PE rats within 30 min, planned comparisons after ANOVA.
Three days following the pre-test, rats underwent 5 sessions of conditioning. After 15 min habituation in the testing room, each animal was injected with amphetamine (0, 0.3 or 0.6 mg/kg, i.p.) and immediately placed in a specific side chamber for 45 min. Rats received amphetamine injection on days 4, 6, and 8 and saline injection on days 5 and 7 (Fig. 2A). Amphetamine- and saline-paired compartments were randomly assigned. The CPP acquisition test was conducted 3 days after the last conditioning session. The procedure was similar to the pre-test except that the duration was 30 min.
One day after the CPP acquisition test, animals conditioned with 0.6 mg/kg amphetamine (at which dose both control and PE rats acquired CPP, shown in Results section) went through extinction training (twice daily for 6 days; 12 sessions in total), during which animals were confined to saline- or amphetamine-paired compartments alternately for 45 min without any injections. One day after the extinction training, animals were tested (30 min) for place preference (extinction test). On the following day, reinstatement test (30 min) was conducted by administering amphetamine (0.3 mg/kg, i.p.) immediately before testing. We used this dose of amphetamine because it could detect group differences in CPP acquisition (see Results section).
2.5. Self-administration test
2.5.1. Pre-training for self-administration.
The purpose of introducing a pre-training procedure was to make animals establish the association between lever presses and reward availability, so that they can rapidly acquire the self-administration behavior, lowering the attrition rate, which, in a previous study, was as higher as 87% in controls and 60% in PE rats [49]. Probably for the same reason, operant training with non-drug reinforcers preceding self-administration experiments is widely applied [50–52]. Additionally, the training process can reveal whether there are group differences in operant learning, which is important information to help accurately interpret potential group differences in drug self-administration. Seven-week-old rats (control: n = 30 from 10 litters; PE: n = 28/10 litters) were 23.5 h/day water deprived to facilitate the pre-raining process (drinking water was accessible for 0.5 h/day after the training session). Training was conducted in the operant chamber on a daily basis. During the initial 30-min shaping session, levers absent, water was delivered under a 1 min variable interval reinforcement schedule, to help animals acquire the association between water availability and the sound of arm movement inside the magazine. From the next day (i.e., in the first operant training session), an active lever was present and lever press resulted in water delivery under a fixed ratio (FR) 1 schedule. The session ended when 100 responses were reached or after 1 h. Rats received training continually until the criterion – 100 responses/session for 3 sessions - was reached. Water ad lib resumed afterwards.
2.5.2. Jugular vein catheterization surgery.
Animals were pretreated with buprenorphine (0.025 mg/kg, s.c.) and then anesthetized with ketamine (60 mg/kg) /xylazine (5 mg/kg, i.p.). A heparin-coated catheter (Instech) was implanted into the right external jugular vein and connected to a back harness with an injection port (Instech). Carprofen (5 mg/kg, s.c.) was administered daily for 3 days after surgery. Catheters were flushed daily with 0.2 ml of heparinized saline (50 IU/ml in 0.9% saline) containing enrofloxacin (4 mg). Animals were allowed to recover for 5-6 days before self-administration experiments.
2.5.3. Self-administration acquisition.
The timeline of the procedures is presented in Fig. 3A. This approach was used previously to limit drug exposure during acquisition of self-administration and thus avoid masking group differences due to intensive drug exposure [27, 35, 53, 54]. Briefly, rats were placed in operant chambers to self-administer amphetamine 3 hours/day under reinforcement schedules of FR1 and FR2, followed by a progressive ratio (PR) schedule. Rats were trained with 0.02 mg/kg/infusion (control: n = 12 from 6 litters; PE: n = 12/6 litters) or 0.1 mg/kg/infusion (control: n = 8/4 litters; PE: n = 8/4 litters) of amphetamine, delivered at the rate of 1.84 ml/s. Each of the FR1 and FR2 sessions was initiated by a priming infusion. After reaching the criterion of 9 infusions/session in one FR1 and one FR2 sessions within 4 days, the rats would proceed to six PR sessions. Otherwise, they would be excluded from later experiments. The chamber house light was illuminated during the entire session. A tone was continuously on during the infusion. A 30 s time-out period was initiated by the onset of drug delivery, signaled by the illumination of a cue light above the lever, during which time, lever press was recorded, with no programmed consequences. Under the PR schedule, ratio values were calculated by rounding 5e(0.25×infusion number)-5 [55, 56]. Reaching the maximal infusions or stopping responding in 1 h would end the session automatically. After the completion of experiments, a catheter patency test was conducted, and animals that failed the test would be excluded from the data. Only 3 rats were excluded due to catheter blockage.
Fig. 3.
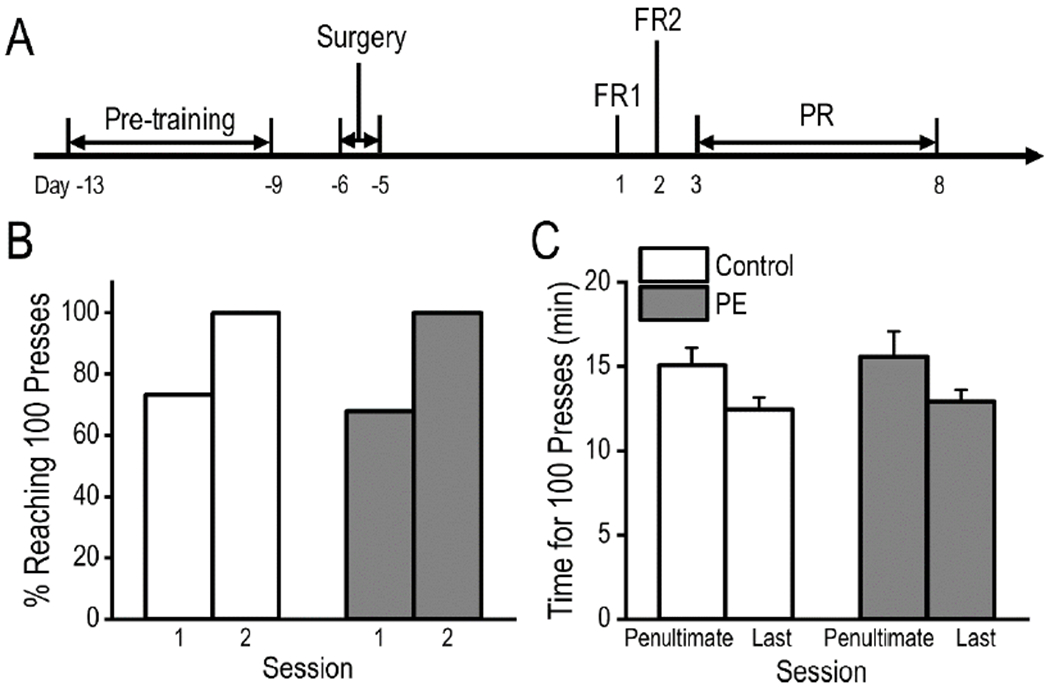
Prenatal ethanol exposure (PE) did not lead to deficits in operant learning. (A) Timeline of the pre-training and acquisition of amphetamine self-administration experiments. (B)There was no group difference in proportion of rats acquiring the lever press behavior (completion of 100 trials in 1 h) in the first 2 sessions of pre-training. (C) No group differences were observed in time spent completing 100 trials/session. Data are presented as Mean ± SEM.
2.5.4. Self-administration extinction and reinstatement.
The timeline of the procedures is presented in Fig. 5A. Briefly, a new group of control and PE rats (control: n = 11 from 5 litters; PE: n = 9/4 litters) were trained to acquire amphetamine self-administration under the FR1 schedule at 0.1 mg/kg/infusion for 3 sessions followed by 0.03 mg/kg/infusion for another 7 sessions (3 h/session, maximal infusions set at 20/session to prevent excessive drug intake). In order to study extinction, we switched to the FR1 schedule because it was found that rats trained under the PR schedule could not extinguish the drug-seeking behavior easily in our preliminary studies (unpublished). Furthermore, we found this training paradigm facilitated fast acquisition without masking group differences. After acquisition training, rats underwent twenty 1-h extinction sessions (2 sessions/day, 3 h apart), during which time lever presses produced no programmed consequences, with house light, cue light, and tone off during the entire session. To study the effects of abstinence on drug-induced reinstatement, amphetamine-induced (0.3 or 0.6 mg/kg, s.c.) reinstatement tests were conducted at time points within a week or 5-8 weeks after extinction training (see Fig. 5A for details). During abstinence, rats were housed in their home cages undisturbed, except for weekly cage changes. Saline (0.9%) was injected (s.c.) on days prior to the amphetamine-induced reinstatement tests to record baseline responses. Each baseline or reinstatement session lasted for 3 h, during which time the house light, cue light, and tone were off. After extended abstinence, more than one baseline session was scheduled before the reinstatement tests, because rats’ reactivity to the contextual cue (testing chamber) might interfere with their reactivity to the drug. As such, they were allowed to habituate to the testing chambers before the tests (see Fig. 5 for details).
Fig. 5.
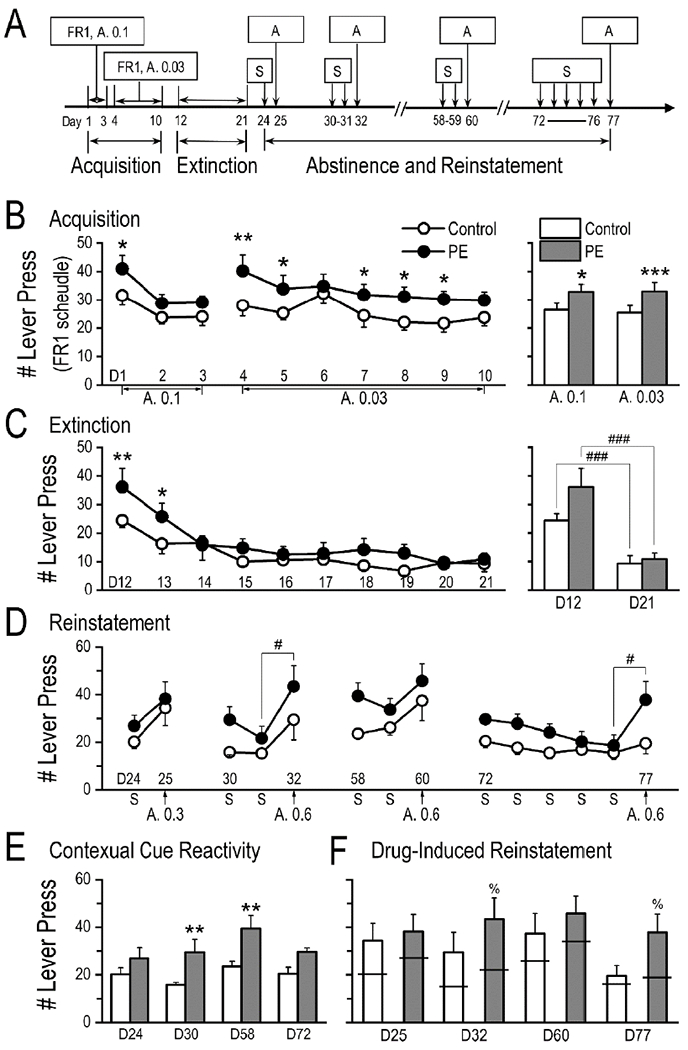
Prenatal ethanol exposure (PE) led to greater reactivity to contextual drug cues after extended abstinence and amphetamine-induced reinstatement of drug seeking. (A) Timeline of the experiments after pre-training. (B) In the self-administration acquisition phase, PE rats responded more than controls under the fixed ratio 1 (FR1) schedule, at both 0.1 mg/kg/infusion and 0.03 mg/kg/infusion doses. Left panel depicts daily sessions; right panel is a summary graph of averages across sessions. (C) Successful extinction of drug seeking was observed in both control and PE rats (right panel, by comparing the first and last days of extinction training), although during the first 2 days of extinction training, PE rats exhibited higher responding than controls (left panel, each circle/dot depicts the average across two daily sessions). (D) Session data from 4 reinstatement tests (and corresponding baselines) after different lengths of drug abstinence. (E) Summary graph showing higher reactivity to the contextual drug cue (the testing chamber) in PE rats than in controls after extended drug abstinence (on days 30 and 58). (F) Amphetamine (0.6 mg/kg, s.c.) -induced reinstatement of self-administration was observed in PE but not control rats on days 32 and 77. The horizontal bars reflect mean baseline responding. Data are presented as Mean ± SEM. Abbreviations: A, amphetamine (the number following A represents the dose applied); D, day (followed by the testing day number, details in Fig. 5A); S, 0.9% saline. *: p < 0.05, **: p < 0.01, ***: p < 0.001, control vs. PE rats; #: p < 0.05; ###: p < 0.001, between 2 sessions in the same group; %: p < 0.05, baseline vs. reinstatement session in PE rats, planned comparisons after ANOVA.
2.6. Statistical Analysis
Results were analyzed, using 2-way or 3-way analysis of variance (ANOVA) and Pearson’s chi-squared tests with SAS 9.4 (SAS Institute, Cary, NC), and the alpha level was set at 0.05. To determine and control for potential litter effects, litter was included as a nested factor in ANOVA. Planned comparisons and contrasts were used to assess pairwise differences and linear trends respectively. In the self-administration reinstatement tests, 5.36% of the 280 datum points (from both baseline and testing sessions) were identified as outliers, using boxplots [57]. These outliers were then brought to the next highest or lowest values within the same group in a given session, prior to statistical analyses. This way of outlier identification and treatment has been used previously [58].
3. Results
3.1. Prenatal ethanol exposure led to lower birthweights but not smaller litter sizes
Thirty-six control and 32 PE dams gave birth to 496 and 417 pups respectively (Table1). Prenatal ethanol exposure did not influence number of pups/litter. However, a sex main effect with fewer pups in the female sex was observed (2-way ANOVA - group: control vs. PE, and sex: male vs. female; sex main effect, F1,66 = 13.42, p < 0.001). Prenatal ethanol exposure led to reduced pup weights on PD 1 (by 3.05% on average; 2-way ANOVA with litter as a nested variable; group main effect, F1,843 = 51.69, p < 0.001). Pup weights were also lower in the female sex (sex main effect, F1,843 = 132.78, p < 0.001). Litter has an effect on pup weights (litter effect: F66,843 = 17.45, p < 0.001). No bodyweight differences between PE and control rats with the same sex were found at weaning (on PD 21) or at the age of 7 weeks. These results indicated that PE did not give rise to major teratogenic effects.
Table 1.
Birth Outcome after Prenatal Ethanol Exposure
| Control: 36 litters (mean ± SEM) |
PE: 32 litters (mean ± SEM) |
p-value | |
|---|---|---|---|
| Litter Size | 13.78 ± .42 | 13.03 ± .42 | 0.318 |
| Number of Male Pups | 7.39 ± .37 | 7.38 ± .36 | 0.979 |
| Number of Female Pups | 6.39 ± .39 | 5.66 ± .35 | 0.167 |
| Pup weight on Postnatal Day 1 | |||
| Average Weight (g) | 6.57 ± .03 | 6.37 ± .04 | <0.001 |
| Average Male Weight (g) | 6.75 ± .04 | 6.53 ± .04 | <0.001 |
| Average Female Weight (g) | 6.35 ± .04 | 6.15 ± .06 | <0.001 |
Note. Data are expressed as mean ± SEM. PE: prenatal ethanol exposure. P-values are based on planned comparisons after ANOVA.
3.2. Prenatal ethanol exposure increased locomotor activity
Rats with PE travelled longer distances (6331 ± 341.7 cm) than controls (5516 ± 317.2 cm) during the 60-min testing session (2-way mixed ANOVA with litter as nested variable - group: control vs. PE, and epoch: 1-12; group main effect: F1,47 = 40.27, p < 0.001; epoch main effect: F11,792 = 216.27, p < 0.001; litter effect: F25,47 = 7.50, p < 0.001, Fig. 1A). Rats with PE also spent more time on movement (2-way mixed ANOVA; group main effect, F1,47 = 25.81, p < 0.001; epoch main effect, F11,792 = 114.31, p < 0.001; litter effect: F25,47 = 4.90, p < 0.001, Fig. 1B). Comparisons in both distance travelled and movement time showed that PE increased rats’ locomotor activity in a novel environment, which is a phenotype of increase addiction risk [32].
3.3. Prenatal ethanol exposure induced amphetamine CPP acquisition
The CPP pre-test results were examined by a 2-way ANOVA (group: control vs. PE; compartment: left vs. right) with litter as a nested factor (control: n = 36; PE: n = 41). There was no difference in time (in seconds) spent between the two side compartments in either control (347.30 ± 10.41 vs. 355.61 ± 14.99) or PE rats (347.44 ± 10.74 vs. 350.72 ± 14.29), indicating that the CPP apparatus was unbiased.
In CPP acquisition test, vehicle (0 mg/kg amphetamine) and 2 different doses of amphetamine (0.3, and 0.6 mg/kg) were applied (Fig. 2B). The vehicle produced no CPP for either side chamber in control or PE rats (control: n = 9, PE: n = 10; 2-way ANOVA with litter as a nested factor - group: control vs. PE, and compartment: saline-paired vs. amphetamine paired). In contrast, 0.3 mg/kg amphetamine produced CPP for the drug-paired compartment only in PE but not control rats (control: n = 15, PE: n = 16; a compartment main effect produced by a 2-way ANOVA, F1,29 = 10.47, p < 0.01; in PE rats, a planned comparison after ANOVA showed CPP, p < 0.01). Amphetamine at 0.6 mg/kg, on the other hand, led to CPP in both control and PE rats (control: n = 12, PE: n = 15; a compartment main effect produced by a 2-way ANOVA, F1,25 = 22.68, p < 0.001; planned comparisons after ANOVA showed CPP in controls, p < 0.001, and in PE rats, p < 0.01).
The extinction training successfully extinguished CPP for the previously drug-paired compartment in both control and PE rats in the high-dose (0.6 mg/kg) group (Fig. 2C). No differences were shown in time spent between the 2 side chambers during the extinction test in either control or PE rats (2-way ANOVA with litter as a nested factor - group: control vs. PE, and compartment: previously saline-paired vs. amphetamine paired). In the following CPP reinstatement test, 0.3 mg/kg amphetamine reinstated CPP in both control and PE rats, with no group differences (a compartment main effect produced by a 2-way ANOVA, F1,25 = 21.06, p < 0.001; planned comparisons after ANOVA showed reinstated CPP in controls, p < 0.01, and in PE rats, p < 0.01; Fig. 2C). Taken together, PE enhanced CPP acquisition but exerted no effects on amphetamine-induced reinstatement of CPP.
3.4. Prenatal ethanol exposure caused no deficits in operant learning
Rats were trained to lever press before the self-administration experiments. Control and PE rats reached the criterion of 100 responses/session at similar rates (Fig. 3B, Chi-squared test). As the training progressed, the time taken to carry out 100 responses continued to decline but no group differences were observed (2-way mixed ANOVA with litter as a nested factor - group: control vs. PE, and session: penultimate vs. last; session main effect, F1,56 = 7.43, p < 0.01; litter effect: F18,38 = 1.94, p < 0.05, Fig. 3C). The results suggested that PE induced no deficits in operant learning. As such, potential group differences in amphetamine self-administration should not be attributed to deficits in operant learning.
3.5. Prenatal ethanol exposure enhanced acquisition of amphetamine self-administration
Rats were trained to acquire amphetamine self-administration at 0.02 or 0.1 mg/kg/infusion. No group differences were observed in proportion of rats reaching the FR criterion within 4 days at the 0.02 mg/kg/infusion dose (chi-squared test; control: 9/12, PE: 11/12). During the 6 PR sessions, PE rats self-administered more amphetamine (infusions) than controls at the 0.02 mg/kg/infusion dose (control: n = 8, PE: n = 10; 2-way mixed ANOVA with litter as a nested factor - group: control vs. PE, and session: 1-6; group main effect, F1,6 = 52.08, p < 0.001; session main effect, F5,80 = 2.49, p < 0.05; Fig. 4A left panel). Specifically, PE rats self-administered 6.63 ± 0.49 infusions/session (average across 6 PR sessions), greater than controls (4.25 ± 0.35 infusions/session, control vs. PE: p < 0.001, planned comparison, Fig. 4A right panel). Additionally, it has been observed that under a PR schedule, rats could stop responding at a certain time point before the session ended [55]. As such, responding time (from session initiation to the time point at which the last lever press was made) can be used as a measure of motivation for drug seeking. At the dose of 0.02 mg/kg/infusion, PE rats had longer responding time than controls (2-way mixed ANOVA with litter as a nested factor; group main effect, F1,6 = 8.51, p < 0.05; Control: 2.04 ± 0.17 h vs. PE: 2.57 ± 0.13 h, averages across 6 PR sessions, Fig. 4B).
Fig. 4.
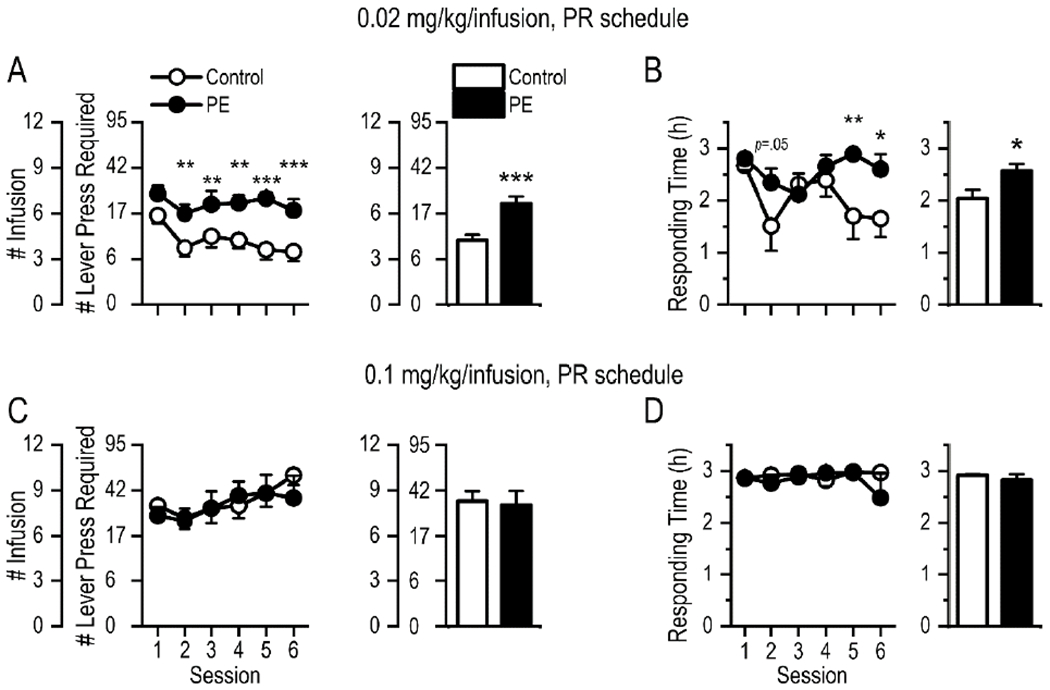
Rats with prenatal ethanol exposure (PE) exerted greater effort to obtain amphetamine under a progressive ratio (PR) schedule. (A) At the dose of 0.02 mg/kg/infusion, PE rats self-administered more amphetamine (infusions) than controls. Each circle or solid dot in the curve graph depicts the data from a specific session; the length of a bar represents the average across 6 sessions. Data are presented as Mean ± SEM number of amphetamine infusions obtained. The number of lever presses required under the PR schedule to obtain successive infusions of amphetamine is also shown (not cumulative requirement). (B) At the dose of 0.02 mg/kg/infusion, the responding time/session (maximum: 3 h) was longer in PE rats than in controls. Responding time: the duration between session initiation and the time point at which the last lever press was made. (C) At the dose of 0.1 mg/kg/infusion, there was no group difference in number of amphetamine infusions. (D) At the dose of 0.1 mg/kg/infusion, there was no group difference in responding time. *: p < 0.05; **: p < 0.01; ***: p < 0.001, control vs. PE rats, planned comparisons after ANOVA.
We also investigated PE effects with a higher amphetamine dose (0.1 mg/kg/infusion). All rats in both groups (control: n = 8; PE: n = 8) reached the FR criterion within 4 days. No group difference between PE and control rats but a session effect was observed in self-administration (infusions) during the 6 PR sessions (2-way mixed ANOVA with litter as a nested factor - group: control vs, PE, and session: 1-6; session main effect, F5,50 = 2.75, p < 0.05; litter effect: F6,4 = 9.52, p < 0.05, Fig. 4C left panel). On average, PE rats self-administered 8.03 ± 0.94 infusions/session, similar to controls (8.31 ± 0.64 infusions, Fig. 4C right panel). Escalations of drug seeking, an indication of addiction liability [59], were observed in both groups, indicated by upward linear trends in number of infusions (controls: p <0.05; PE: p < 0.05, planned contrasts). In addition, at the dose of 0.1 mg/kg/infusion, there is no difference in responding time (2-way mixed ANOVA with litter as a nested factor; Control: 2.92 ± 0.02 h vs. PE: 2.82 ± 0.12 h, averages across 6 PR sessions, Fig. 4D). The average responding time was close to the session duration (3 h) in both groups, indicative of high motivation for drug seeking at this dose. Taken together, PE rats show enhanced amphetamine self-administration at a lower, but not a higher, amphetamine dose. At a higher dose, both control and PE rats showed escalations of drug taking.
3.6. Prenatal ethanol exposure led to greater reactivity to the contextual drug cue after extended abstinence and amphetamine-induced reinstatement of drug seeking
To study extinction and reinstatement of drug seeking, rats (control: n = 11; PE: n = 9) were first trained to acquire amphetamine self-administration under the FR1 schedule at 0.1 mg/kg/infusion for 3 days followed by 0.03 mg/kg/infusion for 7 days for a maximum of 20 infusions/day. Rats in the PE group showed increased number of lever presses at both 0.1 mg/kg/infusion (2-way mixed ANOVA with litter as nested factor - group: control vs. PE, and session: 1-3; group main effect, F1,11 = 6.11, p < 0.05; session main effect, F2,36 = 6.95, p < 0.01; Fig. 5B) and 0.03 mg/kg/infusion doses (2-way mixed ANOVA with litter as a nested variable; group main effect, F1,11 = 28.47, p < 0.001; session main effect, F6,108 = 2.39, p < 0.05; litter effect: F7,11 = 8.44, p < 0.01, Fig. 5B).
The rats then underwent extinction training for 20 sessions (2 sessions/day). Extinction was observed in both groups, based on significant decreases in number of lever presses between the first and the last days of extinction training (control: from 24.41 ± 2.39 to 9.32 ± 2.80, 61.83% decrease, p < 0.001; PE: from 36.17 ± 6.44 to 10.89 ±2.19, 69.89% decrease, p < 0.001, planned comparisons, Fig. 5C right panel), although during the first two days, PE rats exhibited more lever presses than controls (2-way mixed ANOVA with litter as a nested variable; group main effect, F1,11 = 13.61, p < 0.01; session main effect, F9,162 = 9.54, p < 0.001; litter effect: F7,11 = 4.75, p < 0.05; on the first day of extinction training, control vs. PE: p < 0.01, planned comparison; on the 2nd day, control vs. PE: p < 0.05, Fig. 5C left panel).
Furthermore, after extended periods of drug abstinence (9 and 37 days after the extinction training), PE rats showed greater reactivity to the contextual drug cue – the testing chamber, i.e., higher numbers of lever presses than controls (Fig. 5E), when they were re-exposed to the chamber (9 and 37 days after extinction training respectively: p < 0.01; p < 0.01, planned comparisons following a 2-way mixed ANOVA with litter as a nested factor - group: control vs. PE, and session: 24, 30, 58, & 72 in Fig. 5E, showing group main effect, F1,11 = 18.24, p < 0.01; session main effect, F3,54 = 2.97, p < 0.05).
Four amphetamine-induced reinstatement tests were conducted 4, 11, 39, and 56 days after extinction training respectively (Fig. 5D & F), and significant reinstatement was only observed in PE but not control rats 11 and 56 days after extinction training respectively (p < 0.05; p < 0.05, planned comparisons following a 3-way mixed ANOVA with litter as a nested factor - group: control vs. PE, testing day: 4, 11, 39, & 56 days after extinction training, and session: immediate baseline vs. reinstatement session, showing a group main effect, F1,11 = 9.64, p < 0.05, a session main effect, F1,18 = 24.02, p < 0.001, and the litter effect, F7,11 = 4.42, p < 0.05). Specifically, amphetamine did not induce reinstatement 4 days after the extinction training, perhaps because the priming drug dose was too low (0.3 mg/kg). Therefore, another reinstatement test was performed 1 week later with a priming dose of 0.6 mg/kg. We observed successful reinstatement of drug seeking in PE but not control rats. Furthermore, to study reinstatement following prolonged abstinence, we conducted reinstatement test 5 weeks after extinction, in which test we did not observe reinstatement of drug seeking in either control or PE rats. This could be due to high baselines caused by reactivity to the contextual drug cue. So an additional reinstatement test was performed 8 weeks after extinction, following more saline sessions, which allowed animals to habituate to the testing box. We found PE but not control rats showed reinstatement of drug seeking, indicating persistence of PE effects on drug-induced reinstatement.
Taken together, PE led to greater reactivity to the contextual drug cue after extended abstinence and amphetamine-induced reinstatement of drug seeking. These results support that PE induces an increase in drug addiction risk.
4. Discussion
4.1. Increased risk of psychostimulant addiction in PE rats
Animals with PE display greater locomotor activity in a novel environment, a behavioral phenotype associated with increased addiction risk. In addition, we have observed that PE enhances acquisition of amphetamine CPP and self-administration. Prenatal ethanol exposure also leads to greater reactivity to the contextual drug cue after extended abstinence and amphetamine-induced reinstatement of drug seeking. These results support that PE increases psychostimulant addiction risk.
The increase in locomotor response to novelty in adult PE rats is consistent with many previous studies [11, 13, 14, 18, 60], but different from others that report decreased [61] or unchanged [21, 62, 63] locomotor activity after PE. The discrepancy could be attributed to differences in PE paradigms (e.g., exposure time, dose, and route of ethanol administration) and animals (e.g., sex, age, strain, vendor, and rearing condition). A review of alterations in locomotor activity caused by PE can be found in [64]. These constraints indicate that locomotor response to novelty alone might not be a consistent and reliable predictor of addiction risk.
In the present study, experiments were conducted only in male rats to limit the scope of the project. This was necessitated by the need to test different amphetamine doses in behavioral experiments involving sophisticated, time-consuming procedures to investigate multiple elements of addictive behaviors. Nonetheless, sex is an important factor [65, 66] that we will pursue in future studies. Indeed, previous human studies have shown that females progress to drug dependence more rapidly than males after initial drug use [67, 68]. Earlier animal studies have also shown that females are more vulnerable than males to drug reinforcing effects [69], drug craving [70, 71], and relapse, especially when estrogen levels are high [72–75]. It remains unclear whether PE effects on addiction risk interact with sex [76].
We only employed a single dose of PE (6 g/kg via two gavages of 3 g/kg ethanol/day, 15% w/v) in the present study to model heavy PE in humans, as mentioned above. This dose is commonly used in preclinical PE studies, and the peak BAC of dams in our paradigm was actually lower than that in many previous studies [77]. Nonetheless, the peak BAC level should not be the only consideration when choosing a rodent model of PE. Other factors including timing of treatment, route of administration, administration of nutritional supplements, and stress need to be considered as do species differences in genetics, metabolic rate, food consumption and tolerance [77]. These considerations suggest that caution be exercised when comparing BACs between rodents and humans [78, 79]. Importantly, the PE regimen used in the present study does not cause major teratogenic effects in that it leads only to a 3% decrease in birth weight. We have not observed major motor dysfunction in PE rats in the locomotor test. In addition, the results from pre-training for amphetamine self-administration show no deficits in learning of the operant procedure in PE rats. We recognize that it will also be important to investigate whether moderate PE could increase addiction risk. Based on our previous study showing that moderate PE (3 g/kg/day) alters excitatory synaptic function in VTA DA neurons [53], it is possible that it may also increase addiction risk.
4.2. Prenatal ethanol exposure enhances the rewarding properties of psychostimulants
The CPP paradigm is used in the present study to directly investigate the rewarding effects of psychostimulants and assess addiction risk in PE rats. We have applied an unbiased box design in which animals do not show pre-existing place preference, which is also the preferred CPP approach [34]. In addition, the unequal numbers in amphetamine (3 pairings) and saline (2 pairings) pairings [48] do not contribute to biased results either. We show that when both compartments are paired with saline (in the 0 mg/kg amphetamine group), animals do not develop place preference to the compartment with more pairings. Therefore, any place preference after conditioning should be attributed to the association of the rewarding/incentive value of amphetamine with the amphetamine-paired compartment.
The doses of amphetamine (0.3 or 0.6 mg/kg) used in our CPP experiments are much lower (~ 10 fold) compared to those used in most other studies, in which amphetamine doses often range from 2 to 5 mg/kg [80–83]. A previous study has shown distinct brain mechanisms mediating amphetamine CPP and amphetamine-induced locomotor sensitization. Amphetamine at > 1 mg/kg doses could induce both CPP and locomotor sensitization, while amphetamine at < 1 mg/kg doses induce CPP only [83]. Therefore, we use low doses in the present study to avoid confounding effects of motor sensitization on CPP acquisition. The second reason for using low amphetamine doses is that higher doses of psychostimulants often mask group differences in addiction risk. Results from many self-administration studies [27, 50, 84, 85] show that group differences in addiction risk can be detected only when lower but not higher doses of psychostimulants are given to the animals, which has been further confirmed by the present study. Specifically, amphetamine at 0.3 mg/kg produces CPP only in PE but not control rats, whereas the same drug at 0.6 mg/kg produces CPP in both groups. Similarly, group differences in acquisition of self-administration are observed only at the lower (0.02 mg/kg/infusion) but not the higher amphetamine dose (0.1 mg/kg) under the PR schedule. In fact, a previous study indicates that group differences in self-administration could be detected when an even lower amphetamine dose (0.01 mg/kg/infusion) was employed [86].
It is worth noting that despite the difference in amphetamine CPP acquisition between control and PE rats at the dose of 0.3 mg/kg, we observed that the two groups of rats spent approximately the same time in the previously amphetamine-paired chambers during the acquisition test (Fig. 2B). Because the center chamber was inaccessible during the pairing sessions, it could represent a relatively novel distraction in the acquisition test. Indeed, novelty-seeking has been a major confounding factor in the CPP paradigm [33]. In the present study, we observed PE rats trained at the dose of 0.3 mg/kg spent more time, albeit non-significantly, than controls in the center chamber during the CPP acquisition test. Therefore, it is possible that CPP observed in PE rats in the 0.3 mg/kg amphetamine condition was influenced by novelty of the center chamber during testing.
The CPP paradigm is an important approach in studying drug addiction, which can quickly detect addiction liability of drugs without surgery or extensive training [33]. Moreover, drug cue preference is observable in the CPP paradigm when drugs are passively administered [42]. Nevertheless, in the CPP paradigm, animals do not perform operant behavior that leads to contingent drug administration, a hallmark of addictive behavior in humans [87]. Therefore, we also utilize the self-administration paradigm, which directly resembles human drug taking/seeking behavior [41, 42]. In addition, this method provides sophisticated manipulations of drug delivery and reinforcement schedule, etc. to assess different elements of addictive behavior. In the present study, we have examined acquisition of drug seeking, reactivity to contextual drug cues after extended abstinence, and drug priming-induced reinstatement of drug seeking, using this paradigm.
Under the PR schedule, we assess addiction liability by observing the effort level the animal is willing to exert to gain one more drug infusion [55]. We show that PE rats exert more effort (lever presses) than controls to acquire extra amphetamine infusions at the lower unit dose. Moreover, PE rats are less likely to stop responding before the session ends, showing higher motivation for drug seeking. Both observations support increased addiction risk in PE rats, similar to our previous findings using slightly different operant paradigms [49, 53].
It is important to examine animals’ drug seeking behavior when drugs become unavailable after acquisition of self-administration because the strong urge, or craving, to obtain drugs, is a major cause for relapse [88, 89]. In the present study, PE rats exhibit more lever presses than controls when re-exposed to the operant chambers after periods of drug abstinence, showing recovery of responses activated by the contextual cue – the testing chamber. Nonetheless, we did not perform traditional cue-induced reinstatement tests, in which animals are re-exposed to previously drug-paired response-contingent cues (light and/or tone). Results from previous studies, however, have shown that other than response-contingent cues, contextual cues can also induce reinstatement of extinguished self-administration [40, 90, 91]. We believe that the operant chamber in our test serves as a contextual cue, which is sufficient to induce recovery of responses in PE rats.
We have also examined drug priming-induced reinstatement of drug seeking behavior [28]. We find that amphetamine at the 0.3 mg/kg dose (s.c.) cannot induce reinstatement in either control or PE rats. However, at 0.6 mg/kg (s.c.), reinstatement is observed in PE but not control rats. Taken together, PE rats exhibit greater reactivity to contextual drug cues after extended abstinence and drug-induced restatement. These results indicate that PE-induced increase in addiction risk is persistent, which can be observed after very limited psychostimulant exposure. In the present study, most of the PE effects in addictive behaviors are detected with the self-administration paradigm, which in our opinion is the more powerful methodology for studying PE-induced increase in addiction risk.
4.3. Prenatal ethanol exposure may increase risk of drug addiction in general
Increased addiction risk after PE is well-documented in clinical and animal studies. However, as mentioned earlier, most of the studies focus on the risk of ethanol dependence or preference. Only a few studies investigate the effects of PE on preference of or risk of addiction to other drugs of abuse. One of these studies shows that ethanol exposure during both pre- and early postnatal development leads to enhanced acquisition of cocaine CPP [21]. Other studies show that PE does not enhance nicotine self-administration [92] but neonatal ethanol exposure gives rise to nicotine CPP [93]. The discrepancies in these results could be mediated by the differences in the method of ethanol exposure and details in the behavioral paradigms employed. The present study uses the same PE procedure and both CPP and self-administration paradigms to investigate different aspects of addictive behaviors. This approach has provided clear evidence that PE indeed increases psychostimulant addiction risk. We speculate that PE may increase risk of addiction to all drugs of abuse. Actually, existing evidence from both clinical and animal studies show prenatal drug exposure increases addiction risk to drugs in general [22, 23].
4.4. Neural mechanisms of increased addiction risk caused by PE
The observations from the present study support the conclusion that PE leads to increased addiction risk. What could be the underlying neuronal mechanisms? Interestingly, many previous studies have shown that PE persistently alters the response of midbrain DA neurons to psychostimulants. These neurons constitute the origin of the brain reward pathway. First, PE leads to a persistent and profound reduction in VTA DA neuron impulse activity [30,45, 46, 94]. This effect is not caused by cell loss [46]. Increasing inhibitory tone to DA neurons by either pharmacological means or by stimulating inhibitory inputs can reverse (normalize) the reduced number of spontaneously active VTA DA neurons [46, 94]. These observations – reduced number of spontaneously active DA neurons and inhibition-induced reversal – resemble what has been reported after chronic antipsychotic treatment in previous studies [95, 96]. It has been suggested that such effects are caused by over-excitation, leading to impairment in impulse flow (depolarization block) in VTA DA neurons [97]. Therefore, a reduction in the number of spontaneously active VTA DA neurons in PE rats could also be due to over-excitation/depolarization block [46, 94]. This hypothesis is supported by our recent study demonstrating increased strength and plasticity of excitatory synapses onto VTA DA neurons in PE rats [49] and a reduction in endocannabinoid-mediated long-term depression to weaken synapses [53]. An interesting observation is that psychostimulants (e.g., amphetamine and methylphenidate) can reverse the reduced number of spontaneously active DA neurons in PE rats [29, 30, 94]. In control animals, psychostimulants exert inhibitory effects on VTA DA neurons by increasing dendritic DA release and the activation of D2-like somatodendritic DA receptors [29, 30, 94]. We attribute the reversal effects of psychostimulants on reduced number of spontaneously active VTA DA neurons in PE rats to the ability of amphetamine to increase the inhibitory tone to these neurons. The reversal effects of psychostimulants in PE rats reflect a net increase in impulse activity, which is likely to enhance impulse-dependent DA release and amplify already enhanced DA neurotransmission in nucleus accumbens/prefrontal cortical areas by psychostimulants due to their direct effects on DA terminals.
Results from previous studies have also shown that PE-induced changes in VTA DA neurons and their responses to psychostimulants are not unique consequences. For example, a reduction in spontaneously active VTA DA neurons due to depolarization block is also observed following repeated exposure to ethanol, cocaine, amphetamine, methylphenidate, and nicotine during adulthood [98], or prenatal stress exposure [5], conditions known to increased addiction risk [5, 20, 22, 84]. Furthermore, PE significantly enhances the excitatory synaptic strength in VTA DA neurons [49], which is a critical cellular mechanism for increased addiction risk or addictive behaviors [27, 99–101]. These observations demonstrate that PE leads to changes in the mesolimbic/cortical DA systems in a manner resembling other conditions leading to increased addiction risk, further supporting the conclusion that PE increases addiction risk.
5. Conclusions
To summarize, we have shown that PE, without causing major teratogenic effects, leads to a behavioral phenotype associated with increased addiction risk and enhanced acquisition of drug cue preference and drug taking behavior. We have also found that PE leads to greater reactivity to contextual drug cues after extended abstinence and drug priming-induced reinstatement of drug seeking behavior. These effects are observable with very limited psychostimulant exposure and thus provide unequivocal evidence that PE gives rise to increased psychostimulant addiction risk. We also posit that PE might increase risk of addiction to all drugs of abuse, as it leads to cellular changes in VTA DA neurons critical to addictive behaviors. We believe that these results provide important insights into development of prevention strategies for drug addiction in individuals with FASD.
Highlights.
Rats with prenatal ethanol exposure display higher locomotor responses to novelty.
Rats with prenatal ethanol exposure show amphetamine conditioned place preference.
Rats with prenatal ethanol exposure self-administer more amphetamine.
No group differences are observed at higher amphetamine doses.
Rats with prenatal ethanol exposure exhibit amphetamine-induced reinstatement.
Acknowledgements
This work was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health (Grants AA12435 and AA019482 to R.S.)· The authors thank Dr. Jerry B. Richards for his suggestions on the experimental design, and Amy Hilburger, Marita Paredez, Jiaobao Gao, and Alvin Wen for their help for conducting experiments and collecting data
Abbreviations:
- FR1
fixed ratio 1
- FR2
fixed ratio 2
- PR
progressive ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wagner FA, Anthony JC, From first drug use to drug dependence: developmental periods of risk for dependence upon marijuana, cocaine, and alcohol, (2002). [DOI] [PubMed] [Google Scholar]
- [2].Volkow ND, What do we know about drug addiction?, American Journal of Psychiatry 162(8) (2005) 1401–1402. [DOI] [PubMed] [Google Scholar]
- [3].Perera F, Herbstman J, Prenatal environmental exposures, epigenetics, and disease, Reproductive toxicology 31(3) (2011) 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lanphear BP, The impact of toxins on the developing brain, Annual review of public health 36 (2015). [DOI] [PubMed] [Google Scholar]
- [5].Hausknecht K, Haj-Dahmane S, Shen RY, Prenatal stress exposure increases the excitation of dopamine neurons in the ventral tegmental area and alters their reponses to psychostimulants, Neuropsychopharmacology 38(2) (2013) 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sokol RJ, Delaney-Black V, Nordstrom B, Fetal alcohol spectrum disorder, JAMA 290(22) (2003) 2996–9. [DOI] [PubMed] [Google Scholar]
- [7].Riley EP, McGee CL, Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior, Exp Biol Med (Maywood) 230(6) (2005) 357–65. [DOI] [PubMed] [Google Scholar]
- [8].Alati R, Al Mamun A, Williams GM, O’Callaghan M, Najman JM, Bor W, In utero alcohol exposure and prediction of alcohol disorders in early adulthood: a birth cohort study, Arch Gen Psychiatry 63(9) (2006) 1009–16. [DOI] [PubMed] [Google Scholar]
- [9].Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP, A21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking, Arch Gen Psychiatry 60(4) (2003) 377–85. [DOI] [PubMed] [Google Scholar]
- [10].Famy C, Streissguth AP, Unis AS, Mental illness in adults with fetal alcohol syndrome or fetal alcohol effects, Am J Psychiatry 155(4) (1998) 552–4. [DOI] [PubMed] [Google Scholar]
- [11].Bond NW, Prenatal Alcohol Exposure In Rodents: A Review Of Its Effects On Offspring Activity And Learning Ability, Australian Journal of Psychology 33(3) (1981) 331–344. [Google Scholar]
- [12].Abel EL, Prenatal effects of alcohol., Drug & Alcohol Dependence 14(1) (1984) 1–10. [DOI] [PubMed] [Google Scholar]
- [13].Kelly SJ, Goodlett CR, Hulsether SA, West JR, Impaired spatial navigation in adult female but not adult male rats exposed to alcohol during the brain growth spurt, Behav Brain Res 27(3) (1988) 247–57. [DOI] [PubMed] [Google Scholar]
- [14].Thomas JD, Sather TM, Whinery LA, Voluntary exercise influences behavioral development in rats exposed to alcohol during the neonatal brain growth spurt, Behav Neurosci 122(6) (2008) 1264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Weinberg J, Sliwowska JH, Lan N, Hellemans KG, Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome, J Neuroendocrinol 20(4) (2008) 470–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hellemans KG, Verma P, Yoon E, Yu W, Weinberg J, Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood, Ann N Y Acad Sci 1144 (2008) 154–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Blanchard BA, Hannigan JH, Riley EP, Amphetamine-induced activity after fetal alcohol exposure and undernutrition in rats, Neurotoxicol Teratol 9(2) (1987) 113–9. [DOI] [PubMed] [Google Scholar]
- [18].Barbier E, Houchi H, Warnault V, Pierrefiche O, Daoust M, Naassila M, Effects of prenatal and postnatal maternal ethanol on offspring response to alcohol and psychostimulants in long evans rats, Neuroscience 161(2) (2009) 427–440. [DOI] [PubMed] [Google Scholar]
- [19].Lee S, Choi I, Kang S, Rivier C, Role of various neurotransmitters in mediating the long-term endocrine consequences of prenatal alcohol exposure, Ann N Y Acad Sci 1144 (2008) 176–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Spear NE, Molina JC, Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: a theoretical review, Alcohol Clin Exp Res 29(6) (2005) 909–29. [DOI] [PubMed] [Google Scholar]
- [21].Barbier E, Pierrefiche O, Vaudry D, Vaudry H, Daoust M, Naassila M, Long-term alterations in vulnerability to addiction to drugs of abuse and in brain gene expression after early life ethanol exposure, Neuropharmacology 55(7) (2008) 1199–211. [DOI] [PubMed] [Google Scholar]
- [22].Malanga CJ, Kosofsky BE, Does drug abuse beget drug abuse? Behavioral analysis of addiction liability in animal models of prenatal drug exposure, Brain Res Dev Brain Res 147(1–2) (2003) 47–57. [DOI] [PubMed] [Google Scholar]
- [23].Glantz MD, Chambers JC, Prenatal drug exposure effects on subsequent vulnerability to drug abuse, Development and Psychopathology 18(3) (2006) 893–922. [DOI] [PubMed] [Google Scholar]
- [24].Day NL, Goldschmidt L, Thomas CA, Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14, Addiction 101(9) (2006) 1313–1322. [DOI] [PubMed] [Google Scholar]
- [25].Koob GF, Le Moal M, Drug abuse: hedonic homeostatic dysregulation, Science 278(5335) (1997) 52–58. [DOI] [PubMed] [Google Scholar]
- [26].Kalivas PW, Stewart J, Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity, Brain Research - Brain Research Reviews 16(3) (1991) 223–244. [DOI] [PubMed] [Google Scholar]
- [27].Suto N, Tanabe LM, Austin JD, Creekmore E, Vezina P, Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in an NMDA, AMPA/kainate, and metabotropic glutamate receptor-dependent manner, Neuropsychopharmacology 28(4) (2003) 629–39. [DOI] [PubMed] [Google Scholar]
- [28].Shaham Y, Shalev U, Lu L, De Wit H, Stewart J, The reinstatement model of drug relapse: history, methodology and major findings, Psychopharmacology (Berl) 168(1–2) (2003) 3–20. [DOI] [PubMed] [Google Scholar]
- [29].Choong K, Shen RY, Methylphenidate restores ventral tegmental area dopamine neuron activity in prenatal ethanol-exposed rats by augmenting dopamine neurotransmission, Journal of Pharmacology & Experimental Therapeutics 309(2) (2004) 444–451. [DOI] [PubMed] [Google Scholar]
- [30].Xu C, Shen RY, Amphetamine normalizes the electrical activity of dopamine neurons in the ventral tegmental area following prenatal ethanol exposure, The Journal of Pharmacology and Experimental Therapeutics 297(2) (2001) 746–752. [PubMed] [Google Scholar]
- [31].Piazza PV, Deminiere JM, Le Moal M, Simon H, Factors that predict individual vulnerability to amphetamine self-administration, Science 245(4925) (1989) 1511–3. [DOI] [PubMed] [Google Scholar]
- [32].Gancarz AM, San George MA, Ashrafioun L, Richards JB, Locomotor activity in a novel environment predicts both responding for a visual stimulus and self-administration of a low dose of methamphetamine in rats, Behav Processes 86(2) (2011)295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bardo MT, Bevins RA, Conditioned place preference: what does it add to our preclinical understanding of drug reward?, Psychopharmacology (Berl) 153(1) (2000) 31–43. [DOI] [PubMed] [Google Scholar]
- [34].Tzschentke TM, Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade, Addict Biol 12(3–4) (2007) 227–462. [DOI] [PubMed] [Google Scholar]
- [35].Suto N, Austin JD, Tanabe LM, Kramer MK, Wright DA, Vezina P, Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in a D1 dopamine receptor dependent manner, Neuropsychopharmacology 27(6) (2002) 970–9. [DOI] [PubMed] [Google Scholar]
- [36].Panlilio LV, Goldberg SR, Self-administration of drugs in animals and humans as a model and an investigative tool, Addiction 102(12) (2007) 1863–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].O’Connor EC, Chapman K, Butler P, Mead AN, The predictive validity of the rat self-administration model for abuse liability, Neuroscience & Biobehavioral Reviews 35(3) (2011)912–938. [DOI] [PubMed] [Google Scholar]
- [38].Balster RL, Bigelow GE, Guidelines and methodological reviews concerning drug abuse liability assessment, Drug and alcohol dependence 70(3) (2003) S13–S40. [DOI] [PubMed] [Google Scholar]
- [39].Johanson CE, The evaluation of the abuse liability of drugs, Drug Safety 5(1) (1990) 46–57. [DOI] [PubMed] [Google Scholar]
- [40].Perry CJ, Zbukvic I, Kim JH, Lawrence AJ, Role of cues and contexts on drug-seeking behaviour, British journal of pharmacology 171(20) (2014) 4636–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Carroll ME, Acquisition and reacquisition (relapse) of drug abuse: modulation by alternative reinforcers, NIDA Res Monogr 169 (1998) 6–25. [PubMed] [Google Scholar]
- [42].Prus AJ, James JR, Rosecrans JA, Conditioned place preference, in: Buccafusco J (Ed.), Methods of Behavior Analysis in Neuroscience, CRC Press/Taylor & Francis, Boca Raton, FL, 2009. [PubMed] [Google Scholar]
- [43].Fernández-Teruel A, Giménez-Llort L, Escorihuela RM, Gil L, Aguilar R, Steimer T, Tobeña A, Early-life handling stimulation and environmental enrichment: are some of their effects mediated by similar neural mechanisms?, Pharmacology Biochemistry and Behavior 73(1) (2002) 233–245. [DOI] [PubMed] [Google Scholar]
- [44].Gursky ZH, Klintsova AY, Wheel Running and Environmental Complexity as a Therapeutic Intervention in an Animal Model of FASD, Journal of visualized experiments: JoVE (120) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Choong K, Shen R, Prenatal ethanol exposure alters the postnatal development of the spontaneous electrical activity of dopamine neurons in the ventral tegmental area, Neuroscience 126(4) (2004) 1083–1091. [DOI] [PubMed] [Google Scholar]
- [46].Shen RY, Hannigan JH, Kapatos G, Prenatal ethanol reduces the activity of adult midbrain dopamine neurons, Alcoholism: Clinical & Experimental Research 23(11) (1999) 1801–1807. [PubMed] [Google Scholar]
- [47].Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B, Effects of moderate alcohol consumption on the central nervous system, Alcoholism: Clinical and Experimental Research 22(5) (1998) 998–1040. [DOI] [PubMed] [Google Scholar]
- [48].Mueller D, Stewart J, Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction, Behav Brain Res 115(1) (2000) 39–47. [DOI] [PubMed] [Google Scholar]
- [49].Hausknecht K, Haj-Dahmane S, Shen YL, Vezina P, Dlugos C, Shen RY, Excitatory synaptic function and plasticity is persistently altered in ventral tegmental area dopamine neurons after prenatal ethanol exposure, Neuropsychopharmacology 40(4) (2015) 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cain ME, Denehy ED, Bardo MT, Individual differences in amphetamine self-administration: the role of the central nucleus of the amygdala, Neuropsychopharmacology 33(5) (2008) 1149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ahmed SH, Walker JR, Koob GF, Persistent increase in the motivation to take heroin in rats with a history of drug escalation, Neuropsychopharmacology 22(4) (2000) 413. [DOI] [PubMed] [Google Scholar]
- [52].Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O, Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens, Proceedings of the National Academy of Sciences 97(8) (2000) 4321–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hausknecht K, Shen Y-L, Wang R-X, Haj-Dahmane S, Shen R-Y, Prenatal Ethanol Exposure Persistently Alters Endocannabinoid Signaling and Endocannabinoid-Mediated Excitatory Synaptic Plasticity in Ventral Tegmental Area Dopamine Neurons, Journal of Neuroscience 37(24) (2017) 5798–5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cortright JJ, Lorrain DS, Beeler JA, Tang W-J, Vezina P, Previous exposure to Δ9-tetrahydrocannibinol enhances locomotor responding to but not self-administration of amphetamine, Journal of Pharmacology and Experimental Therapeutics 337(3) (2011) 724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Richardson NR, Roberts D, Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy, Journal of Neuroscience Methods 66 (1996) 1–11. [DOI] [PubMed] [Google Scholar]
- [56].Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N, Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine, J Neurosci 22(11) (2002) 4654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vandervieren E, Hubert M, An adjusted boxplot for skewed distributions, COMPSTAT 2004, proceedings in computational statistics. Springer, Heidelberg: (2004) 1933–1940. [Google Scholar]
- [58].Wang R, Hausknecht KA, Haj-Dahmane S, Shen R-Y, Richards JB, Decreased environmental complexity during development impairs habituation of reinforcer effectiveness of sensory stimuli, Behavioural brain research 337 (2018) 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Edwards S, Koob GF, Escalation of drug self-administration as a hallmark of persistent addiction liability, Behavioural pharmacology 24 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Abel EL, Resddy RR, Prenatal high saturated fat diet modifies behavioral effects of prenatal alcohol exposure in rats, Alcohol 14(1) (1997) 25–9. [DOI] [PubMed] [Google Scholar]
- [61].Carneiro LM, Diogenes JP, Vasconcelos SM, Aragao GF, Noronha EC, Gomes PB, Viana GS, Behavioral and neurochemical effects on rat offspring after prenatal exposure to ethanol, Neurotoxicol Teratol 27(4) (2005) 585–92. [DOI] [PubMed] [Google Scholar]
- [62].Randall S, Hannigan JH, In utero alcohol and postnatal methylphenidate: locomotion and dopamine receptors, Neurotoxicology & Teratology 21(5) (1999) 587–593. [DOI] [PubMed] [Google Scholar]
- [63].Allan AM, Chynoweth J, Tyler LA, Caldwell KK, A mouse model of prenatal ethanol exposure using a voluntary drinking paradigm, Alcohol Clin Exp Res 27(12) (2003) 2009–16. [DOI] [PubMed] [Google Scholar]
- [64].Marquardt K, Brigman JL, The impact of prenatal alcohol exposure on social, cognitive and affective behavioral domains: Insights from rodent models, Alcohol 51 (2016) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Clayton JA, Collins FS, NIH to balance sex in cell and animal studies, Nature 509(7500) (2014) 282–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Guizzetti M, Davies DL, Egli M, Finn DA, Molina R, Regunathan S, Robinson DL, Sohrabji F, Sex and the Lab: An Alcohol-Focused Commentary on the NIH Initiative to Balance Sex in Cell and Animal Studies, Alcoholism: Clinical and Experimental Research 40(6) (2016) 1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hernandez-Avila CA, Rounsaville BJ, Kranzler HR, Opioid-, cannabis-and alcohol-dependent women show more rapid progression to substance abuse treatment, Drug & Alcohol Dependence 74(3) (2004) 265–272. [DOI] [PubMed] [Google Scholar]
- [68].Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A, Neuroimaging of gender differences in alcohol dependence: are women more vulnerable?, Alcoholism: Clinical and Experimental Research 29(5) (2005) 896–901. [DOI] [PubMed] [Google Scholar]
- [69].Lynch WJ, Roth ME, Carroll ME, Biological basis of sex differences in drug abuse: preclinical and clinical studies, Psychopharmacology 164(2) (2002) 121–137. [DOI] [PubMed] [Google Scholar]
- [70].Kennedy AP, Epstein DH, Phillips KA, Preston KL, Sex differences in cocaine/heroin users: drug-use triggers and craving in daily life, Drug & Alcohol Dependence 132(1) (2013) 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hitschfeld MJ, Schneekloth TD, Ebbert JO, Hall-Havin DK, Karpyak VM, Abulseoud OA, Patten CA, Geske JR, Frye MA, Female smokers have the highest alcohol craving in a residential alcoholism treatment cohort, Drug & Alcohol Dependence 150 (2015) 179–182. [DOI] [PubMed] [Google Scholar]
- [72].Becker JB, Hu M, Sex differences in drug abuse, Frontiers in neuroendocrinology 29(1) (2008) 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Rubonis AV, Colby SM, Monti PM, Rohsenow DJ, Gulliver S, Sirota A, Alcohol cue reactivity and mood induction in male and female alcoholics, Journal of Studies on Alcohol 55(4) (1994) 487–494. [DOI] [PubMed] [Google Scholar]
- [74].Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE, Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus, Psychopharmacology 182(2) (2005) 245–252. [DOI] [PubMed] [Google Scholar]
- [75].Becker JB, Sex differences in addiction, Dialogues in clinical neuroscience 18(4) (2016) 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Terasaki LS, Gomez J, Schwarz JM, An examination of sex differences in the effects of early-life opiate and alcohol exposure, Phil. Trans. R. Soc. B 371(1688) (2016) 20150123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Gil-Mohapel J, Boehme F, Kainer L, Christie BR, Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models, Brain research reviews 64(2) (2010) 283–303. [DOI] [PubMed] [Google Scholar]
- [78].Hannigan JH, What research with animals is telling us about alcohol-related neurodevelopmental disorder, Pharmacol Biochem Behav 55(4) (1996) 489–99. [DOI] [PubMed] [Google Scholar]
- [79].Abel EL, Fetal alcohol syndrome: behavioral teratology, Psychological Bulletin 87(1) (1980) 29. [PubMed] [Google Scholar]
- [80].Campbell J, Spear LP, Effects of early handling on amphetamine-induced locomotor activation and conditioned place preference in the adult rat, Psychopharmacology (Berl) 143(2) (1999) 183–9. [DOI] [PubMed] [Google Scholar]
- [81].Parker LA, Burton P, Sorge RE, Yakiwchuk C, Mechoulam R, Effect of low doses of delta9-tetrahydrocannabinol and cannabidiol on the extinction of cocaine-induced and amphetamine-induced conditioned place preference learning in rats, Psychopharmacology (Berl) 175(3) (2004) 360–6. [DOI] [PubMed] [Google Scholar]
- [82].Sakurai S, Yu L, Tan SE, Roles of hippocampal N-methyl-D-aspartate receptors and calcium/calmodulin-dependent protein kinase II in amphetamine-produced conditioned place preference in rats, Behav Pharmacol 18(5–6) (2007) 497–506. [DOI] [PubMed] [Google Scholar]
- [83].Shen F, Meredith GE, Napier TC, Amphetamine-induced place preference and conditioned motor sensitization requires activation of tyrosine kinase receptors in the hippocampus, J Neurosci 26(43) (2006) 11041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Vezina P, Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs, Neurosci Biobehav Rev 27(8) (2004) 827–39. [DOI] [PubMed] [Google Scholar]
- [85].Pierre PJ, Vezina P, Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug, Psychopharmacology (Berl) 129(3) (1997) 277–84. [DOI] [PubMed] [Google Scholar]
- [86].Vezina P, Pierre PJ, Lorrain DS, The effect of previous exposure to amphetamine on drug-induced locomotion and self-administration of a low dose of the drug, Psychopharmacology (Berl) 147(2) (1999) 125–34. [DOI] [PubMed] [Google Scholar]
- [87].Feltenstein MW, See RE, The neurocircuitry of addiction: an overview, Br J Pharmacol 154(2) (2008) 261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ludwig AM, Wikler A, “ Craving” and relapse to drink, Quarterly journal of studies on alcohol (1974). [PubMed] [Google Scholar]
- [89].Killen JD, Fortmann SP, Craving is associated with smoking relapse: findings from three prospective studies, Experimental and clinical psychopharmacology 5(2) (1997) 137. [DOI] [PubMed] [Google Scholar]
- [90].Bossert JM, Liu SY, Lu L, Shaham Y, A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking, Journal of Neuroscience 24(47) (2004) 10726–10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Crombag HS, Bossert JM, Koya E, Shaham Y, Context-induced relapse to drug seeking: a review, Philosophical Transactions of the Royal Society of London B: Biological Sciences 363(1507) (2008) 3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Matta SG, Elberger AJ, Combined exposure to nicotine and ethanol throughout full gestation results in enhanced acquisition of nicotine self-administration in young adult rat offspring, Psychopharmacology (Berl) 193(2) (2007) 199–213. [DOI] [PubMed] [Google Scholar]
- [93].Rogers JL, See RE, Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats, Neurobiol Learn Mem 87(4) (2007) 688–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Shen RY, Choong KC, Different adaptations in ventral tegmental area dopamine neurons in control and ethanol exposed rats after methylphenidate treatment, Biol Psychiatry 59(7) (2006) 635–42. [DOI] [PubMed] [Google Scholar]
- [95].Bunney BS, Grace AA, Acute and chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity, Life Sciences 23(16) (1978) 1715–1727. [DOI] [PubMed] [Google Scholar]
- [96].Grace AA, Bunney BS, Moore H, Todd CL, Dopamine-cell depolarization block as a model for the therapeutic actions of antipsychotic drugs, Trends Neurosci 20(1) (1997)31–7. [DOI] [PubMed] [Google Scholar]
- [97].Tanabe LM, Suto N, Creekmore E, Steinmiller CL, Vezina P, Blockade of D2 dopamine receptors in the VTA induces a long-lasting enhancement of the locomotor activating effects of amphetamine, Behav Pharmacol 15(5–6) (2004) 387–95. [DOI] [PubMed] [Google Scholar]
- [98].Shen RY, Choong KC, Thompson AC, Long-term reduction in ventral tegmental area dopamine neuron population activity following repeated stimulant or ethanol treatment, Biol Psychiatry 61(1) (2007) 93–100. [DOI] [PubMed] [Google Scholar]
- [99].Luscher C, Malenka RC, Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling, Neuron 69(4) (2011) 650–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bowers MS, Chen BT, Bonci A, AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future, Neuron 67(1) (2010) 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Carlezon WA Jr., Haile CN, Coppersmith R, Hayashi Y, Malinow R, Neve RL, Nestler EJ, Distinct sites of opiate reward and aversion within the midbrain identified using a herpes simplex virus vector expressing GluR1, J Neurosci 20(5) (2000) RC62. [DOI] [PMC free article] [PubMed] [Google Scholar]


