Abstract
Purpose
This randomized parallel study aims to investigate the azelaic acid (AA), and pyruvic acid (PA) peels treatment effect on health-related quality of life (QOL) in young adult women with acne vulgaris.
Patients and Methods
The participants were 120 female undergraduate students, with mild to moderate facial acne and an average age of 22 years old (M = 22.2, SD = 16.1). Eligibility criteria were as follows: female gender, 18–25 years of age, no dermatological treatment within the last 12 months and mild to moderate papulopustular acne. Patients were randomly divided into two groups, the first group was treated with AA, and the second group was treated with PA. Both groups received treatment every 2 weeks, for a total of 12 weeks. The Hellgren–Vincent scale was used to assess acne severity, and the Dermatology Life Quality Index (DLQI) and Skindex-29 were used to evaluate the quality of life of each patient. These scores were calculated before treatment, and after finishing the final treatment.
Results
All scoring systems used (Hellgren–Vincent scale, DLQI, and Skindex-29) demonstrated improvement in both groups. QOL scores were slightly better in the group using pyruvic acid compared with azelaic acid.
Conclusion
Both AA and PA have a significant impact on the objective assessment of acne symptoms, as well as the subjectively measured quality of life of young adult women with acne. There is a slightly greater improvement in QOL scores with PA compared with AA peeling treatment.
Keywords: azelaic acid, acid peels, DLQI, pyruvic acid, Skindex-29, women
Introduction
Acne vulgaris is a disease that occurs in up to 80% of adolescent patients, around 50–60% of women aged 20–25, and 12% of women over 25 years of age.1–5 Acne may present as non-inflammatory comedones (ie, whiteheads and blackheads), pustules, nodules and cysts, or inflammatory papules.6,7 Scars or facial deformities affect up to 20% of teenagers.5,7 Irritation, itching, and local pain are physical symptoms that can contribute to reduced quality of life. The condition can be associated with severe psychological and social impairment, including low self-esteem, altered body image, dysmorphia, psychosomatic symptoms, shame, embarrassment, social isolation, anxiety, depression, and even suicidal ideation.1,2,4,5,8,9 Around 30% of dermatology patients present with psychosocial and psychiatric comorbidity.9
In addition, a lower quality of life; some patients report losing confidence, which negatively affects social activities and workplace functioning.1,4 Furthermore, the stress associated with an unsightly skin condition can increase stress-reactive dermatoses, such as eczema or psoriasis.10 The successful management of psychodermatological disorders includes improving the patient’s self-esteem, providing mechanisms to cope with stress, improving social functioning, and treating anxiety and depression associated with their skin disease.11,12
Chemical peels are one of the most frequently used agents for acne treatment.13,14 The repeated peeling of the epidermis layer of the skin stimulates the upper dermis to produce new collagen, leading to regeneration and remodeling of the skin, improved texture and reduction in surface abnormalities.14,15 AA (azelaic acid) is also helpful to reduce the hyperpigmentation that may occur following inflammation, without inducing bacterial resistance.16,17 AA has an established history of effectiveness; however, PA is a newer, safer, and potentially more effective peeling agent.13,14 However, to our best knowledge, no study compares the effectiveness of treatment with AA and PA peeling on subjective QOL in young adult women. There is also scarce evidence comparing systemic and local therapies.5 The study’s objective is to parallel the efficacy of acne treatment using AA and PA peels in the subjective assessment of QOL in young adult women.
Materials and Methods
Study Design
A prospective study with follow-up analysis was conducted to compare the impact of two acid peels treatments on subjective quality of life in adult young women. The parallel trial study design consisted of two completely separate treatments: azelaic acid peel (AA sample) and pyruvic acid peel (PA sample), in a 1:1 ratio. All patients participated voluntarily in the 12-week randomized parallel study. The research was approved by the Human Research Ethics Committee of the Opole Medical School (No. BC 1/2018), according to the principles of the Declaration of Helsinki. The study was registered at http://www.isrctn.com/(No. ISRCTN79716614). Patients signed voluntary written consent before starting the study. The subjects were informed that they could withdraw from the study at any time, without giving a reason.
Participants
Participants in the study were undergraduate students of cosmetology at Opole Medical School in south Poland. Primary exclusion criteria for treatment using acid peels were; active inflammation of the skin, bacterial or viral infection, fungal relapsing skin diseases, disturbed skin integrity, any recent surgical procedures in the treatment area, active herpes, treatment with isotretinoin, reduced immunity, allergy to peeling ingredients, pregnancy, and lactation. The study was performed in cosmetology department of Opole Medical School, Opole, Poland, from January to April 2020.
Intervention
Severity of acne was measured using the Hellgren–Vincent scale (mean score = 2.58, SD = 0.50, range 2–3). The QOL measurement (the DLQI and Skindex-29) was conducted twice; at baseline (before treatment) and 12 weeks later (after six sessions of peeling treatment). Peel treatment was performed, every 2 weeks for 12 weeks (total of 6 treatments). The ARKANA COSMETICS provided the AA and PA acids for testing and research and to gain feedback on effectiveness. Use of other cosmetic treatments and new cosmetics was prohibited until the end of the study. Before applying the acid, make-up removal using micellar fluid was performed, followed by a skin de-grease using a pre-peel lotion soaked swab. A layer of petroleum jelly was applied to nasal wings and lips for protection. The eyes were protected with a cotton swab moistened with water.
In the AA group, Azelaic Peel 1 (16% AA, 10% Almond acid, and 2% salicylic acid, pH 1.0) was applied twice with a swab (1.25 mL per application). Secondly, Azelaic Peel 2 (16% AA, pH 2.5) was applied twice with a swab (1.25 mL per application). After drying, the peel forms a matte, white coat. After 6–8 hours, the patient washed off the acid at home with a cotton swab dipped in cold water. This washing was repeated three times, taking care that the peel does not get into the eyes. Finally, cream with SPF 30 was applied on the skin. Cream with SPF 30 was also recommended for everyday use.
The cleansing and degreasing procedures were the same as for azelaic acid peel. In the PA group, Pyruvic Peel (50% PA, pH 0.8) was applied with a cotton swab three times (1 mL per application) for approximately 1 minute until erythema appeared. The average application time of each layer was 2 minutes or less, dependent on skin redness. Next, neutralizer (5 mL of pH Indicators) was applied to the skin for 1 to 2 minutes and then rinsed with cold water. Finally, cream with SPF 30 was applied.
During the 12 weeks of the peel sessions, none of the patients were treated with any other dermatological agents apart from AA or PA peels. The typical reaction after application of AA and PA was redness, tingling, itching, or stinging, which persisted for up to an hour after application. Patients were asked to use a mild micellar liquid to remove make-up and to use cream with SPF 30 for everyday use throughout the 12-week trial.
Measures
Hellgren–Vincent Scale
This scale was used to estimate the number of imperfections (papules, pustules, blackheads) and assess the level of seborrhea. There are five degrees of symptom severity:
1. erythema, blackheads, 1–5 pustules or papules;
2. erythema, blackheads, 6–10 pustules or papules;
3. erythema, blackheads, 11–20 pustules or papules;
4. erythema, blackheads, 21–30 pustules or papules;
5. erythema, blackheads, over 30 pustules or papules.
Dermatology Life Quality Index (DLQI)
The DLQI measures the impact of skin problems on patients' QOL. It consists of ten items divided into six groups: symptoms and feelings, daily activities, recreation, work or school, personal relationships, and treatment.19 The questionnaire can be completed in 5 minutes, and the participants were asked to evaluate their QOL over the last seven days. Each item is scored on a 4-point Likert scale (from 0 = not at all ⁄not relevant; to 3 = very much). Aguilar-Duran et al10 suggest that higher scores correlate with higher impairment of QOL (DLQI < 10 = low; 10–20 = moderate; and > 20 = high levels of distress). The internal consistency (Cronbach’s α) of the DLQI ranged from 0.75 to 0.92 in previous studies.20 In the present study, Cronbach’s α = 0.82.
Skindex-29
The Skindex-29 contains 29 items divided into three parts: physical symptoms (7 items), emotional experiences (10 items), and psychosocial functioning (12 items).21 Responses are on a 5-point Likert scale (from 1 = never; to 5 = always), the final result is based on the average. The Skindex-29 has been shown to be a useful scoring system, with Cronbach’s α coefficient ranging from 0.87 to 0.96 in previous studies.22 In the present study, Cronbach’s α was 0.95 for the total score, and for physical symptoms, emotional experiences and psychosocial functioning were 0.80, 0.89, and 0.89, respectively.
Procedure
The sample size was determined using the Cochran formula with correction for the small sample size.18 Block randomization was used to assign participants into one of two parallel groups (AA or PA). This was achieved by sequentially numbering the participants and assigning those with odd numbers to the AA group, and those with even numbers to the PA group. Both AA and PA groups consisted of 60 women (50% of the total sample). Eligible participants were adult women aged 18–25 with mild to moderate papulopustular acne who had not undergone any dermatological treatment within the last 12 months. A single-blind method was used; both AA and PA groups of students were informed that they would receive acid peeling treatment, but they were unaware which type of acid peel (azelaic or pyruvic) was used. Care providers were aware of which treatment each participant received.
Statistical Analysis
Analysis of variance (ANOVA) was performed to examine changes following AA and PA treatment. The dependent variables were acne symptoms (assessed by the Hellgren–Vincent scale) and subjective assessment of QOL (assessed by the DLQI and Skindex-29 scales). The factor variables were acid peels (pyruvic, azelaic) and treatment time (test = at baseline, retest = after 12 weeks). The ANOVA analysis was conducted separately for each of the Hellgren–Vincent scale, DLQI, and Skindex-29 total scales, as well as for three subscales: physical symptoms, emotional experiences, and psychosocial functioning. In all of the following analyses, the effects of the treatment over time in comparable groups (AA and PA) were examined in a two-tailed test, with no direct hypotheses. Tukey’s honestly significant difference (HSD) test was conducted to find means that are significantly different from each other. Effect sizes were calculated using partial eta squared (ηp2).
Results
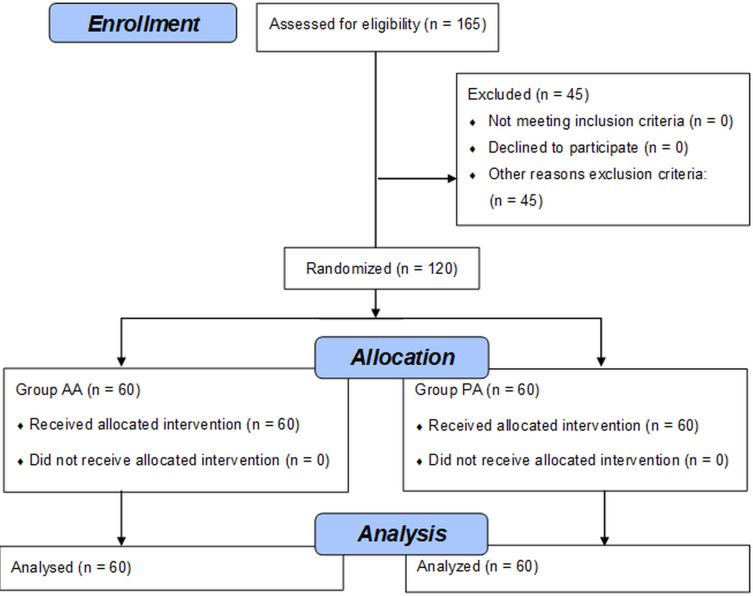
Our initial estimate of sample size included an assumption of the prevalence of acne vulgaris of around 60% of among young adult women. We estimated that a total of 165 female students would be needed to detect a difference between groups, assuming an α of 0.05 (a 95% confidence level and ±5% precision). There was a total population of 300 undergraduates studying cosmetology at the university. The study initially recruited 165 female students (Figure 1). However, 45 did not fulfill the above criteria and were excluded from further study (active inflammation of the skin: n = 15; disturbed skin integrity: n = 20; active herpes: n = 10). Therefore, the final study sample included 120 women aged between 20 and 24 years old (M = 22.2, SD = 16.1). Descriptive statistics are shown in Table 1.
Figure 1.
Flow chart of the study.
Table 1.
Descriptive Statistics for AA and PA Groups (Number [N], Percent [%], Range, Mean [M] and Standard Deviation [SD])
| Characteristics | AA Group (n = 60) | PA Group (n = 60) | ||||
|---|---|---|---|---|---|---|
| n (%) | Range | M (SD) | n (%) | Range | M (SD) | |
| Age | 20–24 | 22.23 (1.55) | 20–24 | 22.13 (1.17) | ||
| Hellgren–Vincent scale | 2–3 | 2.57 (0.50) | 2–3 | 2.60 (0.49) | ||
| 1 | 0 (0) | 0 (0) | ||||
| 2 | 26 (43) | 24 (40) | ||||
| 3 | 34 (57) | 36 (60) | ||||
| 4 | 0 (0) | 0 (0) | ||||
| DLQI | 18–30 | 22.33 (2.87) | 19–30 | 23.30 (3.02) | ||
| Mildly impaired | 0 (0) | 0–10 | 0 (0) | 0–10 | ||
| Moderately impaired | 23 (38) | 11–20 | 19 (32) | 11–20 | ||
| Severely impaired | 37 (62) | 21–30 | 41 (68) | 21–30 | ||
| Skindex-29 Total | 2.62–4.66 | 3.64 (0.41) | 3.00–4.48 | 3.87 (0.36) | ||
| Physical symptoms | 2.57–4.71 | 3.70 (0.44) | 3.00–4.57 | 3.88 (0.35) | ||
| Psychosocial functioning | 2.70–4.80 | 3.63 (0.44) | 3.00–4.50 | 3.90 (0.40) | ||
| Emotional experiences | 2.58–4.58 | 3.63 (0.42) | 3.00–4.50 | 3.85 (0.38) | ||
Abbreviations: AA, azelaic acid; PA, pyruvic acid; Hellgren–Vincent scale; DLQI, Dermatology Life Quality Index.
The number of participants who were randomly assigned and received the acid peels was 60 in both AA and PA groups. There were no losses or exclusions following randomization. The degree of acne severity symptoms, assessed using the Hellgren–Vincent scale, reduced significantly when comparing the mean scores before (MAA = 2.57, MPA = 2.60) and after acid peels treatments (MAA = 1.50, MPA = 1.47), F(1, 118) = 467.28, p < 0.000, ηp2 = 0.80. The effect size of the acid peels treatment is large and explains 80% of the variance of the Hellgren–Vincent scale. There was no statistically significant difference in the objective measurement of acne symptoms using the Hellgren–Vincent scale when comparing the two groups, F(1, 118) = 0.00, p <1.0, ηp2 = 0.00. There was no interaction effect between acid peels and treatment, F(1, 118) = 0.43, p < 0.513, ηp2 = 0.10.
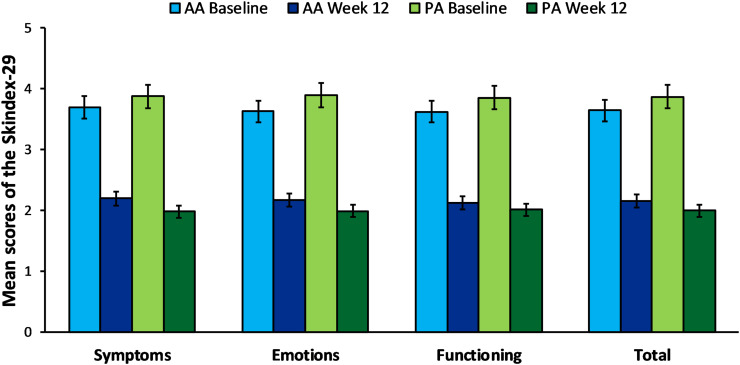
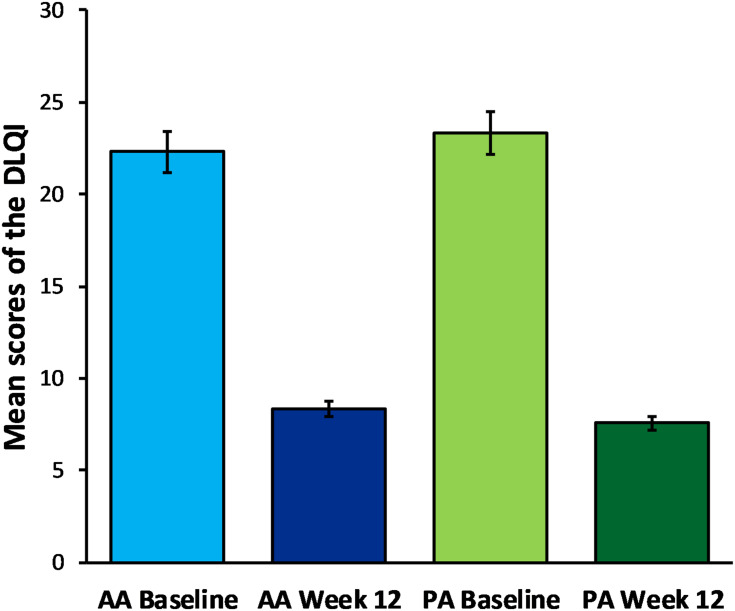
The treatment effects are shown in Figure 2A and B for the AA peeling and Figure 2C and D for the PA peeling. Mean scores and 95% confidence intervals for individual scales to assess the QOL associated with skin problems are shown in Table 2. The repeated measures AVOVA showed that the average scores of the Skindex-29 (Figure 3) and DLQI (Figure 4) both decreased after treatment using acid peels. The effect size estimated by ηp2 for the main effect of treatment (test, retest) was large and can explain from 89% to 94% of the QOL variance. These parameters indicate a great improvement in participant’s quality of life with acne following peel treatment (Table 3). Although the analysis did not show significant differences between the AA and PA groups in QOL, there was a statistically significant interaction effect between the two factors: acid (AA, PA) and treatment (test, retest) in all scales of the Skindex-29 as well as in the DLQI. However, it is important to note that the effect size of the interaction effect was very weak, and can explain between 5% and 12% of the QOL variance. Further analysis of the Tukey’s HSD post hoc test revealed that the level of physical symptoms in the Skindex-29 significantly decreased after 12 weeks of treatment by using PA peels, in comparison to AA peels (p = 0.024). There were no significant differences in psychosocial functioning (p = 0.416), emotional experiences (p = 0.086) or total symptoms (p = 0.120) in the Skindex-29, or the DLQI scores (p = 0.349), when comparing AA and PA groups.
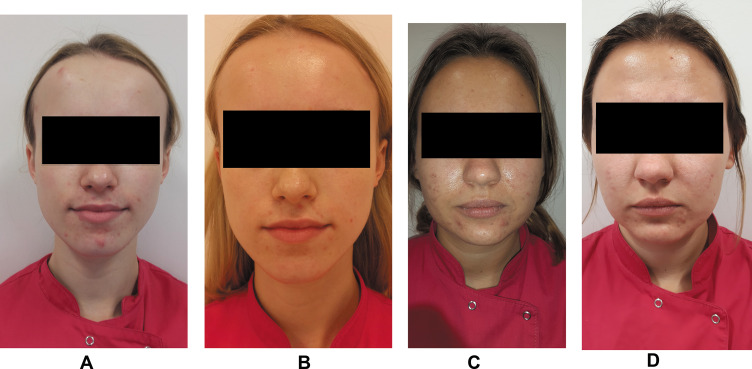
Figure 2.
Example participant: (A) AA group, before treatment; (B) AA group, after treatment (C) PA group, before treatment; (D) PA group, after treatment.
Table 2.
Descriptive Statistics (Mean [M], Standard Deviation [SD], Lower Limit [LL], Upper Limit [UL] and Confidence Interval [CI]) in DLQI and Skindex-29 Scales (Total, Physical Symptoms, Psychosocial Functioning, and Emotional Experiences) for the AA and PA Groups, at Baseline and 12 Weeks After Treatment
| Variables | AA Group (n = 60) | |||||||
|---|---|---|---|---|---|---|---|---|
| Before Treatment | After Treatment | |||||||
| M | SD | 95% CI | M | SD | 95% CI | |||
| LL | UL | LL | UL | |||||
| Skindex-29 Total | 3.64 | 0.05 | 3.54 | 3.74 | 2.16 | 0.05 | 2.06 | 2.26 |
| Physical symptoms | 3.70 | 0.05 | 3.59 | 3.80 | 2.20 | 0.05 | 2.09 | 2.30 |
| Psychosocial functioning | 3.62 | 0.05 | 3.52 | 3.73 | 2.13 | 0.05 | 2.02 | 2.24 |
| Emotional experiences | 3.63 | 0.05 | 3.52 | 3.74 | 2.17 | 0.05 | 2.07 | 2.28 |
| DLQI | 22.33 | 0.38 | 21.58 | 23.09 | 8.33 | 0.44 | 7.45 | 9.21 |
| Variables | PA Group (n = 60) | |||||||
| Before Treatment | After Treatment | |||||||
| M | SD | 95% CI | M | SD | 95% CI | |||
| LL | UL | LL | UL | |||||
| Skindex-29 Total | 3.87 | 0.05 | 3.77 | 3.97 | 2.00 | 0.05 | 1.90 | 2.10 |
| Physical symptoms | 3.88 | 0.05 | 3.77 | 3.98 | 1.99 | 0.05 | 1.88 | 2.09 |
| Psychosocial functioning | 3.85 | 0.05 | 3.75 | 3.95 | 2.02 | 0.05 | 1.91 | 2.12 |
| Emotional experiences | 3.90 | 0.05 | 3.79 | 4.00 | 1.99 | 0.05 | 1.89 | 2.10 |
| DLQI | 23.30 | 0.38 | 22.55 | 24.05 | 7.58 | 0.44 | 6.70 | 8.46 |
Abbreviations: AA, azelaic acid; PA, pyruvic acid; DLQI, Dermatology Life Quality Index.
Figure 3.
Comparison of the mean score in the Skindex-29 scales (physical symptoms, emotional experiences, psychosocial functioning and total result) for AA and PA groups, at baseline and 12 weeks after peeling treatment. Error bars represent 95% confidence intervals (CI).
Figure 4.
Comparison of the mean scores in the DLQI for the AA and PA groups, at baseline and 12 weeks after peelings treatment. Error bars represent 95% confidence intervals (CI).
Table 3.
Results of ANOVA with Repeated Measurement for Quality of Life
| Variables | df | F | p | ηp2 |
|---|---|---|---|---|
| Skindex-29 total (A x T) | 1, 118 | 13.54 | 0.000 | 0.10 |
| Acid (Pyruvic x Azelaic) | 1 | 0.50 | 0.482 | 0.00 |
| Treatment (Test x Retest) | 118 | 1006.75 | 0.000 | 0.90 |
| Symptoms (A x T) | 1, 118 | 12.99 | 0.000 | 0.10 |
| Acid (Pyruvic x Azelaic) | 1 | 0.08 | 0.777 | 0.00 |
| Treatment (Test x Retest) | 118 | 979.49 | 0.000 | 0.89 |
| Emotions (A x T) | 1, 118 | 16.43 | 0.000 | 0.12 |
| Acid (Pyruvic x Azelaic) | 1 | 0.68 | 0.411 | 0.01 |
| Treatment (Test x Retest) | 118 | 943.56 | 0.000 | 0.89 |
| Functioning (A x T) | 1, 118 | 9.73 | 0.002 | 0.08 |
| Acid (Pyruvic x Azelaic) | 1 | 1.21 | 0.273 | 0.01 |
| Treatment (Test x Retest) | 118 | 916.21 | 0.000 | 0.89 |
| DLQI (A x T) | 1, 118 | 6.44 | 0.012 | 0.05 |
| Acid (Pyruvic x Azelaic) | 1 | 0.05 | 0.821 | 0.00 |
| Treatment (Test x Retest) | 118 | 1929.48 | 0.000 | 0.94 |
Abbreviations: A, acid; T, treatment; DLQI, Dermatology Life Quality Index.
Discussion
Acne vulgaris patients often experience reduced quality of life. Therefore, dermatology-specific health-related QOL assessment tools such as DLQI and Skindex-29 are useful to aid in the evaluation of treatment response in people suffering from acne.5,8,23,24 In this study, DLQI and Skindex-29 were used to evaluate changes in QOL following 12 weeks of treatment with either AA or PA peels. The results indicate that acne severity and negative QOL symptoms significantly decreased in both groups. However, the PA peels produced a statistically significant greater improvement in the subjective evaluation of physical symptoms using the Skindex-29 scale, when compared with the AA peels. It is important to note that the effect size was small (ηp2 = 0.10); thus, this result should be interpreted with caution. Wambier25 suggests that PA may penetrate the skin faster and more deeply than other medium peels. Therefore, the visible effects of a PA peel may be more profound in a subjective sense compared with AA peeling. However, choice of peeling agent should be based on the patient’s preferences as well as skin type, acne activity, type of acne scars and whether the patient is pregnant or lactating.4
Tan et al4 recommend AA treatment for adult women, because of its comedolytic, antimicrobial, and anti-inflammatory effects and also because of the safety of use during pregnancy and lactation. However, AA should be used with caution in patients with sensitive skin, as it can cause side effects such as redness, burning, and irritation. On the other hand, the side effects of PA peels may appear on the skin with a disrupted barrier, such as with ongoing dermatitis, including retinoid irritation, seborrheic atopic dermatitis, and perioral dermatitis.25 In general, the selection between AA or PA peeling treatment of acne in adult women should be based on patients’ preferences, and whether they are pregnant or lactating.4
It is important to observe that the mean level of negative psychological symptoms assessed by the DLQI decreased following therapy from high levels of distress (DLQI > 20) to low levels (DLQI < 10) in both the AA and PA groups. A recent study found that DLQI is useful to detect a change in QOL in adult female acne patients treated with AA 15% gel twice daily over 24 weeks.26 The results of previous work and the present study are consistent with Fabbrocini et al8 which showed topical treatment is effective for people suffering from acne vulgaris. However, the appropriate peeling should be chosen based on the patient’s preferences as well as on the skin type, acne activity, and type of acne scars. It is also important to note, that positive results and reduction in symptoms of chronic acne in adult females may only be maintained by the ongoing use of peeling treatments.
It is crucial to evaluate QOL in acne patients during dermatological therapy. Recent research evaluated the effect on the acne-specific quality of life in adult women treated for six months with topical 15% azelaic acid (AA) gel versus combined oral contraceptive therapy.27 The research found that both therapies had a significant impact on the acne-related quality of life. Although women treated with an oral contraceptive showed greater improvement in self-perception and acne symptoms, in comparison to those treated with azelaic acid, quality of life scores were higher in both samples. This result is consistent with the present study and indicates that azelaic acid is effective in acne therapy.
Anxiety in patients can cause emotional effects that are not always visible from the beginning.5 Acne treatment should include counseling, informative support, and participation in support groups. Effective treatments for acne can require months to work; therefore, health-care providers should assess psychological symptoms such as lack of confidence, distress level, decreased self-esteem, depression and suicidal thoughts.5,28 According to Aguilar-Duran et al10 patients with mental health problems require a structured plan of psychotherapy, such as schema-focused therapy, relationship counseling, or cognitive behavioral therapy (CBT). This model shows that management of many dermatological diseases depends on the complex interaction of social, biological, and psychological factors. This multi-factor interaction can have a predisposing role in the development of some dermatological disorders as well.9
Study Limitations
There is little scientific research regarding the clinical value of cosmetic acids in improving the quality of life in people with acne. Our research results are promising; however, it would be useful to verify the effectiveness of acid peels in a larger sample. All of the participants were students in the cosmetology department at one university; therefore, attitude towards acne peels could be a source of potential bias. Thus, this study’s relevance may be limited to young adult women (aged between 20 and 24 years old) who are undergraduate students of cosmetology. Further studies should include undergraduates of both sexes, studying at various faculties, people of the same age who do not study, teenagers and older adults. We have compared two acid peels in women with acne but without additional control samples. Further research could consider the comparison of people with acne and those without any skin problems, and also evaluate other clinical treatments, besides acid peels. The placebo effect may also be considered in the future.
Conclusion
Both PA and AA have a positive impact on decreasing acne severity and improving QOL in young women undergoing treatment. PA should be considered, however, as more effective than AA, with regard to the subjective assessment of physical symptoms affecting QOL. The choice of peeling agent should be based on the patient’s preferences as well as on the skin type, acne activity, and type of acne scars. Many dermatological disorders are the result of a complex and sometimes reciprocal interaction between biological, psychiatric/psychological, and social factors that can have a predisposing, precipitating, and/or perpetuating role for dermatological disorders.9 Treatment of acne, therefore, should involve multiple disciplines including psychiatry and neuroscience, endocrinology, and immunology.27,28
Acknowledgments
This work was the part of the research grant (no. ZB3/FI/2020) funded by Ministry of Science in Poland. We thank the ARKANA COSMETICS for support for providing us with the AA and PA peels used in this research.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available in the Mendeley Data http://dx.doi.org/10.17632/x79j759dkc.3.
Disclosure
The authors report no conflicts of interest in this work. We also declare no conflicts of interests with the cosmetics manufacturer.
References
- 1.Fabbrocini G, De Padova MP, Cacciapuoti S, et al. In Tosti A, Grimes PE, De Padova MP, editors. In: Color Atlas of Chemical Peels. Berlin-Heidelberg: Springer-Verlag; 2011:95–105. [Google Scholar]
- 2.Katsambas A, Dessinioti C. New and emerging treatments in dermatology. Acne Dermatol Ther. 2008;21(2):86–95. doi: 10.1111/j.1529-8019.2008.00175.x [DOI] [PubMed] [Google Scholar]
- 3.Rivera R, Guerra A. Management of acne in women over 25 years of age. Actas Dermosifiliogr. 2009;100:33–37. doi: 10.1016/S0001-7310(09)70054-7 [DOI] [PubMed] [Google Scholar]
- 4.Tan AU, Schlosser BJ, Paller AS. A review of diagnosis and treatment of acne in adult female patients. Int J Women's Dermatol. 2018;4:56–71. doi: 10.1016/j.ijwd.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet. 2012;379(9813):361–372. doi: 10.1016/S0140-6736(11)60321-8 [DOI] [PubMed] [Google Scholar]
- 6.Mahto A. Acne vulgaris. Med. 2017;45(6):386–389. doi: 10.1016/j.mpmed.2017.03.003 [DOI] [Google Scholar]
- 7.Nguyen R, Su J. Treatment of acne vulgaris. Paediatr Child Health. 2010;21(3):119–123. doi: 10.1016/j.paed.2010.09.012 [DOI] [Google Scholar]
- 8.Fabbrocini G, Cacciapuoti S, Monfrecola G. A qualitative investigation of the impact of acne on Health-Related Quality of Life (HRQL): development of a conceptual model. Dermatol Ther. 2018;8:85–99. doi: 10.1007/s13555-018-0224-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta MA, Gupta AK. A practical approach to the assessment of psychosocial and psychiatric comorbidity in the dermatology patient. Clin Dermatol. 2013;31:57–61. doi: 10.1016/j.clindermatol.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 10.Aguilar-Duran S, Ahmed A, Taylor R, et al. How to set up a psychodermatology clinic. Clin Exp Dermatol. 2014;39:577–582. doi: 10.1111/ced.12360 [DOI] [PubMed] [Google Scholar]
- 11.Harth W. Psychosomatic dermatology (psychodermatology). JDDG. 2008;1. doi: 10.1111/j.1610-0387.2007.06313.x. [DOI] [PubMed] [Google Scholar]
- 12.Jafferany M, Franca K. Psychodermatology: basics concepts. Acta Derm-Venereol. 2016;217:S35–7. [DOI] [PubMed] [Google Scholar]
- 13.Khunger N. Facial peels In: Prendergast PM, Shiffman MA, editors. Aesthetic Medicine. Berlin-Heidelberg: Springer-Verlag; 2011:157–177. [Google Scholar]
- 14.Kontochristopoulos G, Platsidaki E. Chemical peels in active acne and acne scars. Clin Dermatol. 2017;35:179–182. doi: 10.1016/j.clindermatol.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 15.Hofmeister H. Innovations in superficial chemical peels In: Issa MCA, Tamura B, editors. Chemical and Physical Procedures. Clinical Approaches and Procedures in Cosmetic Dermatology. Vol. 2 Cham: Springer; 2018:141–151. [Google Scholar]
- 16.Kosmadaki M, Katsambas A. Topical treatments for acne. Clin Dermatol. 2017;35:173–178. doi: 10.1016/j.clindermatol.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 17.Leccia MT, Auffret N, Poli F, et al. Topical acne treatments in Europe and the issue of antimicrobial resistance. JEADV. 2015;29:1485–1492. doi: 10.1111/jdv.12989 [DOI] [PubMed] [Google Scholar]
- 18.Cochran WG. Sampling Techniques. New York: John Wiley and Sons; 1963. [Google Scholar]
- 19.Finlay AY, Khan GK. Dermatology life quality index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x [DOI] [PubMed] [Google Scholar]
- 20.Basra MKA, Fenech R, Gatt RM, et al. The dermatology life quality index 1994–2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159:997–1035. doi: 10.1111/j.1365-2133.2008.08832.x [DOI] [PubMed] [Google Scholar]
- 21.Chren MM, Lasek RJ, Flocke SA, et al. Improved discriminative and evaluative capability of a refined version of Skindex, a quality-of-life instrument for patients with skin diseases. Arch Dermatol. 1997;133(11):1433–1440. doi: 10.1001/archderm.1997.03890470111018 [DOI] [PubMed] [Google Scholar]
- 22.Chren MM. The Skindex instruments to measure the effects of skin disease on quality of life. Dermatol Clin. 2012;30(2):231–236. doi: 10.1016/j.det.2011.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Both H, Essink-Bot M-L, Busschbach J, et al. Critical review of generic and dermatology-specific health-related quality of life instruments. J Invest Dermatol. 2007;127:2726–2739. doi: 10.1038/sj.jid.5701142 [DOI] [PubMed] [Google Scholar]
- 24.Marron SE, Chernyshov PV, Tomas-Aragones L. Quality-of-life research in acne vulgaris: current status and future directions. Am J Clin Dermatol. 2019;20(4):527–538. doi: 10.1007/s40257-019-00438-6 [DOI] [PubMed] [Google Scholar]
- 25.Wambier CG. Pyruvic acid peel In: Issa MCA, Tamura B, editors. Chemical and Physical Procedures. Clinical Approaches and Procedures in Cosmetic Dermatology. Vol. 2 Cham: Springer International Publishing AG; 2018:25–34. [Google Scholar]
- 26.Richter C, Trojahn C, Hillmann K, et al. Sensitivity to change of the dermatology life quality index in adult females with facial acne vulgaris: a validation study. JEADV. 2017;31:169–174. doi: 10.1111/jdv.13757 [DOI] [PubMed] [Google Scholar]
- 27.Rocha M, Sanudo A, Bagatin E. The effect on acne quality of life of topical azelaic acid 15% gel versus a combined oral contraceptive in adult female acne: a randomized trial. Dermato-Endocrinology. 2017;9(1):e1361572. doi: 10.1080/19381980.2017.1361572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta MA. Psychiatric dermatology: management. Clin Dermatol. 2018;36:687–690. doi: 10.1016/j.clindermatol.2018.09.013 [DOI] [PubMed] [Google Scholar]