Abstract
Background
Emerging research indicates that CXXC finger protein 5 (CXXC5) is involved in the development of various cancers. Besides, KN motif and ankyrin repeat domains 1 (KANK1) was proved as a tumor suppressor in multiple cancers. Our study aimed to illustrate the functional role and mechanism of CXXC5 and KANK1 in gastric cancer (GC) pathogenesis.
Methods
The tissues of 55 GC patients and six GC cell lines were used to investigate CXXC5 and KANK1 expression using RT-qPCR. Western blot assay was conducted to measure the protein levels of CXXC5, KANK1, epithelial-mesenchymal transformation (EMT) proteins (Vimentin, E-cadherin) and Wnt signaling proteins (β-catenin, Axin2). The correlation between KANK1 and CXXC5 was estimated by Pearson’s correlation analysis. The results of Transwell assays showed the migration and invasion abilities of GC cells, while the apoptosis rate was detected by flow cytometry.
Results
The expressions of CXXC5 and KANK1 were both decreased in GC tissues and cells, compared with the normal ones (P < 0.01). Overexpressing CXXC5 significantly induced apoptosis (P < 0.05) and inhibited EMT, migration (P < 0.05) and invasion (P < 0.01) in GC cells. Wnt/β-catenin/Axin2 signaling was suppressed by CXXC5 overexpression, and activating Wnt/β-catenin/Axin2 signaling reversed the effects of CXXC5. The expression of KANK1 was found to be positively correlated with CXXC5 (r2 = 0.4024). KANK1 presented similar effects with CXXC5 on GC cells; however, silencing CXXC5 or activating Wnt/β-catenin/Axin2 signaling antagonized the effects of KANK1 overexpression on EMT and apoptosis in GC (P < 0.05).
Conclusion
Our study suggested that CXXC5 was downregulated in GC and participated in EMT and apoptosis regulations via the Wnt/β-catenin/Axin2 pathway. Besides, the decreased expression of CXXC5 in GC was caused by KANK1 dysregulation.
Keywords: CXXC5, KANK1, EMT, apoptosis, gastric cancer, Wnt/β-catenin/Axin2 signaling
Introduction
Gastric cancer (GC), one of the most common malignant tumors, is globally considered as a highly lethal digestive tract disease.1 GC is prevalent in Asian countries and its incidence increases year by year.2 Although researchers have succeeded at finding improved strategies for the treatment at an early stage, GC is still imperceptible until an advanced stage because of its inconspicuous clinical features and high rates of metastasis, causing intractable difficulty for the diagnosis and therapy of GC.3,4 There remain many limitations on modulating the pathogenesis of GC.5 Therefore, to understand the potential mechanism and identify biomarkers of GC is of great importance.
Metastasis is the leading cause of GC recurrence and a great hindrance to the treatment of GC.5 In recent decades, emerging studies suggested that dysregulated epithelial-mesenchymal-transition (EMT), an important process involved in wound healing and embryonic development, had critical relevance to cell invasion and metastasis in cancer, GC included.6,7 It is widely acknowledged that EMT enhances the motility of cancer cells.8,9 Researchers further demonstrated that stimulating the EMT process facilitated invasion and metastasis in GC.10,11 Vimentin and E-cadherin are hallmark proteins of EMT. Investigations proposed that elevated expressions of Vimentin and E-cadherin suppression are underlying mechanisms of EMT in tumor metastasis.12,13
As an effective mechanism of programmed cell death, apoptosis is crucial for homeostasis modulation, by eliminating unfavorable cells.14 Dysregulation of apoptosis could result in numerous diseases, cancer included.15 It is widely known that apoptosis evasion is a hallmark of tumor disease, along with uncontrolled cell growth and angiogenesis.16 Increasing evidence indicates that promoting apoptosis of tumor cells inhibits cancer development. Weng et al demonstrated that inducing apoptosis depressed EMT metastasis and development of GC.17 Evidence also indicated that GC pathogenesis could be mediated by regulating apoptosis.18
The family of CXXC-type zinc-finger proteins was delineated to contain a specialized CXXC domain. As a member of this family, CXXC finger protein 5 (CXXC5) was also identified as a retinoid-inducible nuclear factor.19,20 Located in the chromosomal region of 5q31.3, the human CXXC5 gene is mainly expressed in the nucleus of various tissues.21 Studies showed that CXXC5 could directly bind to and regulate the transcription of target DNA.22 Furthermore, CXXC5 was proved to regulate gene expression epigenetically by binding to the CpG-rich sequence of the promoter region.23 Although little is known about the mechanism, the pivotal role of CXXC5 in cell migration and differentiation, tissue homeostasis, embryonic development and pathological changes had been demonstrated.19,24 Initially, investigations confirmed that the expression of CXXC5 was usually absent in patients with hematological diseases, such as myeloid leukemia.21 Recent studies confirmed the controversial functions of CXXC5 in multiple cancers. Fedorko et al suggested CXXC5 as a tumor promoter in endometrial cancer.25 Also, there was evidence presented that CXXC5 could act as a suppressor of hepatocellular carcinoma.26 A ChIP microarray assay discovered abnormal expression of CXXC5 in GC.4 However, we still knew little about the role of CXXC5 in GC pathogenesis.
Recently, KN motif and ankyrin repeat domains 1 (KANK1) was verified as a suppressor in a variety of tumors. Yousif and colleagues indicated that high expression of KANK1 was associated with an improved outcome of breast cancer survival.27 Research workers also found that KANK1 had a poor expression in oral squamous cell carcinoma, and overexpression of KANK1 inhibited the proliferation and elevated the apoptosis rate of tumor cells.28 Besides, the Wnt/β-catenin signaling was known as an important pathway which regulated many cellular progresses in human body, and the aberrant modulation of Wnt/β-catenin pathway had been validated in GC.29 Xu et al proposed that blocking the Wnt/β-catenin signaling could effectively inhibit the proliferation, EMT and chemo-resistance of GC cells.30
In this study, we explored the expressions of CXXC5 and KANK1 in GC and validated their functions on the EMT and apoptosis of GC cells. In addition, the regulatory mechanisms of CXXC5 on GC metastasis and apoptosis were verified in vitro based on Wnt/β-catenin/Axin2 signaling and KANK1.
Materials and Methods
GC Samples and Ethics Approval
In our study, the tumor and adjacent tissues were obtained from GC patients who underwent surgery at the hospital from 2015 to 2018. The exclusion and inclusion criteria were based on the clinical pathology features and classification standard delivered by WHO, and patients who suffered from other primary cancers and received radiation or chemotherapy were excluded.31,32 Finally, 55 cases of GC patients were enrolled in our study. After being excised from the patient’s stomach, tissue specimens were immediately cryopreserved at −80°C. Additionally, our study obtained approval from the clinical research ethics committee the Second Hospital of Jilin University (No. SY201906011). Written informed consents were provided by the participants and their families were also informed. Investigations involving humans were performed in accordance with the principles of Declaration of Helsinki.
Cell Culture and Treatments
We selected several commonly used GC cell lines, SNU-1, HGC-27, MKN-7, MKN-45, MKN-74 and AGS, in recent years with different differentiations, and they were supplied by BeNa Culture Collection (Beijing, China), also was the normal human gastric mucosal epithelial cells (GES-1). We supplemented the RPMI-1640 medium (Sigma-Aldrich, Darmstadt, Germany) with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, Waltham, USA) and 1% penicillin/streptomycin (Gibco, Carlsbad, USA) for cell culture. The incubation environment was 5% CO2 and 37°C with saturated humidity.
Sequence design and synthesis services were provided by GeneChem (Shanghai, China). The pcDNA3.1 plasmid (YouBio, Changsha, China) was used to construct CXXC5 and KANK1 overexpression vectors. Based on Lipofectamine 3000 (Invitrogen, Waltham, USA), the transfection assay was performed with a concentration of 50 nM for small interfering RNA (siRNA) and 0.5 μg for overexpression plasmids. SKL2001 was purchased from MedChemExpress (New Jersey, USA) and was used to treat cells after transfection at a concentration of 40 μM.
RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
The extraction and RT-qPCR assays were conducted according to a previous study.33 TRIzol® reagent (CW Biotech, Beijing, China) was used for total RNA extraction, which should be performed on ice. The quality and quantity of the extracted RNA were determined with NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, Waltham, USA). In line with the protocol of EcoDry™ Premix (Oligo dT) kit (Takara, Dalian, China), 1 μg of RNA sample was reverse transcribed to cDNA. Then, the synthesized cDNA was utilized for qPCR. According to the manufacturers’ instruction, the SYBR Premix Ex Taq Kit (Takara, Dalian, China) was used to quantify the expression levels of mRNAs. The amplification procedure of qPCR was conducted on a StepOnePlus™ System (Applied Biosystems, Waltham, USA) and using the following cycling parameters: 30 s at 95°C for 1 cycle, 15 s at 95°C and 60 s at 60°C for 40 cycles, and a melting curve was generated. GAPDH was the housekeeping gene used for normalization in this study, and the sequences of primers used were as follows: CXXC5 (forward primer: 5`-CGGTGGACAAAAGCAACCCTAC-3`; reverse primer: 5`-CGCTTCAGCATCTCTGTGGACT-3`), KANK1 (forward primer: 5`-CTTGACACAGTATTTTCACGCTTTTG-3`; reverse primer: 5`-AAGTAAATGTGACACGGTAAAAAGG-3`), GAPDH (forward primer: 5`-‑TGAACGGGAAGCTCACTGG-3`; reverse primer: 5`-‑TCCACCACCCTGTTGCTGTA-3`). Relative levels of mRNA expression were quantified by using 2−ΔΔCt method.
Western Blotting
To determine the protein levels of CXXC5, KANK1, EMT proteins (Vimentin and E-cadherin) and signaling proteins (β-catenin and Axin2), Western blotting was conducted followed the description of a previous study.34 It is worth mentioning that the compositions of cell lysis buffer we used in the present study were radioimmunoprecipitation assay buffer (Thermo Fisher Scientific, Waltham, USA) and 1% phenylmethanesulfonyl fluoride (Seebio, Shanghai, China). Besides, the primary antibodies included anti-GAPDH (normalization protein), anti-CXXC5, anti-KANK1, anti-Vimentin, anti-E-cadherin, anti-β-catenin and anti-Axin2 (Cell Signaling Technology, Danvers, USA) with a dilution of 1:500. Horseradish peroxidase-secondary antibody (Cell Signaling Technology, Danvers, USA) was diluted at 1:1000. The expression bands of target proteins were visualized using chemiluminescence detection. ImageJ software version 1.6 (Bethesda, MD, USA) was the platform used to quantify the final data.
Transwell Assay
Transwell chambers with 8 μm pores (Corning Life Sciences, New York, USA) were pre-coated using Matrigel (Corning, NY, USA) in the upper chamber to estimate cell invasion ability. For migration analysis, the upper chambers did not receive any pre-treatment. Cultured cells were seeded in a 24-well plate and allowed to grow to 100% confluency, a concentration of about 2 x 105 cells/well. Then, we starved the cells in serum-free medium in the upper chamber, while the lower one was supplemented with medium containing 10% FBS. Migration and invasion assays were performed after 16 h and 24 h incubation, respectively. Finally, methanol and crystal violet were used to fix and stain the cells in the lower chamber. The numbers of cells in five random areas were counted under an inverted microscope (Optical Instrument Factory, Shanghai, China).
Flow Cytometry
Flow cytometry was conducted to measure the apoptosis rate of cells, according to a previous description.26 We washed 2 x 105 cells with pre-cold phosphate buffer saline buffer after transfection or treatment. Then, Annexin V binding buffer was supplemented into the cells, followed by staining with 5 μL Annexin V/FITC and 5 μL propidium iodide solutions. The incubation lasted for 15 min. After that, a flow cytometer and Cell Quest software were used to analyze the results. All the reagents and equipment were purchased from BD Biosciences (CA, US).
Statistical Analysis
GraphPad Prism package 5 was the statistical analysis software used in this study. To present the final results, data was calculated as mean ± standard error of mean. All the experiments were repeated for at least three times, independently. Pearson’s correlation analysis was conducted to evaluate the relevance between KANK1 and CXXC5. For the assessment of the differences between groups, one‑way analysis of variance was performed. Student’s t-test was utilized for the detection of the statistical significance between two groups. Moreover, paired t-test was conducted for the difference comparison of gene expression in normal and GC tissues. Differences were considered as significant at the P value less than 0.05.
Results
CXXC5 Was Downregulated in GC Tissues and Cells
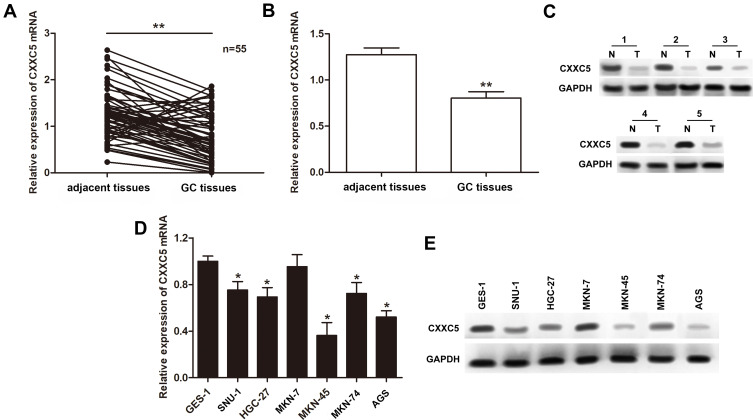
To investigate the dysregulation of CXXC5 in GC, we evaluated CXXC5 expression in 55 paired GC and adjacent normal tissues, as well as six GC cell lines. As shown in Figure 1A and B, the expression of CXXC5 mRNA was significantly downregulated in GC tissues (P < 0.01). We also found that the expression of CXXC5 protein in GC tissues of five randomly selected specimens was obviously decreased compared with normal tissues (Figure 1C). Moreover, the expression levels of CXXC5 mRNA and protein in most GC cell lines were prominently lower than those in normal gastric cell lines (P < 0.05), except MKN-7 (Figure 1D and E).
Figure 1.
Expressions of CXXC5 in GC tissues and cells.
Notes: (A and B) The relative expressions of CXXC5 in GC tissue samples were measured by RT-qPCR; **P < 0.01 versus adjacent tissues. (C) Western blotting assay presented the visualized protein expression of CXXC5. (D and E) The results of RT-qPCR and Western blotting showed the expression levels of CXXC5 mRNA and protein; *P < 0.05 versus GES-1.
Overexpressing CXXC5 Attenuated EMT and Promoted Apoptosis of GC Cells
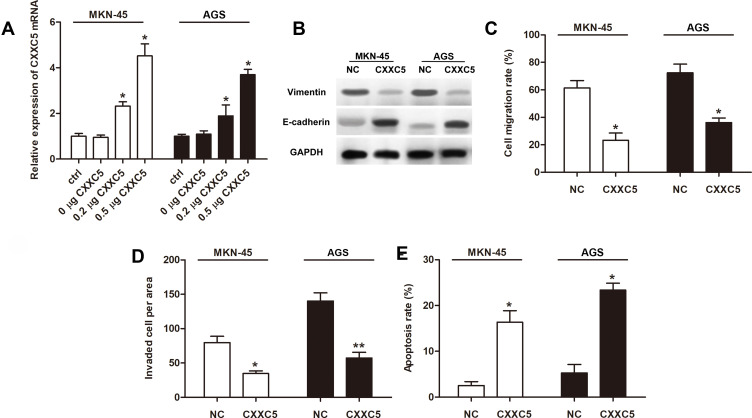
Overexpression experiments were conducted to assess the function of CXXC5 in GC development. Firstly, CXXC5 overexpression significantly increased the mRNA level of CXXC5 in a dose-dependent manner (P < 0.05) (Figure 2A). The expression of Vimentin was inhibited by CXXC5 while E-cadherin expression was promoted, which meant that overexpressed CXXC5 hindered the EMT of GC cells (Figure 2B). In addition, the migration rate and number of invasive GC cells were significantly decreased (P < 0.05), but the apoptosis of cell was promoted (P < 0.05) when cells were transfected with pcDNA-CXXC5 (Figure 2C–E). These results suggested that CXXC5 overexpression attenuated EMT and promoted apoptosis during GC development.
Figure 2.
Overexpressed CXXC5 attenuated EMT and apoptosis in GC.
Notes: MKN-45 and AGS were transfected using pcDNA-CXXC5 (CXXC5) before measurements. (A) RT-qPCR presented the expression of CXXC5 mRNA; *P < 0.05 versus ctrl. (B) The protein levels of vimentin and E-cadherin were detected by Western blotting. (C and D) Transwell assay was performed for the migration and invasion of GC cells; *P < 0.05 and **P < 0.01 versus NC. (E) The apoptosis rate of cell was analyzed by Flow cytometry; *P < 0.05 versus NC.
CXXC5 Regulated EMT and Apoptosis of GC Cells via Wnt/β-Catenin/Axin2 Signaling
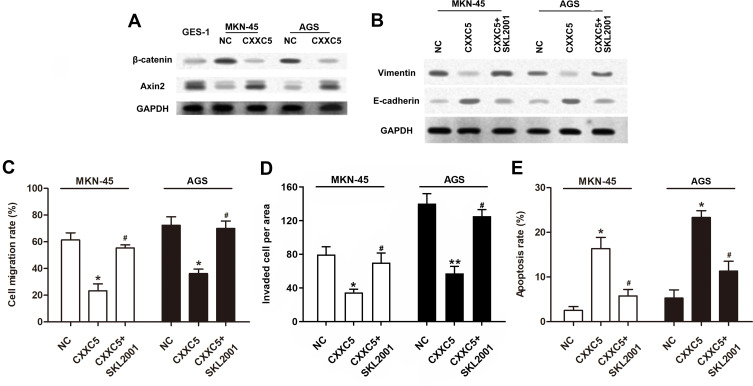
Emerging evidence indicates that Wnt/β-catenin/Axin2 signaling is usually activated in cancer tissues and involved in regulating cancer development.35,36 To further illustrate the mechanism of CXXC5 in GC pathogenesis regulation, Western blotting assay was performed to detect the activation of Wnt/β-catenin/Axin2 signaling. As presented in Figure 3A, Wnt/β-catenin/Axin2 signaling was obviously activated in MNK-45 and AGS cells compared with normal gastric cell line; besides, the expression of β-catenin was prominently suppressed and Axin2 was upregulated by CXXC5 overexpression in GC cells; in other words, CXXC5 participated in regulating the activation of Wnt/β-catenin/Axin2 pathway in GC. Moreover, treatment with SKL2001, an agonist of Wnt/β-catenin/Axin2 signaling, reversed the inhibition of CXXC5 exerted on EMT in GC (Figure 3B). We also found that activating Wnt/β-catenin/Axin2 neutralized the decreased migration and invasion rates and the increased apoptosis rate of GC cells induced by CXXC5 overexpression (P < 0.05) (Figure 3C–E).
Figure 3.
CXXC5 inhibited the activation of Wnt/β-catenin/Axin2 signaling in GC.
Notes: Cells were transfected by 0.5 μg pcDNA-CXXC5 with or without SKL2001 treatment before detections. (A and B) Using Western blotting method, the activation of Wnt/β-catenin/Axin2 pathway and expressions of EMT proteins were measured. (C and D) The migration and invasion of MKN-45 and AGS were detected by Transwell analysis. (E) The results of Flow cytometry presented apoptosis rate. *P < 0.05 and **P < 0.01 versus NC; #P < 0.05 versus CXXC5 group.
KANK1 Positively Mediated CXXC5 Expression in GC
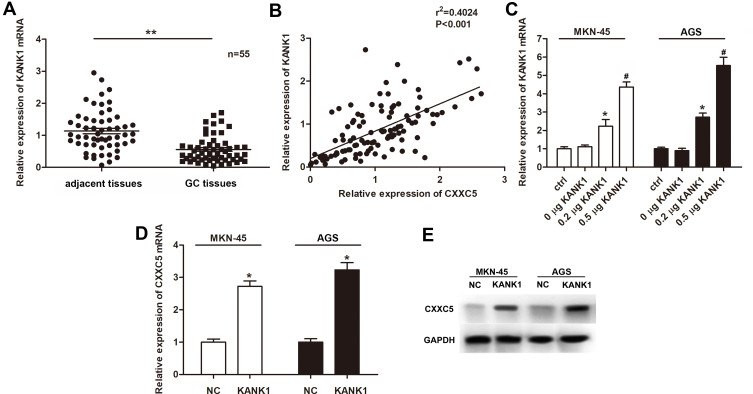
First, we measured the expression of KANK1 in GC tissues and cells. Our findings revealed that KANK1 was a significantly downregulated factor in GC (P < 0.01) (Figure 4A). Furthermore, we discovered the positive correlation between KANK1 and CXXC5 expressions (r2 = 0.4024, P < 0.001) (Figure 4B). In MKN-45 and AGS cells, the transfection of KANK1 overexpression vector dramatically increased the expression of KANK1 mRNA (P < 0.05) (Figure 4C), and the mRNA and protein levels of CXXC5 were both elevated (P < 0.05) (Figure 4D and E). Taken together, the present results indicate that KANK1 positively modulates CXXC5 expression.
Figure 4.
KANK1 was downregulated in GC and positively related to CXXC5.
Notes: (A) RT-qPCR showed the expression profile of KANK1 mRNA in GC tissues; **P < 0.01 versus adjacent tissues. (B) The correlation between KANK1 and CXXC5 expressions was estimated by Pearson’s correlation assay. (C) Cells were transfected with KANK1 overexpression plasmids at different concentrations; then, the expressions of KANK1 mRNA were measured using RT-qPCR; *P < 0.05 versus ctrl; #P < 0.05 versus 0.2 μg KANK1 group. (D and E) Relative expressions of CXXC5 mRNA and protein in KANK1-overexpressed cells were detected by RT-qPCR and Western blotting; *P < 0.05 versus NC.
KANK1 Modulated EMT and Apoptosis in GC by Targeting CXXC5
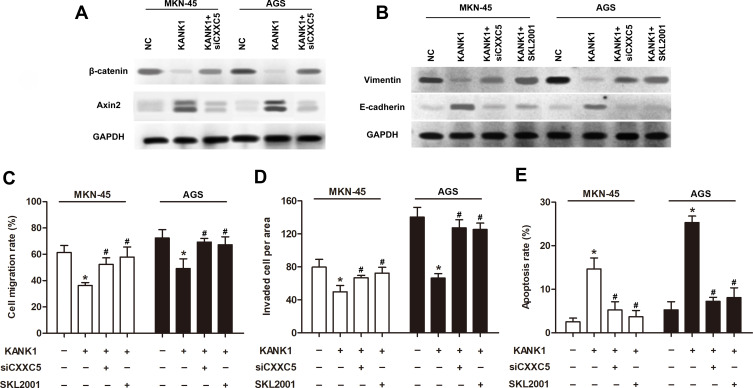
Sequentially, we discovered that overexpression of KANK1 significantly inhibited the expression of β-catenin while elevating Axin2 expression; however, knockdown of CXXC5 antagonized the effect of KANK1 overexpression (Figure 5A). The suppression and facilitation on Vimentin and E-cadherin expressions, respectively, induced by KANK1 overexpression were both reversed by transfection with siCXXC5 and treatment with SKL2001 (Figure 5B). Although overexpressing KANK1 repressed the migration and invasion abilities of GC cells, inhibiting CXXC5 or activating the Wnt/β-catenin/Axin2 pathway could also counteract the effects of KANK1 overexpression (P < 0.05) (Figure 5C and D). Similarly, siCXXC5 and SKL2001 had repressive effects on KANK1-overexpression-stimulated apoptosis in MKN-45 and AGS cells (P < 0.05) (Figure 5E).
Figure 5.
KANK1 mediated the regulation of CXXC5 on EMT and apoptosis in GC.
Notes: Cells were transfected with 0.5 μg pcDNA-KANK1 and 50 nM siCXXC5, then treated by SKL2001. (A and B) Using Western blotting, the protein expressions of β-catenin, Axin2, Vimentin and E-cadherin were measured. (C and D) The migration and invasion of GC cells were detected by Transwell analysis. (E) Flow cytometry was utilized for apoptosis detection. *P < 0.05 versus NC; #P < 0.05 versus the group only treated with KANK1.
Discussion
Previously, CXXC5, a gene encoding retinoid-inducible nuclear factor, was identified to be frequently deleted in hematological diseases.21 Researchers also suggested that CXXC5 was crucial for a series of cell processes such as energy metabolism, signal transduction, apoptosis and angiogenesis.37,38 In recent years, CXXC5 was found to be abnormally expressed in various tumor diseases. Meanwhile, there were differences in CXXC5 expression between different cancers: increased CXXC5 expression was verified in prostate cancer, breast cancer and endometrial cancer,25,39,40 while the expression of CXXC5 was decreased in human malignant peripheral nerve sheath tumors.37 Sui and colleagues discovered that CXXC5 might be downregulated in GC through microarray analysis.4 Consistently, the present study documented that the expression of CXXC5 was decreased in both GC tissues and cells.
To illustrate the function of CXXC5 in GC development, we performed overexpression assays and discovered that elevating the expression of CXXC5 significantly blocked the EMT process, as well as cell migration and invasion in GC, while the apoptosis ability was accelerated. Therefrom, we confirmed the inhibitory effect of CXXC5 on GC metastasis and promotion on apoptosis. Similarly, CXXC5 was validated as a positive regulator of apoptosis and cell cycle arrest of tumor cells leading to cancer suppression in hepatocellular carcinoma.26 There was also evidence suggested that regulating CXXC5 was an effective way to stimulate apoptosis and inhibit cell growth in human malignant peripheral nerve sheath tumors.37 However, the role of CXXC5 in cancer development was controversial. Evidence indicated that the migration and invasion abilities of ovarian cancer cells were enhanced by CXXC5, while apoptosis was depressed.41 Liu et al demonstrated that silencing CXXC5 expression dramatically inhibited the migration, invasion and adhesion in esophageal squamous cell carcinoma.38 Therefore, more in-depth research should be carried out to specify the effect of CXXC5 on GC pathogenesis, and that has been included in our future study.
The Wnt/β-catenin pathway is acknowledged as a fundamental signal transduction pathway for modulating cell proliferation, survival, apoptosis and homeostasis in human tissues.42 Numerous investigations have delineated that the activation of Wnt/β-catenin signaling participates in and contributes to various processes during tumorigenesis of multiple cancers, including GC.35,36,43 According to an in vitro study, Wnt/β-catenin and its downstream target Axin2 were involved in the regulation of stemness in colorectal cancer.43 Also, c-Myb exerted its facilitated effect on invasion and metastasis of breast cancer via Wnt/β-catenin/Axin2 signaling activation.36 Yanaka et al demonstrated that activating the Wnt/β-catenin pathway by miR-544a promoted EMT in GC.35 Interestingly, recent studies discovered that Wnt signaling, as well as the TGF-β, ATM/p53 and BMPs pathways, were regulated and coordinated by CXXC5.44 However, it was still obscure how CXXC5 modulated GC pathogenesis and whether the Wnt/β-catenin/Axin2 pathway was involved in. Hence, we activated Wnt/β-catenin/Axin2 signaling in CXXC5-overexpressed GC cells and found that the suppression of EMT and metastasis was reversed. That is to say, the regulatory role of CXXC5 in GC development relies on the Wnt/β-catenin/Axin2 pathway.
According to a previous study, KANK1 was proved to be a tumor inhibitor in a variety of carcinomas.33 Fan et al validated KANK1 as a dysregulated gene in OSCC, which modulates proliferation and apoptosis through YAP.28 There was also evidence exhibited that elevating KANK1 expression suppressed lung cancer progression by regulating invasion, migration and apoptosis of tumor cells.34 Consistent with our study, Chen and colleagues documented that the expression of KANK1 was decreased and associated with the growth and apoptosis of GC tumor both in vitro and in vivo.33 Another research focusing on human malignant peripheral nerve sheath tumors suggested that KANK1 activated apoptosis and inhibited tumor cell growth via CXXC5 modulation.37 Indeed, our research validates that the abnormal expression of KANK1 gene is associated with CXXC5 dysregulation in GC. To be exact, KANK1 positively regulates CXXC5 expression and is involved in the regulation of CXXC5 in GC EMT and apoptosis.
Conclusion
The present study identified CXXC5 as a downregulated gene in GC tissues and cells and illustrated that the overexpression of CXXC5 induced cell apoptosis and hindered EMT, migration and invasion in GC. Moreover, dysregulation of KANK1 accounted for CXXC5 downregulation in GC. Our research may provide a theoretical basis for diagnosis and therapy for GC. However, this study is based on in vitro studies at the cellular level. In order to further illustrate the role of CXXC5 in the pathological development of GC, in vivo experiments and related clinical detections are important.
Funding Statement
The study was aupported by Natural Science Foundation of Jilin Province(20200201596JC).
Disclosure
The authors report no funding and no conflicts of interest for this work.
References
- 1.Yu Z-H, Wang Y-M, Jiang Y-Z, et al. NID2 can serve as a potential prognosis prediction biomarker and promotes the invasion and migration of gastric cancer. Pathol Res Pract. 2019;10(10):152553. doi: 10.1016/j.prp.2019.152553 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 3.Sitarz R, Skierucha M, Mielko J, et al. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–248. doi: 10.2147/CMAR.S149619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sui W, Shi Z, Xue W, et al. Circular RNA and gene expression profiles in gastric cancer based on microarray chip technology. Oncol Rep. 2017;37(3):1804–1814. doi: 10.3892/or.2017.5415 [DOI] [PubMed] [Google Scholar]
- 5.Hess LM, Michael D, Mytelka DS, et al. Chemotherapy treatment patterns, costs, and outcomes of patients with gastric cancer in the United States: a retrospective analysis of electronic medical record (EMR) and administrative claims data. Gastric Cancer. 2016;19(2):607–615. doi: 10.1007/s10120-015-0486-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao M, Ang L, Huang J, et al. MicroRNAs regulate the epithelial-mesenchymal transition and influence breast cancer invasion and metastasis. Tumour Biol J Int Soc Oncol Dev Biol Med. 2017;39(2):1010428317691682. doi: 10.1177/1010428317691682 [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Wu X, Xiao Y, et al. Coexpression of FOXK1 and vimentin promotes EMT, migration, and invasion in gastric cancer cells. J Mol Med. 2019;97(2):163–176. doi: 10.1007/s00109-018-1720-z [DOI] [PubMed] [Google Scholar]
- 8.Nauseef JT, Henry MD. Epithelial-to-mesenchymal transition in prostate cancer: paradigm or puzzle? Nat Rev Urol. 2011;8(8):428–439. doi: 10.1038/nrurol.2011.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong J, Wang R, Ren G, et al. HMGA2-FOXL2 axis regulates metastases and epithelial-to-mesenchymal transition of chemoresistant gastric cancer. Clin Cancer Res. 2017;23(13):3461–3473. doi: 10.1158/1078-0432.CCR-16-2180 [DOI] [PubMed] [Google Scholar]
- 10.Cao QH, Liu F, Li CZ, et al. Testes-specific protease 50 (TSP50) promotes invasion and metastasis by inducing EMT in gastric cancer. BMC Cancer. 2018;18(1):94. doi: 10.1186/s12885-018-4000-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Zhu Z, Wu H, et al. PODXL, negatively regulated by KLF4, promotes the EMT and metastasis and serves as a novel prognostic indicator of gastric cancer. Gastric Cancer. 2019;22(1):48–59. doi: 10.1007/s10120-018-0833-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu Y, Elble RC. Homeostatic signaling by cell–cell junctions and its dysregulation during cancer progression. J Clin Med. 2016;5(2):26. doi: 10.3390/jcm5020026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaquero J, Guedj N, Clapéron A, et al. Epithelial-mesenchymal transition in cholangiocarcinoma: from clinical evidence to regulatory networks. J Hepatol. 2016;66(2):424–441. doi: 10.1016/j.jhep.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 14.Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:150845. doi: 10.1155/2014/150845 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Arbiser JL, Bonner MY, Gilbert LC. Targeting the duality of cancer. NPJ Precis Oncol. 2017;1(1):1–7. doi: 10.1038/s41698-017-0026-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saralamma VVG, Lee HJ, Raha S, et al. Inhibition of IAP’s and activation of p53 leads to caspase-dependent apoptosis in gastric cancer cells treated with Scutellarein. Oncotarget. 2018;9(5):5993. doi: 10.18632/oncotarget.23202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weng J, Xiao J, Mi Y, et al. PCDHGA9 acts as a tumor suppressor to induce tumor cell apoptosis and autophagy and inhibit the EMT process in human gastric cancer. Cell Death Dis. 2018;9(2):1–21. doi: 10.1038/s41419-017-0189-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin VY, Siu MT, Liu X, et al. MiR-92 suppresses proliferation and induces apoptosis by targeting EP4/Notch1 axis in gastric cancer. Oncotarget. 2018;9(36):24209. doi: 10.18632/oncotarget.24819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long HK, Blackledge NP, Klose RJ. ZF-CxxC domain-containing proteins, CpG islands and the chromatin connection. Biochem Soc Trans. 2013;41(3):727–740. doi: 10.1042/BST20130028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackledge NP, Thomson JP, Skene PJ. CpG island chromatin is shaped by recruitment of ZF-CxxC Proteins. Cold Spring Harb Perspect Biol. 2013;5(11):a018648. doi: 10.1101/cshperspect.a018648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pendino F, Nguyen E, Jonassen I, et al. Functional involvement of RINF, retinoid-inducible nuclear factor (CXXC5), in normal and tumoral human myelopoiesis. Blood. 2009;113(14):3172–3181. doi: 10.1182/blood-2008-07-170035 [DOI] [PubMed] [Google Scholar]
- 22.Ma S, Wan X, Deng Z, et al. Epigenetic regulator CXXC5 recruits DNA demethylase Tet2 to regulate TLR7/9-elicited IFN response in pDCs. J Exp Med. 2017;214(5):1471–1491. doi: 10.1084/jem.20161149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mi-Yeon K, Hyun-Yi K, Jiso H, et al. CXXC5 plays a role as a transcription activator for myelin genes on oligodendrocyte differentiation. Glia. 2016;64(3):350–362. doi: 10.1002/glia.22932 [DOI] [PubMed] [Google Scholar]
- 24.Myunggon K, Jungeun A, Bandukwala HS, et al. Modulation of TET2 expression and 5-methylcytosine oxidation by the CXXC domain protein IDAX. Nature. 2013;497(7447):122–126. doi: 10.1038/nature12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fedorko A, Chandramouli GV, Im Kim H, et al. Abstract LB-111: elevated CXXC5 is associated with recurrence, poor overall survival and cell viability in endometrial cancer. Am Assoc Cancer Res. 2016. [Google Scholar]
- 26.Yan X, Wu J, Jiang Q, et al. CXXC5 suppresses hepatocellular carcinoma by promoting TGF-β-induced cell cycle arrest and apoptosis. J Mol Cell Biol. 2017;10(1):48–59. doi: 10.1093/jmcb/mjx042 [DOI] [PubMed] [Google Scholar]
- 27.Kariri YA, Joseph C, Kurozumi S, et al. Prognostic significance of KN motif and ankyrin repeat domains 1 (KANK1) in invasive breast cancer. Breast Cancer Res Treat. 2020;179(2):349–357. doi: 10.1007/s10549-019-05466-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan H, Tian H, Cheng X, et al. Aberrant Kank1 expression regulates YAP to promote apoptosis and inhibit proliferation in OSCC. J Cell Physiol. 2020;235(2):1850–1865. doi: 10.1002/jcp.29102 [DOI] [PubMed] [Google Scholar]
- 29.Chiurillo MA. Role of the Wnt/β-catenin pathway in gastric cancer: an in-depth literature review. World J Exp Med. 2015;5(2):84. doi: 10.5493/wjem.v5.i2.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu W, He L, Li Y, et al. Silencing of lncRNA ZFAS1 inhibits malignancies by blocking Wnt/β-catenin signaling in gastric cancer cells. Biosci Biotechnol Biochem. 2018;82(3):456–465. doi: 10.1080/09168451.2018.1431518 [DOI] [PubMed] [Google Scholar]
- 31.Li A, Li J, Lin J, et al. COL11A1 is overexpressed in gastric cancer tissues and regulates proliferation, migration and invasion of HGC-27 gastric cancer cells in vitro. Oncol Rep. 2017;37(1):333–340. doi: 10.3892/or.2016.5276 [DOI] [PubMed] [Google Scholar]
- 32.Hu SF, Meng FM, Yin XK, et al. NT5E is associated with unfavorable prognosis and regulates cell proliferation and motility in gastric cancer. Biosci Rep. 2019;39(5):1–11. doi: 10.1042/BSR20190101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen T, Wang K, Tong X. In vivo and in vitro inhibition of human gastric cancer progress by upregulating Kank1 gene. Oncol Rep. 2017;38(3):1663–1669. doi: 10.3892/or.2017.5823 [DOI] [PubMed] [Google Scholar]
- 34.Gu Y, Zhang M. Upregulation of the Kank1 gene inhibits human lung cancer progression in vitro and in vivo. Oncol Rep. 2018;40(3):1243–1250. doi: 10.3892/or.2018.6526 [DOI] [PubMed] [Google Scholar]
- 35.Yanaka Y, Muramatsu T, Uetake H, Kozaki K-I, Inazawa J. miR-544a induces epithelial–mesenchymal transition through the activation of WNT signaling pathway in gastric cancer. Carcinogenesis. 2015;36(11):1363–1371. doi: 10.1093/carcin/bgv106 [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Jin K, van Pelt GW, et al. c-Myb enhances breast cancer invasion and metastasis through the Wnt/β-catenin/Axin2 pathway. Cancer Res. 2016;76(11):3364–3375. doi: 10.1158/0008-5472.CAN-15-2302 [DOI] [PubMed] [Google Scholar]
- 37.Cui Z, Shen Y, Chen KH, et al. KANK1 inhibits cell growth by inducing apoptosis through regulating CXXC5 in human malignant peripheral nerve sheath tumors. Sci Rep. 2017;7:40325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu YT, Zong D, Jiang XS, et al. miR‐32 promotes esophageal squamous cell carcinoma metastasis by targeting CXXC5. J Cell Biochem. 2019;120(4):6250–6263. doi: 10.1002/jcb.27912 [DOI] [PubMed] [Google Scholar]
- 39.Benedetti I, De Marzo AM, Geliebter J, et al. CXXC5 expression in prostate cancer: implications for cancer progression. Int J Exp Pathol. 2017;98(4):234–243. doi: 10.1111/iep.12241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang L, Wang Y, Gao Y, et al. Overexpression of CXXC5 is a strong poor prognostic factor in ER+ breast cancer. Oncol Lett. 2018;16(1):395–401. doi: 10.3892/ol.2018.8647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Ren Y, Zhang R, et al. Effects of CXXC finger protein 5 up-regulated expression in epithelial ovarian cancer. China Oncol. 2015;25(4):260–268. [Google Scholar]
- 42.Croce JC, Mcclay DR. Evolution of the Wnt Pathways. Methods Mol Biol. 2008;469:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H-Y, Lang Y-D, Lin H-N, et al. miR-103/107 prolong Wnt/β-catenin signaling and colorectal cancer stemness by targeting Axin2. Sci Rep. 2019;9(1):1–13. doi: 10.1038/s41598-018-37186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiong X, Tu S, Wang J, Luo S, Yan X. CXXC5: A novel regulator and coordinator of TGF-β, BMP and Wnt signaling. J Cell Mol Med. 2019;23(2):740–749. doi: 10.1111/jcmm.14046 [DOI] [PMC free article] [PubMed] [Google Scholar]