To the Editor:
In volume 78, No. 7, July 2019 of the Journal of Neuropathology and Experimental Neurology, Iverson, Luoto, Karhunen, and Castellani report mild changes of chronic traumatic encephalopathy (CTE) in 6 of 8 men who were autopsied as part of the Tampere Sudden Death Study in Tampere, Finland (1). The men were middle-aged or older at the time of death, range 56–82 years, mean 71. Through a family health survey, none were determined to have a history of contact or collision sports participation, although 2 had a history of traumatic brain injury (TBI) documented in their medical records. Neuropathological examination performed by a single neuropathologist (R.J.C.) found that 6 of the 8 cases (75%) had “sparse” “pathognomonic lesions” of CTE defined as “p-tau in neurons, astrocytes, and cell processes around small blood vessels in an irregular pattern at the depths of the cortical sulci.” The authors presented representative images of the “pathognomonic CTE lesion” in their cases as their Figure 1 (1) , as well as other diagnostic features of CTE as their Figures 2 and 3 (1). The authors concluded that 5 of 6 cases with no known history of brain trauma appeared to meet consensus criteria for CTE (2), and suggested that the study “adds to the emerging literature indicating that CTE pathology is present in people not known to have experienced multiple concussions or subconcussive blows to the head.”
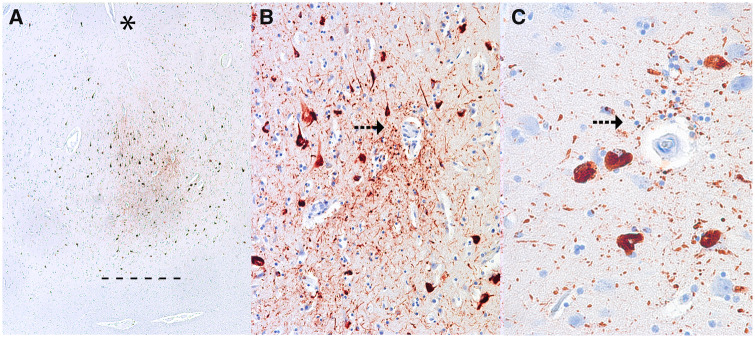
FIGURE 1.
Representative images of the pathognomonic perivascular lesion of chronic traumatic encephalopathy. Ten-μm paraffin-embedded tissue sections immunostained for phosphorylated tau (AT8) (Pierce Endogen). (A) Shows several perivascular clusters of p-tau-positive NFTs and neurites at the depths of the sulcus. Magnification: x40. The depth of the sulcus is indicated with an asterisk (*), the junction between gray and white matter is demarcated as a series of short lines (-). (B, C) There is markedly increased density of dotlike p-tau immunoreactive neurites surrounding the vessel wall (arrows) and the surrounding NFTs show clear vasculocentricity. Magnifications: B, x200, C, x400.
We respectfully disagree with their conclusions and, for the reasons outlined below, believe the authors have mistaken nonspecific hyperphosphorylated tau (p-tau) pathology and/or aging-related tau astrogliopathy (ARTAG) (3–7) for diagnostic CTE pathology (1).
The images provided for Case 1 (their Figure 1) show subpial astrocytic p-tau pathology at the sulcal depth with scattered sparse neurofibrillary tangles (NFTs) and neurites in the deeper cortical layers. Subpial astrocytic p-tau, which often involves thorn-shaped astrocytes or TSA, is a form of ARTAG (3–6). Subpial p-tau pathology may be found in CTE as a supportive feature, but in isolation, it is nondiagnostic for CTE (2). The images provided for Cases 2, 3, 6, and 7 show a sparse distribution of p-tau pathology in the form of neurites and sparse NFTs in brain regions containing a blood vessel; however, in the supplied images, there is no accentuation of p-tau pathology around the vessel or focal vasculocentricity of the neurites, as occurs in CTE. Instead, the p-tau pathology appears diffusely distributed throughout the fields of view. The first 2 images for Case 5 show strictly subpial TSA p-tau pathology, a supportive, but nondiagnostic feature of CTE; the third image shows diffusely distributed NFTs and neurites in a field of view containing a blood vessel, but there is no focal localization to the region around the vessel. None of these images meet minimum criterion for CTE. Images for Cases 4 and 8 were not supplied but were said to not have p-tau pathology at the sulcal depths suggesting that the diagnosis of CTE was made on the presence of still other, nondiagnostic features. The authors observed tau immunoreactivities (presented in their Figures 2–4), which they interpreted as supportive feature lesions. Indeed, Figure 2 shows examples of p-tau pathology in superficial laminae of Cases 3 and 5, a nonspecific feature of several tauopathies; a supportive, yet nondiagnostic feature of CTE. Their Figure 3 shows prominent “granular-fuzzy” astrocytic p-tau pathology in the amygdala of Cases 3 and 7, this pathology is characteristic of ARTAG and is not a feature of CTE (3–7). For reference on what constitutes the pathognomonic lesion of CTE in cases with a low burden of pathology, we enclose several representative images of mild CTE pathology (Figure 1). We also include several representative images of ARTAG pathology that might be confused for CTE (Figure 2).
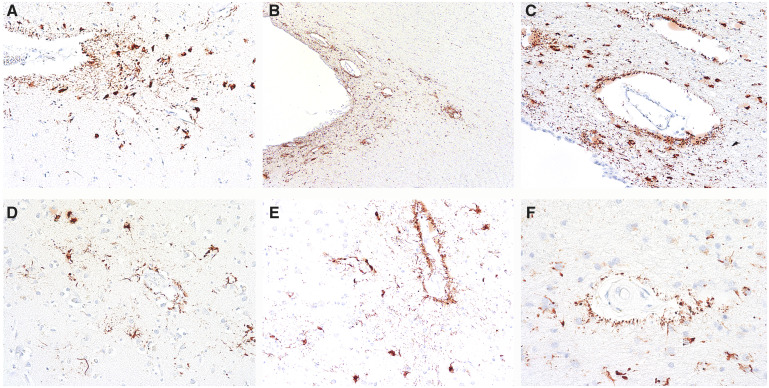
FIGURE 2.
Representative images of ARTAG that might be mistaken for CTE. Ten-μm paraffin-embedded tissue sections immunostained for phosphorylated tau (AT8) (Pierce Endogen). (A, B) P-tau-immunoreactive thorn-shaped astrocytes found at glial limitans at the depths of the sulcus are features of ARTAG that may be found in CTE, but are not diagnostic for CTE. Magnifications: A, x200, B, x100. (C) Perivascular clusters of p-tau positive astrocytes surrounding thin-walled vessel in the superficial regions of the sulcal depths also represent ARTAG. Magnification: x200. (D–F) Common forms of perivascular p-tauimmunoreactive astrocytic pathology (ARTAG) in white matter. Magnification: x200.
Prior to wide recognition of ARTAG as an age-related p-tau pathology (3–5), there was some ambiguity in early publications as to whether astrocytic p-tau pathology in subpial regions, temporal lobe white matter, and brainstem was a feature of CTE (8–11). By 2016, with the publication of the NINDS criteria for CTE, it became clear that clusters of p-tau-immunoreactive astrocytes in the subpial region at the depths of the cortical sulci, white matter of the frontal and temporal cortex, basal ganglia, lateral, and medial brainstem in isolation were not pathognomonic for CTE, but were part of the spectrum of pathology collectively known as ARTAG (2).
Further evidence that the p-tau pathology shown by Iverson and colleagues in their small case series represents non-CTE-specific p-tau pathology or aging-related astrocytic p-tau pathology of ARTAG, comes from a recent large study conducted by Forrest and colleagues. Forrest and colleagues assessed the frontal, temporal, and parietal cortices, the regions most involved in mild CTE, of 310 aged participants in a European community-based population for the presence of CTE and ARTAG (7). Of the 310, none satisfied current diagnostic criteria for CTE and 117 were diagnosed with ARTAG. Isolated p-tau pathologies occurring at the depths of cortical sulci were found in 25 cases (8%) (7), but none reached diagnostic criteria for CTE (2). These findings indicate that ARTAG is a common age-related pathology in community populations but CTE is not (7). Together with a further study, (12) these observations corroborated that isolated p-tau immunoreactivities suggestive of CTE pathology, in the correct context, could be interpreted only as one possible feature or component (7) of CTE pathology. Thus, without fitting all parts of the criteria verbatim (2), in particular, without neuronal tau accumulation in a pathognomonic location, these isolated features should not warrant the diagnosis of CTE. Currently, there is insufficient evidence to support the position that perivascular p-tau-immunoreactive astrocytes alone are diagnostic for CTE. If present in isolation, astrocytic p-tau most likely represents a distinct pathological entity, aging-related tau astrogliopathy (ARTAG) (3–7). Further studies are needed to clarify common pathogenic aspects of CTE-related astroglial tau accumulation and ARTAG seen in nontraumatic aging brains.
The misclassification of isolated ARTAG pathology as CTE by Iverson and colleagues is not compatible with the current criteria of CTE (2) and thus invalidates their conclusion that “the results of this study are striking in that 75% of our small case series met neuropathological criteria for CTE, but none of the men had a known history of participation in contact sports, collision sports, or multiple concussions.” We hope that this clarification between the p-tau pathology of CTE, which currently is emphasized to include neuronal tau accumulation, and isolated ARTAG will prove useful in future studies involving the neuropathological diagnosis of mild CTE.
FUNDING
This work was supported by grant funding from: NIA (AG057902, AG06234), NINDS (U54NS115266, U01NS086659), National Institute of Aging Boston University AD Center (P30AG13846); the Concussion Legacy Foundation and the Nick and Lynn Buoniconti Foundation.
CONFLICT OF INTEREST
Dr McKee, Dr Stein, Dr Crary, and Dr Bieniek receive grants from the National Institutes of Health and Department of Veteran Affairs (McKee, Stein) and funding from Buoniconti Foundation (McKee). Dr McKee is a member of the Mackey-White Committee of the National Football League Players Association. Dr Cantu is a paid consultant to the National Football League Head Neck and Spine Committee, a vice president and chair of the scientific advisory committee of the National Operating Committee on Standards for Athletic Equipment, and a consultant to the Concussion Legacy Foundation; he also receives royalties from Houghton Mifflin Harcourt and compensation for expert legal opinion to the National Collegiate Athletic Association and National Hockey League and is a member of the Mackey-White Committee of the National Football League Players Association.
ACKNOWLEDGMENTS
The views, opinions, and/or findings contained in this article are those of the authors and should not be construed as an official Veterans Affairs position, policy, or decision, unless so designated by other official documentation.
REFERENCES
- 1. Iverson GL, Luoto TM, Karhunen PJ, et al. Mild chronic traumatic encephalopathy neuropathology in people with no known participation in contact sports or history of repetitive neurotrauma. J Neuropathol Exp Neurol 2019;78: 615–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McKee AC, Cairns NJ, Dickson DW, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 2016;131:75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kovacs GG, Molnar K, Laszlo L, et al. A peculiar constellation of tau pathology defines a subset of dementia in the elderly. Acta Neuropathol 2011;122:205–22 [DOI] [PubMed] [Google Scholar]
- 4. Kovacs GG, Milenkovic I, Wohrer A, et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: A community-based autopsy series. Acta Neuropathol 2013;126:365–84 [DOI] [PubMed] [Google Scholar]
- 5. Kovacs GG, Ferrer I, Grinberg LT, et al. Aging-related tau astrogliopathy (ARTAG): Harmonized evaluation strategy. Acta Neuropathol 2016;131:87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kovacs GG, Xie SX, Lee EB, et al. Multisite assessment of aging-related tau astrogliopathy (ARTAG). J Neuropathol Exp Neurol 2017;76:605–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forrest SL, Kril JJ, Wagner S, et al. Chronic traumatic encephalopathy (CTE) is absent from a European community-based aging cohort while cortical aging-related tau astrogliopathy (ARTAG) is highly prevalent. J Neuropathol Exp Neurol 2019;78:398–405 [DOI] [PubMed] [Google Scholar]
- 8. McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 2009;68:709–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013;136:43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ling H, Holton JL, Shaw K, et al. Histological evidence of chronic traumatic encephalopathy in a large series of neurodegenerative diseases. Acta Neuropathol 2015;130:891–3 [DOI] [PubMed] [Google Scholar]
- 11. Puvenna V, Engeler M, Banjara M, et al. Is phosphorylated tau unique to chronic traumatic encephalopathy? Phosphorylated tau in epileptic brain and chronic traumatic encephalopathy. Brain Res 2016;1630:225–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bieniek KF, Blessing MM, Heckman MG, et al. Association between contact sports participation and chronic traumatic encephalopathy: A retrospective cohort study. Brain Pathol 2020;30:63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]