Abstract
Upon recognition of dsRNA, toll-like receptor 3 (TLR3) recruits the adaptor protein TRIF to activate IRF3 and NF-κB signaling, initiating innate immune responses. The ubiquitination of TLR3 downstream signaling molecules and their roles in the innate response have been discovered; however, whether TLR3 itself is ubiquitinated and then functionally involved remains to be elucidated. By immunoprecipitating TLR3-binding proteins in macrophages, we identified ring finger protein 170 (RNF170) as a TLR3-binding E3 ligase. RNF170 mediated the K48-linked polyubiquitination of K766 in the TIR domain of TLR3 and promoted the degradation of TLR3 through the proteasome pathway. The genetic ablation of RNF170 selectively augmented TLR3-triggered innate immune responses both in vitro and in vivo. Our results reveal a novel role for RNF170 in selectively inhibiting TLR3-triggered innate immune responses by promoting TLR3 degradation.
Keywords: TLR3, RNF170, Ubiquitination, Degradation, Innate immunity
Subject terms: Mechanisms of disease, Toll-like receptors, Phosphoinositol signalling
Introduction
As a toll-like receptor (TLR), TLR3 recognizes double-stranded RNA (dsRNA) and plays a critical role in innate immune responses.1 TLR3 consists of a leucine-rich repeat (LRR) domain, a transmembrane domain, and a toll/interleukin-1 receptor (TIR) domain.2 Inactive TLR3 is located in the endoplasmic reticulum (ER) and maintains a closed conformation that keeps its TIR domain inaccessible to cytoplasmic proteins. Upon the recognition of dsRNA, TLR3 undergoes a translocation from the ER to the endolysosome.3 Then, dsRNA binds to the LRR domain in the endolysosome, causing conformational changes in TLR3 and dimerization.4,5 Then, the TIR domain in the cytoplasm recruits the adaptor protein TRIF, initiating downstream IRF3 and NF-κB signaling, and consequently leads to the transcription of type I interferons (IFNs) and inflammatory cytokines.1,6 It has been found that TLR3 has a protective role in controlling virus infection7–9 and that TLR3 deficiency is related to severe infectious diseases, such as herpesvirus encephalitis.10 However, the overexpression of TLR3 can also cause some autoimmune disorders due to TLR3-mediated inflammatory responses;11,12 thus, the precise regulation of TLR3 expression and function is pivotal to maintaining immune homeostasis and preventing related diseases. However, the detailed mechanism regulating TLR3 expression and function is not well understood.
Ubiquitination is an important way to alter protein expression or function at the post-translational level and has been found to participate in the regulation of innate immune responses at many stages.13,14 The attachment of ubiquitin to specific protein substrates is achieved through the action of E3 ligases. In addition, ubiquitin attached to substrates can also form different polylinkages and affect the expression or function of the substrates via different mechanisms. K48-linked polyubiquitination mediates the degradation of substrates through the proteasome pathway, while K63-linked polyubiquitination promotes the activation of substrates.15,16 Many E3 ligases have been reported to be involved in the regulation of signaling molecules downstream of TLR3,17–19 but the potential regulatory effects of ubiquitination on TLR3 expression or function and the related TLR3-bound E3 ligases remain largely unknown.
In the present study, by immunoprecipitating TLR3-interacting proteins in macrophages, we identified ring finger protein 170 (RNF170) as a TLR3-binding E3 ligase. RNF170 was previously identified as an ER membrane-located E3 ligase that mediates the ubiquitination and degradation of inositol 1,4,5-trisphosphate receptors.20 However, the substrates and function of RNF170 in innate immune responses have not yet been identified. Here, we demonstrated that RNF170 promoted the K48-linked polyubiquitination of K766 in TLR3, which led to TLR3 degradation through the proteasome pathway. Consequently, RNF170 inhibited TLR3 signaling and suppressed TLR3-triggered innate immune responses.
Materials And Methods
Mice and cells
Rnf170−/− mice on the C57BL/6J background were generated using CRISPR/Cas9 technology. A mixture of plasmids encoding mCas9 and sgRNAs containing RNF170 A and RNF170 B were injected into fertilized eggs, which were then implanted into pseudopregnant mice. The oligonucleotides for the sgRNAs were as follows: A, GGGTGGATGACATGGCTCTGTGG, and B, GGCTGGCCTGGCACTTGTTGGGG. All mice were housed under specific pathogen-free conditions. All mice experiments were performed under the supervision of the Institutional Animal Care and Use Committee (IACUC), Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, Beijing.
Rnf170−/− RAW264.7 cells were generated using the CRISPR/Cas9 system, and PCR followed by sequencing and immunoblots were used to determine the knockout efficiency. Human embryonic kidney (HEK) 293T cells, RAW264.7 cells and L929 cells were obtained from American Type Culture Collection (Manassas, VA). RNF170-overexpressing cells were generated by transfecting the V5-RNF170 plasmid into RAW264.7 cells and then applying puromycin (Sigma, 2 µg/ml) selection. Bone marrow cells were maintained in granulocyte macrophage-colony stimulating factor (GM-CSF; 10 ng/ml) and interleukin-4 (IL-4; 2 ng/ml) for 7 days to generate bone marrow-derived dendritic cells (BMDCs). Cells were maintained in DMEM (Corning) supplemented with 10% (vol/vol) fetal bovine serum (Invitrogen-Gibco).
Plasmids and reagents
Myc-tagged TLR3 and V5 or Flag-tagged RNF170 full-length sequences were obtained from mouse peritoneal macrophage cDNA and then cloned into the pcDNA3.1 vector. HA-tagged TLR3 was purchased from InvivoGen. Truncation and mutation plasmids were generated based on the full-length plasmids. The antibodies specific for RNF170 were obtained from ABclonal, and the antibody against TLR3 was purchased from NOVUS. Anti-p-P65 (Ser536) (3031), anti-p-IRF3 (Ser396) (4D4G), anti-p-JNK (Thr183/Tyr185) (4668), anti-HA-tag (6E2), anti-Myc-tag (71D10; HRP conjugated), anti-Flag-tag (2368), anti-V5-tag (ab9116), anti-GAPDH and anti-β-actin (6D1) antibodies were used as previously described.21
LPS (1 μg/ml), poly(I:C) (40 μg/ml) and cycloheximide were purchased from Sigma. Poly(I:C) HMW (2 μg/ml) and CpG ODN (2 μg/ml) were purchased from InvivoGen. MG132 was obtained from Calbiochem (133407-82-6), and chloroquine and bafilomycin A1 were obtained from Selleck. VSV, SeV and HSV were used as described.21
IP-mass spectrometry analysis
Mouse peritoneal macrophages were stimulated with poly(I:C) for 3 h. Then, the same amounts of anti-TLR3 antibody and IgG were used for immunoprecipitation. Immunoprecipitated proteins were subjected to electrophoresis by sodium dodecyl sulfate polyacrylamide gel electrophoresis and silver staining. Proteins that were only enriched in the TLR3 sample, not in the IgG control sample, were further analyzed for species identification by mass spectrometry.
Quantitative reverse transcription PCR (qRT-PCR)
RNA was extracted from cells or organs using Trizol reagent (Ambion). ReverTra Ace qPCR RT master mix (Toyobo) was used to synthesize cDNA. Samples were assayed by quantitative real-time PCR using a Light Cycler (Roche) and SYBR RT-PCR kit (Takara). Data were normalized to Actb or Gapdh expression. The sequences for the primers are shown below: 5′-GTGAGATACAACGTAGCTGACTG-3′ and 5′-TCCTGCATCCAAGATAGCAAGT-3′ for Tlr3; 5′-CGATCAAGACGGCCATGAGTC-3′ and 5′-CTCGTCGGTGTCATCTTCTGC-3′ for Trif; 5′-CTGCCTCACAGCTAGTGACC-3′ and 5′-CCGGCGCTGGAGATTATTG-3′ for Mavs; 5′-GGCTTGACACTCCTGGTACAAATGAG-3′ and 5′CAGCACATTGGCAGAGGAAGACAG-3′ for Ifna4; 5′-ATGAGTGGTGGTTGCAGGC-3′ and 5′-TGACCTTTCAAATGCAGTAGATTCA-3′ for Ifnb1; 5′-ACAACCACGGCCTTCCCTAC-3′ and 5′-CATTTCCACGATTTCCCAGA-3′ for Il6; 5′-CCCTCACACTCAGATCATCTTCT-3′ and 5′- GCTACGACGTGGGCTACAG-3′ for Tnf; 5′-TGTTACCAACTGGGACGACA-3′ and 5′-CTGGGTCATCTTTTCACGGT-3′ for Actb; and 5′- AGGTCGGTGTGAACGGATTTG-3′ and 5′-TGTAGACCATGTAGTTGAGGTCA-3′ for Gapdh.
EMCV titer quantification
Heart and liver samples from infected Rnf170+/+ and Rnf170−/− mice were homogenized in 500 μl DMEM, and then the supernatants were titered with tenfold serial dilutions on BHK cells by the 50% Tissue Culture Infective Dose (TCID50) Assay.
Dual-luciferase reporter assay
Cells were seeded in 48-well plates and then cotransfected with luciferase reporter plasmids, TLR3 and RNF170 plasmids, or empty plasmids, as well as Renilla as a control. After transfection, the cells were stimulated with poly(I:C) for different times, and then whole-cell lysates were collected for measuring luciferase activity with the Dual-luciferase Reporter Assay System (Promega) according to the manufacturer’s instructions and for measuring protein expression with immunoblot assays.
Immunoprecipitation and immunoblot assays
Immunoprecipitation and immunoblot assays were performed as described previously.21 For ubiquitination analysis, cell lysates were treated with 1% SDS and incubated at 95 °C for 5 min to disturb protein–protein interactions. Samples were then diluted tenfold with a lysis buffer before immunoprecipitation and immunoblotting using relevant antibodies.
Immunofluorescence assay
Cells were seeded on glass coverslips and stimulated with poly(I:C) for the indicated times. Then, the cells were washed three times in chilled PBS, fixed with 4% (v/v) paraformaldehyde for 15 min, permeabilized with 0.2% Triton X-100 for 10 min and blocked with 1% BSA for 1 h. The cells were incubated overnight at 4 °C with the indicated antibodies, washed and incubated with a fluorescent dye-conjugated secondary antibody. Nuclei were stained with DAPI for 5 min, and confocal images were captured using an Olympus laser scanning confocal microscope.
Flow cytometry
Splenocytes obtained from 6- to 8-week-old Rnf170+/+ and Rnf170−/− mice were labeled with fluorescently labeled antibodies as described previously.21 Then, the cells were washed with PBS and analyzed by an LSRFortessa flow cytometer (BD Biosciences).
Enzyme-linked immunosorbent assay (ELISA)
Cells were seeded in plates and stimulated as indicated. The culture medium was evaluated to measure the protein levels of IFN-α and IFN-β (PBL Interferon Source), as well as IL-6 and TNF-α (R&D Systems) according to the manufacturer’s instructions.
GST pull-down assay
GST and GST-RNF170 were expressed in BL21DE Escherichia coli and purified by GST agarose beads. The relevant assay was described previously.21
Statistical analysis
For comparisons between two groups, Student’s two-tailed paired t-test was used. Mouse survival data were plotted as Kaplan–Meier curves and compared by the log-rank (Mantel-Cox) test. P < 0.05 was considered significant.
Results
RNF170 directly interacts with TLR3
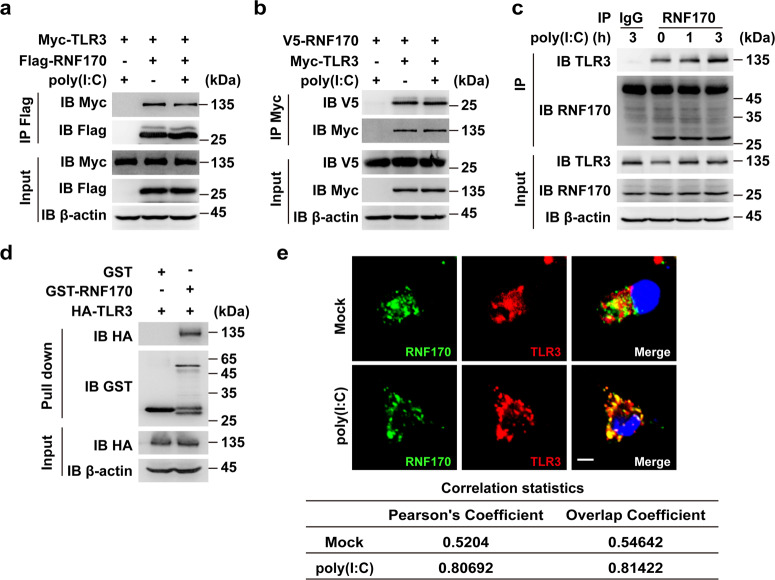
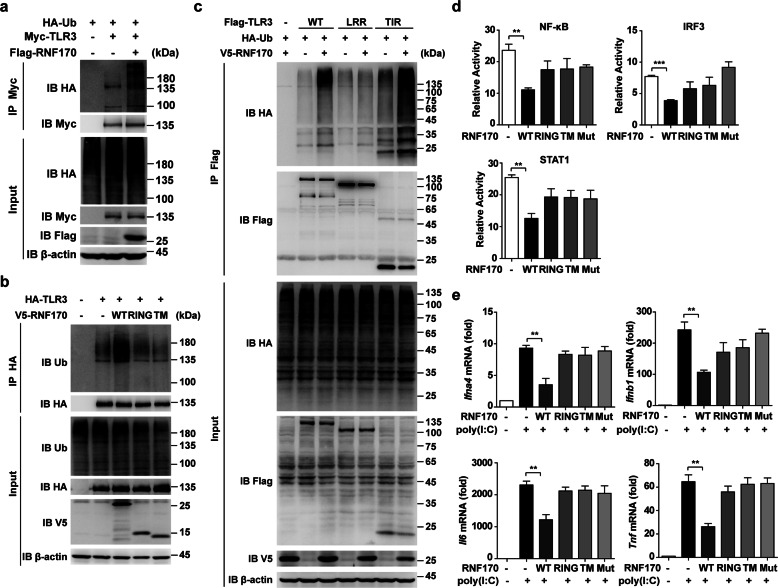
To explore whether ubiquitination participates in the regulation of TLR3 expression or function, we first identified TLR3-interacting E3 ligases in mouse peritoneal macrophages upon dsRNA analog poly(I:C) stimulation by mass spectrometry analysis. Among the proteins identified, we found the E3 ubiquitin ligase RNF170 (Supplementary Table S1). To detect the interaction between TLR3 and RNF170, we performed immunoprecipitation analysis and demonstrated that RNF170 interacted with TLR3 in HEK293T cells cotransfected with TLR3 and RNF170 (Fig. 1a, b). In addition, by using an RNF170-specific antibody (Supplementary Fig. S1a), we also found that endogenous RNF170 interacted with TLR3 in RAW264.7 cells with or without poly(I:C) stimulation (Fig. 1c). Of note, we observed endogenous RNF170 had a different size than that noted in previous reports20,22 (Fig. 1c), probably due to different post-translational modifications or splicing forms in different cells. A GST pull-down assay demonstrated that RNF170 directly interacted with TLR3 (Fig. 1d). To further confirm this interaction, we performed immunofluorescence staining analysis and found that RNF170 colocalized with TLR3 with or without poly(I:C) stimulation (Fig. 1e). Moreover, both RNF170 and TLR3 colocalized with the ER marker KDEL in resting cells and colocalized with the early endosome marker EEA1 after poly(I:C) stimulation (Supplementary Fig. S1b), indicating that both proteins were transferred from the ER to the early endosome after poly(I:C) stimulation. Taken together, these data demonstrated that RNF170 directly bound to TLR3 in resting and activated innate immune cells, suggesting that RNF170 might contribute to the regulation of TLR3-triggered innate immune responses.
Fig. 1.
RNF170 directly interacts with TLR3. a, b HEK293T cells were transfected with Myc-TLR3, Flag-RNF170, or V5-RNF170 for 24 h, stimulated with poly(I:C) for 3 h and treated with MG132 (3 μM) for 7 h. Cell lysates were immunoprecipitated using an anti-Flag or anti-Myc antibody, followed by immunoblot analysis using the indicated antibodies. c RAW264.7 cells were stimulated with poly(I:C) for the indicated times. Cell lysates were immunoprecipitated using an anti-RNF170 or control IgG antibody, followed by immunoblot analysis using the indicated antibodies. d HEK293T cells were transfected with an HA-tagged TLR3 plasmid. After 24 h, cell lysates were incubated with purified GST or GST-RNF170 proteins and subjected to GST pull-down assays. The immunoprecipitated proteins were analyzed by immunoblot analysis with the indicated antibodies. e Peritoneal macrophages were stimulated with or without poly(I:C) for 1.5 h, and then the cells were immunostained with the indicated antibodies and analyzed by fluorescence microscopy. Scale bar, 5 μm. The statistical analysis of the signal correlation between RNF170 and TLR3 was shown in the lower part of the panel. Data are representative of three independent experiments (a–e)
RNF170 selectively inhibits TLR3-induced signaling in vitro
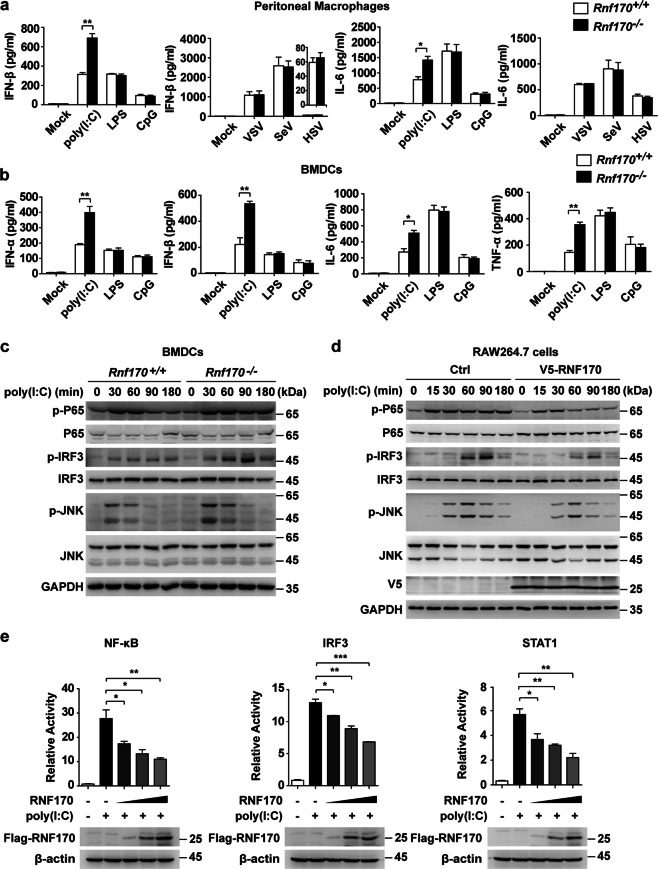
To elucidate the regulatory role of RNF170 in TLR3-triggered innate immune responses, we first generated Rnf170-deficient (Rnf170−/−) RAW264.7 cells and mice via the deletion of exon 4 in RNF170 using the CRISPR/Cas9 system (Supplementary Fig. S2a) and then showed that the expression of RNF170 was efficiently abrogated in the treated RAW264.7 cells and different cells from the Rnf170−/− mice (Supplementary Fig. S2b). Moreover, compared with wild-type (Rnf170+/+) mice, the Rnf170−/− mice were viable, fertile, and normal in size, and the numbers of natural killer cells, dendritic cells (DCs), neutrophils, and F4/80+CD11b+ macrophages in the spleen were also similar between these two mouse strains (Supplementary Fig. S2c). However, we found that both the protein and mRNA levels of IFN-β and IL-6 in peritoneal macrophages from Rnf170−/− mice were increased compared to those in cells from Rnf170+/+ mice in response to poly(I:C) stimulation but not LPS or CpG stimulation or DNA (HSV) or RNA (VSV and SeV) virus infection (Fig. 2a and Supplementary Fig. S3a). Considering that TLR3 is also highly expressed in dendritic cells, we further investigated the effect of RNF170 on innate immune signaling in bone marrow-derived DCs (BMDCs) from Rnf170+/+ and Rnf170−/− mice. We found that the expression of type I IFNs (IFN-α and IFN-β) and proinflammatory cytokines (IL-6 and TNF-α) was increased in the Rnf170−/− BMDCs compared to the Rnf170+/+ cells upon poly(I:C) stimulation (Fig. 2b and Supplementary Fig. S3b). The phosphorylation levels of IRF3, P65, and JNK in the Rnf170−/− BMDCs were also significantly increased compared to those in the Rnf170+/+ cells upon poly(I:C) stimulation (Fig. 2c). In addition, the overexpression of RNF170 decreased these signaling pathways in RAW264.7 cells upon poly(I:C) stimulation (Fig. 2d) and inhibited the promoter activity driven by IRF3, NF-κB, or STAT1 in L929 cells upon poly(I:C) stimulation (Fig. 2e).
Fig. 2.
RNF170 inhibits TLR3-triggered innate immune responses in vitro. a Peritoneal macrophages from Rnf170+/+ and Rnf170−/− mice were stimulated with poly(I:C), LPS or CpG for 6 h or infected with HSV, SeV, or VSV for 12 h. The concentrations of IFN-β and IL-6 in the supernatants were measured by ELISA. b BMDCs from Rnf170+/+ and Rnf170−/− mice were stimulated with poly(I:C), LPS, or CpG for 6 h. The concentrations of IFN-α, IFN-β, IL-6, and TNF-α in the supernatants were measured by ELISA. c BMDCs from Rnf170+/+ and Rnf170−/− mice were stimulated with poly(I:C) for the indicated times, and cell lysates were subjected to immunoblot analysis with the indicated antibodies. d RAW264.7 cells stably expressing V5-RNF170 were stimulated with poly(I:C) for the indicated times. Cell lysates were subjected to immunoblot analysis with the indicated antibodies. e L929 cells were cotransfected with luciferase reporter plasmids and an increasing amount of a RNF170 expression plasmid or control plasmid and then were treated with poly(I:C) for 9 h. Data are representative of three independent experiments (a–e). Data are shown as the mean ± SEM in a, b, and e (n = 3). *P < 0.05, **P < 0.01, and ***P < 0.001; paired Student’s t-test
Given that poly(I:C) activated not only TLR3 but also the cytosolic receptors RIG-I and MDA5, we further investigated whether the RIG-I and MDA5 signaling pathways are regulated by RNF170. We used poly(I:C) LMW stimulation (activation of TLR3), poly(I:C) HMW transfection (activation of MDA5), and VSV infection (activation of RIG-I) to detect the effect of RNF170 on peritoneal macrophages from Rnf170+/+ and Rnf170−/− mice. We found that compared to those in the Rnf170+/+ cells, the phosphorylation levels of IRF3 and P65 in the Rnf170−/− macrophages were significantly increased upon poly(I:C) LMW stimulation but not in response to poly(I:C) HMW transfection or VSV infection (Supplementary Fig. S4a–c). Furthermore, we silenced Mavs or Trif expression in peritoneal macrophages from Rnf170+/+ and Rnf170−/− mice and found that silencing the expression of Trif but not that of Mavs blocked the increased expression of IFN-β and IL-6 observed in the Rnf170−/− macrophages upon poly(I:C) stimulation (Supplementary Fig. S4d–f), suggesting that RNF170 was involved in the regulation of TLR3-TRIF signaling but not the regulation of RIG-I-MAVS signaling. Taken together, these data demonstrated that RNF170 selectively inhibited TLR3-triggered innate immune responses in vitro.
RNF170 inhibits TLR3-triggered innate immune responses in vivo
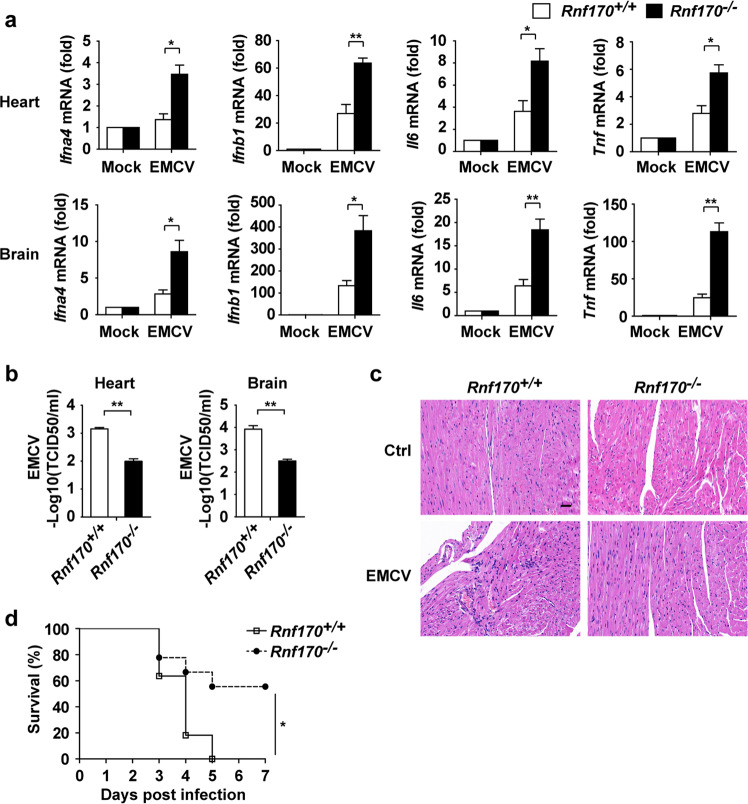
To demonstrate the function of RNF170 in vivo, we infected Rnf170+/+ and Rnf170−/− mice with EMCV, which generates dsRNA in the cytoplasm as part of its life cycle. We found that the expression of type I IFNs (Ifna4 and Ifnb1) and proinflammatory cytokines (Il6 and Tnf) in peritoneal macrophages from the Rnf170−/− mice was increased compared with that in peritoneal macrophages from the Rnf170+/+ mice (Supplementary Fig. S5a). The mRNA levels of type I IFNs and proinflammatory cytokines in the heart and brain of the Rnf170−/− mice were significantly higher than those in the Rnf170+/+ mice (Fig. 3a). Consistently, EMCV titers and viral RNA replicates were decreased in the heart and brain from the Rnf170−/− mice compared to those from the Rnf170+/+ mice (Fig. 3b and Supplementary Fig. S5b), indicating more efficient clearance of the virus in the Rnf170−/− mice. In addition, we also found that the heart tissue of the Rnf170−/− mice had attenuated inflammatory cell infiltration and tissue damage compared with that of the Rnf170+/+ mice upon EMCV infection (Fig. 3c). As a result, the Rnf170−/− mice showed improved survival compared with the Rnf170+/+ mice upon EMCV infection (Fig. 3d). To further detect the function of RNF170 in TLR3 signaling in vivo, we injected Rnf170+/+ and Rnf170−/− mice with poly(I:C) and d-galactosamine. We found that the levels of IFN-β, IL-6, and TNF-α in the serum of the Rnf170−/− mice were significantly higher than those in the serum of the Rnf170+/+ mice (Supplementary Fig. S5c). The Rnf170−/− mice were much more susceptible to inflammation-induced death than the Rnf170+/+ mice after poly(I:C) injection (Supplementary Fig. S5d). Altogether, these results suggested that RNF170 inhibited TLR3-triggered innate immune responses in vivo.
Fig. 3.
RNF170 inhibits TLR3-triggered innate immune responses in vivo. a Sex- and age-matched Rnf170+/+ and Rnf170−/− mice (n = 4 per genotype) were injected intraperitoneally (i.p.) with EMCV (1 × 105 pfu per mouse) and sacrificed after 48 h. Ifna4, Ifnb1, Il6, and Tnf mRNA expression in the heart and brain was measured by qRT-PCR analysis. b EMCV titers in the heart and brain of the mice described in a were determined by a TCID50 assay. c Hematoxylin and eosin staining of heart sections from the mice described in a was shown. Scale bar, 50 μm. d Sex- and age-matched Rnf170+/+ (n = 11) and Rnf170−/− mice (n = 9) were injected intraperitoneally (i.p.) with EMCV (1 × 105 pfu per mouse). The survival rate of the injected mice was monitored every 24 h. Data are representative of three independent experiments (a–d). Data are shown as the mean ± SEM in a and b. *P < 0.05 and **P < 0.01; paired Student’s t-test
RNF170 functions through E3 ligase activity
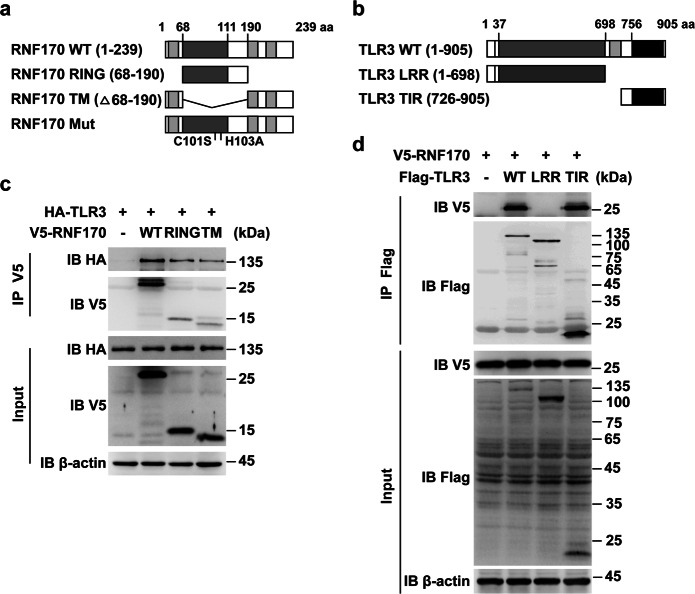
We next investigated how RNF170 negatively regulates TLR3-induced innate immune responses. We first detected which domains of RNF170 and TLR3 associated with each other. We constructed different RNF170 and TLR3 truncations (Fig. 4a, b) and found that both the RING and TM domains of RNF170 bound to the TIR domain of TLR3 (Fig. 4c, d). We then detected whether RNF170 can promote TLR3 ubiquitination to regulate TLR3 signaling. Immunoprecipitation analysis showed that the overexpression of full-length RNF170 but not the overexpression of a single RING or TM domain significantly increased the ubiquitination level of TLR3 (Fig. 5a, b). We further found that the TIR domain but not the LRR domain of TLR3 was ubiquitinated by RNF170 (Fig. 5c). As a double-amino acid point mutation (C101S and H103A) in the RING domain of RNF170 has been reported to abolish the E3 ligase activity of RNF17020, we then generated an RNF170 C101SH103A mutant to detect whether the RNF170-mediated ubiquitination of TLR3 depended on the E3 ligase activity of RNF170. We found that this mutant interacted with TLR3 (Supplementary Fig. S6a) but could not promote the ubiquitination of TLR3 (Supplementary Fig. S6b). In addition, we found that only full-length RNF170 inhibited the promoter activity driven by IRF3, NF-κB or STAT1 in L929 cells and suppressed the mRNA expression of Ifna4, Ifnb1, Il6, and Tnf in RNF170-deficient cells (Fig. 5d, e), suggesting that RNF170 promoted the ubiquitination of TLR3 and inhibited TLR3-triggered innate immune responses via its E3 ligase activity.
Fig. 4.
RING and TM domains of RNF170 interact with the TIR domain of TLR3. a, b A schematic presentation of full-length, truncation, and mutant forms of RNF170 (a) and TLR3 (b) was shown. c, d HEK293T cells were transfected with the indicated plasmids for 24 h, stimulated with poly(I:C) for 3 h and then treated with MG132 (3 μM) for 7 h. Cell lysates were immunoprecipitated using different antibodies. The precipitates were analyzed by immunoblot analysis using the indicated antibodies. Data are representative of three independent experiments (c, d)
Fig. 5.
RNF170 functions through E3 ligase activity. a–c HEK293T cells were transfected with the indicated plasmids, stimulated with poly(I:C) for 3 h and then treated with MG132 (3 μM) for 7 h. Cell lysates were immunoprecipitated using different antibodies. The precipitates were analyzed by immunoblot analysis using the indicated antibodies. d A dual-luciferase reporter assay was used to assess the promoter activity in L929 cells cotransfected with reporter vectors and different RNF170 truncations and stimulated with poly(I:C) for 9 h. e The mRNA expression of Ifna4, Ifnb1, Il6, and Tnf in Rnf170−/− RAW264.7 cells transfected with different RNF170 truncations and then stimulated with poly(I:C) for 3 h was assessed. Data are representative of three independent experiments (a–e). Data are shown as the mean ± SEM in d and e (n = 3). *P < 0.05, **P < 0.01, and ***P < 0.001; paired Student’s t-test
RNF170 promotes the K48-linked polyubiquitination and degradation of TLR3
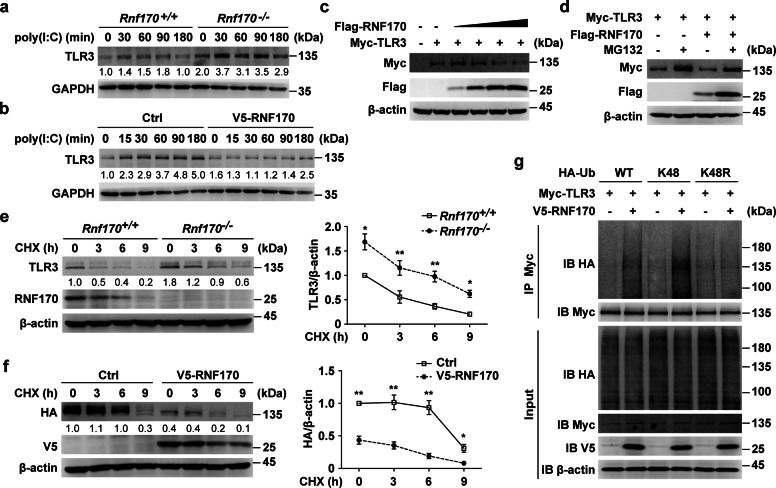
We further investigated the mechanism by which the RNF170-mediated ubiquitination of TLR3 inhibits TLR3-triggered innate immune responses. As silencing the expression of Trif blocked the regulatory function of RNF170 (Supplementary Fig. S4d–f), we hypothesized that RNF170 functions upstream of TRIF. However, we found that RNF170 did not interact with TRIF (Supplementary Fig. S7a), indicating that RNF170 mainly functioned through TLR3. Then, we detected whether RNF170 regulates TLR3 expression. The mRNA level of TLR3 was not affected by RNF170 deficiency (Supplementary Fig. S7b); however, the protein level of TLR3 in Rnf170−/− cells was dramatically increased compared to that in Rnf170+/+ cells (Fig. 6a). Consistently, the overexpression of RNF170 in RAW264.7 cells decreased the protein level of TLR3 (Fig. 6b), indicating that RNF170 might promote the degradation of TLR3. Indeed, we found that RNF170 decreased the protein level of TLR3 in a dose-dependent manner (Fig. 6c), and this effect was diminished in the presence of the proteasome inhibitor MG132 but not in the presence of the lysosome inhibitor chloroquine or bafilomycin A1 (Fig. 6d and Supplementary Fig. S7c). In addition, RNF170 deficiency significantly decreased the protein degradation rate of TLR3, while the overexpression of RNF170 significantly increased the TLR3 degradation rate in L929 cells in the presence of cycloheximide (Fig. 6e, f). Moreover, the overexpression of RNF170 did not affect the proteolysis of TLR3 (Supplementary Fig. S7d), suggesting that RNF170 promoted the degradation of TLR3 through the proteasome pathway. Since K48-linked polyubiquitination promotes substrate degradation via the proteasome pathway, we then detected whether RNF170 promotes the K48-linked polyubiquitination of TLR3. We found that RNF170 substantially increased the K48-linked polyubiquitination of TLR3 and that when K48 of ubiquitin was replaced with arginine (K48R), RNF170 no longer increased the polyubiquitination level of TLR3 (Fig. 6g). Taken together, these data demonstrated that RNF170 promoted the K48-linked polyubiquitination and degradation of TLR3 through the proteasome pathway.
Fig. 6.
RNF170 promotes the K48-linked ubiquitination and proteasomal degradation of TLR3. a Rnf170+/+ and Rnf170−/− RAW264.7 cells were stimulated with poly(I:C) for the indicated time. Cell lysates were immunoblotted with the indicated antibodies. b RAW264.7 cells stably expressing V5-RNF170 were stimulated with poly(I:C) for the indicated time. Cell lysates were immunoblotted with the indicated antibodies. c HEK293T cells were transfected with a Myc-TLR3 plasmid and increasing amounts of Flag-RNF170 for 24 h. Cell lysates were immunoblotted with the indicated antibodies. d HEK293T cells were transfected with the indicated plasmids for 24 h and treated with MG132 (3 μM) for 7 h. Cell lysates were immunoblotted with the indicated antibodies. e Rnf170+/+ and Rnf170−/− RAW264.7 cells were stimulated with poly(I:C) for 1.5 h and then treated with cycloheximide (CHX) for the indicated times, and then cell lysates were immunoblotted with the indicated antibodies. The image intensity of TLR3 was quantified on the right. f L929 cells were transfected with the indicated plasmids for 36 h, stimulated with poly(I:C) for 9 h and then treated with CHX for the indicated times. Cell lysates were immunoblotted with the indicated antibodies. The image intensity of HA-TLR3 was quantified on the right. g HEK293T cells were transfected with the indicated plasmids for 24 h and treated with MG132 (3 μM) for 7 h. Cell lysates were immunoprecipitated using anti-Myc antibodies. The precipitates were analyzed by immunoblot analysis using the indicated antibodies. Data are representative of three independent experiments (a–g). Data are shown as the mean ± SEM in e and f (n = 3). *P < 0.05 and **P < 0.01; paired Student’s t-test
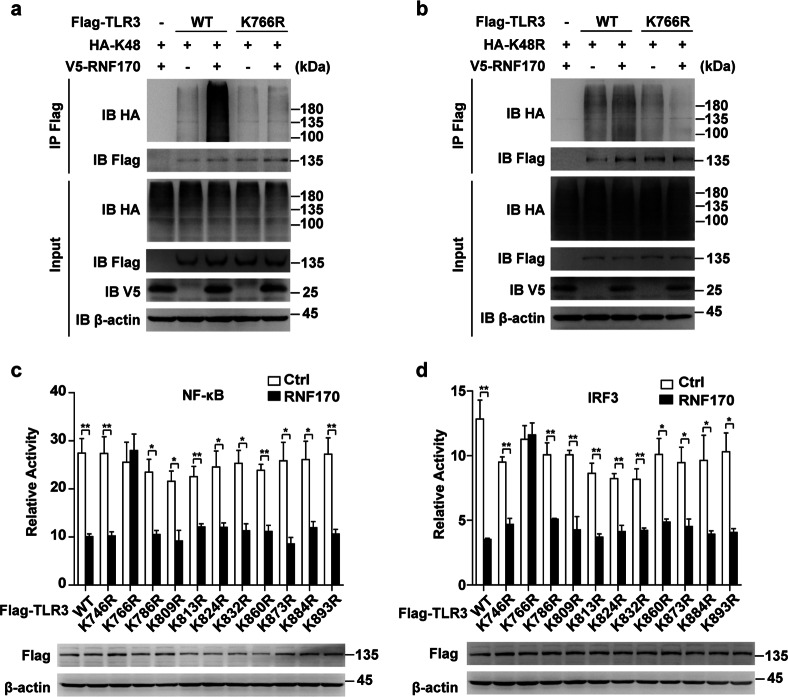
RNF170 promotes the K48-linked polyubiquitination of TLR3 at K766
As RNF170 promoted the ubiquitination of the TLR3 TIR domain, we next mutated 11 lysine residues in the TLR3 TIR domain to arginine (K746R, K766R, K786R, K809R, K813R, K824R, K832R, K860R, K873R, K884R, and K893R) to identify the ubiquitination site in TLR3 modified by RNF170. Immunoprecipitation analysis showed that only the mutation of K766 (K766R) completely blocked the RNF170-mediated ubiquitination of TLR3 (Supplementary Fig. S8a–d). Moreover, RNF170 could not promote the K48- or K48R-linked polyubiquitination on TLR3 K766R (Fig. 7a, b), indicating that the RNF170-mediated ubiquitination of TLR3 was mainly dependent on the K766 residue in TLR3. Consistently, RNF170 could not promote the degradation of TLR3 K766R and could not inhibit the promoter activity driven by IRF3 or NF-κB in TLR3 K766R-overexpressing L929 cells upon poly(I:C) stimulation (Fig. 7c, d and Supplementary Fig. S8e), suggesting that RNF170 inhibited TLR3-induced innate immune responses by promoting the K48-linked polyubiquitination of TLR3 at K766.
Fig. 7.
RNF170 promotes the K48-linked polyubiquitination of K766 in TLR3. a, b HEK293T cells were transfected with the indicated plasmids for 24 h and treated with MG132 (3 μM) for 7 h. Cell lysates were immunoblotted with the indicated antibodies. c, d A dual-luciferase reporter assay was used to assess the promoter activity in L929 cells cotransfected with reporter vectors and different TLR3 mutations and stimulated with poly(I:C) for 9 h. Data are representative of three independent experiments (a–d). Data are shown as the mean ± SEM in c and d (n = 3). *P < 0.05 and **P < 0.01; paired Student’s t-test
Discussion
The ubiquitination-mediated degradation of TLRs is an important mechanism of the host that negatively regulates TLR-triggered innate immune responses.23–25 In addition to degradation through the proteasome pathway, TLRs are also degraded in the lysosome.26 However, the degradation mechanism of TLR3 is largely unknown. TLR3 is considered to be an important pattern recognition receptor (PRR) in antiviral innate immune responses that recognizes dsRNAs that are mainly generated during the life cycles of different kinds of viruses.27 In addition to inducing type I IFNs and inflammatory cytokines, TLR3 participates in antiviral responses in many other ways, such as regulating the maturation of DCs and promoting the cross-priming of cytotoxic T cells (CTLs) by infected cells.28,29 Therefore, it is necessary to elucidate the mechanisms regulating TLR3 to control TLR3-related diseases, such as inflammatory bowel disease and pulmonary inflammation.8,30 Here, we demonstrated that the E3 ligase RNF170 promotes TLR3 degradation by directly associating with TLR3 in both resting and poly(I:C)-stimulated cells, indicating that RNF170 may be a new target to control TLR3 expression or function under both physiological and pathological conditions. Moreover, according to the available data in the Gene Expression Omnibus database, RNF170 expression is increased dramatically in monocytes during HIV infection (accession no. GSE5220),31 and it is first increased and then decreased in fibroblasts during cytomegalovirus infection (accession no. GSE3194),32 further supporting the potential role of RNF170 in antiviral innate immune responses. Thus, we demonstrated that the inhibitory effects of RNF170 on TLR3 and the TLR3-induced signaling pathway provide a potential target for controlling TLR3-related inflammatory diseases. Thus, it will be of great value to investigate the function of RNF170 in TLR3 deregulation-related diseases.
We also demonstrated that RNF170 promotes the K48-linked polyubiquitination of K766 in the TLR3 TIR domain. The TIR domain of TLR3 contributes to triggering downstream signaling by interacting with the adaptor TRIF. Although we did not detect an interaction between RNF170 and TRIF, there might be some other mechanism by which RNF170 inhibits TLR3-induced innate immune responses. Ubiquitination of the TIR domain might change the structure of the TLR3 protein, leading to a failure in translocation, dsRNA recognition or even TLR3 dimerization. Certain experiments remain to be performed to clarify whether RNF170 functions through these possible mechanisms. Furthermore, patients with genetic mutations that lead to a deficiency in the TLR3-TRIF pathway have been found to develop recurrent herpes simplex encephalitis.33 Since the K766 residue in TLR3 is conserved between mice and humans, it will be worthwhile to further examine whether this mutation is related to aberrant RNF170-mediated TLR3 ubiquitination in different diseases.
Translocation from the ER to the endolysosome has been shown to be essential for the signaling of nucleotide-sensing TLRs, such as TLR3, TLR7, and TLR9.34 An ER-located transmembrane protein, UNC93B1, has been reported to interact with the transmembrane domains of these receptors in the ER and deliver the receptors to the endolysosome.35,36 Here, we showed that RNF170, which is another integral ER membrane protein,20 interacts with TLR3 in both the ER membrane and the endolysosome. However, unlike UNC93B1, RNF170 interacts with the TIR domain of TLR3 and negatively regulates TLR3 signaling by promoting the degradation of TLR3. Furthermore, RNF170 selectively targets TLR3, as RNF170 cannot regulate signaling triggered by other PRRs.
The RNF170-mediated inhibition of TLR3 signaling is dependent on the E3 ligase activity of RNF170 within the RING domain. Consistent with a previous report,20 the mutation of two residues (C101 and H103) in the RING domain of RNF170 prevents the ubiquitination of TLR3 and thus promotes TLR3 signaling. However, although both the RING and TM domains of RNF170 could associate with TLR3, a single RING domain of RNF170 could not mediate TLR3 ubiquitination or inhibit TLR3 signaling, indicating that the TM domain of RNF170 is also necessary for ubiquitination. Clarifying how the TM domain assists in mediating ubiquitination might contribute to understanding how RNF170 translocates from the ER to the early endosome, which is an important process for the RNF170-mediated degradation of TLR3 and the negative regulation of TLR3 downstream signaling. In addition, RNF170 is predicted to contain three transmembrane regions,20 and a point mutation in the third transmembrane region of RNF170 has been found to be associated with autosomal-dominant sensory ataxia (ADSA) characterized by an age-dependent increase in walking abnormalities,22,37,38 supporting the important role of the TM domain in RNF170 function. Thus, the function and mechanism of the TM domain of RNF170 need to be further investigated.
Supplementary information
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (grant 81788101 to X.C.; grant 81871236 to M.J.), the National 135 Mega Program of China (grant 2017ZX10202203-002 to M.J.; grant 2017ZX10203206-001 to X.C.), the CAMS Innovation Fund for Medical Sciences (grant 2016-12M-1-003 to X.C.) and the Medical Epigenetics Research Center, Chinese Academy of Medical Sciences (2018PT31015).
Author contributions
M.J. and X.C. designed the research; X.S., M.J., S.L., W.W. and Z.M. performed the experiments; and X.S., M.J. and X.C. analyzed the data and wrote the paper.
Competing interests
The authors declare no competing interests.
Supplementary information
The online version of this article (10.1038/s41423-019-0236-y) contains supplementary material.
References
- 1.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, et al. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science. 2008;320:379–381. doi: 10.1126/science.1155406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGettrick AF, O’Neill LA. Localisation and trafficking of Toll-like receptors: an important mode of regulation. Curr. Opin. Immunol. 2010;22:20–27. doi: 10.1016/j.coi.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Cattaneo A, et al. Cleavage of Toll-like receptor 3 by cathepsins B and H is essential for signaling. Proc. Natl Acad. Sci. USA. 2012;109:9053–9058. doi: 10.1073/pnas.1115091109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leonard JN, et al. The TLR3 signaling complex forms by cooperative receptor dimerization. Proc. Natl Acad. Sci. USA. 2008;105:258–263. doi: 10.1073/pnas.0710779105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roers A, Hiller B, Hornung V. Recognition of endogenous nucleic acids by the innate immune system. Immunity. 2016;44:739–754. doi: 10.1016/j.immuni.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Daffis S, Samuel MA, Suthar MS, Gale M, Diamond MS. Toll-Like receptor 3 has a protective role against West nile virus infection. J. Virol. 2008;82:10349–10358. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guillot L, et al. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 2005;280:5571–5580. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- 9.Lafaille FG, et al. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature. 2012;491:769–773. doi: 10.1038/nature11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang SY, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 11.Harii N, et al. Thyrocytes express a functional toll-like receptor 3: overexpression can be induced by viral infection and reversed by phenylmethimazole and is associated with Hashimoto’s autoimmune thyroiditis. Mol. Endocrinol. 2005;19:1231–1250. doi: 10.1210/me.2004-0100. [DOI] [PubMed] [Google Scholar]
- 12.Roelofs MF, et al. The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. 2005;52:2313–2322. doi: 10.1002/art.21278. [DOI] [PubMed] [Google Scholar]
- 13.Kondo T, Kawai T, Akira S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012;33:449–458. doi: 10.1016/j.it.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Qian C, Cao X. Post-translational modification control of innate immunity. Immunity. 2016;45:15–30. doi: 10.1016/j.immuni.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Cao X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat. Rev. Immunol. 2016;16:35–50. doi: 10.1038/nri.2015.8. [DOI] [PubMed] [Google Scholar]
- 16.Jiang X, Chen ZJ. The role of ubiquitylation in immune defence and pathogen evasion. Nat. Rev. Immunol. 2011;12:35–48. doi: 10.1038/nri3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko R, Park JH, Ha H, Choi Y, Lee SY. Glycogen synthase kinase 3beta ubiquitination by TRAF6 regulates TLR3-mediated pro-inflammatory cytokine production. Nat. Commun. 2015;6:6765. doi: 10.1038/ncomms7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siednienko J, et al. Pellino3 targets the IRF7 pathway and facilitates autoregulation of TLR3- and viral-induced expression of type I interferons. Nat. Immunol. 2012;13:1055–1062. doi: 10.1038/ni.2429. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, et al. E3 ligase WWP2 negatively regulates TLR3-mediated innate immune response by targeting TRIF for ubiquitination and degradation. Proc. Natl Acad. Sci. USA. 2013;110:5115–5120. doi: 10.1073/pnas.1220271110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu JP, Wang Y, Sliter DA, Pearce MM, Wojcikiewicz RJ. RNF170 protein, an endoplasmic reticulum membrane ubiquitin ligase, mediates inositol 1,4,5-trisphosphate receptor ubiquitination and degradation. J. Biol. Chem. 2011;286:24426–24433. doi: 10.1074/jbc.M111.251983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, et al. RNF122 suppresses antiviral type I interferon production by targeting RIG-I CARDs to mediate RIG-I degradation. Proc. Natl Acad. Sci. USA. 2016;113:9581–9586. doi: 10.1073/pnas.1604277113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright FA, et al. A point mutation in the ubiquitin ligase RNF170 that causes autosomal dominant sensory ataxia destabilizes the protein and impairs inositol 1,4,5-trisphosphate receptor-mediated Ca2+signaling. J. Biol. Chem. 2015;290:13948–13957. doi: 10.1074/jbc.M115.655043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chuang TH, Ulevitch RJ. Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat. Immunol. 2004;5:495–502. doi: 10.1038/ni1066. [DOI] [PubMed] [Google Scholar]
- 24.Kumazoe M, et al. Green Tea polyphenol epigallocatechin-3-gallate suppresses toll-like receptor 4 expression via up-regulation of E3 ubiquitin-protein ligase RNF216. J. Biol. Chem. 2017;292:4077–4088. doi: 10.1074/jbc.M116.755959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKelvey AC, et al. RING finger E3 ligase PPP1R11 regulates TLR2 signaling and innate immunity. Elife. 2016;5:e18496. doi: 10.7554/eLife.18496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, et al. Lysosome-associated small Rab GTPase Rab7b negatively regulates TLR4 signaling in macrophages by promoting lysosomal degradation of TLR4. Blood. 2007;110:962–971. doi: 10.1182/blood-2007-01-066027. [DOI] [PubMed] [Google Scholar]
- 27.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Schroder M, Bowie AG. TLR3 in antiviral immunity: key player or bystander? Trends Immunol. 2005;26:462–468. doi: 10.1016/j.it.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Schulz O, et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 30.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect. Immun. 2000;68:7010–7017. doi: 10.1128/IAI.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tilton JC, et al. Diminished production of monocyte proinflammatory cytokines during human immunodeficiency virus viremia is mediated by type I interferons. J. Virol. 2006;80:11486–11497. doi: 10.1128/JVI.00324-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hertel L, Mocarski ES. Global analysis of host cell gene expression late during cytomegalovirus infection reveals extensive dysregulation of cell cycle gene expression and induction of Pseudomitosis independent of US28 function. J. Virol. 2004;78:11988–12011. doi: 10.1128/JVI.78.21.11988-12011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Netea MG, Wijmenga C, O’Neill LA. Genetic variation in Toll-like receptors and disease susceptibility. Nat. Immunol. 2012;13:535–542. doi: 10.1038/ni.2284. [DOI] [PubMed] [Google Scholar]
- 34.Tabeta K, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat. Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 35.Brinkmann MM, et al. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J. Cell Biol. 2007;177:265–275. doi: 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 37.Kim Y, et al. Age-dependent gait abnormalities in mice lacking the Rnf170 gene linked to human autosomal-dominant sensory ataxia. Hum. Mol. Genet. 2015;24:7196–7206. doi: 10.1093/hmg/ddv417. [DOI] [PubMed] [Google Scholar]
- 38.Valdmanis PN, et al. A mutation in the RNF170 gene causes autosomal dominant sensory ataxia. Brain. 2011;134:602–607. doi: 10.1093/brain/awq329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.