Abstract
IL-6 plays important and pleiotropic roles in infection and inflammatory diseases, and its production needs to be tightly regulated. However, the epigenetic mechanism underlying Il6 gene transcription remains to be fully elucidated. Here, we report that lysine-specific demethylase 2b (KDM2B), which demethylates H3K4me3 and H3K36me2, is required in macrophages and dendritic cells for the induction of IL-6 but not TNF-α, IL-1, and IFN-β. Compared to wild-type mice, KDM2B-deficient mice were more resistant to endotoxin shock and colitis, with a less severe inflammatory pathogenesis phenotype and decreased IL-6 production in sera. KDM2B selectively bound the Il6 promoter but did not alter histone demethylation; instead, KDM2B interacted with Brahma-related gene 1 (Brg1), the core ATPase subunit of SWI/SNF chromatin remodeling complexes, to facilitate chromatin accessibility of the Il6 promoter. Furthermore, KDM2B directly recruited RNA Polymerase II to further initiate and promote Il6 transcription. Thus, our finding identifies a novel nonclassical function of KDM2B in gene-specific transcription initiation and enhancement of Il6 independent of its demethylase activity and adds new insight into the specific epigenetic modification mechanism of inflammatory immune responses.
Keywords: KDM2B, IL-6, Brg1, Chromatin remodeling, Inflammation
Subject terms: Chronic inflammation, Epigenetics in immune cells, Interleukins
Introduction
Proinflammatory cytokines are not only essential regulators of immune cell development and differentiation but also crucial mediators of inflammatory responses and immune pathology.1 On the one hand, the components of exogenous pathogens, known as pathogen-associated molecular patterns (PAMPs), are recognized by pattern recognition receptors (PRRs) and then activate their downstream signaling pathways, resulting in the production of various cytokines, including proinflammatory cytokines, in host for defense against pathogens.2–4 On the other hand, damaged and dying cells induced by acute stress and tissue damage can release many cellular contents called damage-associated molecular patterns (DAMPs), including mitochondrial DNA, heat shock proteins and high mobility group box 1 (HMGB1), which also leads to proinflammatory cytokine generation.5,6 Among these proinflammatory cytokines, IL-6 plays a central role in the appropriate orchestration of innate and adaptive immune responses, including the population expansion and activation of T cells, the differentiation of B cells, and regulation of the acute-phase response.7–9 However, dysregulated IL-6 production contributes to the pathogenesis of some inflammatory and autoimmune diseases, such as rheumatoid arthritis and inflammatory bowel disease.10,11 Thus, IL-6 has been regarded as a prominent target for the clinical intervention in inflammation, autoimmunity, and cancer.12 Although the host exerts some transcriptional and post-transcriptional mechanisms to strictly control IL-6 production, the epigenetic regulators involved in IL-6 production remain largely unknown.
Gene transcription in eukaryotes is a highly controlled process with various regulatory factors involved. Genomic genes and histone proteins are subjected to reversible epigenetic modifications, defining the cell status and affecting cell differentiation and development by strictly controlling gene expression.13,14 The condensed chromatin is usually packed in the nucleosome, which is inaccessible and repressive for gene transcription, while chromatin remodeling turns the transcriptionally inactive heterochromatin into the transcriptionally active euchromatin, enabling its “fining” expression.15 Histone modifications, including acetylation, methylation, ubiquitination, ADP-ribosylation, and phosphorylation, are required for the appropriate orchestration of gene transcription silencing and activation, coupling with chromatin remodeling and recruitment of transcription factors. The methylation of histone lysine residues is dynamically controlled by lysine methyltransferases (KMTs) and lysine demethylases (KDMs).16 KDMs have been identified to consist of two major families that are evolutionarily conserved, exerting their demethylase activity through two different reaction mechanisms.17 One is the LSD1 demethylases, utilizing flavin adenine dinucleotide (FAD)-dependent amine oxidation reactions to catalyze the demethylation of their substrates; the other is the Jumonji C (JmjC) demethylases, which perform their catalytic activity via a shared JmjC domain dependent on Fe(II) and α-ketoglutarate.18,19 Although some histone modification proteins have been shown to regulate inflammatory cytokine production,4 the effects of the interaction of histone modification proteins and chromatin remodeling regulators on cytokine production and inflammatory responses need to be uncovered.
The lysine-specific demethylase 2 (KDM2) family of proteins was the first Jmjc-domain-containing family to be identified.20 KDM2B (also known as Jhdm1b or Fbxl10) is a relatively conserved and ubiquitously expressed family member that specifically mediates the removal of methyl groups from H3K4me3 and H3K36me2. KDM2B possesses additional functional domains in addition to JmjC, including a ZF-CXXC domain, a plant homeodomain finger (PHD) domain, an F-box, and several leucine-rich repeats (LRRs). KDM2B has been confirmed to participate in multiple fundamental biological and pathological processes, such as the cell cycle, senescence, and tumorigenesis, which is dependent or independent on its demethylase activity.21 KDM2B promotes induced pluripotent stem cell (iPSC) generation via its demethylase activity.22 Direct binding of KDM2B to the promoter of its target gene, for example, noncoding RNA Xist, is accompanied by a significant decrease in H3K4me3.23 In addition, KDM2B negatively regulates cell proliferation by targeting c-Fos for ubiquitylation and degradation upon mitogenic stimulations.24 However, the role of KDM2B in inflammatory responses remains unclear.
Here, we reported that KDM2B preferentially promoted IL-6 production in response to inflammatory stimuli both in vivo and in vitro by facilitating chromatin accessibility and transcription activation of the Il6 gene through an association with Brahma-related gene 1 (Brg1). Our study identifies a previously unknown nonclassical role of KDM2B in promoting IL-6 production independent of its demethylase activity in inflammatory responses.
Materials and methods
Reagents and antibodies
LPS (E. coli 0111:B4) and poly(I:C) were obtained from Merck Millipore. Antibodies against KDM2B (09-864) (for immunoblot), H3K4me3 (17-614) and H3K36me2 (17-10136) were from Merck Millipore. Antibodies against KDM2B (NBP2-14954) (for immunoprecipitation and ChIP) were from Novus Biologicals. Antibodies against Brg1 (ab110641) were from Abcam. Antibodies against ERK (9102), phospho-ERK (9106), JNK (9258), phospho-JNK (4668), p38 (9212), phospho-p38 (9211), p65 (8242), phospho-p65 (3033), IKKβ (8943), phosphor-IKKα/β (2697), TBK1 (3013), phospho-TBK1 (5483), IRF3 (4302), phospho-IRF3 (4947), β-Actin (4967), Flag-tag (2044) and V5-tag (13202) were from Cell Signaling Technology. Vesicular stomatitis virus (VSV) was a gift from Prof. Wei Pan (Second Military Medical University, Shanghai, China). Herpes simplex virus-1 (HSV-1) was a gift from Prof. Qihan Li (Chinese Academy of Sciences, Beijing, China).
Endotoxin shock model and colitis model
Kdm2b−/− and Kdm2b+/+ littermate mice (6-week-old to 8-week-old) were injected intraperitoneally with LPS (12 mg per kg body weight) or poly(I:C) (10 mg per kg body weight). Sera were collected 2 h after injection. For the colitis model, Kdm2b−/− and Kdm2b+/+ littermate mice were given drinking water supplemented with filter-sterilized 3% dextran sodium sulfate (DSS) (w/v) (molecular weight 36,000–50,000; MP Biomedicals).25 Sera and colons were harvested after 7 days. Cytokines in sera were measured by ELISA kits (R&D Systems). Lung or colon tissues were examined by hematoxylin and eosin (HE) staining.
Cell culture and RNA interference
HEK 293T cell lines were obtained from the American Type Culture Collection. Mouse primary peritoneal macrophages, bone marrow-derived macrophages (BMDMs) and bone marrow-derived dendritic cells (BMDCs) were prepared and cultured as described previously.26 The two different specific siRNAs targeting the Kdm2b gene were from Dharmacon (M-065655-01, Kdm2b siRNA-1) and Santa Cruz (sc-75006, Kdm2b siRNA-2). The two different specific siRNAs targeting Brg1 were from Santa Cruz (sc-29830, Brg1 siRNA-1) and Dharmacon (M-041135-01, Brg1 siRNA-2). The siRNA duplexes were transfected into macrophages using Lipofectamine RNAiMAX (Thermo Fisher) according to the manufacturer’s protocol.
RNA isolation and quantitative-PCR (Q-PCR) assays
Total RNA was extracted from cells using TRIzol reagent (Thermo Fisher) according the instructions. Q-PCR assays were performed using a LightCycler 480 (Roche) and SYBR RT-PCR kit (Takara). Data were normalized to β-actin expression.
RNA sequencing
Total RNA was isolated using an RNeasy mini kit (Qiagen). Paired-end libraries were synthesized by using a TruSeq RNA sample preparation kit (Illumina). Clusters were generated by cBot and then sequenced on an Illumina HiSeq 2500 (Illumina). Hisat2 (version: 2.0.4) was used to map cleaned reads to the mouse GRCm38.p4 (mm10) reference genome, with two mismatches. After genome mapping, Stringtie (version: 1.3.0) was run with a reference annotation to generate FPKM (fragments per kilobase of exon model per million mapped reads) values for known gene models. Differentially expressed genes (DEGs) were analyzed using edgeR and identified with a cutoff of absolute fold change of 2.0. The P value significance threshold in multiple tests was set by the false discovery rate (FDR). RNA-sequencing data were deposited into the Gene Expression Omnibus (GSE120651).
Immunoprecipitation (IP) and immunoblot (IB) analysis
Total macrophage proteins were extracted with cell lysis buffer (Cell Signaling Technology) with addition of a protease inhibitor “cocktail” (Calbiochem) and 1 mM phenylmethylsulfonyl fluoride (PMSF). The extracted protein concentration was measured by a BCA protein assay reagent kit (Thermo Fisher). Immunoprecipitation and immunoblot analyses were performed as described previously.27
Plasmid construction
Vectors encoding mouse Kdm2b (NM-001003953.2) or Brg1 (NM-011417.3) were constructed by PCR-based amplification from cDNA of mouse macrophages and then cloned into the pcDNA3.1 V5-His eukaryotic expression vector (Invitrogen) or p3xFLAG-CMV-14 (Sigma) as described. Mutants of KDM2B were constructed by PCR-based amplification from the wild-type plasmid. Each construct was confirmed by DNA sequencing. The wild-type KDM2B construct or KDM2B mutants were transfected into mouse macrophages through nucleofection using a Macrophage Nucleofector kit and 4D-Nucleofector System (Lonza).
Liquid chromatography-tandem mass spectrometry analysis
Nuclear proteins from macrophages were extracted using an NE-PER nuclear and cytoplasmic protein extraction reagent kit (Thermo Fisher). Protein complexes immunoprecipitated with KDM2B antibody were separated by SDS-PAGE electrophoresis. After silver staining, proteins were excised, digested, and then analyzed by reverse-phase nanospray liquid chromatography-tandem mass spectrometry (LC-MS). Data were processed through ProteinLynx Global Server version 2.4 (PLGS 2.4). The resulting peak lists were used for searching the NCBI protein database with the Mascot search engine.
Chromatin immunoprecipitation (ChIP) and Re-ChIP assays
Macrophages were cross-linked with 1% (vol/vol) methanol-free formaldehyde for 10 min and then subjected to ChIP assay according to the protocol of a ChIP assay kit (Millipore). A Re-ChIP assay was performed as described previously using a Re-ChIP-IT kit (Active Motif).27 Data were acquired through Q-PCR and then normalized to the corresponding input DNA for each sample. The primers used for amplification and quantification in ChIP and Re-ChIP assays are shown in Supplementary Table 1.
DNase I sensitivity analysis and formaldehyde-assisted isolation of regulatory elements (FAIRE) assay
Nuclei of macrophages were treated with DNase I (0.1 U/μl, Promega) at 37 °C for 30 min and then treated with EDTA (50 mM). Genomic DNA was extracted and purified using a QIAamp DNA micro kit (QIAGEN). The primers used for DNase I sensitivity analysis were the same as those described in the ChIP assay. In the FAIRE assay, as described,28 macrophages were cross-linked briefly with formaldehyde, then lysed and sonicated. The sheared chromatins were subjected to phenol/chloroform extraction. DNA in the aqueous phase was then precipitated and purified by ethanol. FAIRE-enriched chromatin was detected and amplified by Q-PCR using the primers presented in Supplementary Table 2.
Luciferase reporter assay
HEK 293T cells were transfected with a mixture of appropriate luciferase reporter plasmid, pRL-TK-Renilla-luciferase plasmid, various adapter plasmids and wild-type KDM2B plasmid or KDM2B mutants with JetPEI reagents (Polyplus Transfection) following the manufacturer’s instructions. The pcDNA3.1 vector was used as an empty control, and the total amount of plasmid DNA was equalized with it as well. After 24 h, luciferase reporter assays were performed using a Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s protocols. Data were normalized for transfection efficiency by the division of firefly luciferase activity values with Renilla luciferase activity values.
Statistical analysis
The statistical significance of comparisons between two groups was determined with an unpaired Student’s t test. For comparison of more than two groups, one-way ANOVA was used. P values of less than 0.05 were considered statistically significant.
Results
KDM2B deficiency attenuates IL-6 production and inflammatory tissue injury
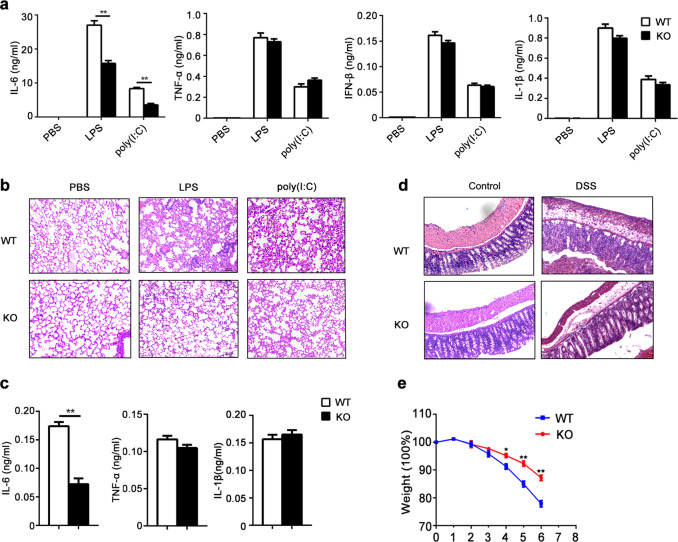
To investigate the physiological role of KDM2B in inflammation, we generated Kdm2b knockout mice (Kdm2b−/−) using the CRISPR-Cas9 system. Seventeen bases of the Jmjc domain responsible for its demethylase activity were deleted from the Kdm2b gene (Supplementary Fig. 1a, b), which resulted in a frameshift mutation in the Kdm2b gene such that the KDM2B protein could not be normally expressed in Kdm2b-deficient cells, such as macrophages (Supplementary Fig. 1c). Kdm2b−/− mice and the control wild-type littermates (Kdm2b+/+) were fertile and normal in size as well as weight. Furthermore, KDM2B deficiency did not affect the development of innate immune cells, including macrophages and dendritic cells (Supplementary Fig. 1d, e). We then intraperitoneally injected the Toll-like receptor 4 (TLR4) ligand lipopolysaccharide (LPS) or the TLR3 ligand polyinosinic-polycytidylic acid (poly(I:C)) into Kdm2b−/− and Kdm2b+/+ mice and found that compared with Kdm2b+/+ mice, Kdm2b−/− mice produced significantly less IL-6 but not TNF-α, IFN-β, or IL-1β in sera (Fig. 1a). Furthermore, compared with Kdm2b+/+ mice, Kdm2b−/− mice exhibited alleviated tissue damage and infiltration of less inflammatory cells in their lungs (Fig. 1b). Considering that excessive production of IL-6 in innate immune cells contributes to the development of inflammatory bowel diseases (IBDs), we further investigated the role of KDM2B in a colitis model induced by dextran sodium sulfate (DSS). Compared with KDM2B-sufficient mice, KDM2B-deficient mice showed less production of IL-6 but not TNF-α or IL-1β in sera, alleviated disruption of colon mucosal structures, decreased infiltration of inflammatory cells in colon tissues, and slowed weight loss (Fig. 1c–e). These results indicate that KDM2B deficiency inhibits inflammatory responses and attenuates tissue injuries, which is attributed to the decreased production of the proinflammatory cytokine IL-6 in vivo.
Fig. 1.
KDM2B deficiency attenuates IL-6 production and inflammatory injury in tissue. a, b ELISA of cytokines in sera (a) and HE staining of lung tissues (b) from Kdm2b−/− (KO) and Kdm2b+/+ (WT) mice (n = 5 per group) intraperitoneally injected with LPS or poly(I:C). c–e ELISA of cytokines in sera (c), HE staining of colonic tissue sections (d), and changes in body weights (e) of Kdm2b−/− and Kdm2b+/+ mice (n = 5 per group) after treatment with 3% DSS for 6 days. The data are from three independent experiments (a, c, e; mean ± s.e.m.) or are representative of three independent experiments with similar results (b, d). *P < 0.05; **P < 0.01
KDM2B selectively promotes IL-6 production in innate immune cells
To further confirm the role of KDM2B in inflammatory responses mediated by innate immune cells, we screened the expression difference of inducible inflammation-related genes triggered by LPS in macrophages from Kdm2b−/− and Kdm2b+/+ mice. RNA sequencing analysis showed that the expression level of the Il6 gene but not that other proinflammatory cytokine genes, such as Tnf, was significantly decreased in KDM2B-deficient macrophages (Fig. 2a). We then challenged peritoneal macrophages with different kinds of TLR ligands or viruses to confirm the effect of KDM2B deficiency on cytokine production and found that IL-6 expression at both the mRNA and protein levels was markedly decreased in KDM2B-deficient peritoneal macrophages (Fig. 2b). However, the expression levels of other cytokines, including TNF-α, IFN-β, IL-1β, IL-1α, and IL-12, showed no significant difference between Kdm2b−/− and Kdm2b+/+ peritoneal macrophages upon treatment with TLR ligands or viruses (Supplementary Fig. 2a, b). KDM2B expression remained unchanged in peritoneal macrophages stimulated with LPS or poly(I:C) (Supplementary Fig. 2c). Silencing of KDM2B with two specific siRNAs both significantly inhibited the production of IL-6 but not TNF-α or IFN-β induced by TLR ligands or viruses in peritoneal macrophages (Fig. 2c; Supplementary Fig. 3). In addition, the reduced production of IL-6 but not TNF-α or IFN-β was also found in KDM2B-deficient BMDMs or BMDCs stimulated with TLR ligands or infected with viruses (Fig. 2d, e; Supplementary Fig. 4a, b). These data confirm that KDM2B selectively facilitates IL-6 production in innate immune cells in response to innate stimuli.
Fig. 2.
KDM2B deficiency decreases IL-6 production in response to LPS or poly(I:C) in innate immune cells. a Heat map of mRNA expression in Kdm2b−/− (KO) and Kdm2b+/+ (WT) peritoneal macrophages 3 h after LPS stimulation. b, c Q-PCR analysis of Il6 mRNA expression or ELISA of IL-6 production in supernatants of Kdm2b−/− and Kdm2b+/+ peritoneal macrophages (b) or mouse peritoneal macrophages transfected with control siRNA (Ctrl siRNA-1) or Kdm2b-specific siRNA (Kdm2b siRNA-1) (c) followed by stimulation with LPS or poly(I:C) for the indicated times. d, e Q-PCR analysis of Il6 mRNA expression or ELISA of IL-6 production in supernatants of Kdm2b−/− and Kdm2b+/+ BMDMs (d) and BMDCs (e) stimulated with LPS or poly(I:C) for 4 h. The data are from three independent experiments (b–e, mean ± s.e.m.). *P < 0.05; **P < 0.01
KDM2B binds to the Il6 promoter and does not alter its methylation modification
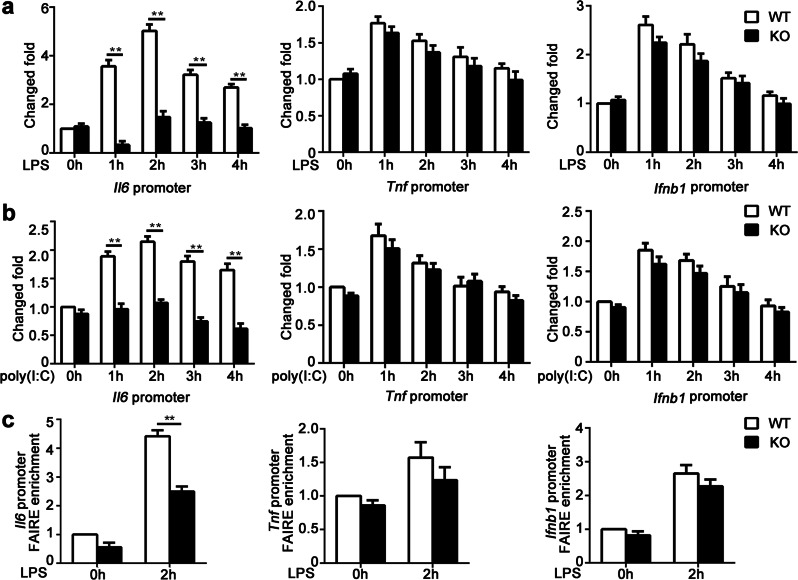
Upon recognition by exogenous or endogenous ligands, most TLRs recruit the adapter myeloid differentiation primary response gene 88 (MyD88) and then activate the MyD88-dependent downstream signaling cascade, ultimately mediating the activation of MAPK and NF-κB that results in transcription of inflammatory cytokine genes. In addition, TLR4, TLR3 or retinoic acid-induced gene I (RIG-I) can activate the IRF3-dependent signaling pathway to induce type I interferon production in innate immune cells.2 However, KDM2B deficiency did not affect the activation of the MAPK, NF-κB and IRF3 pathways induced by LPS in macrophages (Supplementary Fig. 5a). Overexpression of KDM2B also had no effect on MyD88-mediated NF-κB reporter activation or RIG-I-triggered IRF3 reporter activation (Supplementary Fig. 5b), suggesting that other mechanisms rather than the above signaling pathways were involved in the enhanced IL-6 production mediated by KDM2B. Since KDM2B is a histone lysine-specific demethylase, we wondered whether KDM2B regulated gene expression by binding to the promoters of cytokine genes and altering the methylation levels of H3K4 or H3K36 at their promoter regions. An increased recruitment of KDM2B to the promoter of Il6 but not the Tnf or Ifnb1 genes was found in macrophages stimulated with LPS or poly(I:C) (Fig. 3a, b). Nevertheless, KDM2B deficiency did not alter the level of H3K4me3 or H3K36me2 at the promoter of the Il6, Tnf, or Ifnb1 genes in macrophages upon stimulation with LPS or poly(I:C) (Fig. 3c; Supplementary Fig. 5c, d). In addition, an Il6 promoter luciferase reporter assay showed that wild-type KDM2B enhanced MyD88-mediated Il6 promoter reporter activation in a dose-dependent manner, while a demethylase activity-inactivating Jmjc domain-deleted mutant of KDM2B (KDM2B ΔJmjc) also increased Il6 reporter activity (Fig. 3d). These results demonstrate that KDM2B selectively binds the Il6 promoter and enhances Il6 transcription in a manner independent of its histone demethylase activity.
Fig. 3.
KDM2B selectively binds the Il6 promoter but does not affect its methylation modification. a, b ChIP followed by quantitative PCR (ChIP-qPCR) analysis of KDM2B occupancy at the Il6, Tnf and Ifnb1 promoters in Kdm2b−/− (KO) and Kdm2b+/+ (WT) peritoneal macrophages stimulated with LPS (a) or poly(I:C) (b) for the indicated times. c ChIP-qPCR analysis of H3K4me3 modification at the Il6 promoter in Kdm2b−/− (KO) and Kdm2b+/+ (WT) peritoneal macrophages stimulated with LPS or poly(I:C) for 2 h or left untreated. d Luciferase activity assay of Il6 promoter reporter activated by MyD88 in lysates of HEK 293T cells transfected with mock vector, wild-type KDM2B or a KDM2B mutant with a Jmjc domain deletion (KDM2B ΔJmjc) as indicated. The data are from three independent experiments (a–d, mean ± s.e.m.). **P < 0.01
KDM2B increases the chromatin accessibility of the Il6 promoter
Since KDM2B did not alter the methylation modification of the Il6 promoter, we wondered whether KDM2B affected the chromatin conformation of the Il6 promoter. Interestingly, DNase I sensitivity assays showed that the chromatin accessibility of the Il6 promoter but not the Tnf or Ifnb1 promoter was significantly decreased in KDM2B-deficient macrophages stimulated with LPS or poly(I:C) (Fig. 4a, b). Furthermore, FAIRE assays showed that the chromatin conformation of the Il6 promoter but not the Tnf or Ifnb1 promoter was less open in KDM2B-deficient macrophages than in KDM2B-sufficient macrophages upon LPS stimulation (Fig. 4c). Together, these data demonstrate that KDM2B enhances Il6 transcription by increasing the chromatin accessibility of the Il6 promoter.
Fig. 4.
KDM2B increases the chromatin accessibility of the Il6 promoter. a, b Chromatin accessibility of the Il6, Tnf and Ifnb1 promoter regions assayed by DNase I sensitivity analysis in Kdm2b−/− (KO) and Kdm2b+/+ (WT) macrophages stimulated with LPS (a) or poly(I:C) (b) for the indicated times. c FAIRE assays of the Il6, Tnf, and Ifnb1 promoters in Kdm2b−/− and Kdm2b+/+ macrophages stimulated with LPS for the indicated times. The data are from three independent experiments (a–c, mean ± s.e.m.). **P < 0.01
KDM2B interacts with Brg1 to remodel chromatin of the Il6 promoter
Next, we investigated the mechanism by which KDM2B affected the chromatin accessibility of the Il6 promoter. Protein complexes immunoprecipitated by an antibody against KDM2B from lysates of LPS-stimulated macrophages were subjected to mass spectrometry analysis to identify possible KDM2B-associated proteins involved in chromatin remodeling (Supplementary Fig. 6a). Among the KDM2B-interacting proteins detected in the immunoprecipitates, Brahma-related gene 1 (Brg1) (also known as Smarca4) attracted our attention (Supplementary Fig. 6a–c). Brg1 is the core catalytic subunit of the SWI/SNF chromatin remodeling complex, which has been reported to be necessary for the transcriptional activation of secondary response genes, such as Il6.29 Immunoprecipitation assays further confirmed that KDM2B interacted with Brg1 in macrophages at the early phase after stimulation with LPS (Fig. 5a, b). We then determined the domain required for the interaction between KDM2B and Brg1 and found that KDM2B associated with Brg1 via its LRR domain (Fig. 5c, d). Furthermore, wild-type KDM2B but not a KDM2B mutant with an LRR domain deletion (KDM2B ΔLRR) enhanced Il6 promoter reporter activation (Supplementary Fig. 7a). Both wild-type KDM2B and the demethylase activity-deficient KDM2B mutant (KDM2B-ΔJmjc) rescued IL-6 production induced by LPS in Kdm2b−/− macrophages, while the KDM2B-ΔLRR mutant could not (Supplementary Fig. 7b), suggesting that the LRR domain of KDM2B was required for the enhancement of IL-6 production mediated by KDM2B. To further reveal the in vivo recruitment of Brg1 to the Il6 promoter by KDM2B, we performed sequential ChIP (Re-ChIP) assays and found that KDM2B and Brg1 formed a complex to bind the Il6 promoter in macrophages stimulated by LPS (Fig. 5e). Furthermore, ChIP assays confirmed that KDM2B deficiency reduced the recruitment of Brg1 to the Il6 promoter in macrophages upon TLR activation (Fig. 5f) while the associations of Brg1 with the promoters of Tnf and Ifnb1 genes were not altered by KDM2B deficiency (Supplementary Fig. 8a). To verify the role of Brg1 in Il6 transcription, we knocked down Brg1 expression in macrophages using two specific siRNAs and found that Brg1 silencing impaired the chromatin accessibility of the Il6 promoter region upon LPS stimulation (Fig. 5g; Supplementary Fig. 8b–d). Furthermore, Brg1 silencing significantly inhibited IL-6 production at the mRNA and protein levels induced by LPS or poly(I:C) in macrophages (Fig. 5h, i; Supplementary Fig. 8e). These results indicate that KDM2B recruits Brg1 to the Il6 promoter and enhances Il6 transcription through Brg1-mediated chromatin remodeling.
Fig. 5.
KDM2B interacts with Brg1 to increase the chromatin accessibility of the Il6 promoter. a, b Immunoblot analysis of KDM2B and Brg1 in protein complexes immunoprecipitated with an anti-KDM2B antibody (a) or anti-Brg1 antibody (b) from lysates of peritoneal macrophages stimulated with LPS for the indicated times. c, d HEK 293T cells were cotransfected with Flag-Brg1 and V5-KDM2B-WT (wild type), V5-KDM2B-ΔJmjc (Jmjc domain deletion), V5-KDM2B-ΔCXXC (CXXC domain deletion), V5-KDM2B-ΔPHD (PHD domain deletion), V5-KDM2B-ΔFBOX (F-box domain deletion), or V5-KDM2B-ΔLRR (LRR domain deletion), followed by immunoprecipitation with an anti-V5 antibody (c) or anti-Flag antibody (d) and immunoblot analysis with an anti-Flag antibody (c) or anti-V5 antibody (d). e Re-ChIP-PCR assay of KDM2B-Brg1 interaction at the Il6 promoter in peritoneal macrophages stimulated with LPS for 1 h. First-round ChIP (1st antibody) was performed with an anti-KDM2B antibody or IgG. Eluted samples were subjected to second-round ChIP (2nd antibody). Line 5 was the input. f ChIP-qPCR analysis of Brg1 occupancy at the Il6 promoter in Kdm2b−/− (KO) and Kdm2b+/+ (WT) peritoneal macrophages stimulated with LPS or poly(I:C) for the indicated times. g Chromatin accessibility of the Il6 promoter region assayed by DNase I sensitivity analysis in macrophages transfected with Brg1 siRNA (Brg1 siRNA-1) or control siRNA (Ctrl siRNA-1) and, 48 h later, stimulated with LPS for the indicated times. h, i Q-PCR analysis of Il6 mRNA expression and ELISA of IL-6 production in supernatants of peritoneal macrophages transfected with Brg1 siRNA (Brg1 siRNA-1) or control siRNA (Ctrl siRNA-1) and, 48 h later, stimulated with LPS (h) or poly(I:C) (i) for the indicated times. The data are from three independent experiments (f–i, mean ± s.e.m.) or are representative of three independent experiments with similar results (a–e). *P < 0.05; **P < 0.01
KDM2B recruits RNA polymerase II for Il6 gene transcriptional activation
Chromatin with a more open conformation of the Il6 promoter, mediated by the KDM2B and Brg1 complex, might provide more access for recruitment of the basal transcription machinery. Interestingly, we identified an interaction of KDM2B and RNA Polymerase II (RNA Pol II) in macrophages upon LPS stimulation (Fig. 6a). Brg1 silencing did not affect the interaction between KDM2B and RNA Pol II in LPS-stimulated macrophages, suggesting that their interaction was independent of Brg1 (Supplementary Fig. 8f). Moreover, KDM2B deficiency impaired the binding of RNA Pol II to the Il6 promoter in LPS-stimulated macrophages, which further resulted in attenuated Il6 mRNA transcription (Fig. 6b). Thus, our data indicate that KDM2B selectively binds the Il6 promoter and recruits Brg1 to make the chromatin state of the Il6 promoter more open. The enhanced binding of RNA Pol II mediated by KDM2B to the Il6 promoter with an open chromatin conformation further promotes Il6 transcription and production (Supplementary Fig. 9).
Fig. 6.
KDM2B recruits RNA Pol II for Il6 gene transcriptional activation. a Immunoblot analysis of RNA Pol II and KDM2B in protein complexes immunoprecipitated with an anti-KDM2B antibody from lysates of peritoneal macrophages stimulated with LPS for the indicated times. b ChIP-qPCR analysis of RNA Pol II occupancy at the Il6 promoter in Kdm2b−/− (KO) and Kdm2b+/+ (WT) peritoneal macrophages stimulated with LPS for the indicated times. Data are from three independent experiments (b, mean ± s.e.m.) or are representative of three independent experiments with similar results (a). **P < 0.01
Discussion
Innate immune cells, including macrophages and dendritic cells, play critical roles in the production and secretion of IL-6 in response to infections and tissue injuries.30 Dysregulated excessive production of IL-6 often drives disease progression and maintains aberrant immunological reactions in infection, inflammation and cancers.12,31 Hence, IL-6 production must be strictly controlled at the level of both transcription and translation. Here, we report a novel function of KDM2B in epigenetically positively regulating inflammation by promoting Il6 gene expression at the transcriptional level.
Transcriptional induction of proinflammatory cytokines involves several significant epigenetic modifications, including DNA methylation, histone modification, and chromatin remodeling. The inducible transcription programs triggered by the innate immune system consist of primary and secondary response genes that differ in their kinetics of expression and in their requirement for new protein synthesis and chromatin remodeling at their promoters.32,33 The majority of primary response genes (PRGs), for example, Tnf and Il1b, are rapidly induced upon innate stimulus and do not require de novo protein synthesis. They are characterized by the presence of CpG islands (CGIs) in their gene promoters and constitutively active chromatin. The promoters of most PRGs are preassociated with RNA Polymerase II in unstimulated cells and exhibit high levels of H3K4me3 and H3Ac even prior to stimulation, and then innate stimuli immediately induce the transcription of these genes.34,35 However, the molecular mechanisms underlying the transcription of secondary response genes (SRGs), such as Il6, are more complicated than PRGs and remain obscure. The inducible expression of SRGs is much slower in response to stimulation owing to high nucleosome occupancy at the promoter, for which activation requires remodeling of promoter-associated nucleosomes by ATP-dependent SWI/SNF chromatin remodeling complexes. For example, the Il6 promoter is cleaved at a low efficiency in unstimulated cells and exhibits increased cleavage with increased chromatin accessibility following LPS stimulation.29,32 The Il6 gene is a SWI/SNF-dependent secondary response gene in macrophages, and a previous study demonstrated that simultaneous knockdown of Brg1 and Brahma (Brm), the core ATPase subunit of SWI/SNF complexes, inhibits IL-6 production.29 Nevertheless, considering that the recruitment of the SWI/SNF complex to a proinflammatory gene promoter is not a sequence-specific procedure,36 what mediates selective nucleosome remodeling at the Il6 promoter is unknown.
Our study confirms that histone demethylase KDM2B is required for Brg1-mediated chromatin remodeling at the Il6 promoter. KDM2B selectively binds to the promoter of the Il6 gene but not gene promoters of other proinflammatory cytokines, including Ifnb1 or Tnf, which is necessary for Il6 transcription. However, this modulating function is independent of its histone demethylase activity. We prove that KDM2B associates with Brg1-containing SWI/SNF complexes to catalyze chromatin remodeling of the Il6 promoter and then provide access for additional RNA Pol II recruitment as well as transcription initiation. Thus, KDM2B plays an important role in the coupling of chromatin remodeling and recruitment of Pol II as well as the general transcription machinery. This study uncovers the transcriptional regulatory mechanisms of KDM2B underlying the activation and fine tuning of Il6 gene expression for inflammation initiation and amplification, independent of its histone demethylase activity.
In regard to its classical demethylase function, KDM2B catalyzes the demethylation of H3K4me3, which is the biological marker of active genes that is permissive for transcription. Thus, KDM2B usually functions as a transcription repressor in multiple gene transcription processes. For example, variant poly-comb repressive complex 1.1 (PRC1.1) is recruited to CpG islands of lineage-specific genes through DNA-binding activity of KDM2B, which represses expression of these genes and maintains the undifferentiated state of mouse embryonic fibroblasts.21 To date, no report has addressed the role of KDM2B in promoting the gene transcription program. In our study, KDM2B is required for Il6 gene transcription activation in a demethylase-independent manner. Our study uncovers a novel function of KDM2B in chromatin remodeling and transcription initiation of the Il6 gene.
In conclusion, our study identifies KDM2B as a positive regulator of inflammation and provides a novel gene-specific manner of epigenetic regulation of proinflammatory IL-6 production. Since excessive production of IL-6 is involved in the pathogen processes of several chronic inflammatory and autoimmune diseases, this gene-specific epigenetic regulation of IL-6 makes it possible to effectively inhibit IL-6 production in inflammatory tissues by using a selective KDM2B inhibitor.
Supplementary information
Acknowledgements
We thank X. Sun and M. Jin for technical assistance. This work was supported by the National Natural Science Foundation of China (31570871, 81571541, 81771695, 31770970, and 81770094), Program of Shanghai Chief Scientist of Medical and Health Subject (2018BR16), and Shuguang Program sponsored by the Shanghai Education Development Foundation and Shanghai Municipal Education Commission (18SG33).
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Qingqing Zhou, Yunkai Zhang, Bo Wang
Contributor Information
Xingguang Liu, Phone: +86 21 5562 0605, Email: liuxg@immunol.org.
Zhenzhen Zhan, Phone: +86 21 6156 9870, Email: zhanzz@tongji.edu.cn.
Supplementary information
The online version of this article (10.1038/s41423-019-0251-z) contains supplementary material.
References
- 1.O’Shea JJ, Ma A, Lipsky P. Cytokines and autoimmunity. Nat. Rev. Immunol. 2002;2:37–45. doi: 10.1038/nri702. [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 4.Cao X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat. Rev. Immunol. 2016;16:35–50. doi: 10.1038/nri.2015.8. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q, Raoof M, Chen Y, Sumi Y, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat. Rev. Rheum. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 7.Mills KH. TLR-dependent T cell activation in autoimmunity. Nat. Rev. Immunol. 2011;11:807–822. doi: 10.1038/nri3095. [DOI] [PubMed] [Google Scholar]
- 8.Rosser EC, et al. Regulatory B cells are induced by gut microbiota-driven interleukin-1beta and interleukin-6 production. Nat. Med. 2014;20:1334–1339. doi: 10.1038/nm.3680. [DOI] [PubMed] [Google Scholar]
- 9.Kopf M, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka Y, Martin Mola E. IL-6 targeting compared to TNF targeting in rheumatoid arthritis: studies of olokizumab, sarilumab and sirukumab. Ann. Rheum. Dis. 2014;73:1595–1597. doi: 10.1136/annrheumdis-2013-205002. [DOI] [PubMed] [Google Scholar]
- 11.Atreya R, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat. Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 12.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J. Clin. Invest. 2011;121:3375–3383. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat. Rev. Genet. 2013;14:204–220. doi: 10.1038/nrg3354. [DOI] [PubMed] [Google Scholar]
- 14.Ventham NT, Kennedy NA, Nimmo ER, Satsangi J. Beyond gene discovery in inflammatory bowel disease: the emerging role of epigenetics. Gastroenterology. 2013;145:293–308. doi: 10.1053/j.gastro.2013.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narlikar GJ, Sundaramoorthy R, Owen-Hughes T. Mechanisms and functions of ATP-dependent chromatin-remodeling enzymes. Cell. 2013;154:490–503. doi: 10.1016/j.cell.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black JC, Van Rechem C, Whetstine JR. Histone lysine methylation dynamics: establishment, regulation, and biological impact. Mol. Cell. 2012;48:491–507. doi: 10.1016/j.molcel.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kooistra SM, Helin K. Molecular mechanisms and potential functions of histone demethylases. Nat. Rev. Mol. Cell. Biol. 2012;13:297–311. doi: 10.1038/nrm3327. [DOI] [PubMed] [Google Scholar]
- 18.Park SY, Park JW, Chun YS. Jumonji histone demethylases as emerging therapeutic targets. Pharm. Res. 2016;105:146–151. doi: 10.1016/j.phrs.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y. Histone lysine demethylases: emerging roles in development, physiology and disease. Nat. Rev. Genet. 2007;8:829–833. doi: 10.1038/nrg2218. [DOI] [PubMed] [Google Scholar]
- 20.Tsukada YI, Fang J, Erdjument-Bromage H, Warren ME, Tempst P. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 21.Jin H, et al. Kdm2b maintains murine embryonic stem cell status by recruiting PRC1 complex to CpG islands of developmental genes. Nat. Cell Biol. 2013;15:373–384. doi: 10.1038/ncb2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang G, He J, Zhang Y. Kdm2b promotes induced pluripotent stem cell generation by facilitating gene activation early in reprogramming. Nat. Cell Biol. 2012;14:457–466. doi: 10.1038/ncb2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janzer A, et al. The H3K4me3 histone demethylase Fbxl10 is a regulator of chemokine expression, cellular morphology, and the metabolome of fibroblasts. J. Biol. Chem. 2012;287:30984–30992. doi: 10.1074/jbc.M112.341040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han XR, et al. KDM2B/FBXL10 targets c-Fos for ubiquitylation and degradation in response to mitogenic stimulation. Oncogene. 2016;35:4179–4190. doi: 10.1038/onc.2015.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, et al. Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase Btk. Nat. Immunol. 2011;12:416–424. doi: 10.1038/ni.2015. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature. 2015;525:389–393. doi: 10.1038/nature15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon JM, Giresi PG, Davis IJ, Lieb JD. Using formaldehyde-assisted isolation of regulatory elements (FAIRE) to isolate active regulatory DNA. Nat. Protoc. 2012;7:256–267. doi: 10.1038/nprot.2011.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirezcarrozzi VR, et al. Selective and antagonistic functions of SWI/SNF and Mi-2beta nucleosome remodeling complexes during an inflammatory response. Genes Dev. 2006;20:282–296. doi: 10.1101/gad.1383206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. CSH Perspect. Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hargreaves DC, Horng T, Medzhitov R. Control of inducible gene expression by signal-dependent transcriptional elongation. Cell. 2009;138:129–145. doi: 10.1016/j.cell.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iglesias MJ, et al. Combined chromatin and expression analysis reveals specific regulatory mechanisms within cytokine genes in the macrophage early immune response. PLoS One. 2012;7:e32306. doi: 10.1371/journal.pone.0032306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fowler T, Sen R, Roy AL. Regulation of primary response genes. Mol. Cell. 2011;44:348–360. doi: 10.1016/j.molcel.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramirez-Carrozzi VR, et al. A unifying model for the selective regulation of inducible transcription by CpG islands and nucleosome remodeling. Cell. 2009;138:114–128. doi: 10.1016/j.cell.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Euskirchen G, Auerbach RK, Snyder M. SWI/SNF chromatin-remodeling factors: multiscale analyses and diverse functions. J. Biol. Chem. 2012;287:30897–30905. doi: 10.1074/jbc.R111.309302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.