Abstract
Maternal sepsis is “a life-threatening condition defined as organ dysfunction resulting from infection during pregnancy, childbirth, post-abortion, or postpartum period.” (World Health Organisation, 2017). Serious infection during, or immediately after, pregnancy may go initially unrecognized in an otherwise young and healthy group, who nevertheless do have a compromized immune system. Secondly, whilst malaise, flushes, nausea, vomiting and abdominal pain are common in pregnancy, each can herald sepsis with rapid demise for mother and baby. The MBRRACE-UK report in 20171 found an overall sepsis-related maternal mortality rate of 0.56 per 100,000 maternities with a mortality rate from genital tract sepsis of 0.28 per 100,000 maternities. This review will focus on the major causes, recognition, differentiation and microbiological management of sepsis in pregnancy, using two detailed cases to illustrate.
Keywords: covid-19, enterovirus, exotoxins, influenza, PVL-SA, sepsis, toxic shock
Introduction
Assessment of sepsis
Clinical approach: as well as the traditional systems-based history and Sepsis-6 approach, it is a useful skill to be able to evaluate a patient with some basic microbiological principles in mind, rather than simply following unit guidelines. This approach helps with direct questioning to pertinent areas, consideration of infections that are common pitfalls for the unwary or inexperienced clinician, and ensuring nothing is overlooked.
A targeted history – the following are a series of questions which focus on infection, the answers to which go a long way towards narrowing the differential diagnosis of the underlying site and type of organism;
-
1.
Recent febrile illnesses; chills, rigors, with or without myalgia suggest staphylococcal/streptococcal bacteraemia, or influenza
-
2.Exposure history
-
a.Have there been any infectious contacts; e.g. children/family with tonsillitis, scarlet fever or impetigo (Group A beta-haemolytic streptococci, GAS)
-
b.Has there been contact with animals, particularly ones that have given birth recently; e.g. cats and dogs (Pasteurella multocida, Q fever, Chlamydophila).
-
c.Has there been recent foreign travel, or hospital admission (consider multi resistant pathogens).
-
a.
-
3.
Recurrent boils or abscesses; Staphylococcus aureus is a very likely organism. PVL-SA (Panton Valentine Leukocidin S. aureus) is a toxin produced by certain types of S. aureus. Recurrent skin infections may occur, including boils (furunculosis), carbuncles, folliculitis and cellulitis. Cutaneous lesions can be more than 5 cm in size, and may be associated with necrosis. Pain and erythema may be out of proportion to the severity of signs.
-
4.
Prior infections in pregnancy and sensitivities; e.g. urinary - consider Extended Spectrum Beta lactamase (ESBL) producing coliforms.
-
5.Allergies – ask exactly what happens.
-
a.If beta-lactam allergic, treatment with penicillins is not necessarily precluded if it was only weak reaction or only a possible allergy (e.g. vomiting with co-amoxiclav is usually an intolerance, not an allergy)
-
b.If a rash was reported as part of a previous beta-lactam allergic reaction then there is a 3%–10% chance of cross reaction with cephalosporins
-
c.If anaphylaxis reported to penicillins previously, also avoid cephalosporins and carbapenems
-
d.Check if related antibiotics were tolerated without a problem
-
a.
-
6.Diarrhoea and vomiting
-
•Gastroenteritis; suggestive of food borne pathogens and other family members may be affected
-
•Early toxic shock should be considered (exotoxins acting as enterotoxins) if there is also a rash
-
•Consider Clostridium difficile if there has been recent use of broad spectrum antibiotics
-
•Think of norovirus if vomiting predominates and others are affected
-
•
-
7.
Recent antimicrobials; the current infection is more likely to be resistant to recently used antibiotics and other options should be chosen.
Gram positive and Gram negative sepsis are usually very different – traditionally, infections are managed according to their likely site of origin given the symptomatology; respiratory or gut, for example. However, using a more ‘microbiological’ approach, namely whether infections are more likely due to ‘Gram positive ‘or ‘Gram negative’, can be very helpful in pointing to the focus of infection and thus pertinent ancillary questions more precisely.
The Gram stain demystified: bacteria stain blue or pink. Hans Gram invented the quickest and most useful test we still do in bacteriology. Broadly speaking, ‘blue’ stained bacteria are skin/mucus membrane based – (“cold and blue”) whereas Gram negatives-pink stained are ‘warm’ and so more likely found inside the body, e.g. gut and urinary pathogens.
There is some overlap, but broadly, a patient with skin related sepsis will have ‘Gram positive’ infection and urosepsis or abdominal sepsis mainly ‘Gram negative ‘infection.
The shape of the stained bacteria can also enable provisional identification in minutes. For example, pus showing Gram positive (blue) cocci (spherical or berry shaped; kokkus means berry) in clusters (staphyle (Gr) meaning bunch of grapes) would suggest Staphylococci as the cause of the infection. Gram positive cocci in chains, (Streptos (Gr) meaning twisted chain) are Streptococci. Gram negative cocci, especially if in pairs, are not part of the gut flora except for Neisseria spp (e.g. N. gonorrhoeae).
Not all gut bacteria are Gram negative. Some colonic bacteria include Gram positive anaerobes such as Clostridium spp, and also enteral streptococci (known as enterococci or faecal streptococci).
Gram negative ‘rods’ or bacilli include coliforms and Pseudomonas spp. “Coliforms” is an umbrella term including Enterobacter coli, Klebsiella spp and Enterobacter spp, associated with urinary or abdominal sepsis.
Anaerobic Gram-negative rods, such as Bacteroides spp are sometimes associated with preterm premature rupture of membranes, and cerclage.
Empiric therapy: patients with skin sepsis, abscess or toxic shock (confluent rash, shock, organ failure) will almost always be Gram positive in origin, probably staphylococcal, and empirical flucloxacillin/vancomycin should be started after cultures.
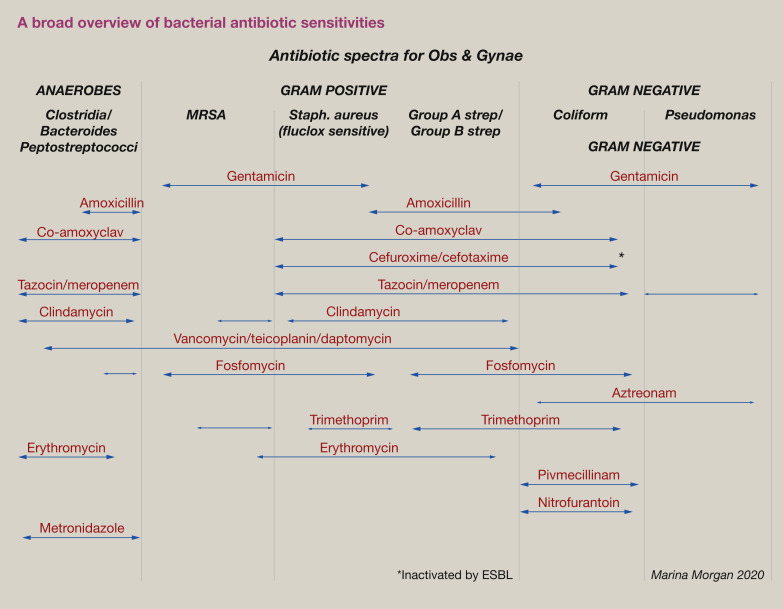
In contrast, urinary or gut sepsis is predominantly Gram negative. For empiric treatment, aminoglycosides/piperacillin-tazobactam or meropenem are most appropriate. Figure 1 provides a broad overview of the antibiotic sensitivity of different bacterial groups.
Figure 1.
A broad overview of bacterial antibiotic sensitivities.
Case 1
A 29-year-old woman in the 18thweek of her first pregnancy is admitted with a temperature of 38 °C, loin pain, back pain, abdominal pain, rigors, severe diarrhoea, and vomiting. She has a history of recurrent pyelonephritis, with two bouts in early pregnancy. A renal scan found no calculi on the last admission. She is now in her third week of prophylactic cephalexin. Her partner was well and no other contacts were unwell. She is reportedly allergic to penicillin but experienced only a mild rash in childhood.
She is not confused but has a respiratory rate of 26 breaths per minute, an oxygen saturation of 90% on room air, and a heart rate of 140/min.Her systolic blood pressure is 100 mmHg, and she has not passed urine for 4 h.There is a fetal tachycardia of 170/min. Her chest is clear on auscultation, and only mild abdominal tenderness is elicited on palpation.
What are the four most likely causes of her sepsis?
The differential diagnosis should include;
-
1.
Urosepsis
-
2.
Gastroenteritis
-
3.
C. difficile infection
-
4.
Early toxic shock (exotoxins acting as enterotoxins)
Acute pyelonephritis is the most common cause of septic shock in pregnancy.
What specimens would you send to the laboratory?
‘Routine’ blood tests should include venous blood gas for glucose and lactate, full blood count, clotting studies, urea, electrolytes, creatinine, and C-reactive protein (CRP).
Two sets of blood cultures should be obtained prior to immediate antibiotic administration, and a midstream urine specimen for culture. A stool sample should be sent for C. difficile testing and routine culture when it becomes available.
With a working diagnosis of sepsis, she is commenced on intravenous fluids, catheterized, and the sepsis 6 protocol is applied. Her serum lactate is 2.2 mmol/L. She is isolated with enteric (blood and body fluid) precautions in a single room. Her diarrhoea continues and is noted to be particularly foul smelling, and a sample is sent for culture and C. difficile toxin testing. Her case is discussed with the consultant and the intensive care unit but the decision is made to monitor urinary output, observe closely and manage as per the unit protocol for sepsis on the obstetric high dependency unit.
The empirical obstetric unit antibiotic guideline for urosepsis recommends cefuroxime with additional gentamicin if in septic shock. However, the most recent urinary culture results (7 days previously) reveal an ‘Extended Spectrum Beta-Lactamase’ (ESBL) producingEnterobacter cloacaeresistant to trimethoprim, cephalexin, co-amoxiclav, piperacillin-tazobactam and gentamicin. TheE. cloacaeis, however, sensitive to ciprofloxacin, fosfomycin, pivmecillinam and meropenem.
What antimicrobial agent would you use?
Table 1 summarises the antibiotic choices for pregnancy related urinary tract infection. Neither empirical agent would cover the likely E. cloacae in this case. ESBL producers are resistant to cephalosporins, and cephalosporins are likely to encourage C. difficile infection.
Table 1.
Antibiotic treatment of urinary tract infection during pregnancy
| Oral agent | Intravenous agent | |
|---|---|---|
| Simple UTI (cystitis) with non ESBL producing Gram negatives |
See your local guidelines
|
N/A |
| Simple UTI (cystitis) with ESBL producing Gram negatives |
|
N/A |
| Complex UTI (pyelonephritis) | N/A |
|
| Complex UTI (history of, or suspected, ESBL producing organism) | N/A | Carbapenem (e.g. meropenem) then consider prophylaxis – e.g. nitrofurantoin |
| Severe sepsis and pyelonephritis | N/A | If no previous gentamicin resistant organisms, a stat dose of gentamicin (3 mg/kg or see your local policy) plus piperacillin-tazobactam |
In this case meropenem would be the broadest agent to cover the likely diagnosis of urosepsis and the recent E. cloacae is fortunately sensitive to it.
Gentamicin is currently considered the safest aminoglycoside for use in Gram negative septicaemia, however it never provides cover for anaerobes or streptococci. Meropenem is not renally toxic and has the necessary spectrum of activity. For pyelonephritis, the treatment course should be for a total of 14 days, followed by oral nitrofurantoin prophylaxis (upper renal tract infection is not responsive to nitrofurantoin).2
Treatment of acute pyelonephritis and sepsis in pregnancy
Following urine and blood cultures, pyelonephritis should be treated with intravenous empirical antimicrobials for at least 48 h. Hydronephrosis, renal stones and abscess should be excluded with an ultrasound scan. Usually the empirical guideline should be followed, but confirm that there is no need to amend more usual prescribing to cover resistant uro-pathogens (as in this case).
Given its potential for renal toxicity, regular gentamicin is best avoided unless the risk of renal damage is outweighed by the likelihood of death from sepsis. A single dose lessens the risk of toxicity.
Resolution of CASE 1:the blood cultures and urine yieldE. cloacaesensitive to meropenem, fosfomycin and pivmecillinam, and theC. difficiletoxin test proved positive. The patient improved dramatically on meropenem therapy and was discharged home well after 7 days with the pregnancy intact, to continue intravenous outpatient ertapenem therapy for a further 7 days to complete a 14 days course.
What are the infection control implications of this case?
In this case, diarrhoea itself warranted isolation with enteric precautions in a side room and communication with hospital ‘Infection Control’ also would have been appropriate for the ESBL carriage and likelihood of C. difficile infection.
Fortunately, the E. cloacae was not resistant to carbapenems. ‘Carbapenemase Producing Enterobacteriaceae’, or CPE producers, can be resistant to all antimicrobials, occasioning usage of uncommon, potentially toxic agents or combinations thereof. Only really case reports of CPE have been reported to date in pregnancy. CPEs are particularly associated with foreign travel, and easily spread, with many hospital outbreaks causing closure of units to admissions. Most hospitals actively screen high risk patients for CPE using rectal swabs, and barrier nurse patients until proven negative.
Why might this lady have a positive C. difficile toxin test?
Particularly offensive diarrhoea is a feature of C. difficile infection. Diarrhoea is often associated with sepsis, but is especially common in exotoxin-related sepsis. The prime example is Toxic Shock Syndrome (TSS). Produced by Gram positive organisms such as staphylococci, streptococci, or C. difficile superinfection, exotoxins act on gut receptors stimulating peristalsis and vomiting, initially commonly misdiagnosed as simple gastroenteritis.
In this case, many weeks of antimicrobials had disrupted the gut microbiome, denuding the protective layer of bacteria (anaerobic wallpaper) lining the gut adjacent to the enterocytes. Overgrowth of more resistant anaerobe C. difficile follows, producing exotoxins and massive inflammation, very offensive diarrhoea and ultimately enterocytes slough off. Delayed diagnosis or incorrect treatment with antiperistalsis agents results in colonic perforation due to toxic megacolon.
Diagnosis is by toxin-testing of stool, not culture. Presence of actual toxin means the diarrhoea is likely due to C. difficile. Mere PCR (polymerase chain reaction) positive stools only prove the presence of the gene capable of producing toxin and does not necessarily need treatment.
Treatment of C. difficile in pregnancy
Stopping intercurrent meropenem is impossible here so has to continue, along with C. difficile therapy. Oral metronidazole can be very nauseating and increasing resistance is reported. Treatment with oral vancomycin 125 mg 6-hourly for 10 days is more pleasant, effective and causes less disruption to the residual microbiome anaerobes, and a quicker recovery. Mostly occurring peri-partum, especially after caesarean prophylaxis with cephalosporins, C. difficile infection can present at any time, with toxic megacolon necessitating colectomy in up to 5% of patients.
What future prophylaxis may be considered?
There is no easy option. Amoxicillin and cephalexin, the most commonly used prophylactic antibiotics in pregnancy, will be inactivated by the ESBLS and therefore ineffective. The gut will remain a reservoir for future infection, as well as a possible focus around any renal stones which may have been seeded during the bacteraemia or the ascending infection.
The case should be discussed with a microbiologist as there may be some extra sensitivities possible. Examples would be fosfomycin and pivmecillinam, but fosfomycin is only effective in lower tract infections and pivmecillinam cannot be used in penicillin allergic patients. This lady has to be assumed to be penicillin allergic although her history is of a seemingly mild allergy. After pregnancy it may be possible to consider penicillin challenge and skin testing since penicillins are useful for so many infections and most patients are not in fact truly penicillin allergic. Nitrofurantoin should be avoided in patients with G6PD deficiency or after 37 weeks in pregnancy (because of the effect on immature neonatal erythrocytes).
Case 2
A 36-year-old mother of three becomes very unwell with rigors and temperature of 38 °C 9 h following an instrumented vaginal delivery. The baby was well at delivery and the mother complains of feeling intensely cold, alternating with bouts of sweating. Agitated, and mildly confused, with a severe headache, and generalized severe myalgia (all limbs and back) she has been vomiting with worsening diarrhoea and abdominal pain.
On examination, she looks unwell, with visible sweating. She dislikes being touched and complains of whole-body pain. She is thirsty and mildly dehydrated. There is no meningism or photophobia. Her temperature is 38.5 °C, blood pressure is 90/65 mmHg and her respiratory rate 30 bpm. Her venous oxygen saturation is 95% on air. A fine erythematous rash is noted on her trunk and limbs, with some bilateral conjunctival reddening.
What diagnoses might you consider in the differential diagnosis?
-
1.
Toxic shock (staphylococcal or streptococcal)
-
2.
Influenza
-
3.
COVID-19
-
4.
Enterovirus infection
This case presents with overlapping features of viral infection and bacterial toxic shock syndromes and a more detailed history together with appropriate laboratory tests are essential to help differentiate between them (Table 2 and Figure 2, Figure 3, Figure 4, Figure 5 ).
Table 2.
The differential presentations of influenza, COVID-19, Toxic Shock and Streptococcal Toxic Shock syndromes
| Toxic Shock Syndrome (TSS) (S. aureus) |
Streptococcal Toxic Shock Syndrome (STSS) due to GAS | Influenza | COVID-19 | Enterovirus |
|---|---|---|---|---|
| Often associated with only a small ‘insignificant’ wound infection Rarely now related to menstruation |
May be of vaginal origin Often contact history of GAS (sore throat/impetigo/scarlet fever) |
Seasonal; autumn-winter | Not known | Seasonal; Spring/summer |
| General malaise, myalgia | Rapid illness and prostration, myalgia | Very acute onset, severe myalgia Headache, prostration |
‘Influenza like illness’ | Fatigue, myalgia and may have gastrointestinal symptoms |
| Usually no cough or sore throat | Usually no cough Recent sore throat common |
Cough, sore throat upper respiratory tract symptoms, runny nose | Cough Shortness of breath with pneumonia later Sore throat uncommon |
Cough, [pneumonia uncommon] |
| Headache | Headache | Headache | Headache not common | Severe headache and viral meningitis common |
| High temp/low temp, confusion, prostration | High temp/low temp, confusion, prostration | Fever, severe prostration | Fever, weakness | Fever |
| Vomiting; due to exotoxin production (acting as enterotoxins) | Exotoxins causing diarrhoea and vomiting | Diarrhoea (especially H1N1 ‘swine flu’) Viral gut replication |
Diarrhoea 5%–10% | May or may not have diarrhoea |
| No loss of taste/smell | No loss of taste/smell | No loss of taste/smell | Loss of taste/smell common | No loss of taste/smell |
| All cases of TSS (i.e. staphylococcal TSS) have confluent erythematous rash Multi-organ failure |
Multi-organ failure 10% patients have a rash | No rash May have multi-organ failure late in illness |
Rarely, chillblain like rash some days into the illness Multi-organ failure late in illness |
Variable rash, usually maculopapular but may be vesicular and involve mouth |
| Haemoptysis not associated with TSS, (usually associated with PVL-S. aureus pneumonia) | Primary GAS pneumonia rare but haemoptysis a feature | Haemoptysis (if severe influenza pneumonia) | Haemoptysis rare | |
| Very high or rapidly climbing CRP and creatine kinase, lymphopenia | Very high or rapidly climbing CRP and creatine kinase, lymphopenia | Low CRP (unless bacterial superinfection), lymphopenia | Low CRP (unless cytokine storm or bacterial superinfection) lymphopenia | Low CRP lymphopenia |
| Treat with anti- exotoxin antimicrobials and IVIG if not responding | Treatment with anti- exotoxin antimicrobials And IVIG if not responding |
Oseltamivir | No known effective drug therapy | IVIG for neonate if becomes septic |
Figure 2.
The rash of toxic shock syndrome (TSS) affecting chest and shoulders.
Figure 3.
Typical post-STSS peeling and desquamation.
Figure 4.
Group A beta-haemolytic streptococcal septicaemia with the rash of STSS plus thrombocytopenia. The origin of the infection was the vagina. Note the bleeding from the central line access point, secondary to disseminated intravascular coagulation.
Figure 5.
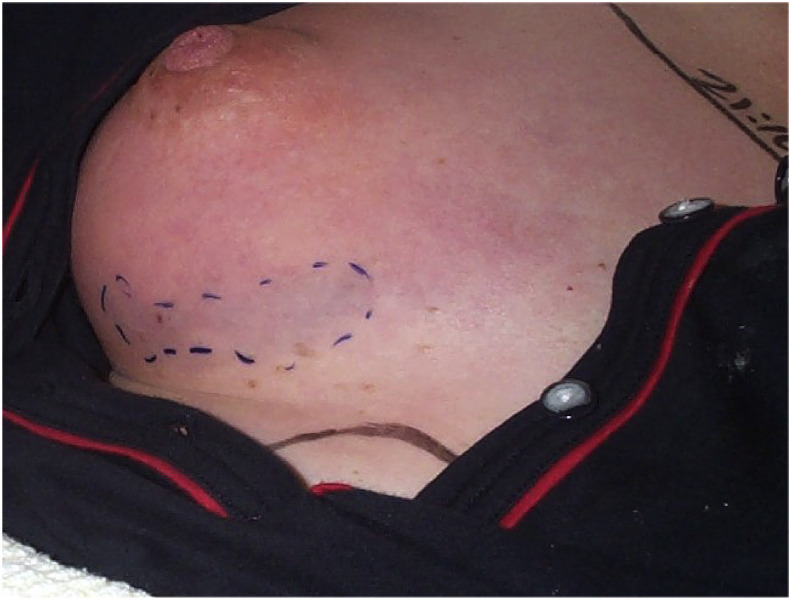
Group A streptoccal (GAS) necrotizing fsasciitis (NF) of the breast 24 h post partum. Note the area of discolouration overlying necrotic breast tissue.
With conjunctival suffusion, sepsis and a developing rash the most likely diagnosis is early streptococcal or staphylococcal toxic shock syndrome.
The developing maculopapular or blanching erythema is exotoxin-related, although sometimes erroneously attributed to beta-lactam allergy. Overall 50% of Group A beta-haemolytic streptococcal (GAS) infections in pregnancy develop into septic shock with very rapid progression within 2 h.
A sudden onset of prostration and severe myalgia is especially typical of influenza. Seasonal influenzas typically occur in late Autumn/winter months. Diarrhoea is occasionally a feature of influenza, particularly associated especially with the H1N1 strain (“swine flu”).
Other viruses, including parainfluenza, enterovirus and more recently the SARS-CoV2 (COVID-19), can also present with an influenza-like illness. Generally, patients with COVID-19 admitted to hospital are more likely to exhibit a non-productive cough with prominent fatigue, gastrointestinal symptoms, and prevalence in the elderly. With chest symptoms, imaging more commonly shows ground-glass opacities in patients with COVID-19, than with influenza.
Myalgia, fever, chills and gastroenteritis can also be a feature of bacterial infections such as streptococcal or staphylococcal bacteraemia and toxic shock.
Enteroviral infections can produce a severe headache and viral meningitis. Rashes are a common feature, and incidence is seasonal, usually in warmer months of the year (late Spring or early Summer).
Primary bacterial gastroenteritis with septicaemia is possible, but is not associated with a rash. Campylobacter and salmonella are the most common bacterial causes of gastroenteritis, and severe systemic infection is rare but is associated with miscarriage and preterm labour. Uncomplicated gastroenteritis should be managed symptomatically unless features of bacteraemia are present, in which case advice from an infection specialist should be sought.
Purely viral gastroenteritis (e.g. norovirus) is extremely contagious and likely to have presented already in family contacts or be prevalent in the community. Vomiting is more predominant than diarrhoea.
What infection control issues are raised?
The case should be discussed with Infection Control since toxic shock, influenza, COVID-19 or enteroviral infection all necessitate barrier nursing, i.e. isolation in a single room with ensuite facilities. Appropriate Personal Protective equipment (PPE) should be worn.
Streptococcal toxic shock syndrome (STSS) has a mortality rate as high as 80%, far higher than TSS, which is more readily diagnosed because of the characteristic rash (Table 3 ). Skin contact and respiratory secretions are the usual mode of spread, and numerous GAS outbreaks and deaths have occurred in maternity units, some involving shared toilet and shower facilities.
Table 3.
Staphylococcal and Streptococcal toxic shock syndrome clinical disease definitions
| Staphylococcal toxic shock syndrome (TSS) | Streptococcal toxic shock syndrome (STSS) (Modified CDC definition 2010) |
|---|---|
|
|
|
Multisystem involvement Three or more of the following systems affected:
|
B. Clinical case definition Multi-organ involvement characterized by two or more of the following:
|
|
Case classification: Probable – 4 of the 5 clinical findings positive Confirmed – case with all 5 clinical findings |
Case classification: Probable – meets clinical case definition (above) plus isolation from non-sterile site Definite – meets clinical case definition (above) plus isolation of Group A streptococcus from a normally sterile site |
| Overall better prognosis – mortality < 10% | Poor prognosis especially if associated with NF- mortality >40% |
Respiratory routes of infection are the primary modes of spread for influenza and COVID-19, but also through direct contact and contamination of surfaces.
Enteroviruses are common in pregnant women, causing fever in >10% of pregnant women in one study. Spread mainly through close family contact or faecal-oral routes with poor hygiene, the most dangerous time is around delivery, when there is a significant risk to the neonate of enteroviral meningitis, myocarditis and death.
What features of the history may help differentiate between these causative agents?
Answers to the following questions may help to focus the differential diagnosis towards, or away, from viral or bacterial causes;
•Are there cases of influenza, COVID-19 or enterovirus currently circulating in the community?
-
•
Have there been any family or close contacts with similar illnesses?
-
•
Has the patient been immunized against influenza?
-
•
Have there been any contacts with tonsillitis, impetigo or scarlet fever? (consider Group A beta-haemolytic streptococci)
-
•
Recent exposure to contacts with recurrent skin infections, boils or abscesses raises the possibility of PVL S. aureus.
-
•
Consumption of undercooked chicken/barbeque? – (salmonella, campylobacter)
-
•
Foreign travel/inpatient stays/multiple courses antimicrobials? (Increased risk of multi-resistant organisms)
What features of the examination may help differentiate between these potential causative agents?
The presence of conjunctival suffusion and a widespread rash strongly suggests toxic shock (Figure 2, Figure 4). In its most typical form, the rash typically blanches with pressure leaving an imprint of fingers on the skin.
Influenza is not associated with a rash or conjunctival suffusion. The enterovirus rash is not usually associated with conjunctival suffusion. COVID-19 very rarely produces odd rashes such as chilblains, and conjunctivitis.
With the most likely diagnosis being streptococcal or staphylococcal TSS, a primary focus of the organism should be sought in the skin or soft tissues, e.g. mastitis, cannula-related infection, urinary, episiotomy wounds, cellulitis.
Skin should be carefully examined for inflamed or purulent injection sites or infected cannulae. Pus should be aspirated from any abscesses and cultured. Swabs should be taken of any discharge, pre-moistened with sterile water when sampling dry or scabby skin.
Cellulitis and blisters suggest deep streptococcal infection (staphylococci usually cause brawny cellulitis and rarely blisters.)
Some 1% of women have perineal carriage of GAS, hence post-delivery ascending infection is equally likely. An ensuing bacteraemia may have seeded elsewhere causing deep infection, such as necrotizing fasciitis (NF) (see Figure 5).
Any skin discolouration or bruising together with a history of disproportionate pain (pain score 7–10/10) or rapidly escalating need for analgesia, culminating in opiates, suggests necrotizing fasciitis (NF). Early in the natural history of this condition there is nothing to see, as infection spreads along the fascia below the surface, visible only when ascending infection causes skin discolouration (see Figure 5).
Differentiating ‘after pains’ from deep infection is difficult, but other features of sepsis are usually emerging and can be found if they are sought after.
What investigations should be performed?
Blood should be taken for venous gas analysis, glucose and lactate levels, full blood count, clotting, urea, electrolytes, creatinine, liver function tests, C-reactive protein (CRP) and creatinine kinase.
Repeating the blood tests 4–6 h later is helpful in serious bacterial infection. Administration of intravenous fluids will cause some haemodilution, but the haemoglobin will fall disproportionately to the haematocrit if intravascular haemolysis occurs as part of streptococcal septicaemia, and lymphopenia may be prominent, with counts as low as 0.1–0.2 x 109/L, in streptococcal toxic shock.
The peripheral blood neutrophil count may be very high or very low in severe sepsis. Severe neutropenia suggests the action of leucocidal toxins, e.g. S. aureus producing Panton Valentine Leucocidin.
The CRP usually remains low in purely viral infections, and rises extremely rapidly (doubling within hours) and is significantly raised in severe bacterial infections such as toxic shock or necrotizing fasciitis.
The creatinine kinase (CK) rises significantly in muscle inflammation and necrosis, but post-delivery may be moderately raised naturally. A significant rise in CK over a short time is suggestive of deep ongoing infection and should prompt examination for possible necrotizing fasciitis (the deep fascia being adjacent to muscle, superficial adjacent to fat.) An exception is the breast where, with little underlying muscle, the CK may well be normal even in NF (see Figure 5).
From a microbiological point of view, investigations should include;
•Two sets of blood cultures
-
•
Throat and vaginal swabs
-
•
Other appropriate wound swabs
-
•
Urine microscopy and culture
-
•
Breast milk microscopy and culture if there is mastitis
Early Gram stain aids empirical therapy.
If viral infection is likely, perform nasal swabs (influenza, COVID-19) and throat swabs (enterovirus PCR, COVID-19) using viral transport medium.
The CRP on admission is 320 mg/L, rising to 428 mg/L 4 h later. The haemoglobin falls from 93 g/L to 65 g/L over the same time period, suggesting intravascular haemolysis. There is a thrombocytopenia of 99 x109/L and lymphopenia of 0.2 x 109/L. She sustains a significant rise in her creatine kinase levels and there is no clinical evidence of metastatic spread of infection to her limbs, or elsewhere. Her high vaginal swab yields GAS. An ultrasound scan excludes retained products. Her condition stabilises with antibiotics and supportive measures and she is noted to have an acute kidney injury, with a GFR of 30, but not sufficient to necessitate a reduction in the dose of flucloxacillin.
What antimicrobial agents should be commenced?
In sepsis, whether primary bacterial, or possibly secondary to viral infection, empirical treatment should be started immediately after taking two sets of blood cultures.
The combination of piperacillin-tazobactam plus clindamycin, recommended for serious sepsis in pregnancy/puerperium (RCOG Green top guideline), covers S. aureus (but not MRSA), streptococci, Gram negatives and anaerobes.
The recommended therapy for TSS is flucloxacillin plus an agent such as clindamycin to switch off ribosomal production of exotoxins. If MRSA is suspected then vancomycin can be added until sensitivities are available. Flucloxacillin has reasonable activity against GAS but if GAS is confirmed, changing flucloxacillin to benzyl penicillin 2.4 g 6-hourly and continuing clindamycin is recommended.
Check recent swab results to guide empirical therapy in case there are clindamycin-resistant staphylococci or streptococci. Alternative antimicrobials to stop exotoxin production include linezolid and daptomycin but these should be discussed with a microbiologist because of potential side effects and interactions.
If there was ongoing deterioration, warranting intensive care admission, then intravenous immunoglobulin 2 g/kg may be lifesaving.3 It is thought to directly antagonise the effects of exotoxins and cytokines. Commercial IVIG is imported from the USA, and contains exotoxin-neutralising antibodies from pooled donors. Only effective in Gram positive sepsis, IVIG has been used successfully in pregnancy in cases of exotoxin related disease, including TSS, STSS, GAS, MRSA necrotizing fasciitis, and PVL-S. aureus necrotizing pneumonia. The main contraindication to IVIG use is a congenital deficiency of immunoglobulin A.
Resolution Case 2
She recovers well, responding to 4 L of i.v. fluid resuscitation and a combination of intravenous flucloxacillin (2 g 6-hourly) and clindamycin (1.2 g 6-hourly). The CK remains stable, suggesting no myonecrosis and the pains settle. Her observations improve steadily and the CRP falls to 300 mg/L by the following morning. Lymphocytes rise to 1 x 109/L, suggesting infection is being controlled.
The following day, GAS is grown from her vaginal swab and blood cultures, sensitive to penicillin and clindamycin. The assumed portal of entry is the vagina. Therapy with benzyl penicillin 2.4 g 6-hourly replaces the flucloxacillin, and clindamycin is continued. By day 7, the CRP has fallen to 50 mg/L when she is considered well enough to be discharged home to complete a 10-day course of oral amoxicillin.
Although appearing well, and only positive for GAS on the umbilicus, the neonate was treated empirically as per national guidelines with oral amoxicillin.4
Practice Points.
-
•
Always check results carefully to ensure any previous organisms are sensitive to the proposed antimicrobials
-
•
Gastroenteritis symptoms may be due to exotoxins and reflect sepsis distant from the bowel
-
•
Cell wall active antimicrobials do not switch off exotoxin production so dual therapy is usually necessary, and least to begin with, if an exotoxin producing infection is the cause
-
•
STSS and TSS are similar except STSS rarely presents with the classic sunburn rash and has a far worse prognosis
References
- 1.Knight M., Nair M., Tuffnell D., Shakespeare J., Kenyon S., Kurinczuk J.J., editors. Saving lives, improving mothers' care - lessons learned to inform maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2013–15. National Perinatal Epidemiology Unit, University of Oxford; Oxford: 2017. On behalf of MBRRACE-UK. [Google Scholar]
- 2.Michelim L., Bosi G.R., Comparsi E. Urinary tract infection in pregnancy: review of clinical management. J Clin Nephrol Res. 2017;3:1030–1037. [Google Scholar]
- 3.Linnér A., Darenberg J., Sjölin J., Henriques-Normark B., Norrby-Teglund A. Clinical efficacy of polyspecific intravenous immunoglobulin therapy in patients with streptococcal toxic shock syndrome: a comparative observational study. Clin Infect Dis. 2014;59:851–857. doi: 10.1093/cid/ciu449. [DOI] [PubMed] [Google Scholar]
- 4.Steer J.A., Lamagni T., Healy B., Morgan M., Dryden M., Rao B., et al. GAS Guideline Development Working Group Guidelines for prevention and control of group A streptococcal infection in acute healthcare and maternity settings in the UK. J Infect. 2012;64:1e18. doi: 10.1016/j.jinf.2011.11.001. [DOI] [PubMed] [Google Scholar]