Abstract
Type 2 diabetes mellitus (T2DM) is associated with both poorer clinical outcomes during the COVID-19 pandemic and an increased risk of death in such hospitalized patients. While the role of glucose control has been emphasized to improve the prognosis, the impact of different glucose-lowering agents remains largely unknown. Metformin remains the first-line pharmacological choice for the management of hyperglycaemia in T2DM. Because metformin exerts various effects beyond its glucose-lowering action, among which are anti-inflammatory effects, it may be speculated that this biguanide might positively influence the prognosis of patients with T2DM hospitalized for COVID-19. The present concise review summarizes the available data from observational retrospective studies that have shown a reduction in mortality in metformin users compared with non-users, and briefly discusses the potential underlying mechanisms that might perhaps explain this favourable impact. However, given the potential confounders inherently found in observational studies, caution is required before drawing any firm conclusions in the absence of randomized controlled trials.
Keywords: Inflammation, Meta-analysis, Metformin, Mortality, SARS-CoV-2, Type 2 diabetes
Introduction
During the coronavirus disease 2019 (COVID-19) pandemic due to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, several observational studies around the world have shown that type 2 diabetes mellitus (T2DM) is a risk factor for more severe disease, leading to acute respiratory distress syndrome and artificial ventilation in intensive care units, and almost doubling the death rate [1], [2], [3]. Among hospitalized patients with both T2DM and COVID-19, several prognostic factors have been identified, among which are older age and obesity [4]. However, while the role of the quality of glucose control has been repeatedly emphasized [5], [6], the potential influence of the class of glucose-lowering medication remains unclear [2], [7], [8].
The pharmacotherapy of T2DM now comprises several new drugs that compete with older oral antidiabetic agents. Nevertheless, metformin remains the first-choice drug for the management of hyperglycaemia in T2DM patients [9]. Indeed, it has been the most popular glucose-lowering agent during the past two decades worldwide, and around two-thirds to three-quarters of patients with T2DM are treated with metformin alone or in combination. The United Kingdom Prospective Diabetes Study (UKPDS) [10] showed that metformin reduces mortality in obese patients with newly diagnosed T2DM, and obesity has been reported to be a major risk factor for developing severe COVID-19 [11], [12]. Thus, it is of major importance to know the effect of metformin on the clinical outcomes of patients with T2DM during the COVID-19 outbreak. Inflammation due to cytokine storms is recognized to play a crucial role in the poor prognosis of patients infected with the SARS-CoV-2 [13] and, as metformin has been shown to exert some anti-inflammatory effects irrespective of diabetes status [14], [15], it may be speculated that this glucose-lowering agent could favourably influence the clinical outcomes of patients with T2DM hospitalized for COVID-19 beyond its effects on glucose control [16], [17], [18].
The aim of this short narrative review was to summarize the available data from observational studies in patients with T2DM comparing rates of death in metformin users vs non-users who were hospitalized for COVID-19, and to discuss the potential underlying mechanisms that might positively influence outcomes.
Possible mechanisms leading to beneficial effects
Over the past few weeks, several authors have written letters to editors and commentaries to speculate about the possible beneficial effects of metformin in diabetes patients with COVID-19 [17] based on the pleiotropic effects of this medication, characterized by a complex mode of action [19], and the multiple pathogenic mechanisms that contribute to worsen the prognosis of patients with COVID-19, especially when T2DM is present [13]. The most important mechanisms of metformin activity in relation to COVID-19 are summarized in Table 1 [16], [17], [20], [21], [22], [23], [24], [25]. It is noteworthy, however, that all such proposed mechanisms remain, at present, theoretical.
Table 1.
Suggested mechanisms to explain the possible positive effects of metformin on COVID-19 outcomes.
| Mechanisms | References |
|---|---|
| Improved glucose control | Ceriello et al. [5], [6] |
| Reduction in body weight | El-Arabey and Abdalla [20] |
| Reduction in insulin resistance | Penlioglou et al. [16] Sharma et al. [17] |
| AMPK activation leads to phosphorylation of ACE2, thereby inhibition of virus penetration | Sharma et al. [17] |
| Inhibition of mTOR pathway and prevention of immune hyperactivation | Sharma et al. [17] Bolourian and Mojtahedi [21] Lehrer [22] |
| Action on endosomal Na+/H+ exchanger, thereby increasing cellular pH and interference with viral endocytic cycle | Esam [23] |
| Anti-inflammatory properties | Kumar Singh and Singh [18] |
| Reduction in neutrophils | Dalan [24] |
| Inhibition of mitochondrial complex 1, suppression of mitochondrial reactive oxygen species (ROS) signalling, ROS prevention: CRAC-mediated interleukin-6 release | Menendez [25] |
AMPK: AMP-activated protein kinase; ACE2: angiotensin-converting enzyme 2; mTOR: mammalian target of rapamycin; CRAC: Ca2+ release-activated Ca2+ channels.
In contrast, some authors have challenged the prescription of metformin and raised concerns about its use in patients with T2DM hospitalized for COVID-19, in particular by pointing out the possible risk of lactic acidosis in cases of multiple organ failure [26], [27], [28].
Retrospective analyses of observational human studies
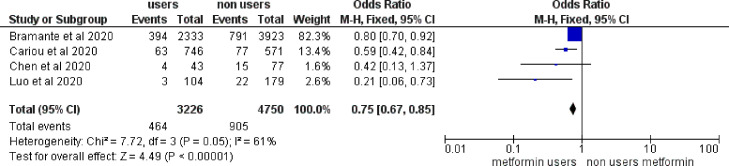
A few retrospective analyses of observational studies in patients with T2DM hospitalized for severe COVID-19 suggested that the use of metformin might be associated with a reduction in mortality [18]. The main characteristics of those studies are presented in Table 2 , and the relative effects of metformin on mortality rates are summarized in a meta-analysis (Fig. 1 ). A positive effect (whether significant or not) was observed in all four studies with an overall reduction of death by 25% (P < 0.00001), but with moderate-to-high heterogeneity (I 2 = 61%). The size of the four studies varied considerably, with two big cohorts from the US [29] and France [4], but two more limited samples from China [30], [31]. Mean ages ranged from 65 to 75 years and the gender ratio showed a slight predominance of males. Unfortunately, detailed information on body mass index (BMI) was missing in most of the studies. Greater reductions of mortality in metformin users vs non-users were observed in the two trials that recruited smaller numbers of patients [30], [31]. The results of these four studies have been briefly discussed elsewhere [18].
Table 2.
Characteristics of four observational studies comparing death rates in metformin users vs non-users among patients with type 2 diabetes hospitalized for COVID-19.
| References | Country | Endpoint | Metformin users/non-users |
|||
|---|---|---|---|---|---|---|
| Patients (n) |
Mean age (years) |
Gender (% male) |
BMI (kg/m2) |
|||
| Bramante et al. [29] | US | In-hospital mortality | 2333/3923 | 73/76 | 52/45 | NA |
| Cariou et al. [4] | France | Death at ≤7 days of admission | 746/571 | 70 (both groups) |
65 (both groups) |
28.5 (both groups) |
| Chen et al. [30] | China | In-hospital mortality | 43/77 | 62/67 | NA | NA |
| Luo et al. [31] | China | In-hospital mortality | 104/179 | 63/65 | 51/57 | NA |
BMI: body mass index; NA: not available.
Fig. 1.
Mortality rates in metformin users compared with non-users among patients with type 2 diabetes hospitalized for COVID-19 infection. M–H: Mantel–Haenszel (test).
In one large US study of 6256 subjects (>95% T2DM, 52.8% female, mean age 75 years), metformin was significantly associated with reduced mortality in women (but not in men) in an observational analysis of claims data from individuals hospitalized with COVID-19 with an odds ratio (OR) of 0.759 [95% confidence interval (CI): 0.601–0.960] after propensity matching [29].
The Coronavirus-SARS-CoV-2 and Diabetes Outcomes (CORONADO) study, as with many other observational studies, was not specifically designed to investigate the potential influence of previous antidiabetic medications [4]. Nevertheless, in this large observational multicentre French study, metformin users prior to hospital admission had a lower death rate at day 7 compared with metformin non-users in an unadjusted analysis (OR: 0.59, 95% CI: 0.42–0.84). After full adjustment, a non-statistically significant trend for fewer deaths still remained in metformin users compared with non-users (OR: 0.80, 95% CI: 0.45–1.43), as previously described elsewhere [18].
In a smaller retrospective study of T2DM patients hospitalized for COVID-19, a reduced trend for in-hospital deaths was observed in metformin users (n = 43) compared with non-users (n = 77): 9.3% vs 19.5%, respectively. A noteworthy finding was that metformin users had a significantly lower increase in interleukin (IL)-6 compared with non-users: 4.1 pg/mL vs 11.1 pg/mL, respectively (P = 0.02) [30].
In another study, despite having similar baseline characteristics, laboratory parameters and treatment interventions between metformin users (n = 104) and metformin non-users (n = 179), there was a significantly lower in-hospital mortality rate in patients treated with metformin vs not (2.9% vs 12.3%, respectively; P = 0.01). Interestingly, this difference was observed despite significantly higher baseline fasting plasma glucose levels in metformin users compared with non-users. In a multivariate analysis, a more than fourfold reduction in in-hospital mortality was noted in metformin users compared with non-users (OR: 4.36, 95% CI: 1.22–15.59; P = 0.02) [31].
Finally, one study could not be included in the meta-analysis because it did not provide enough precise data regarding the relationship between the use of metformin and death [32]. In this comparative analysis that mainly focused on the effect of glucose control (1:1 propensity-matching for other comorbidities), a significant reduction in all-cause mortality was observed in the well-controlled compared with poorly controlled T2DM patients (adjusted HR: 0.13, 95% CI: 0.04–0.44; P < 0.001). Of potential interest, a significantly greater proportion of patients were taking metformin in the well-controlled arm vs poorly controlled arm (39.2% vs 26.4%, respectively; P = 0.003). While the beneficial outcome was attributed to good glycaemic control, a potential benefit resulting from metformin use itself could not be excluded when considering the data reported in the four above-mentioned studies [32].
Discussion
The available retrospective studies, albeit still limited in number, have all shown a reduction in mortality in metformin users compared with non-users among patients with T2DM hospitalized for COVID-19. However, caution is required before drawing any firm conclusions. Indeed, as with every observational study, potential confounders could influence the final results, and it is not possible to eliminate all of them even after multiple adjustments and propensity matching. Of note, metformin non-users may represent patients with relevant contraindications to the prescription of metformin, such as old age, renal insufficiency and/or cardiovascular disease, even though these contraindications have been alleviated in recent years [33]. These conditions all represent risk factors that could trigger poorer prognoses in cases of COVID-19 [11]. On the other hand, metformin is more commonly used in obese patients with T2DM [10], and obesity has been demonstrated to be associated with a greater risk of severe COVID-19 infection and a higher mortality rate [11], [12].
The findings of observational studies showing a reduction of death rates in patients treated with metformin while exposed to COVID-19 are in agreement with previous observations reporting significantly lower mortality rates in patients treated with metformin compared with metformin non-users during bacterial infections such as sepsis (OR: 0.59, 95% CI: 0.43–0.79; P = 0.001) [34] and active tuberculosis [35]. Animal data have also shown a higher survival rate when mice exposed to experimental sepsis were treated with metformin [36]. As discussed in a previous review, in addition to its traditional effects on glucose metabolism, metformin also offers antimicrobial benefits in a very wide range of infections whether in vitro and in vivo [37]. Metformin has also been found to be associated with a markedly lower mortality rate in patients with respiratory pathologies such as chronic obstructive pulmonary disease [38], [39]. Finally, in a recent randomized, double-blind, placebo-controlled, proof-of-concept phase-II trial, metformin reduced metabolic complications and inflammation in non-diabetes patients receiving systemic glucocorticoid therapy for inflammatory diseases. The results of this study further emphasize the anti-inflammatory effects of metformin independently of glucose control [15].
Both metformin therapy and COVID-19 can also share similar adverse gastrointestinal events. The most commonly reported side-effects of metformin are nausea, dyspepsia and diarrhoea, usually occurring within the first few days or weeks of starting therapy [40]. COVID-19 can also induce gastrointestinal disturbances beyond the classic systemic (fever) and respiratory (cough, dyspnoea) symptoms [41]. Of course, metformin should be stopped in cases of severe gastrointestinal symptoms. The risk of lactic acidosis, pointed out by some authors as a possible complication [26], [27], [28], is in fact rarely seen with metformin and, even then, the drug may not necessarily be responsible for it, as previously explained [42]. However, lactic acidosis may arise in cases of multiple organ failure, particularly renal insufficiency and hepatic failure, and especially in the context of hypoxia triggering anaerobic glycolysis [26], [27].
However, firm conclusions about the impact of metformin therapy can only be drawn from double-blind randomized controlled trials (RCTs), and such trials are almost impossible in the context of COVID-19, considering the known difficulties encountered in carrying out such RCTs (for example, the ambitious European DISCOVERY trial). Because metformin is out of patent and very inexpensive, no pharmaceutical company is likely to be interested in planning a study to demonstrate the benefits of metformin on COVID-19-related clinical outcomes. Nevertheless, it is worth noting that such an RCT is currently ongoing with dapagliflozin, a sodium–glucose cotransporter type-2 (SGLT2) inhibitor, in the Dapagliflozin in Respiratory Failure in Patients with COVID-19 (DARE-19) trial (ClinicalTrials.gov identifier: NCT04350593) [43]. Of potential interest, SGLT2 inhibitors have also demonstrated anti-inflammatory effects [44] that are somewhat similar to those described with metformin [14].
In addition, an intriguing finding was recently reported by Bramante et al. [29]: in their large observational study of patients hospitalized due to COVID-19, the reduction in mortality in metformin users compared with non-users was highly significant in women, but was not observed in men. According to those authors, this gender-specific finding is consistent with the greater reduction of tumour necrosis factor (TNF)-α by metformin in women than in men, and suggests that metformin conveys protection against COVID-19 through TNF-α-mediated effects. Nevertheless, the authors also recognize that prospective studies are now needed to understand the mechanisms and causality behind these effects. Other authors have pointed out the reduction in IL-6 levels in metformin-treated patients [30]. Whatever the case may be, these findings confirm the potential anti-inflammatory activity of metformin [14], which could be beneficial in an infection like COVID-19 where cytokine storms play such a deleterious role and markedly worsen the prognosis, including survival [13].
Conclusion
Metformin, the marketplace leader among glucose-lowering agents for the management of T2DM, has a complex mechanism of action involving multiple mechanisms, some of which lead to anti-inflammatory activity that may help in reducing the risk of severe COVID-19 beyond the effects of glucose control. Some preliminary data from retrospective studies have confirmed a reduction in death rates in metformin users compared with non-users in patients with T2DM hospitalized for COVID-19. Caution is, however, required when interpreting these observational findings, as only RCTs can provide definitive conclusions. Nevertheless, there are at least no negative safety indications, so there is no reason to stop metformin therapy during COVID-19 infection except in cases of severe gastrointestinal symptoms, hypoxia and/or multiple organ failure.
Declaration of interest
None declared.
References
- 1.Targher G., Mantovani A., Wang X.B., Yan H.D., Sun Q.F., Pan K.H. Patients with diabetes are at higher risk for severe illness from COVID-19. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.05.001. Epub: May 13; S1262-3636(20)30075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar Singh A., Khunti K. Assessment of risk, severity, mortality, glycemic control and antidiabetic agents in patients with diabetes and COVID-19: a narrative review. Diabetes Res Clin Pract. 2020;165 doi: 10.1016/j.diabres.2020.108266. Epub: 108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14:535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I. Phenotypic characteristics and prognosis of in-patients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceriello A. Hyperglycemia and the worse prognosis of COVID-19. Why a fast blood glucose control should be mandatory. Diabetes Res Clin Pract. 2020;163 doi: 10.1016/j.diabres.2020.108266. Epub: 108186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceriello A., De Nigris V., Prattichizzo F. Why is hyperglycemia worsening COVID-19 and its prognosis? Diabetes Obes Metab. 2020;28 doi: 10.1111/dom.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pal R., Bhadada S.K. Should anti-diabetic medications be reconsidered amid COVID-19 pandemic? Diabetes Res Clin Pract. 2020;163 doi: 10.1016/j.diabres.2020.108146. Epub: 108146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloomgarden Z. Is the type of diabetes treatment relevant to outcome of COVID-19? J Diabetes. 2020;12:486–487. doi: 10.1111/1753-0407.13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buse J.B., Wexler D.J., Tsapas A., Rossing P., Mingrone G., Mathieu C. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2020;63:221–228. doi: 10.1007/s00125-019-05039-w. [DOI] [PubMed] [Google Scholar]
- 10.United Kingdom Prospective Diabetes Study Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34), UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 11.Scheen A.J., Marre M., Thivolet C. Prognostic factors in patients with diabetes hospitalized for COVID-19: findings from the CORONADO study and recent reports. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.05.008. S1262-3636(20)30085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vas P., Hopkins D., Feher M.D., Rubino F., Martin M.B. Diabetes, obesity and COVID-19: a complex interplay. Diabetes Obes Metab. 2020 doi: 10.1111/dom.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron A.R., Morrison V.L., Levin D., Mohan M., Forteath C., Beall C. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ Res. 2016;119:652–665. doi: 10.1161/CIRCRESAHA.116.308445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pernicova I., Kelly S., Ajodha S., Sahdev A., Bestwick J.P., Gabrovska P. Metformin to reduce metabolic complications and inflammation in patients on systemic glucocorticoid therapy: a randomised, double-blind, placebo-controlled, proof-of-concept, phase 2 trial. Lancet Diabetes Endocrinol. 2020;8:278–291. doi: 10.1016/S2213-8587(20)30021-8. [DOI] [PubMed] [Google Scholar]
- 16.Penlioglou T., Papachristou S., Papanas N. COVID-19 and diabetes mellitus: may old anti-diabetic agents become the new philosopher's stone? Diabetes Ther. 2020;11:1–3. doi: 10.1007/s13300-020-00830-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma S., Ray A., Sadasivam B. Metformin in COVID-19: a possible role beyond diabetes. Diabetes Res Clin Pract. 2020;164 doi: 10.1016/j.diabres.2020.108183. Epub: 108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar Singh A., Singh R. Is metformin ahead in the race as a repurposed host-directed therapy for patients with diabetes and COVID-19? Diabetes Res Clin Pract. 2020;165 doi: 10.1016/j.diabres.2020.108268. Epub: 108268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rena G., Hardie D.G., Pearson E.R. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Arabey A.A., Abdalla M. Metformin and COVID-19: a novel deal of an old drug. J Med Virol. 2020;29 doi: 10.1002/jmv.25958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolourian A., Mojtahedi Z. Obesity and COVID-19: the mTOR pathway as a possible culprit. Obes Rev. 2020 doi: 10.1111/obr.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehrer S. Inhaled biguanides and mTOR inhibition for influenza and coronavirus (Review) World Acad Sci J. 2020;2(3):1. doi: 10.3892/wasj.2020.42. Epub 29 March 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esam Z. A proposed mechanism for the possible therapeutic potential of metformin in COVID-19. Diabetes Res Clin Pract. 2020;164 doi: 10.1016/j.diabres.2020.108282. Epub: 108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalan R. Metformin, neutrophils and COVID-19 infection. Diabetes Res Clin Pract. 2020;164 doi: 10.1016/j.diabres.2020.108230. Epub: 108230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menendez J.A. Metformin and SARS-CoV-2: mechanistic lessons on air pollution to weather the cytokine/thrombotic storm in COVID-19. Aging (Albany NY) 2020;12:8760–8765. doi: 10.18632/aging.103347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kow C.S., Hasan S.S. Metformin use amid coronavirus disease 2019 pandemic. J Med Virol. 2020 doi: 10.1002/jmv.26090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ursini F., Ciaffi J., Landini M.P., Meliconi R. COVID-19 and diabetes: is metformin a friend or foe? Diabetes Res Clin Pract. 2020;164 doi: 10.1016/j.diabres.2020.108167. Epub: 108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cure E., Cumhur Cure M. Comment on “Should anti-diabetic medications be reconsidered amid COVID-19 pandemic?.”. Diabetes Res Clin Pract. 2020;164 doi: 10.1016/j.diabres.2020.108184. Epub: 108184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bramante C., Ingraham N., Murray T., Marmor S., Hoversten S., Gronski J. Observational study of metformin and risk of mortality in patients hospitalized with Covid-19. medRxiv. 2020;28 doi: 10.1101/2020.06.19.20135095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y., Yang D., Cheng B., Chen J., Peng A., Yang C. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43:1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 31.Luo P., Qiu L., Liu Y., Liu X.L., Zheng J.L., Xue H.Y. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am J Trop Med Hyg. 2020;103:69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheen A.J., Paquot N. Metformin revisited: a critical review of the benefit-risk balance in at-risk patients with type 2 diabetes. Diabetes Metab. 2013;39:179–190. doi: 10.1016/j.diabet.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Liang H., Ding X., Li L., Wang T., Kan Q., Wang L. Association of preadmission metformin use and mortality in patients with sepsis and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Crit Care. 2019;23:50. doi: 10.1186/s13054-019-2346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang M., He J.Q. Impacts of metformin on tuberculosis incidence and clinical outcomes in patients with diabetes: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76:149–159. doi: 10.1007/s00228-019-02786-y. [DOI] [PubMed] [Google Scholar]
- 36.Gras V., Bouffandeau B., Montravers P.H., Lalau J.D. Effect of metformin on survival rate in experimental sepsis. Diabetes Metab. 2006;32:147–150. doi: 10.1016/s1262-3636(07)70261-6. [DOI] [PubMed] [Google Scholar]
- 37.Malik F., Mehdi S.F., Ali H., Patel P., Basharat A., Kumar A. Is metformin poised for a second career as an antimicrobial? Diabetes Metab Res Rev. 2018;34:e2975. doi: 10.1002/dmrr.2975. [DOI] [PubMed] [Google Scholar]
- 38.Mendy A., Gopal R., Alcorn J.F., Forno E. Reduced mortality from lower respiratory tract disease in adult diabetic patients treated with metformin. Respirology. 2019;24:646–651. doi: 10.1111/resp.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho T.W., Huang C.T., Tsai Y.J., Lien A.S., Lai F., Yu C.J. Metformin use mitigates the adverse prognostic effect of diabetes mellitus in chronic obstructive pulmonary disease. Respir Res. 2019;20:69. doi: 10.1186/s12931-019-1035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonnet F., Scheen A. Understanding and overcoming metformin gastrointestinal intolerance. Diabetes Obes Metab. 2017;19:473–481. doi: 10.1111/dom.12854. [DOI] [PubMed] [Google Scholar]
- 41.Ma C., Cong Y., Zhang H. COVID-19 and the digestive system. Am J Gastroenterol. 2020;115:1003–1006. doi: 10.14309/ajg.0000000000000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lalau J.D., Kajbaf F., Protti A., Christensen M.M., De Broe M.E., Wiernsperger N. Metformin-associated lactic acidosis (MALA): moving towards a new paradigm. Diabetes Obes Metab. 2017;19:1502–1512. doi: 10.1111/dom.12974. [DOI] [PubMed] [Google Scholar]
- 43.Scheen A.J. SGLT2 inhibition during the COVID-19 epidemic: friend or foe? Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.06.003. S1262-3636(20)30091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonnet F., Scheen A.J. Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: potential contribution for diabetic complications and cardiovascular disease. Diabetes Metab. 2018;44:457–464. doi: 10.1016/j.diabet.2018.09.005. [DOI] [PubMed] [Google Scholar]