Abstract
Type 1 and type 2 diabetes mellitus increase the risk of atherosclerotic cardiovascular disease (CVD), resulting in acute cardiovascular events, such as heart attack and stroke. Recent clinical trials point toward new treatment and prevention strategies for cardiovascular complications of type 2 diabetes. New antidiabetic agents show unexpected cardioprotective benefits. Moreover, genetic and reverse translational strategies have revealed potential novel targets for CVD prevention in diabetes, including inhibition of apolipoprotein C3. Modeling and pharmacology-based approaches to improve insulin action provide additional potential strategies to combat CVD. The development of new strategies for improved diabetes and lipid control fuels hope for future prevention of CVD associated with diabetes.
Keywords: Angiopoietin-like, Apolipoprotein C3, Atherosclerosis, Diabetes, Insulin, Genetics
Cardiovascular Complications in People With Diabetes
Atherosclerotic cardiovascular disease (CVD), including coronary artery disease (CAD) and its complications, are leading causes of death and disability worldwide [1]. The risk of CVD is markedly increased in both type 1 diabetes mellitus (T1DM; See Glossary) and type 2 diabetes mellitus (T2DM), with events occurring at younger ages in patients with diabetes [2–5]. Approximately 10% of all vascular deaths in adults from developed countries have been attributed to diabetes [3], emphasizing the importance of understanding how diabetes increases CVD risk and how CVD associates with diabetes can be prevented.
Optimal glycemic control is beneficial in preventing microvascular complications of diabetes, such as kidney disease and eye complications, but glucose lowering has a less obvious effect in preventing major cardiovascular events, especially in people with established CVD [6–11]. Furthermore, intense glucose lowering regimens are associated with an increased risk of hypoglycemia, which can, in severe cases and without continuous monitoring, lead to seizures, loss of consciousness and even death. In addition to elevated blood glucose levels, diabetes can be associated with dyslipidemia. In people with T2DM, plasma triglyceride levels are often elevated, highdensity lipoprotein (HDL) cholesterol is low and low-density lipoprotein (LDL) levels are normal but the LDL particle is often smaller and denser with triglyceride enrichment. Dyslipidemia in T1DM primarily occurs in people for whom glucose control is suboptimal. In such patients, insulin deficiency results in hypertriglyceridemia [12, 13] (see Box 1). To lower the risk of heart attacks and strokes, people with diabetes are frequently treated with statins, the most effective current intervention for lowering CVD risk in people with and without diabetes. However, even with statin treatment to lower LDL-cholesterol (LDL-C), people with diabetes have a high residual risk for CVD [14]. Furthermore, statin treatment can increase the development of T2DM in people at increased risk for diabetes [15, 16]. Although the benefit of statins in reducing CVD events clearly outweighs the risk of incident diabetes [15], these issues do increase the urgency of finding additional CVD prevention strategies for people with diabetes or at an increased risk of developing diabetes.
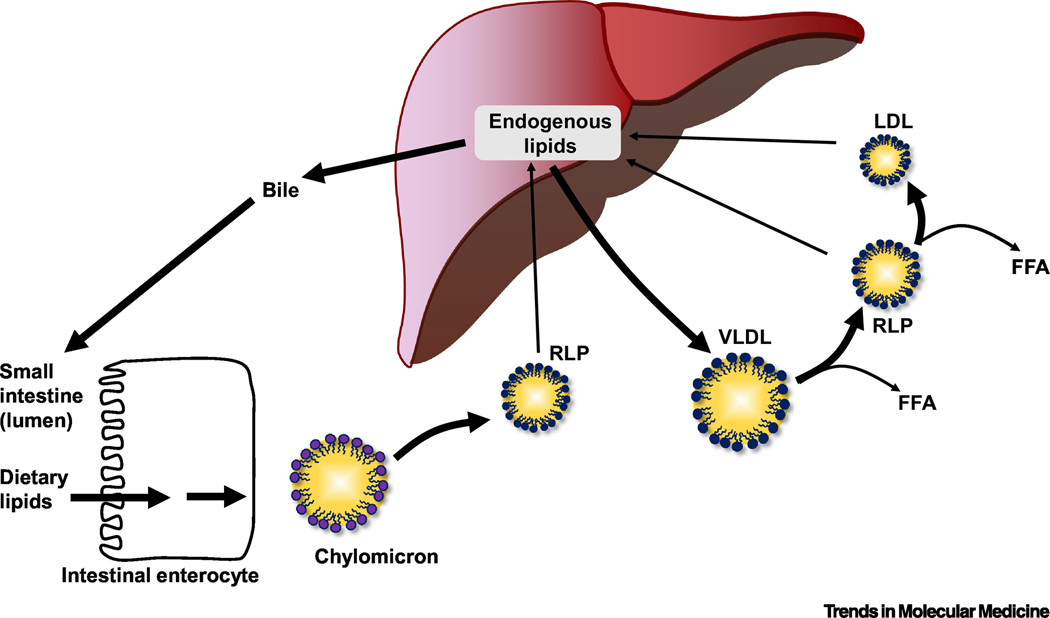
Box 1. Lipoprotein Metabolism and Effects of Diabetes.
Lipids, such as cholesteryl esters and triglycerides, are carried in blood in the core of lipoprotein particles. Lipoprotein particles allow these lipid molecules to be effectively transported between tissues. Following a meal, dietary lipids are taken up in the intestine and form large (1200–75 nm in diameter) triglyceride-rich lipoproteins called chylomicrons (Box 1, lower left) transport fat to peripheral tissues for storage and utilization. The major apolipoprotein associated with chylomicrons is a truncated form of APOB100 termed APOB48. Very low-density lipoproteins (VLDLs) are APOB100-containing smaller (30–80 nm in diameter) triglyceride-rich lipoproteins secreted from the liver (Box 1, center). When VLDL production in the liver is increased, the size of the VLDL particles also increases, allowing the particles to carry more lipid. VLDL transports triglycerides from the liver to peripheral tissues primarily during fasting. Breakdown (hydrolysis) of triglycerides in the core of chylomicrons and VLDL by lipoprotein lipase leads to the formation of triglyceride-depleted particles termed remnant lipoprotein particles (RLP) and free fatty acids (FFA). These remnants are cleared through receptors by the liver, but are also believed to accumulate in the artery wall and contribute to atherosclerosis. Low-density lipoproteins (LDLs) are derived from VLDL and remnants and are smaller than these particles (25–18 nm in diameter). LDL carries mostly cholesterol (and less triglycerides) and is responsible for transporting the largest load of cholesterol in the blood to deliver to tissues. The main apolipoprotein in LDL is APOB100. Although cholesterol over-accumulation in the artery wall leads to atherosclerosis, cholesterol is needed in all cells for proper membrane maintenance, and for production of important hormones. High-density lipoproteins (HDLs) also vary in size (14–7 nm). The main structural apolipoprotein in HDL is APOA1. HDL plays an important role in removing excess cholesterol from the body through a process termed reverse cholesterol transport. This effect of HDL is believed to contribute to its cardioprotective functions.
How does diabetes alter lipoprotein metabolism? Type 2 diabetes is often associated with high levels of plasma triglycerides and low HDL-cholesterol levels. LDLcholesterol concentrations are normal or slightly elevated, but the LDL particles are frequently small and dense with relative enrichment of triglycerides. Dyslipidemia associated with type 1 diabetes occurs primarily when blood glucose control is poor. Absolute insulin deficiency in such cases results in elevated triglycerides with low HDLcholesterol and LDL-cholesterol levels. Well-controlled patients with type 1 diabetes tend to have normal lipid profiles.
Clinician’s Corner
Atherosclerotic cardiovascular disease, including coronary artery disease (CAD) and its complications, are leading causes of death and disability worldwide and risk of CAD is markedly increased in patients with type 1 or type 2 diabetes mellitus.
Recent clinical trials show that two classes of anti-diabetic medications – GLP-1 receptor agonists, which increase insulin secretion, and SGLT2 inhibitors, which lower blood glucose levels by preventing glucose reabsorption in the kidney – appear to reduce cardiovascular events in patients with type 2 diabetes. The main cardioprotective effects of SGLT2 inhibitors are likely mediated by protection from heart failure and renal disease, with a less significant effect on CAD.
Although lowering LDL-cholesterol (LDL-C) with statins is a mainstay of primary and secondary CAD protection, statin therapy increases the risk of developing incident type 2 diabetes in some patients. Human genetic evidence supports the conclusion that lowering LDL-C across a range of mechanisms may lead to increased risk of type 2 diabetes.
Several human genetic studies have found that TRL lowering via LPL activation is associated with reductions in risk of both CAD and type 2 diabetes, making LPL and its endogenous regulators attractive new therapeutic targets.
Humans with loss of function mutations in APOC3, an inhibitor of LPL, have reduced plasma triglycerides and reduced risk of CAD while increased serum levels of APOC3 are associated with CAD, suggesting that APOC3 antagonism may be therapeutically advantageous. Relative insulin deficiency and/or insulin resistance elevate plasma APOC3, and plasma APOC3 is predictive of future cardiovascular events in patients with diabetes. Thus, APOC3 inhibition may be particularly beneficial as a treatment for CAD in patients with diabetes. Evaluation of the effects of APOC3 inhibition in large cardiovascular outcomes studies in people with diabetes and elevated APOC3 levels are needed next.
Here, we review strategies for identifying new approaches to prevent CVD risk associated with diabetes and highlight some of the emerging targets. We focus on i) human genetic approaches; ii) translational approaches, including reverse translation; and iii) modeling and pharmacology-based approaches to improve insulin receptor activation.
Human Genetics to Identify Novel Therapeutic Targets
Human genetic studies are powerfully positioned to provide insights into potential therapeutic targets due to several unique characteristics. First, identifying naturally occurring genetic variations that associate with a disease or trait in humans provides immediate relevance to human biology and pathophysiology [17]. For example, a lowfrequency protein-altering genetic variant in the gene encoding the glucagon-like peptide 1 (GLP1) receptor is associated with lower fasting glucose and reduced risk of T2DM, consistent with the anti-diabetogenic effects of GLP1-receptor agonists [18]. That same GLP1-receptor variant is also associated with reduced CVD risk [18], consistent with the cardioprotective effects that have been observed in clinical trials of GLP-1 receptor agonists [19]. Genome-wide association studies (GWAS) have now identified hundreds of genomic loci that robustly associate with diabetes [20, 21] and CVD [22, 23], many of which do not appear to act through known risk factor pathways [24]. A systematic understanding of which genes are affected by each risk locus has tremendous potential for identifying novel mechanisms and therapeutic targets.
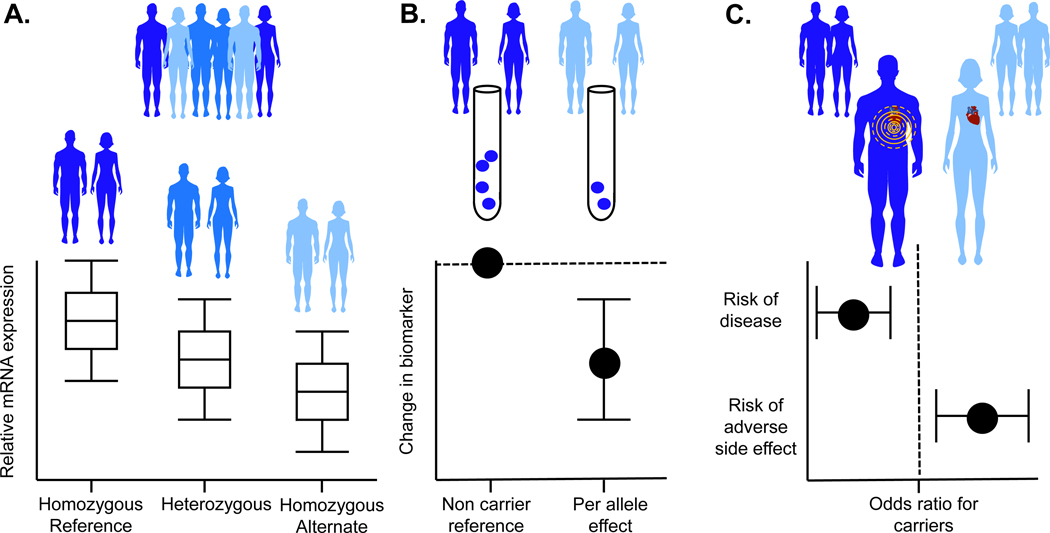
Second, unlike epidemiological correlations between biomarkers and disease, which can be confounded (either by co-correlation with unobserved factors or by reverse causation where biomarkers are altered by the disease itself), genetic variation is randomly allocated at birth, preventing correlation with confounders, and remains fixed throughout life, preventing alteration by disease processes. Thus, genetic variants that associate with altered biomarkers can be used to estimate the likely causal impact of that biomarker on disease (see Figure 1). Termed Mendelian Randomization [25], this approach has provided insights into which lipoprotein fractions are causal for CVD among numerous other therapeutically relevant observations [26, 27]. Finally, some humans naturally inherit alleles that induce loss of function in the encoded protein. When the encoded protein is the target of an inhibitory drug, the phenotypes of humans carrying loss of function mutations in that gene can successfully be used to estimate the beneficial and adverse effects of that drug [28–31].
Figure 1. A human genetic framework to estimate the beneficial and adverse effects of therapeutic agents.
(A) DNA sequence variants resulting in reduced gene expression of therapeutic targets are frequently used as genetic proxies for inhibitory drugs. For example, DNA variation leading to a slight reduction in HMG Co-A reductase is associated with lower plasma LDL-cholesterol; (B) Biomarkers affected by the drug target can be used to validate that the DNA variants are appropriate proxies. The dashed line indicates the reference group; (C) The association between the same DNA variants and disease outcomes can be used to estimate the clinical effects of the drug (for example, DNA variation leading to a slight reduction in HMG Co-A reductase is associated with lower risk of heart attack but increased risk of diabetes). The dashed line indicates an odds ratio of 1 (i.e., no effect).
Human genetic findings related to lipoproteins: CVD benefits vs adverse diabetic effects
Macrovascular complications of diabetes are associated with altered lipoprotein metabolism (Box 1). Lipid lowering therapy with statin medications has been shown to reduce risk of macrovascular events in the setting of diabetes [32] and is now considered standard of care for the prevention of atherosclerotic CVD in adult patients with diabetes [33]. Despite beneficial reductions in cardiovascular events, therapeutic trials have consistently shown an increased risk of T2DM among patients treated with high intensity statins compared with those on placebo and it was debated whether or not this increased risk was due to an off-target effect of statins, an artifact of study design, or other possibilities [34]. Human genetics helped to resolve this question in a study of naturally occurring genetic variants in the gene HMGCR [35], which encodes the protein HMG Co-A reductase, the rate-limiting enzyme in cholesterol biosynthesis that is inhibited by statin medications (Figure 2A). DNA variants in HMGCR that associated with significantly reduced HMGCR expression associated with both lower LDL-C and reduced risk of CVD, confirming that these HMGCR variants mimic the effect of statins. These same genetic variants also associated with increased risk of T2DM [35], suggesting that the increased T2DM risk associated with statin use stems from the drugs’ on-target effect of inhibiting HMG Co-A reductase [36]. Although the mechanism behind this increased risk is still unknown, it might be mediated through secondary effects of inhibiting HMG Co-A reductase, resulting in the inhibition of β-cell glucose transporters [37], or activation of the inflammasome [38]. The conclusion that the increased risk of T2DM is an effect of inhibiting HMG Co-A reductase has important therapeutic implications: development of more specific HMG Co-A reductase inhibitors in an attempt to reduce T2DM risk may not be worthwhile (as it would have been if the increased T2DM risk was not seen in the genetic proxy suggesting it was due to an offtarget effect) [36].
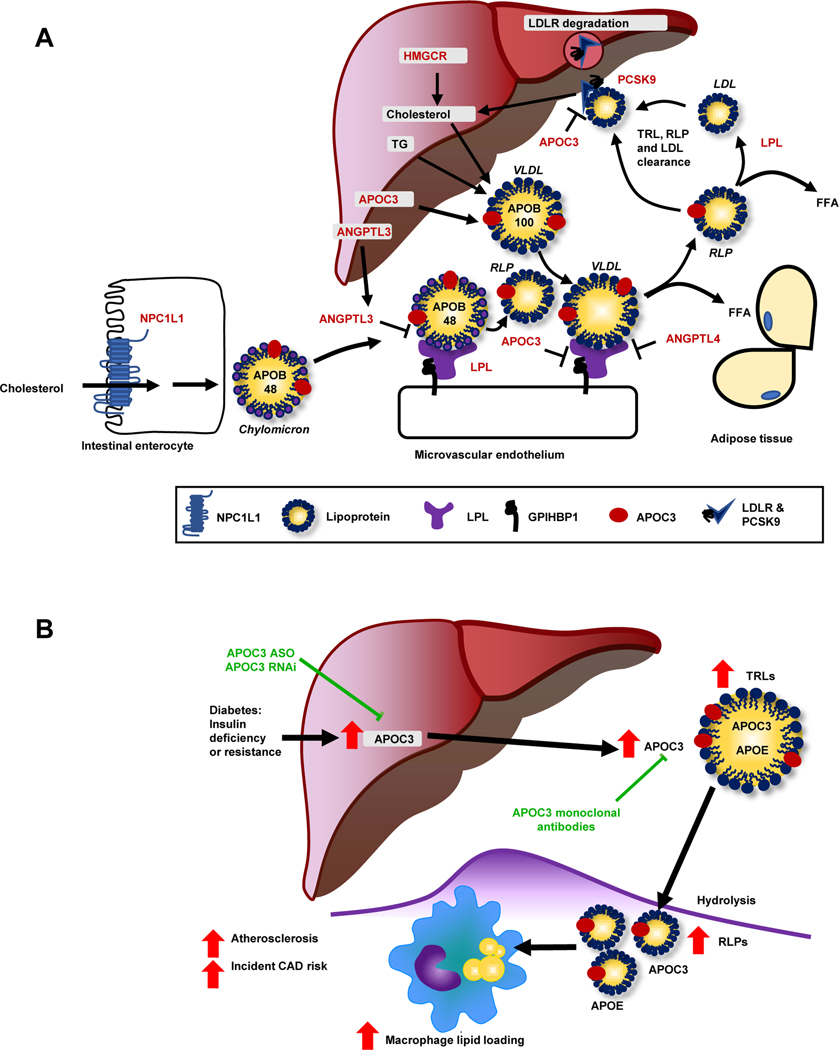
Figure 2. Lipoprotein metabolism at a glance.
(A) This schematic shows the genes discussed in relation to lipid metabolism (highlighted by red font). Cholesterol and sterols are taken up from the intestinal lumen via the Nieman Pick C1-like 1 (NPC1L1) transporter (blue, lower left corner) and are then re-assembled into chylomicrons containing apolipoprotein (APO) B48. The liver produces cholesterol through HMG-CoA reductase (HMGCR; upper center), which is incorporated with triglycerides into APOB100-containing VLDL. LPL (purple) hydrolyzes triglycerides into free fatty acids (FFA), resulting in the formation of LDL and RLPs from VLDL and chylomicrons, respectively, which can enter tissues. Most APOB-containing lipoproteins are taken up via the hepatic LDL receptor (LDLR). Proprotein convertase subtilisin/kexin type 9 (PCSK9) stimulates internalization and degradation of the LDLR, thereby reducing cell surface expression of the LDLR. Angiopoietin-like (ANGPL) 3, ANGPTL4, and APOC3 are endogenous inhibitors of LPL. APOC3 can also suppress hepatic uptake of APOEcontaining lipoprotein particles (found e.g., on VLDL and RLPs). (B) Diabetes enhances hepatic production of APOC3. Elevated levels of APOC3 increase circulating levels of triglyceride-rich lipoproteins (TRLs), resulting in accumulation of RLPs in the artery wall and accelerated atherosclerosis, in part via increased macrophage lipid loading. An antisense oligonucleotide (ASO) to APOC3 completely blocks diabetes-accelerated atherosclerosis in a mouse model. Other emerging strategies to block APOC3 include small interfering RNA (RNAi) and monoclonal antibodies. Figure 2B is reproduced and modified from [80] with permission from the Journal of Clinical Investigation.
This framework (Figure 1) of using DNA variation in a gene encoding a drug target as a proxy for an inhibitory drug has also provided insights into risk of T2DM with other non-statin LDL-C lowering medications. As proxies for other LDL-C lowering medications, DNA sequence variants in NPC1L1, which encodes the protein NiemanPick C1 like intracellular cholesterol transporter 1, the target of ezetimibe (a drug that reduces cholesterol absorption in the small intestine; Figure 2A) and PCSK9, which encodes the target of PCSK9-inhibitors (a class of drugs that increase LDL receptors on the surface of hepatocytes; Figure 2A) can be used to address the question of whether or not these drugs will also increase risk of T2DM. Using this approach, Mendelian randomization suggests that LDL-C lowering by inhibiting either PCSK9 or NPC1L1 will increase T2DM risk [39]. Although the mechanism driving the increased risk of T2DM via NPC1L1 and PCSK9 inhibition is also unknown, this increased T2DM risk has important therapeutic implications, as it suggests that LDL-C lowering, perhaps regardless of the mechanism through which this is achieved, may lead to an increased risk of T2DM.
Similar to the increased risk of T2DM seen with genetically reduced LDL-C, human genetic evidence supports the general conclusion that lower concentrations of triglyceride-rich lipoproteins (TRLs) associate with protection from CVD at a cost of increasing risk for T2DM [40, 41]. However, the metabolic pathway of TRL metabolism involving lipoprotein lipase (LPL) may break the general rule of an association between lipid-lowering and increased diabetes risk. LPL is the major enzyme responsible for the hydrolysis of triglycerides in TRLs and subsequent release of fatty acids for uptake into primarily skeletal muscle and adipose tissue [42]. A series of different genetic variants in LPL and many of its endogenous regulators demonstrate that genetic variation in this pathway, resulting in increased LPL enzymatic activity, is associated with reduced levels of TRLs and risk of CVD [43]. Do these targets also affect T2DM risk? Beyond a reduction in CVD, a partial loss-of-function allele in ANGPTL4 (which encodes angiopoietin-like 4; ANGPTL4; an endogenous inhibitor of LPL) also associated with reduced risk of T2DM [43], contrary to the increased T2DM risk observed with LDL-C lowering. Subsequent studies have confirmed that loss of function in ANGPTL4 is associated with lower fasting glucose, greater insulin sensitivity, and a corresponding reduction in T2DM risk [44], and that TRL-lowering associated with genetic variation in LPL itself protects from development of T2DM [45]. Similar findings have been reported for angiopoietin-like 3 (ANGPTL3), another endogenous inhibitor of LPL [46]. ANGPTL3-deficiency is associated with protection against CVD [47] and lower blood glucose levels [46]. In rodents, a significant part of the beneficial effects on glucose homeostasis by ANGPTL3-deficiency has been attributed to increased fatty acid uptake and oxidation in brown adipose tissue with diminished fatty acid uptake and increased glucose uptake in white adipose tissue [48].
Together, these observations suggest that therapeutic strategies to target the LPL pathway may be beneficial for preventing both CVD and T2DM risk whereas targeting LDL-C has detrimental effects on T2DM risk in some patients.
Translational Approaches to Identify Novel Therapeutic Targets
The traditional bench-to-bedside biomedical research paradigm - in which mechanisms with potential relevance to human disease are discovered using cellular and animal model systems before designing and testing novel therapeutics in clinical trials - has resulted in mixed success; although the number of new drugs approved by the US Food and Drug Administration (FDA) has steadily increased over the last decadeI, only about 10% of agents entering Phase 1 clinical trials eventually achieve FDA approval. “Reverse translation” or “human-first” studies aim to improve this by shifting the paradigm into a bedside-to-bench approach, often harnessing genetic information or other biosamples from patients as the primary discovery tool (e.g. comparing samples from patients with and without diabetic CVD). This approach, when combined with functional studies of identified target genes in animal model systems, has provided insights about new treatment strategies, some of which will be reviewed below. Beyond biosamples, the bedside-to-benchtop approach can also leverage clinical insights. For example, resulting from the FDA recommendation in 2008 to include cardiovascular endpoints in phase 2 and phase 3 clinical trials of new antidiabetic therapies, several new therapeutics have revealed unexpected protective effects on cardiovascular outcomes (see Clinician’s Corner).
Translational approaches and new targets for CVD prevention in type 2 diabetes
Examples of translational “human-first” studies that revealed cardioprotective effects in subjects with diabetes include clinical trials on GLP-1 receptor agonists, sodium-glucose cotransporter 2 (SGLT2) inhibitors, and icosapent ethyl ester. These trials have propelled a large surge in animal- and in vitro studies to search for mechanisms and additional drug targets.
GLP-1 receptor agonists, which increase insulin secretion, and SGLT2 inhibitors, which lower blood glucose levels by preventing glucose reabsorption in the kidney, were both recently shown to prevent CVD events in patients with T2DM [49–53]. The beneficial effects of GLP-1 receptor agonists on myocardial infarction and CVD death are multifactorial, and include anti-inflammatory effects through both direct and indirect effects in the myocardium and vasculature, direct effects on improvement of cardiac function and increased vasodilation, reduced postprandial lipids, increased natriuresis and diuresis, reduced body weight, and reduced coagulation [54].
SGLT2 inhibitors are only modestly effective in reducing atherosclerotic cardiovascular disease, and display the greatest cardiorenal benefit on heart failure and progression of renal disease [55]. Furthermore, the recent DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial (NCT03036124)II indicates that the beneficial effects of this SGLT2 inhibitor are not restricted to people with diabetes but can also be observed in those without diabetes [56]. It has therefore been suggested that the beneficial effects of SGLT2 inhibitors are not directly related to blood glucose-lowering, but instead include natriuresis and osmotic diuresis, reduced inflammation, reduced oxidative stress, reduced arterial stiffness, reductions in blood pressure and body weight, and renoprotective effects [57].
Other clinical trials have shown beneficial effects on CVD outcomes of icosapent ethyl ester (eicosapentaenoic acid; EPA), a highly pure fish oil ester, in populations with and without T2DM [58, 59]. The cardioprotective mechanisms of EPA in people with diabetes are still largely unclear, and are areas of intensive research [60, 61]. The possibility that EPA’s protective effects are not explained by its triglyceride-lowering actions, but perhaps by mechanisms related to production of pro-resolving bioactive lipid mediators, altered membrane fluidity, protective effects in endothelial cells, or antioxidative effects are being investigated [62–64].
Together, the positive findings on GLP1 receptor agonists, SGLT2 inhibitors and EPA supplementation on CVD risk have created a large interest in the search for related mechanisms and new drug targets in these pathways to combat CVD risk associated with diabetes.
Translational approaches have led to identification of APOC3 as a potential target for prevention of atherosclerotic vascular disease in both T1DM and T2DM
Another relevant example of translational approaches to investigate mechanisms of a protein with known adverse CVD effects is apolipoprotein C3 (APOC3). APOC3 prevents the clearance of TRLs and their remnant lipoprotein particles (RLPs) and is produced mainly by the liver. The original interest in APOC3 stems from a human study in which a loss-of-function mutation in the APOC3 gene was associated with reduced plasma triglyceride levels and apparent protection against CVD [65]. The genetic results were later confirmed in large population studies [66, 67]. Furthermore, inhibition of APOC3 improved whole-body insulin resistance in a small study on subjects with T2DM [68] consistent with the concept that targeting the LPL pathway might have a beneficial effect on T2DM risk.
APOC3 is readily exchangeable between lipoprotein populations and is found at varying amounts on chylomicrons, VLDL, LDL, RLPs, and HDL. APOC apolipoproteins are transferred to HDL during lipolysis of TRLs, likely due to the reduction in volume and surface area of the TRLs during lipolysis [69]. APOC3 appears to increase plasma TRL levels by several mechanisms. It inhibits LPL activity, thereby preventing uptake of fatty acids into skeletal muscle and adipose tissue [70, 71]. By inhibiting LPL, it also slows conversion of VLDL to LDL [72], which further contributes to VLDL and RLP accumulation (Figure 2A). In addition, at least in mice and in LPL-deficient humans [73–75], APOC3 hinders the reuptake of TRLs by the liver by blocking the binding of APOE present on TRLs and RLPs to their hepatic receptors; the LDL receptor, LDL receptor related protein 1 (LRP1), and syndecan 1 [73]. A similar mechanism has been proposed to operate in intestinal enterocytes in mice overexpressing APOC3 [76]. The role for this mechanism in humans is unclear. Likewise, the in vivo relevance of in vitro studies suggesting that APOC3 overexpression enhances VLDL assembly and secretion [77] is uncertain. APOC3 is therefore generally believed to increase TRLs in vivo through reduced TRL catabolism. Consistent with an important role of APOC3 in TRL clearance, in normotriglyceridemic individuals, APOE-containing VLDL is rapidly cleared whereas hypertriglyceridemic patients exhibit increased APOC3 in TRLs, reduced clearance of TRLs, and increased levels of dense LDL [78].
In both the general population and in subjects with diabetes, serum levels of APOC3 are associated with an increased risk of CVD [79–81]. Recently, it was shown that serum levels of APOC3 in people with T1DM predict future cardiovascular events in two different prospective studies [80, 81]. Importantly, the increased APOC3 levels were found before CVD was evident. In the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study (NCT00005754)III, serum APOC3 levels predicted future CAD even after adjusting for age, sex, diabetes duration, glycemic control (measured as HbA1c) and LDL-C, suggesting that in T1DM, APOC3 predicts incident CVD independent of traditional risk factors [80]. Consistently, serum samples collected from the Diabetes Control and Complications Trial/ Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort (NCT00360893)IV demonstrated that APOC3 serum levels associated nominally with future CVD events, even after adjusting for age, sex and LDL-C [81]. Importantly, in both studies plasma triglyceride levels were within the normal range or close to normal, in agreement with the fact that people with T1DM who have well-controlled glucose levels often exhibit normal lipid profiles. Furthermore, although APOC3 and triglycerides were not independent predictors of CVD in CACTI (which was expected because APOC3 acts to slow TRL clearance), APOC3 appeared to be a stronger predictor of future CVD than did triglycerides, indicating a particularly important role for APOC3 in predicting incident CVD in T1DM [80].
Causal effects of APOC3 in atherosclerosis associated with diabetes were supported by a mouse model of T1DM-accelerated atherosclerosis [82], which showed elevated plasma APOC3 levels [80]. While several studies have demonstrated that overexpression of APOC3 results in accelerated atherosclerosis in mice, the impact of deleting APOC3 in nondiabetic mice is less clear [83, 84]. However, reducing plasma APOC3 through inhibiting hepatic APOC3 production with an antisense oligonucleotide (ASO) strikingly prevented both early atherosclerosis and progression of more advanced atherosclerosis under diabetic conditions [80]. This highlights the importance of APOC3 in promoting atherosclerosis in diabetes (Figure 2B).
Both hyperglycemia and insulin deficiency have been proposed to increase APOC3 levels [85, 86]. In the mouse model of T1DM discussed above, APOC3 levels were elevated in diabetic mice due to insulin deficiency rather than to hyperglycemia because the elevated APOC3 levels were prevented by an intensive insulin regimen but not by treatment with an SGLT2 inhibitor [80]. The exact mechanism whereby insulin suppresses APOC3 levels needs further investigation. One possibility is that insulin promotes the clearance of APOC3-rich TRLs by increasing membrane localization of LRP1 in the liver [87, 88] and/or by promoting hepatic LDL receptor expression, as discussed below. These observations also raise the possibility that hepatic insulin resistance associated with either T1DM or T2DM could be responsible, at least in part, for increased plasma levels of APOC3.
How do reduced levels of APOC3 result in athero-protection? Again, mechanistic experimentation in animal models sheds insight into this question. It has been postulated that the enrichment of APOC3 on lipoprotein particles associates with more atherogenic properties. For example, a sub-population of HDL devoid of APOC3 is associated with reduced CVD risk whereas APOC3-containing sub-populations of HDL fail to associate with cardioprotection [89]. APOC3 levels in HDL may be a reflection of altered TRL catabolism, rather than reduced HDL function [80]. Similarly, enrichment of LDL with APOC3 results in faster conversion into small, dense LDL [90], a highly proatherogenic particle [91]. Under diabetic conditions, TRLs and their lipolysis products are likely pro-atherogenic, with increased trapping in the artery wall of APOB, APOC3 and APOE [80] (Figure 2B). This mechanism is supported by evidence that LDL and VLDL enriched in APOC3 bind more efficiently to the extracellular matrix present in the artery wall [92]. The notion that APOC3 is atherogenic, through direct or indirect effects, is also highlighted by the population-based Bruneck study in which TRL-associated proteins such as APOC3, APOC2, and APOE were predictors of CVD and were lowered by inhibition of APOC3 [93]. Recently, APOC3 was shown to directly stimulate inflammasome activation in human monocytes by enhancing dimerization of toll-like receptors 2 and 4 [94]. This study suggests a causal link between APOC3 and increased inflammasome activation, which is likely to enhance atherosclerosis and perhaps CVD risk. It also suggests that some of the effects of APOC3 might be mediated through direct actions on vascular cells [95].
Will it be feasible to target APOC3 to combat the increased risk of CVD observed in T1DM or T2DM? A human APOC3 antisense therapeutic (Volanesorsen) has been shown to reduce plasma APOC3 and plasma triglycerides in subjects with familial chylomicronemia syndrome (a condition resulting from LPL-deficiency) [74, 75], in subjects with other forms of hypertriglyceridemia [96], and in a small study in subjects with T2DM [68]. Inhibiting APOC3 in humans, at least short-term, is therefore feasible. A drawback of the currently tested APOC3 ASO therapeutic (a 2′-O-methoxyethylmodified antisense oligonucleotide) is that it induced thrombocytopenia in a subset of subjects [75]. It is uncertain if this effect was due to the chemical class of antisense oligonucleotides, high concentrations of the antisense oligonucleotides, or a targetspecific effect. In either case, no functional effects on platelets were observed [97]. A newer version of the APOC3 antisense uses an N-acetyl galactosamine (GalNAc3)modified ASO to target the ASO to hepatocytes. This allows for lower dosing and thus potentially less systemic off-target effects. Notably this ASO been shown to reduce plasma APOC3 and triglyceride levels in healthy human subjects [98] without a clinically significant effect on hematologic parameters [98]. As an alternative to antisense technologies targeting APOC3, monoclonal antibodies against APOC3 have been generated to reduce plasma APOC3 levels, and have been shown to successfully lower APOC3 and triglycerides in a humanized mouse model [99]. A small interfering RNA (RNAi) targeting APOC3 has also recently been shown to be effective in reducing plasma triglycerides in non-human primates [100] and in humans.
Together, these recent findings suggest that APOC3 is a promising target for prevention of CVD associated with diabetes. Furthermore, because people with diabetes and impaired kidney function are at a particularly increased risk of CVD [101–105], and because decreased renal function is associated with elevated APOC3 levels [106–110], targeting APOC3 in this population might be particularly beneficial. Evaluation of the effects of APOC3 inhibition in large cardiovascular outcomes studies in people with diabetes and elevated APOC3 levels is needed next (see Clinician’s corner).
Modeling and Pharmacology-based Approaches to Improve Insulin Receptor Activation
A third approach to identify novel treatment strategies for prevention of cardiovascular complications of diabetes is to use modeling and pharmacology-based methods to, for example, improve insulin’s action. T2DM is characterized by impaired insulin sensitivity and an inability of islet β-cells to compensate for the decreased insulin sensitivity [111]. Insulin treatment is critical in T1DM and in overt T2DM to prevent ketosis and other detrimental effects of insulin deficiency. Technologies for continuous glucose monitoring and improved insulin administration have developed rapidly, making it easier to optimize insulin regimens. The development of modified insulins that have short- or long-acting properties have also improved glucose control [112]. The effects of these insulin analogs have so far not outperformed the weak effect of conventional insulin on CVD risk [113]. However, new possibilities have emerged over the past several years, including pharmacological methods of targeting insulin delivery to the liver and modeling to create insulin mimetic peptides with biological effects superior to those of human insulin.
Targeting insulin delivery to the hepatic insulin receptor
Insulin exerts pro- or anti-atherosclerotic effects depending on the cell type on which it acts [114–118]. The liver expresses an abundance of insulin receptors, and the liver is also the first organ encountered by insulin after its release from the pancreas through the portal vein. Approaches have therefore attempted to preferentially target insulin administration to the liver. This can be achieved e.g., by tethering insulin to phospholipid vesicles. Liver-targeted lispro insulin (a rapid-acting human insulin analog) was recently shown to lower plasma glucose like conventional lispro insulin, but compared with conventional insulin, it also lowered plasma cholesterol without significantly affecting LDL-C in patients with T1DM [119]. It is possible that liver-directed insulin delivery has a better effect on lipid metabolism than conventionally administered insulin, including perhaps suppression of APOC3, which would in turn be an interesting strategy for CVD prevention (Figure 2).
Mouse studies have revealed the importance of the hepatic insulin receptor in suppressing APOB-containing lipoproteins and atherosclerosis. For example, a mouse model selectively deficient in hepatic insulin receptors but with normal insulin receptor levels in peripheral tissues showed elevated VLDL levels associated with increased atherosclerosis when these mice were fed a high fat sodium cholate-containing diet [116]. Consistently, silencing hepatic insulin receptor levels in obese ob/ob mice or in fat-fed mice resulted in a marked reduction in hepatic LDL receptor levels [115]. Together, these two studies support the concept that defective hepatic insulin receptor signaling mediates impaired clearance of APOB-containing lipoproteins through the LDL receptor, and also perhaps worsened atherosclerosis. Strategies to target insulin delivery to the liver, mimicking the physiological delivery of insulin, could therefore be beneficial.
Modeling to create insulin mimetics with improved anti-atherosclerotic effects
The insulin receptor activates two major pathways of intracellular signaling; the Akt and Erk signaling pathways. In the liver, activation of the Akt pathway predominates over the Erk pathway after insulin injection in mice [120]. Sophisticated modeling of insulin binding to its receptor has provided evidence that insulin uses two different sites to crosslink the two α-subunits of the insulin receptor, inducing a conformational change that activates the receptor [121, 122]. These modeling studies led to screening of peptide display libraries for peptides that bind two distinct hotspots on the insulin receptor. Using this approach, an insulin mimetic peptide (Ac-SLEEEWAQIECEVYGRGCPSESFYDWFERQL-amide), which effectively couples the insulin receptor to Akt signaling, but is less effective in activating the Erk pathway was identified [123]. This peptide lowered blood glucose like insulin, but had a superior ability to prevent atherosclerosis in a mouse model of pre-diabetes and also transiently reduced plasma triglycerides [120], consistent with the triglyceride-lowering effects of this peptide in Zucker diabetic fatty rats [124]. The former study suggested a novel treatment strategy for advanced atherosclerosis associated with metabolic syndrome and T2DM [120], however, it has so far been tested only in mice.
Together, these new approaches suggest that it might be possible to target insulin receptor activation in ways that lead to the desired glucose lowering effects and also to enhanced CVD protection.
Concluding Remarks
New CVD treatment strategies targeting lipids as well as non-lipid pathways are on the horizon for people with T1DM and T2DM and are moving us closer to a more individualized approach for CVD prevention in diabetes. Genetic studies combined with functional studies of identified target genes have provided a strong reason for further investigation of the LPL pathway, a master regulator of TRLs and their remnants, to target residual cardiovascular risk. Although powerful, genetic studies have limitations in that they do not provide detailed mechanistic insight. Furthermore, genetic variation is not equivalent to pharmacologic therapy, as the effect of genetic variation is present over an individual’s lifetime, and pharmacologic inhibition may include off-target effects [30]. Therefore, additional approaches to discovery of strategies to prevent the increased CVD risk associated with diabetes are needed (see Outstanding Questions). The approach of reverse translation or “human-first” studies discussed here is likely to lead to the discovery of new drug targets. Furthermore, it is tempting to speculate that modified insulin receptor activation that targets the liver and/or the Akt signaling pathway might provide benefits. The last decade revealed cardioprotective effects of several new medications that also have beneficial effects on diabetes. This new decade is certain to bring to light additional advances to help people with diabetes stay protected from cardiovascular complications.
Outstanding questions
What mechanism(s) underlie the increased risk of diabetes associated with reducing LDL-cholesterol and why does LPL-mediated reduction of triglyceriderich lipoproteins protect against risk of diabetes?
Despite rich evidence supporting a causative role for APOC3 in atherosclerotic cardiovascular disease, it is not known how APOC3 mediates its pro-atherogenic effects. Does it act through directly affecting vascular cells or are the detrimental effects of APOC3 mediated by its ability to prevent clearance of triglyceride-rich lipoproteins and their remnants?
Is APOC3 a more important cardiovascular risk factor in people with diabetes and/or hepatic insulin resistance than in people without diabetes, given that insulin suppresses APOC3 levels?
Do triglyceride-rich lipoproteins and/or their remnants explain a significant part of the residual cardiovascular disease risk in patients on LDL-cholesterol lowering medications? Will it be possible to reduce residual cardiovascular risk in these patients by medications that target clearance of triglyceride-rich lipoproteins and/or their remnants?
Highlights
“Reverse translation” harnesses genetic information or data from human biosamples as the primary discovery tool. This approach, when combined with mechanistic studies in animal model systems, has provided important insights about emerging cardiovascular disease (CVD) treatment strategies.
Increased catabolism of triglyceride-rich lipoproteins through activation of lipoprotein lipase (LPL) appears to prevent both CVD and type 2 diabetes (T2DM). This pathway includes APOC3, ANGPTL3 and ANGPTL4, all endogenous inhibitors of LPL.
Insulin suppresses APOC3, thus APOC3 inhibition may be beneficial as a CVD prevention strategy in patients with diabetes and impaired insulin levels or insulin resistance.
Modeling and pharmacology-based approaches to improve hepatic insulin receptor activation are other emerging approaches for CVD protection in the setting of diabetes.
Acknowledgments
Research in the authors’ laboratories is supported by the American Diabetes Association grant 1–16-IBS-153 and the National Institutes of Health (NIH) grants U24DK076169 and U24DK115255, subaward 32307–34 to J.E.K., and by NIH grants to N.O.S. (R01HL131961 and UM1HG008853) and K.E.B. (DP3DK108209, R01HL127694, R01HL126028, and P01HL092969), and by the Diabetes Research Center at the University of Washington, P30DK017047.
Glossary
- Angiopoietin-like 3
An endogenous inhibitor of the enzyme lipoprotein lipase (see below). Angiopoietin-like 3 (ANGPTL3) is mainly produced by the liver. Loss-of-function mutations in ANGPTL3 associate with apparent protection against cardiovascular disease and increased insulin sensitivity
- Angiopoietin-like 4
Angiopoietin-like 4 (ANGPTL4), like ANGPTL3, inhibits lipoprotein lipase, and is produced by many different cells in the body. Partial loss-of-function mutations in ANGPTL4 associate with apparent protection against cardiovascular disease and diabetes risk
- Apolipoprotein
A type of protein that binds lipoproteins and have structural functions or functions related to lipoprotein metabolism and clearance or to inflammation. Examples of apolipoproteins include apolipoprotein B100, the main apolipoprotein in low-density lipoprotein, and apolipoprotein A1, the main structural protein of highdensity lipoprotein.
- Apolipoprotein C3
A small apolipoprotein that prevents clearance of plasma triglycerides by inhibiting lipoprotein lipase and by hampering the uptake of triglyceriderich lipoproteins (chylomicrons, very low-density lipoproteins and their remnant lipoproteins) in the liver
- Atherosclerotic cardiovascular disease
Atherosclerosis, the build-up of plaques in susceptible large and medium-sized arteries, is responsible for most cardiovascular events, such as heart attacks and strokes. Atherosclerosis is worsened by diabetes
- Lipoprotein lipase
An enzyme (lipase) bound primarily to the capillary endothelium. Lipoprotein lipase (LPL) hydrolyzes triglycerides in the core of triglyceride-rich lipoproteins to liberate free fatty acids for uptake and utilization in tissues. Because of its localization, LPL primarily stimulates fatty acid uptake into adipose tissue and skeletal muscle
- Mendelian randomization
Mendelian randomization is a method based on the concept that genetic variants that associate with altered biomarkers can be used as instruments to estimate the likely causal impact of that biomarker on disease
- Triglyceride-rich lipoproteins
These lipoproteins include large lipoproteins, such as intestinal-derived chylomicrons, and the smaller very low-density lipoproteins (VLDL) derived from the liver. Triglyceride-rich lipoproteins are enriched in triglycerides and deliver these triglycerides to peripheral tissues where fatty acids are liberated by LPL. Triglyceride-rich lipoproteins are often elevated in type 2 diabetes and in poorly controlled type 1 diabetes
- Type 1 diabetes mellitus (T1DM)
An autoimmune disease targeting the insulinproducing pancreatic beta cells. Approximately 5% of people who have diabetes have type 1 diabetes, which is much less prevalent than type 2 diabetes
- Type 2 diabetes mellitus (T2DM)
Type 2 diabetes is characterized by elevated blood glucose levels, like type 1 diabetes. In type 2 diabetes, the elevated blood glucose is due to organs not responding sufficiently well to insulin, and/or to insufficient insulin production
Footnotes
Conflict of Interest
N.O.S. has received an investigator-initiated grant from Regeneron. K.E.B. and J.E.K. have received research support from Novo Nordisk A/S.
Resources
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ et al. (2019) Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 139 (10), e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Beckman JA et al. (2013) Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Eur Heart J 34 (31), 2444–52. [DOI] [PubMed] [Google Scholar]
- 3.Emerging Risk Factors C. et al. (2010) Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375 (9733), 2215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Ferranti SD et al. (2014) Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation 130 (13), 1110–30. [DOI] [PubMed] [Google Scholar]
- 5.Rawshani A. et al. (2018) Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 392 (10146), 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bebu I. et al. (2019) Mediation of the Effect of Glycemia on the Risk of CVD Outcomes in Type 1 Diabetes: The DCCT/EDIC Study. Diabetes Care 42 (7), 1284–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bebu I. et al. (2017) The relationship of blood glucose with cardiovascular disease is mediated over time by traditional risk factors in type 1 diabetes: the DCCT/EDIC study. Diabetologia 60 (10), 2084–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buse JB (2015) Glycemic Targets in Diabetes Care: Emerging Clarity after Accord. Trans Am Clin Climatol Assoc 126, 62–76. [PMC free article] [PubMed] [Google Scholar]
- 9.Ismail-Beigi F. et al. (2010) Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 376 (9739), 419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Group AC et al. (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358 (24), 2560–72. [DOI] [PubMed] [Google Scholar]
- 11.Zoungas S. et al. (2014) Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med 371 (15), 1392–406. [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg HN (1991) Lipoprotein physiology in nondiabetic and diabetic states. Relationship to atherogenesis. Diabetes Care 14 (9), 839–55. [DOI] [PubMed] [Google Scholar]
- 13.Chait A. et al. (2020) Remnants of the triglyceride-rich lipoproteins, diabetes, and cardiovascular disease. Diabetes 69 (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao C. et al. (2016) Pharmacological Targeting of the Atherogenic Dyslipidemia Complex: The Next Frontier in CVD Prevention Beyond Lowering LDL Cholesterol. Diabetes 65 (7), 1767–78. [DOI] [PubMed] [Google Scholar]
- 15.Laakso M and Kuusisto J. (2017) Diabetes Secondary to Treatment with Statins. Curr Diab Rep 17 (2), 10. [DOI] [PubMed] [Google Scholar]
- 16.Casula M. et al. (2017) Statin use and risk of new-onset diabetes: A meta-analysis of observational studies. Nutr Metab Cardiovasc Dis 27 (5), 396–406. [DOI] [PubMed] [Google Scholar]
- 17.Visscher PM et al. (2017) 10 Years of GWAS Discovery: Biology, Function, and Translation. Am J Hum Genet 101 (1), 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott RA et al. (2016) A genomic approach to therapeutic target validation identifies a glucose-lowering GLP1R variant protective for coronary heart disease. Sci Transl Med 8 (341), 341ra76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen A. et al. (2018) Glucagon-like peptide 1 in health and disease. Nat Rev Endocrinol 14 (7), 390–403. [DOI] [PubMed] [Google Scholar]
- 20.Mahajan A. et al. (2018) Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet 50 (11), 1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue A. et al. (2018) Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat Commun 9 (1), 2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson CP et al. (2017) Association analyses based on false discovery rate implicate new loci for coronary artery disease. Nat Genet 49 (9), 1385–1391. [DOI] [PubMed] [Google Scholar]
- 23.van der Harst P and Verweij N. (2018) Identification of 64 Novel Genetic Loci Provides an Expanded View on the Genetic Architecture of Coronary Artery Disease. Circ Res 122 (3), 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webb TR et al. (2017) Systematic Evaluation of Pleiotropy Identifies 6 Further Loci Associated With Coronary Artery Disease. J Am Coll Cardiol 69 (7), 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith GD and Ebrahim S. (2003) ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 32 (1), 1–22. [DOI] [PubMed] [Google Scholar]
- 26.Holmes MV et al. (2017) Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol 14 (10), 577–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett DA and Holmes MV (2017) Mendelian randomisation in cardiovascular research: an introduction for clinicians. Heart 103 (18), 1400–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plenge RM et al. (2013) Validating therapeutic targets through human genetics. Nat Rev Drug Discov 12 (8), 581–94. [DOI] [PubMed] [Google Scholar]
- 29.Stitziel NO and Kathiresan S. (2016) Leveraging human genetics to guide drug target discovery. Trends Cardiovasc Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young EP and Stitziel NO (2019) Capitalizing on Insights from Human Genetics to Identify Novel Therapeutic Targets for Coronary Artery Disease. Annu Rev Med 70, 19–32. [DOI] [PubMed] [Google Scholar]
- 31.Mullard A. (2015) The phenotypic screening pendulum swings. Nat Rev Drug Discov 14 (12), 807–9. [DOI] [PubMed] [Google Scholar]
- 32.Collins R. et al. (2003) MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 361 (9374), 2005–16. [DOI] [PubMed] [Google Scholar]
- 33.Arnett DK et al. (2019) 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 140 (11), e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sattar N. et al. (2010) Statins and risk of incident diabetes: a collaborative metaanalysis of randomised statin trials. Lancet 375 (9716), 735–42. [DOI] [PubMed] [Google Scholar]
- 35.Swerdlow DI et al. (2015) HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet 385 (9965), 351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frayling TM (2015) Statins and type 2 diabetes: genetic studies on target. Lancet 385 (9965), 310–2. [DOI] [PubMed] [Google Scholar]
- 37.Sampson UK et al. (2011) Are statins diabetogenic? Curr Opin Cardiol 26 (4), 342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henriksbo BD and Schertzer JD (2015) Is immunity a mechanism contributing to statin-induced diabetes? Adipocyte 4 (4), 232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lotta LA et al. (2016) Association Between Low-Density Lipoprotein CholesterolLowering Genetic Variants and Risk of Type 2 Diabetes: A Meta-analysis. JAMA 316 (13), 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White J. et al. (2016) Association of Lipid Fractions With Risks for Coronary Artery Disease and Diabetes. JAMA Cardiol 1 (6), 692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saleheen D. et al. (2016) Disentangling the Causal Association of Plasma Lipid Traits and Type 2 Diabetes Using Human Genetics. JAMA Cardiol 1 (6), 631–3. [DOI] [PubMed] [Google Scholar]
- 42.Goldberg IJ (1996) Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J Lipid Res 37 (4), 693–707. [PubMed] [Google Scholar]
- 43.Stitziel N. et al. (2016) Coding Variation in ANGPTL4, LPL, and SVEP1 and the Risk of Coronary Disease. N Engl J Med 374 (12), 1134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gusarova V. et al. (2018) Genetic inactivation of ANGPTL4 improves glucose homeostasis and is associated with reduced risk of diabetes. Nat Commun 9 (1), 2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lotta LA et al. (2018) Association of Genetically Enhanced Lipoprotein LipaseMediated Lipolysis and Low-Density Lipoprotein Cholesterol-Lowering Alleles With Risk of Coronary Disease and Type 2 Diabetes. JAMA Cardiol 3 (10), 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robciuc MR et al. (2013) Angptl3 deficiency is associated with increased insulin sensitivity, lipoprotein lipase activity, and decreased serum free fatty acids. Arterioscler Thromb Vasc Biol 33 (7), 1706–13. [DOI] [PubMed] [Google Scholar]
- 47.Stitziel NO et al. (2017) ANGPTL3 Deficiency and Protection Against Coronary Artery Disease. J Am Coll Cardiol 69 (16), 2054–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banfi S. et al. (2018) Increased thermogenesis by a noncanonical pathway in ANGPTL3/8-deficient mice. Proc Natl Acad Sci U S A 115 (6), E1249–E1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt AM (2019) Diabetes Mellitus and Cardiovascular Disease. Arterioscler Thromb Vasc Biol 39 (4), 558–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdul-Ghani M. et al. (2017) Cardiovascular Disease and Type 2 Diabetes: Has the Dawn of a New Era Arrived? Diabetes Care 40 (7), 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cavender MA et al. (2018) SGLT-2 Inhibitors and Cardiovascular Risk: An Analysis of CVD-REAL. J Am Coll Cardiol 71 (22), 2497–2506. [DOI] [PubMed] [Google Scholar]
- 52.Zinman B. et al. (2015) Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 373 (22), 2117–28. [DOI] [PubMed] [Google Scholar]
- 53.Neal B. et al. (2017) Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 377 (7), 644–657. [DOI] [PubMed] [Google Scholar]
- 54.Drucker DJ (2016) The Cardiovascular Biology of Glucagon-like Peptide-1. Cell Metab 24 (1), 15–30. [DOI] [PubMed] [Google Scholar]
- 55.Zelniker TA et al. (2019) SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and metaanalysis of cardiovascular outcome trials. Lancet 393 (10166), 31–39. [DOI] [PubMed] [Google Scholar]
- 56.McMurray JJV et al. (2019) Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 381 (21), 1995–2008. [DOI] [PubMed] [Google Scholar]
- 57.Verma S. (2019) Potential Mechanisms of Sodium-Glucose Co-Transporter 2 Inhibitor-Related Cardiovascular Benefits. Am J Cardiol 124 Suppl 1, S36–S44. [DOI] [PubMed] [Google Scholar]
- 58.Yokoyama M. et al. (2007) Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369 (9567), 1090–8. [DOI] [PubMed] [Google Scholar]
- 59.Bhatt DL et al. (2019) Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med 380 (1), 11–22. [DOI] [PubMed] [Google Scholar]
- 60.Lytvyn Y. et al. (2017) Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials. Circulation 136 (17), 1643–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bajaj HS et al. (2018) Glucagon-like peptide-1 receptor agonists and cardiovascular protection in type 2 diabetes: a pathophysiology-based review of clinical implications. Curr Opin Cardiol 33 (6), 665–675. [DOI] [PubMed] [Google Scholar]
- 62.Mason RP et al. (2016) Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. Biochim Biophys Acta 1858 (12), 3131–3140. [DOI] [PubMed] [Google Scholar]
- 63.Sherratt SCR and Mason RP (2018) Eicosapentaenoic acid inhibits oxidation of high density lipoprotein particles in a manner distinct from docosahexaenoic acid. Biochem Biophys Res Commun 496 (2), 335–338. [DOI] [PubMed] [Google Scholar]
- 64.Boden WE et al. (2019) Profound reductions in first and total cardiovascular events with icosapent ethyl in the REDUCE-IT trial: why these results usher in a new era in dyslipidaemia therapeutics. Eur Heart J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pollin TI et al. (2008) A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science 322 (5908), 1702–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tg et al. (2014) Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med 371 (1), 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jorgensen AB et al. (2014) Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med 371 (1), 32–41. [DOI] [PubMed] [Google Scholar]
- 68.Digenio A. et al. (2016) Antisense-Mediated Lowering of Plasma Apolipoprotein CIII by Volanesorsen Improves Dyslipidemia and Insulin Sensitivity in Type 2 Diabetes. Diabetes Care 39 (8), 1408–15. [DOI] [PubMed] [Google Scholar]
- 69.Havel RJ et al. (1973) Interchange of apolipoproteins between chylomicrons and high density lipoproteins during alimentary lipemia in man. J Clin Invest 52 (1), 32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown WV and Baginsky ML (1972) Inhibition of lipoprotein lipase by an apoprotein of human very low density lipoprotein. Biochem Biophys Res Commun 46 (2), 375–82. [DOI] [PubMed] [Google Scholar]
- 71.Ginsberg HN et al. (1986) Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J Clin Invest 78 (5), 1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reyes-Soffer G. et al. (2019) Effects of APOC3 Heterozygous Deficiency on Plasma Lipid and Lipoprotein Metabolism. Arterioscler Thromb Vasc Biol 39 (1), 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gordts PL et al. (2016) ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J Clin Invest 126 (8), 2855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gaudet D. et al. (2014) Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med 371 (23), 2200–6. [DOI] [PubMed] [Google Scholar]
- 75.Witztum JL et al. (2019) Volanesorsen and Triglyceride Levels in Familial Chylomicronemia Syndrome. N Engl J Med 381 (6), 531–542. [DOI] [PubMed] [Google Scholar]
- 76.Li D. et al. (2019) Intestinal basolateral lipid substrate transport is linked to chylomicron secretion and is regulated by apoC-III. J Lipid Res 60 (9), 1503–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sundaram M. et al. (2010) Expression of apolipoprotein C-III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions. J Lipid Res 51 (1), 150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng C. et al. (2010) Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation 121 (15), 1722–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wulff AB et al. (2018) APOC3 Loss-of-Function Mutations, Remnant Cholesterol, Low-Density Lipoprotein Cholesterol, and Cardiovascular Risk: Mediation- and MetaAnalyses of 137 895 Individuals. Arterioscler Thromb Vasc Biol 38 (3), 660–668. [DOI] [PubMed] [Google Scholar]
- 80.Kanter JE et al. (2019) Increased apolipoprotein C3 drives cardiovascular risk in type 1 diabetes. J Clin Invest 130, 4165–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Basu A. et al. (2019) Serum apolipoproteins and apolipoprotein-defined lipoprotein subclasses: a hypothesis-generating prospective study of cardiovascular events in T1D. J Lipid Res 60 (8), 1432–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Renard CB et al. (2004) Diabetes and diabetes-associated lipid abnormalities have distinct effects on initiation and progression of atherosclerotic lesions. J Clin Invest 114 (5), 659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Masucci-Magoulas L. et al. (1997) A mouse model with features of familial combined hyperlipidemia. Science 275 (5298), 391–4. [DOI] [PubMed] [Google Scholar]
- 84.Li H. et al. (2015) Aggravated restenosis and atherogenesis in ApoCIII transgenic mice but lack of protection in ApoCIII knockouts: the effect of authentic triglyceride-rich lipoproteins with and without ApoCIII. Cardiovasc Res 107 (4), 579–89. [DOI] [PubMed] [Google Scholar]
- 85.Caron S. et al. (2011) Transcriptional activation of apolipoprotein CIII expression by glucose may contribute to diabetic dyslipidemia. Arterioscler Thromb Vasc Biol 31 (3), 513–9. [DOI] [PubMed] [Google Scholar]
- 86.Chen M. et al. (1994) Transcriptional regulation of the apoC-III gene by insulin in diabetic mice: correlation with changes in plasma triglyceride levels. J Lipid Res 35 (11), 1918–24. [PubMed] [Google Scholar]
- 87.Laatsch A. et al. (2009) Insulin stimulates hepatic low density lipoprotein receptorrelated protein 1 (LRP1) to increase postprandial lipoprotein clearance. Atherosclerosis 204 (1), 105–11. [DOI] [PubMed] [Google Scholar]
- 88.Haas ME et al. (2013) The regulation of ApoB metabolism by insulin. Trends Endocrinol Metab 24 (8), 391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jensen MK et al. (2018) High-Density Lipoprotein Subspecies Defined by Presence of Apolipoprotein C-III and Incident Coronary Heart Disease in Four Cohorts. Circulation 137 (13), 1364–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mendivil CO et al. (2010) Metabolism of very-low-density lipoprotein and lowdensity lipoprotein containing apolipoprotein C-III and not other small apolipoproteins. Arterioscler Thromb Vasc Biol 30 (2), 239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mendivil CO et al. (2011) Low-density lipoproteins containing apolipoprotein C-III and the risk of coronary heart disease. Circulation 124 (19), 2065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Olin-Lewis K. et al. (2002) ApoC-III content of apoB-containing lipoproteins is associated with binding to the vascular proteoglycan biglycan. J Lipid Res 43 (11), 1969–77. [DOI] [PubMed] [Google Scholar]
- 93.Pechlaner R. et al. (2017) Very-Low-Density Lipoprotein-Associated Apolipoproteins Predict Cardiovascular Events and Are Lowered by Inhibition of APOCIII. J Am Coll Cardiol 69 (7), 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zewinger S. et al. (2019) Apolipoprotein C3 induces inflammation and organ damage by alternative inflammasome activation. Nat Immunol. [DOI] [PubMed] [Google Scholar]
- 95.Ridker PM et al. (2017) Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 377 (12), 1119–1131. [DOI] [PubMed] [Google Scholar]
- 96.Gaudet D. et al. (2015) Antisense Inhibition of Apolipoprotein C-III in Patients with Hypertriglyceridemia. N Engl J Med 373 (5), 438–47. [DOI] [PubMed] [Google Scholar]
- 97.Crooke ST et al. (2017) The Effects of 2’-O-Methoxyethyl Containing Antisense Oligonucleotides on Platelets in Human Clinical Trials. Nucleic Acid Ther 27 (3), 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alexander VJ et al. (2019) N-acetyl galactosamine-conjugated antisense drug to APOC3 mRNA, triglycerides and atherogenic lipoprotein levels. Eur Heart J 40 (33), 2785–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khetarpal SA et al. (2017) A human APOC3 missense variant and monoclonal antibody accelerate apoC-III clearance and lower triglyceride-rich lipoprotein levels. Nat Med 23 (9), 1086–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Butler AA et al. (2019) Fructose-induced hypertriglyceridemia in rhesus macaques is attenuated with fish oil or ApoC3 RNA interference. J Lipid Res 60 (4), 805–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Afkarian M. et al. (2013) Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 24 (2), 302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fox CS et al. (2012) Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 380 (9854), 1662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Groop PH et al. (2009) The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 58 (7), 1651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maahs DM et al. (2013) Impaired renal function further increases odds of 6-year coronary artery calcification progression in adults with type 1 diabetes: the CACTI study. Diabetes Care 36 (9), 2607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Orchard TJ et al. (2010) In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 53 (11), 2312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Attman PO et al. (2011) The effect of decreasing renal function on lipoprotein profiles. Nephrol Dial Transplant 26 (8), 2572–5. [DOI] [PubMed] [Google Scholar]
- 107.Chan DT et al. (2009) Chronic kidney disease delays VLDL-apoB-100 particle catabolism: potential role of apolipoprotein C-III. J Lipid Res 50 (12), 2524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harper CR and Jacobson TA (2008) Managing dyslipidemia in chronic kidney disease. J Am Coll Cardiol 51 (25), 2375–84. [DOI] [PubMed] [Google Scholar]
- 109.Ooi EM et al. (2011) Plasma apolipoprotein C-III metabolism in patients with chronic kidney disease. J Lipid Res 52 (4), 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Attman PO et al. (1992) Dyslipoproteinemia in diabetic renal failure. Kidney Int 42 (6), 1381–9. [DOI] [PubMed] [Google Scholar]
- 111.Kahn SE et al. (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444 (7121), 840–6. [DOI] [PubMed] [Google Scholar]
- 112.Mathieu C. et al. (2017) Insulin analogues in type 1 diabetes mellitus: getting better all the time. Nat Rev Endocrinol 13 (7), 385–399. [DOI] [PubMed] [Google Scholar]
- 113.Schroeder EB, O’Connor PJ, Schmittdiel J, Reynolds K, Desai JR, Ho M, Vazquez Benitez G, Anderson JP, Pimentel N, Loes LM, Neugebauer R. (2018) Impact of Human vs. Analog Insulins on Occurrence of Death and Major Cardiovascular Events. Diabetes 67 (Suppl. 1), Abstract. [Google Scholar]
- 114.Han S. et al. (2006) Macrophage insulin receptor deficiency increases ER stressinduced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab 3 (4), 257–66. [DOI] [PubMed] [Google Scholar]
- 115.Han S. et al. (2009) Hepatic insulin signaling regulates VLDL secretion and atherogenesis in mice. J Clin Invest 119 (4), 1029–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Biddinger SB et al. (2008) Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab 7 (2), 125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rask-Madsen C. et al. (2010) Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab 11 (5), 379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baumgartl J. et al. (2006) Myeloid lineage cell-restricted insulin resistance protects apolipoproteinE-deficient mice against atherosclerosis. Cell Metab 3 (4), 247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Klonoff D. et al. (2019) Divergent Hypoglycemic Effects of Hepatic-Directed Prandial Insulin: A Six-Month Phase 2b Study in Type 1 Diabetes. Diabetes Care. [DOI] [PubMed] [Google Scholar]
- 120.Kanter JE et al. (2018) A Novel Strategy to Prevent Advanced Atherosclerosis and Lower Blood Glucose in a Mouse Model of Metabolic Syndrome. Diabetes 67 (5), 946–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De Meyts P. (1994) The structural basis of insulin and insulin-like growth factor-I receptor binding and negative co-operativity, and its relevance to mitogenic versus metabolic signalling. Diabetologia 37 Suppl 2, S135–48. [DOI] [PubMed] [Google Scholar]
- 122.Schaffer L. (1994) A model for insulin binding to the insulin receptor. Eur J Biochem 221 (3), 1127–32. [DOI] [PubMed] [Google Scholar]
- 123.Schaffer L. et al. (2003) Assembly of high-affinity insulin receptor agonists and antagonists from peptide building blocks. Proc Natl Acad Sci U S A 100 (8), 4435–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Frikke-Schmidt H. et al. (2015) Treatment of diabetic rats with insulin or a synthetic insulin receptor agonist peptide leads to divergent metabolic responses. Diabetes 64 (3), 1057–66. [DOI] [PubMed] [Google Scholar]