Abstract
Individuals with Down syndrome (DS) are at high risk for developing Alzheimer’s disease (AD) pathology and this has provided significant insights into our understanding of the genetic basis of AD. The present review summarizes recent clinical, neuropathologic, imaging, and fluid biomarker studies of AD in DS (DSAD), highlighting the striking similarities as well as some notable differences between DSAD and the more common late-onset form of AD in the general population (LOAD), as well as the much rarer, autosomal-dominant form of AD (ADAD). There has been significant progress in our understanding of the natural history of AD biomarkers in DS and their relationship to clinically meaningful changes. Additional work is needed to clearly define the continuum of AD that has been described in the general population such as the preclinical, prodromal and dementia stages of AD. Multiple therapeutic approaches including those targeting beta-amyloid, but also tau and the amyloid precursor protein itself require consideration. Recent developments in the field are presented within the context of such efforts to conduct clinical trials to treat and potentially prevent AD in DS.
1. Introduction
In 1866, John Langdon Down described patients under his care at the Earlswood Asylum who exhibited intellectual disability along with abnormal physical growth and a distinct appearance [1]. Nearly a century later, in 1959, trisomy for chromosome 21 was identified as the molecular basis for DS [2]. In 1987, mapping of the gene that encodes the amyloid precursor protein to chromosome 21 [3] along with further discovery of mutations on chromosome 21 that cause autosomal-dominant forms of AD [4–5], suggested that overexpression of the amyloid precursor protein is causative for Alzheimer’s disease pathology in both DSAD and ADAD.
Over the past four decades, tremendous strides have been made in addressing treatable medical co-morbidities in DS, which has led to a doubling in life-expectancy [6]. A child with DS born today can expect to live into his or her sixties and often beyond. With this increased lifespan, however, the prevalence of AD dementia in this population has surged. A number of studies have shown that the risk of AD dementia in DS is approximately 23% at 50 years of age, 45% at 55 years of age and 88% or more at 65 years of age [7–10]. Dementia, cardiovascular disease and pneumonia make up the most common causes of death in people with DS, over the age of 36 years [11].
As mentioned, DS is caused by trisomy of chromosome 21. There are in fact three cytogenetic forms of DS: 1) Free Trisomy 21 which of a supplementary chromosome 21 in all cells [12]; 2) Mosaic Trisomy 21 where there are two cell lineages, one with the normal number of chromosomes and another one with an extra number of chromosome 21 [13]; and 3) Robertsonian Translocation Trisomy 21 which occurs only in 2-4% of the cases [14]. All of these result in an increased copy of the APP gene and overexpression of the APP protein. Some individuals in the general population develop an early-onset form of AD as a result of duplication of a small region of chromosome 21 that includes the APP gene (dup-APP). These individuals develop an early-onset form AD and the pathogenic mechanisms in these individuals are thought to be analogous to those in individuals with DS [15–16] since an additional copy of APP is present in both dup-APP and DS forms of AD. This is in contrast to the autosomal dominant forms of AD (ADAD) in which other genes (i.e. PSEN1, which encodes presenilin 1, or PSEN2, which encodes presenilin 2) are mutated and where the processing of APP is altered independently of gene copy number. Both of these mutations also lead to over-production of the neurotoxic APP cleavage product, Aβ (beta-amyloid) [17]. As expected, individuals with Dup-APP share many traits with people with DS, including early age of dementia onset (mean age 52 years for dup-APP), ubiquitous AD neuropathology [18–19] and an increased prevalence of cerebral amyloid angiopathy (CAA) [20]. Although almost all people with DS have AD neuropathology by age 40, the variability in the prevalence of dementia is more marked in DS than in dup-APP. And, although Alzheimer’s disease pathology is virtually inevitable in DS, the age of onset of clinical dementia varies tremendously. Indeed, not all individuals with DS will develop clinical dementia in their lifetime. Furthermore, the manifestation of CAA is less prevalent in DS than in dup-APP [21] but more prevalent than in sporadic AD (22).
Case reports of individuals with DS who have partial trisomy 21 have provided deep insights into the critical role that APP overexpression plays in AD pathogenesis [23–24]. These rare an unusual cases point to the crucial importance of APP overexpression as the key driver of AD in DS. Moreover, the discovery of a mutation in the APP gene that results in diminished production of Aβ and is protective against the development of AD pathology and dementia, further supports the notion that AD can be solely driven by APP dysmetabolism resulting in increased production of Aβ [25]. Subsequent to accumulation of beta-amyloid, the second key neuropathological hallmark of AD, neurofibrillary tangles, begin to develop. The abnormal hyperphosphorylation of tau protein leading to neurofibrillary tangles is seen later in the course of AD both in the general population as well as in ADAD and DSAD and correlates with the constellation of symptoms based on its regional location in the brain [26]. Tau pathology also appears to correlate temporally more closely with symptom onset in addition to spatially with symptom type, whereas Aβ accumulation into fibrillar plaques occurs decades earlier [27–28] To sum up, the discovery of the genetic causes for DSAD and ADAD catalyzed research into the relationship of chromosome 21, the gene for amyloid precursor protein, and the pivotal role of Aβ in AD pathogenesis. In the past decade, the clinical, imaging, pathologic and biochemical relationships of AD in DSAD and ADAD have been individually described by groups from around the world. Nonetheless, although DSAD and ADAD have fundamentally different initial pathways as compared to LOAD, they share a remarkably similar pathophysiology resulting in overproduction and subsequent accumulation of Aβ which sets off a cascade of pathogenic events leading to neurodegeneration and the syndrome of dementia [29–32].
2. Clinical Presentation of AD in DS
In general, the clinical presentation of DSAD is similar to both ADAD and LOAD. DSAD cases present with an insidious onset of episodic memory difficulties followed by inevitable progression of cognitive deficits resulting in loss of function in activities of daily living which is the cornerstone of dementia. Variability in baseline intellectual function in DS makes the diagnosis of dementia challenging. The most obvious difference between DSAD and ADAD with LOAD is the younger age at onset in individuals with DSAD and ADAD. Symptoms typically appear between the ages of 30 and 50 years, average age 52 and approximately 80% have dementia by the age of 65 years [33]. Behavioral symptoms seem to be more prevalent in DSAD as compared to all other forms of AD [34–35] and seem to present more frequently in those with DS who experience an earlier onset of dementia [36–39].
Focal neurological signs and symptoms appear to be more common in ADAD than in DSAD and LOAD. Cerebral amyloid angiopathy, myoclonus and seizures are a key feature of the APP duplication pedigrees [19] and observed less frequently in DSAD. When myoclonus is observed in DSAD, it is more closely correlated with onset of dementia and also appears to be associated with a more rapid cognitive decline in demented individuals with DS [40]. Up to 84% of demented individuals with DS develop seizures [41] with myoclonic epilepsy appearing to be the most common form in DS [42].
3. Neuropathology
For several decades, it has been known that fibrillar Aβ plaque accumulation is nearly universal in people with DS over age 40, as demonstrated by autopsy studies [43]. Autopsy studies have demonstrated that Aβ plaques begin to develop in very young people with DS [44–45] and are found in all adults with DS by 40 years of age —decades earlier than that observed in LOAD within the general population. Aβ initially appears in diffuse deposits that over time progress to form compact neuritic plaques with increasing age. After 40 years of age, the accumulation of brain amyloid is not linear but rather exponential and spreads throughout the cortex [46]. Aβ also accumulates within the cerebral vasculature in DS, just has it has been observed to occur in sporadic LOAD and, to a greater extent, in ADAD resulting in cerebral amyloid angiopathy (CAA) [21]. On a cellular lever, there is observed increases in the endocytic recycling of Aβ, with consequent endosomal enlargement that has been reported in DS as early as 28 weeks of gestation [47].
As mentioned, amyloid pathology begins in late teens with deposits of diffuse plaques initially within temporal lobe, then spreading to neocortical regions and hippocampus, reaching subcortical regions by the late forties. After 50 years of age, every region of the brain, including the cerebellum, is littered with amyloid plaques [48–49]. Cerebral amyloid angiopathy (CAA) begins at approximately 40 years of age, approximately 25 years after initial deposition of Aβ as plaques [49].
Tau is a microtubule-related protein that becomes hyperphosphorylated in AD, leading to a conformational change in its structure, leading to the establishment of neurofibrillary tangles (NFTs) which disrupts the neuronal cytoskeleton [50–51]. The earliest sites of tau pathology include the entorhinal and trans-entorhinal cortex, spreading to hippocampus, then temporal cortex and eventually to other regions of cerebral cortex, finally reaching visual association cortex and primary visual cortex [51] Tau pathology begins at approximately 35 years of age within the hippocampus and spreads to neocortical regions after 45 years of age [51]. Interestingly, critical inflammatory genes on chromosome 21 are triplicated in DS and are believed to influence the inflammatory state of the DS brain [52]. There are two macrophage types in the CNS immune system: M1 macrophages which are pro-inflammatory and responsible for inflammatory signaling, and M2 macrophages, which are anti-and participate in the resolution of the inflammatory process. Indeed, M2 macrophages produce anti-inflammatory cytokines, thereby contributing to tissue healing [53]. As many genes located on chromosome 21 are primarily associated with M1, that is, proinflammatory macrophages, it has been recently been shown that the DS brain has a pro-inflammatory status [54]. It remains unclear to what extent this proinflammatory state affects the manifestation of AD neuropathology in DS.
4. Neuroimaging
4.1. Amyloid PET
Amyloid PET imaging has been performed using various tracers in adults with DS over the past decade [55–58]. Amyloid PET positivity increases dramatically over age 40 reflecting the binding of these tracers to the fibrillar form of Aβ in plaques [59–60]. The progression of amyloid accumulation in the brain appears to be unique in individuals with DS as compared to LOAD. Recent studies have demonstrated that the first area of accumulation is the striatum [61– 62] which is similar to the striatal binding observed in ADAD [63–65]. Rates of accumulation of amyloid appear similar to rates in the sporadic population [62]. The decades-long period between the emergence of amyloid plaques in the brain until the development of symptoms defines the ‘Preclinical stage of AD’ in the sporadic population. The next stage, when symptoms are restricted to memory loss, is referred to as ‘Prodromal stage of AD’ and the final stage, where memory and other cognitive domains affected and contribute to functional decline, is referred to as the ‘dementia stage of AD’ [66]. It is believed that these same stages exist as a continuum DS. Although detection of elevated brain amyloid using PET has helped define the preclinical stage of AD in the general population and thereby refined sample selection for AD clinical trials [66] its applicability to clinical practice (both in the general as well as the DS population) will likely be limited. In the general population, amyloid PET imaging will likely be most useful for determining if AD is the underlying cause in symptomatic individuals. In people with DS, who are genetically pre-determined to develop AD, the utility of amyloid PET imaging in the clinic may have a very limited role since almost everyone will exhibit elevated brain amyloid.
4.2. Tau PET
As mentioned earlier, tau pathology in the form of NFTs is a key hallmark of AD pathology and a more proximal marker of subsequent AD-related cognitive decline than amyloid pathology [67]. Indeed, the regional distribution of tau pathology in the brain mirrors clinical symptoms in the general population [68]. A similar relationship has been seen regarding NFTs and cognitive decline in DS [69]. The PET tracer 18F-AV-1451 has been shown to bind to neurofibrillary tangles [70–71]. Tau pathology in adults with DS has recently been studied using the PET tracer 18F-AV-1451, as well as its relationship to regional amyloid deposition, regional cerebral glucose metabolism, regional brain atrophy, and cognitive and functional status [69]. The relationship between neurofibrillary tangles and amyloid load is consistent with that observed in LOAD. Presence of tau extending beyond the medial temporal lobes appears to correlate with subsequent cognitive decline [70]. It has been noted that higher cognitive scores correlate with lower tau pathology in DS just as has been observed in the general population [69]. Tau PET my therefore represent an appealing outcome measure for clinical trials as it is more closely linked in time with AD-related cognitive decline as compared to amyloid PET,
4.3. FDG PET
FDG-PET is useful in measuring regional neuronal activity based on local glucose metabolism. Various patterns in regional FDG signal (both hypometabolic as well as hyper-metabolic) have been shown to be associated with cognitive function in people with DS [71]. Recently, it has been shown that individuals with DS demonstrate the same regional hypometabolism observed in the posterior cingulate-precuneus as in individuals with AD in the general population [72–73]. There also appears to be an inverse relationship between amyloid accumulation and regional glucose metabolism [57] as well as an inverse relationship between tau pathology and FDG PET signal [69]. More work is needed in studying longitiunual changes in FDG PET in DSAD to better understand its potential role as an outcome measure for clinical trials.
4.4. MRI
Just as in the general population, neuropathological studies indicate that older adults with DS exhibit medial temporal lobe atrophy as part of the development of AD [74–76]. MRI studies demonstrate hippocampal atrophy in DS, just as it has been reported in LOAD. Furthermore, hippocampal atrophy in DS correlates with changes in memory measures but is a late finding of neurodegeneration, just as in the general population [77]. Regional grey matter changes can be detectable before signs of clinical dementia [78]. Individuals with DSAD eventually show atrophy of the amygdala, caudate, posterior cingulate, parietal, temporal, frontal regions, as well as enlargement of cerebrospinal fluid spaces [78]. Although MRI exquisitely depicts brain atrophy, the fact that it reflects neurodegeneration, which defines the final stage of AD, severely limits its use as an AD biomarker. However, MRI will continue a critical role in safety assessments for adverse events in clinical trials.
5. Fluid Biomarkers
Blood-based assays have clear advantages as biomarkers because they are easily accessible and non-invasive. The role of plasma levels of Aβ as a biomarker for DSAD has been studied extensively. People with DS have consistently shown higher plasma Aβ concentrations compared with individuals without DS [79–80]. There have only been a limited number of CSF studies in individuals with DS due to the challenges in collecting CSF from individuals with DS [81–82]. These studies show elevated levels of Aβ42. As these individuals age, however, CSF Aβ42 levels decline (as expected with their deposition into plaques) and CSF tau levels increase [82]. Recent work on CSF and plasma levels of neurofilament light (NfL), a component of the axonal cytoskeleton and marker of neuronal damage and degeneration, has shown strong correlation with cognitive status in adults with DS [83–84]. Specifically, plasma NfL levels appear to increase with age, and can distinguish between normal aging in DS and DSAD [84]. Indeed, there appears to be excellent diagnostic performance of plasma NfL for the asymptomatic versus dementia group, with an AUC of 0.95 (95% CI 0.92–0.98), and a sensitivity of 90% and a specificity of 92% [82]. Plasma NfL levels have also been shown to correlate with standard biomarkers of AD pathology as well as with markers of neurodegeneration (regional cerebral glucose metabolism as assessed with FDGPET and hippocampal atrophy), and even cognitive and functional decline [83].
Beyond standard AD biomarkers such as beta-amyloid species, total-tau and phospho-tau species, and NF-L, metabolomic analyses may represent another biomarker with which we can further characterize AD in the DS population. Specifically, recent work has shown metabolic perturbations characterize individuals with DS who were cognitively stable, have mild cognitive impairment, or DSAD [85]. Whether these metabolomic changes will be more applicable to screening purposes versus outcome measures for clinical trials will be based on longitudinal data on rates of change in metabolomic profiles in conjunction with clinically meaningful cognitive and functional changes.
6. The Alzheimer’s Biomarker Consortium for Down syndrome (ABC-DS)
As the largest AD biomarker study in DS to date, ABC-DS is setting the stage for powering secondary prevention trials for DSAD. Similar to the two largest ongoing longitudinal studies of AD biomarkers: the Alzheimer Disease Neuroimaging Initiative (ADNI) and the Dominantly Inherited Alzheimer Network (DIAN), all data is being made available in an open-access format using a model similar to ADNI. This 5-year longitudinal study launched in 2015, is examining the progression of AD related biomarkers (Aβ-, tau- and fluorodeoxyglucose-PET, structural and functional MRI, cerebrospinal fluid Aβ and tau, plasma Aβ and proteomics, genetics, neuropathology) as well as cognitive and functional measures in over 500 adults with DS [86]. The consortium stages participants based on clinical and neuropsychological assessments as being located on the continuum of AD in DS. Participants were classified as cognitively stable (CS) if they were without cognitive or functional decline, beyond what would be expected with adult aging, per se. Participants were classified as having mild cognitive impairment (MCI-DS) if they demonstrated some cognitive and/or functional decline over and above what would be expected with aging per se, but not severe enough to indicate the presence of dementia. Participants were categorized as having dementia (DS-AD) if there was evidence of substantial progressive declines in cognitive functioning and daily living skills. An “unable to determine” category was utilized to indicate that declines were observed but could be caused by significant life circumstance (e.g., staff changes, family death) or conditions unrelated to AD (e.g., severe sensory loss, poorly resolved hip fracture, psychiatric diagnosis, in particular depression) [86]. These data will allow for a deeper, richer understanding of the natural history of AD biomarkers in DS (Figure 1).
Figure 1. The Natural History of AD Biomarkers in DS.
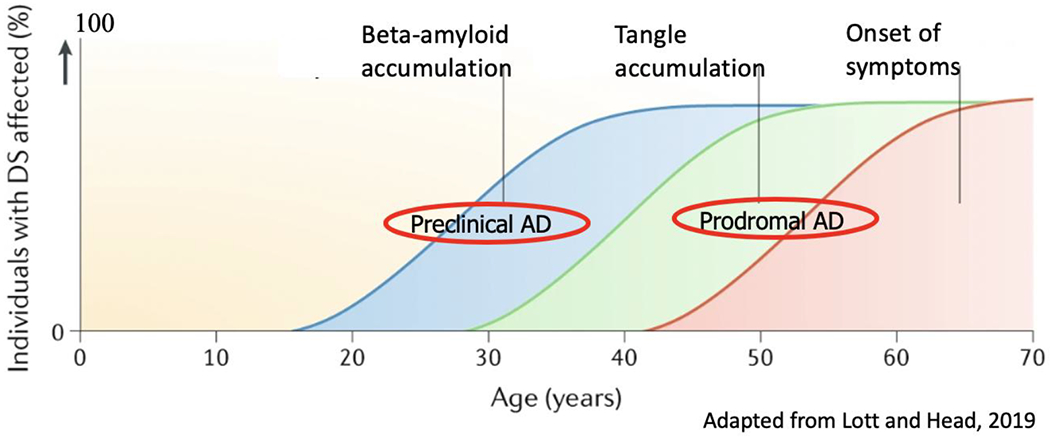
The continuum of AD in DS is thought to be similar to LOAD but beginning at a younger age, much as in ADAD.
7. Clinical Trials
Clinical trials require identifying the appropriate sample population and outcome measures that will best assess safety and efficacy of any given intervention. The US Code of Federal Regulations outlines specific requirements to enhance protections for ‘vulnerable populations’ including individuals DS, which also raises issues that need to be addressed as part of the informed consent process. In developing disease-modifying drugs for DSAD, there is a need to understand the ideal sample (e.g. age range), the interventions (e.g. pharmacologic mechanism of action, PK/PD) and outcome measures (e.g. clinically meaningful and sensitivity to change) for a study-design. In addition, level of intellectual disability as well as language abilities will need to be carefully considered when selecting outcome measures. Specifically, floor-effects may limit feasibility of many cognitive instruments used to assess longitudinal change in memory or the impact of a particular drug on this change. Few empirically evaluated, psychometrically sound outcome measures appropriate for use in clinical trials with individuals with DS have been identified. Moreover, for clinical trials that aim to prevent the dementia stage of AD in DS by intervening during its earliest stages (i.e. preclinical stage), assessment tools will need to be created and validated to assess subtle changes in the asymptomatic or minimally symptomatic stage of AD in DS. The NIH-sponsored meeting on Outcomes Measures for Clinical Trials in Down syndrome resulted in guidelines for the selection of outcome measures for clinical trials for DSAD, though most involve the dementia stage of AD in DS [87].
The DS population will undoubtedly require a unique set of considerations in terms of sensitive and valid assessments of early AD-related cognitive decline. Baseline assessments will be important, as will be the need for carefully planned and consistent testing sessions. Some direct neuropsychological measures will likely only be sensitive and valid in verbal and/or higher functioning adults with DS. Such measures will allow researchers to track individuals with DS prospectively (perhaps as part of a trial ready cohort) and are most likely to be relevant in clinical trials for therapies aimed at delaying the onset of and/or preventing AD-related cognitive decline. Indeed, treatments are likely to be most effective if introduced prior to irreversible losses of critical neural pathways in the later stages of the disease [88–89].
8. The Horizon21 DS Consortium
In addition to the ABC-DS effort, the Horizon21 DS consortium in Europe is leading a parallel project to assess longitudinal change in aging adults with DS. This consortium consists of various existing DS cohorts from the UK (the London Down Syndrome Consortium [LonDownS], the Cambridge Dementia in Down’s Syndrome [DiDS] cohort), Netherlands (the Rotterdam Down syndrome study), Germany (AD21 study group, Munich), France (TriAL21 for Lejeune Institute, Paris), and Spain (the Down Alzheimer Barcelona Neuroimaging Initiative (DABNI). The consortium is providing deep insights into cognitive and clinical outcome measures of AD in DS in over 1,000 participants, setting the stage for clinical trials [90].
9. Current treatment trials
The conduct of clinical trials in the DS population raises many methodological challenges. Given the wide variations in baseline intellectual capabilities and cognitive functioning, these differences must be taken into account for accurate assessment in testing situations. The main defining feature of dementia in the general population is a decline from the baseline level of function and performance of daily skills. Consensus guidelines for the evaluation and management of dementia in DS have been proposed [91]. These guidelines focus on the dementia stage and do not include prodromal AD or preclinical criteria. These criteria are currently lacking in the DS field. The consensus guidelines are meant to serve as recommendations for clinicians evaluating individuals with DS who are experiencing cognitive decline. The guidelines provide a step-by-step and comprehensive approach to clinical evaluation. The key hallmark defining dementia is functional decline as compared to previously attained levels and beyond that which would be explained by age-related changes. However, the earliest signs of AD-related cognitive decline, distinct of normal age-related change in adults with DS may be subtle and will often require an astute observer. In addition, concomitant medications, recent medical illnesses (including laboratory testing such as TSH and Vitamin B12), or recent life events that can impact psychosocial functioning must also be considered when interpreting results. Although some validated measures exist [92–93] there is currently no single, standard clinical instrument for the assessment of mild cognitive impairment or dementia in adults with DS. In this regard, the consensus guidelines are meant to aid clinicians attempting to diagnosis dementia in people with DS and have severely limited utility for defining earlier stages of AD in DS such as preclinical and prodromal AD. The development of research guidelines for the definition of the preclinical, prodromal and dementia stages of AD in DS are critical for consideration of clinical trials to treat or prevent AD in DS. Nonetheless, a few small multicenter clinical trials have been successfully conducted in the DS population and have provided important insights into trial design and implementation in this group.
9.1. Scyllo-inositol
ELND005 (scyllo-inositol), an endogenous myo-inositol isomer, is hypothesized to reduce amyloid toxicity to improve cognitive function in patients with DS. ELND005 (scyllo-inositol; cyclohexane-1,2,3,4,5,6-hexol) has also been studied as a potential disease-modifying treatment of LOAD [94]. In preclinical studies, ELND005 showed amyloid anti-aggregation effects in vitro, reduced oligomer-induced neuronal toxicity, and improved learning in animal models of AD [94–96]. ELND005 showed amyloid-lowering effects in cerebrospinal fluid (CSF) and brain, respectively, in patients with AD in the general population [97]. In addition, ELND005 showed beneficial trends on cognition in mild AD [97]. A phase II randomized, double-blind, placebo-controlled study of oral ELND005 in 26 adults with DS without dementia demonstrated safety and tolerability of the anti-amyloid compound in DS, as well as feasibility of conducting a PK/PD study in adults with DS [98].
9.2. ACI-24
ACI-24 is a liposomal vaccine that elicits an antibody response against aggregated Aβ peptides without concomitant pro-inflammatory T-cell activation [99]. ACI-24 is based on the truncated Aβ1–15 sequence, which is devoid of T-cell epitopes located closer to the peptide’s C-terminus. The peptide sequence is anchored into the surface of liposomes such that that the peptides adopt an aggregated β-sheet structure as a conformational epitope. Previous active vaccines (e.g., AN-1792) elicited a T-cell response, which led to an increased risk of meningoencephalitis [100]. A phase I/II trial of ACI-24 is ongoing in Europe and aims to address safety, immunogenicity, and efficacy in mild to moderate Alzheimer’s dementia in the general population. A phase lb placebo-controlled, multicenter study with ACI-24 for the treatment of AD in individuals with DS was launched in 2016 (NCT02738450). The study enrolled 16 adults with DS aged 25–45 years and treated them with ACI-24 for 1 year with an additional year of follow-up. Primary endpoints included measures of safety, tolerability and immunogenicity. Secondary endpoints of this clinical trial are effects on biomarkers of AD pathology as well as cognitive and clinical function and neuroinflammatory markers. The study is anticipated to be completed in 2020 with topline results expected in the near future.
10. Future directions
Clearly, genetic support for the role of APP in AD in the pathogenesis of AD in DS is undeniably compelling. As described above, the presence of an extra-copy of APP, whether as trisomy 21 or Dup-APP, results in overproduction of Aβ and subsequent development og amyloid-plaques and neurofibrillary tangles and the manifestation of dementia. As well, individuals with DS who have trisomy 21 but are disomic for APP develop no amyloid plaques or neurofibrillary tangles and have no symptoms of dementia. Finally, a mutation in the APP gene that prevents its cleavage to produce Aβ protects against the development of amyloid plaques and neurofibrillary tangles as well as dementia. Therefore, Aβ remains a prime target for therapeutics, including both active and passive immunization, just as it is being actively studied in the ADAD and LOAD populations.
Beyond anti-Aβ drugs, compounds that modulate APP expression in DS may have a great potential for effectiveness. In addition, the role of tau appears to be more closely related to subsequent cognitive decline. Therefore, drugs that prevent hyperphosphorylation of tau and/or drugs that remove hyperphosphorylated species of tau may represent another therapeutic avenue. Perhaps as importantly, understanding protective genetics and epigenetics in DS may help provide novel therapeutic avenues to consider for the prevention of dementia in DS.
11. The Alzheimer’s Clinical Trial Consortium for Down syndrome
The tight link between genetic determinants of AD and the overproduction of Aβ provides compelling support for the amyloid cascade hypothesis and has been the focal point in the development of disease-modifying drugs for AD [89]. It is hypothesized that disease-modifying treatments for AD and DS should begin prior to the onset of cognitive symptoms to prevent extensive neurodegeneration and thus necessitates a clear understanding of biomarker changes throughout the course of the disease. The ABC-DS project is setting the stage for conducting secondary prevention trials for DSAD. The NIH-funded Alzheimer’s Clinical Trial Consortium for Down Syndrome (ACTC-DS) recently launched the Trial Ready-Cohort for Down syndrome (TRC-DS) (Figure 2). TRC-DS will enroll 120 non-demented participants with DS into a longitudinal safety-run in study with MRI, amyloid PET, cognitive testing, and biofluid biomarker analysis in advance of upcoming randomized placebo controlled clinical trials for DSAD. Data from the ABC-DS project is providing key insights into study design including sample-size selection and duration of treatment period.
Figure 2. The Trial Ready Cohort – Down Syndrome.
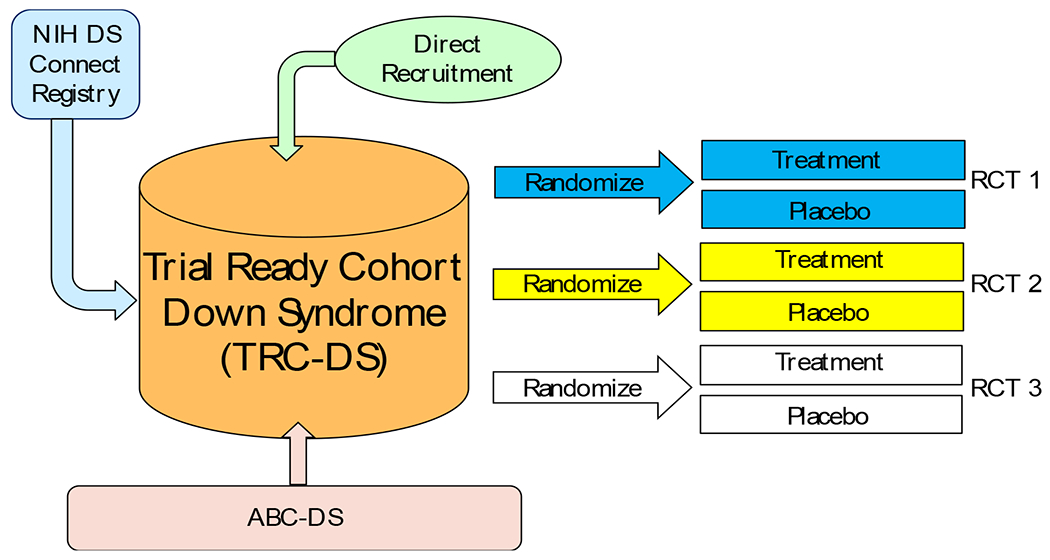
A clinical trial platform has been developed to utilize a collaborative approach to design and conduct clinical trials for AD in DS across expert international sites using widely accepted tools and standards.
12. Conclusions
Over the past decade, great progress has been made in understanding DSAD utilizing brain MRI, amyloid and tau PET and biofluid biomarkers. Indeed, several research groups from around the world have shown that there exist remarkable similarities (and some differences) between LOAD, ADAD and DSAD. We are now poised to ask whether therapies that are currently being tested for LOAD and ADAD can be evaluated in people with DS. Undoubtedly, many questions about DSAD still remain unanswered. We will need to identify which neuropsychological tests (or composite of tests) will provide a reasonable measure of clinical meaningfulness. We will need to understand how removal of amyloid will affect AD-related cognitive decline and to what extent. We will need to consider how early in the course DSAD we should intervene and for how long treatment should last to provide measurable benefit to patients. Data from the ABC-DS and Horizon21 studies are setting the stage for conducting secondary prevention trials to prevent AD in DS. Recent efforts such as the ACTC-DS will utilize a collaborative approach to design and conduct such trials across expert international sites using widely accepted tools and standards.
Key points:
There is a need for novel therapeutics for Alzheimer’s disease in Down syndrome (DSAD).
There is a lack of randomized controlled trials (RCTs) for DSAD therapeutics.
New biomarker strategies may enhance diagnostics and clinical trial designs, and new therapeutic targets may assist in the development of disease-modifying therapies for DSAD.
Acknowledgments
Funding: MSR has received funding support from NIH R61AG066543.
Footnotes
Conflicts of Interest: MSR has served as consultant to AC Immune.
References
- 1.Down JLH. Observations on an ethnic classification of idiots. Lond Hosp Clin Lect Rep. 1866;3:259–62. [Google Scholar]
- 2.Lejeune J, Gautier M, Turpin R. A study of somatic chromosomes in nine infants with mongolism. CR Acad Sci (Paris) 1959;240:1026–27. [Google Scholar]
- 3.Tanzi RE, Gusella JF, Watkins PC, et al. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987;235:880–84. [DOI] [PubMed] [Google Scholar]
- 4.Murrell J, Farlow M, Ghetti B, et al. A mutation in the amyloid precursor protein associated with hereditary Alzheimer’s disease. Science. 1991;254:97–99. [DOI] [PubMed] [Google Scholar]
- 5.Goate A, Chartier-Hardin M- C, Mullan M. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704– 76. [DOI] [PubMed] [Google Scholar]
- 6.Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down’s syndrome in the USA from 1983 to 1997: a population-based study. Lancet. 2002;359(9311): 1019–1025. [DOI] [PubMed] [Google Scholar]
- 7.Rubenstein E, Hartley S, Bishop L. Epidemiology of Dementia and Alzheimer Disease in Individuals with Down Syndrome. JAMA Neurol. 2020;77(2):262–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarron M, McCallion P, Reilly E, et al. A prospective 20-year longitudinal follow-up of dementia in persons with Down syndrome. J. Intellect. Disabil. Res. 61, 843–852 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Hithersay R, Startin CM, Hamburg S, et al. Association of Dementia With Mortality Among Adults With Down Syndrome Older Than 35 Years. JAMA Neurol. 2019;76(2): 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayen E, Possin KL, Chen Y, et al. Prevalence of Aging, Dementia, and Multimorbidity in Older Adults With Down Syndrome. JAMA Neurol. 2018;75(11): 1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landes SD, Stevens JD, Turk MA. Obscuring effect of coding developmental disability as the underlying cause of death on mortality trends for adults with developmental disability: a cross-sectional study using US Mortality Data from 2012 to 2016. BMJ Open. 2019;9(2):e026614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonarakis SE. Human chromosome 21: genome mapping and exploration circa 1993. Trends Genet. 1993;9:142–148. [DOI] [PubMed] [Google Scholar]
- 13.Thuline HC, Pueschel SM. - Cytogenetics in Down syndrome In: Pueschel SM, Rynders JE, Down Syndrome. Advances in Biomedicine and the Behavioral Sciences. Cambridge: Ware Press (pub.) 1982; : 133. [Google Scholar]
- 14.Asim A, Kumar A, Muthuswamy S, et al. - Down syndrome: an insight of the disease. Journal of Biomedical Science. 2015;22–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sleegers K, Brouwers N, Gijselinck I, et al. APP duplication is sufficient to cause early onset Alzheimer’s dementia with cerebral amyloid angiopathy. Brain. 2006; 129:2977– 2983. [DOI] [PubMed] [Google Scholar]
- 16.McNaughton D, Knight W, Guerreiro R, et al. Duplication of amyloid precursor protein (APP), but not prion protein (PRNP) gene is a significant cause of early onset dementia in a large UK series. Neurobiol. Aging. 2012;33:426.el3–426.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rumble B, Retallack R, Hilbich C, et al. Amyloid A4 and its precursor in Down’s syndrome and Alzheimer’s disease. N. Engl. J. Med. 320, 1446–1462 (1989). [DOI] [PubMed] [Google Scholar]
- 18.Rovelet-Lecrux A, Hannequin D, Raux G, et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat. Genet. 38, 24–26 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Cabrejo L, Guyant-Marechal L, Laquerriere A, et al. Phenotype associated with APP duplication in five families. Brain 129, 2966–2976 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Wallon D, Rousseau S, Rovelet-Lecrux A, et al. The French series of autosomal dominant early onset Alzheimer’s disease cases: mutation spectrum and cerebrospinal fluid biomarkers. J. Alzheimers Dis. 2012:30, 847–856. [DOI] [PubMed] [Google Scholar]
- 21.Carmona-Iragui M, Balasa M, Benejam B, et al. Cerebral amyloid angiopathy in Down syndrome and sporadic and autosomal-dominant Alzheimer’s disease. Alzheimers Dement. 2017:13, 1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Head E, Phelan MJ, Doran E, et al. Cerebrovascular pathology in Down syndrome and Alzheimer disease. Acta Neuropathol Commun. 2017:Dec 1;5(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasher VP, Farrer MJ, Kessling AM, et al. Molecular mapping of Alzheimer-type dementia in Down’s syndrome. Ann. Neurol. 1998:43, 380–383.9506555 [Google Scholar]
- 24.Doran E, Keator D, Head E, et al. Down Syndrome, Partial Trisomy 21, and Absence of Alzheimer’s Disease: The Role of APP. J Alzheimers Dis. 2017;56(2):459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonsson T, Atwal JK, Steinberg S, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012. August 2;488(7409):96–9. [DOI] [PubMed] [Google Scholar]
- 26.Brier MR, Gordon B, Friedrichsen K, et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Science Translational Medicine, 2016: 8(338), 338ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ossenkoppele R, Schonhaut DR, Scholl M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139(Pt 5): 1551—1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margallo-Lana ML, Moore PB, Kay DW, et al. Fifteen-year follow-up of 92 hospitalized adults with Down’s syndrome: incidence of cognitive decline, its relationship to age and neuropathology. J Intellect Disabil Res. 2007;51(Pt. 6):463–477. [DOI] [PubMed] [Google Scholar]
- 29.Beyreuther K and Masters CL (1991) Amyloid precursor protein (APP) and beta A4 amyloid in the etiology of Alzheimer’s disease: precursor-product relationships in the derangement of neuronal function. Brain Pathol. 1, 241– 251. [DOI] [PubMed] [Google Scholar]
- 30.Selkoe DJ (1991) The molecular pathology of Alzheimer’s disease. Neuron 6, 487–498. [DOI] [PubMed] [Google Scholar]
- 31.Hardy JA and Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256, 184– 185. [DOI] [PubMed] [Google Scholar]
- 32.Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1): 119—128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiseman FK, Al-Janabi T, Hardy J, et al. A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome. Nat Rev Neurosci. 2015. September;16(9):564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ball SL, Holland AJ, Hon J, et al. Personality and behaviour changes mark the early stages of Alzheimer’s disease in adults with Down’s syndrome: findings from a prospective population-based study. Int. J. Geriatr. Psychiatry. 2006;21:661–673. [DOI] [PubMed] [Google Scholar]
- 35.Ball SL, Holland AJ, Treppner P, et al. Executive dysfunction and its association with personality and behaviour changes in the development of Alzheimer’s disease in adults with Down syndrome and mild to moderate learning disabilities. Br. J. Clin. Psychol. 2008;47:1–29. [DOI] [PubMed] [Google Scholar]
- 36.Dekker AD, Sacco S, Carfi A, et al. The Behavioral and Psychological Symptoms of Dementia in Down Syndrome (BPSD-DS) Scale: Comprehensive Assessment of Psychopathology in Down Syndrome. J Alzheimers Dis. 2018;63(2):797–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lautarescu BA, Holland AJ, Zaman SH. The Early Presentation of Dementia in People with Down Syndrome: a Systematic Review of Longitudinal Studies. Neuropsychol Rev. 2017;27(l):31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dekker AD, Strydom A, Coppus AM, et al. Behavioural and psychological symptoms of dementia in Down syndrome: Early indicators of clinical Alzheimer’s disease?. Cortex. 2015;73:36–61. [DOI] [PubMed] [Google Scholar]
- 39.Holland AJ, Hon J, Huppert FA, et al. Incidence and course of dementia in people with Down’s syndrome: findings from a population-based study. J. Intellect. Disabil. Res. 2000;44:138–146. [DOI] [PubMed] [Google Scholar]
- 40.Lott IT, Doran E, Nguyen VQ, et al. Down syndrome and dementia: seizures and cognitive decline. J Alzheimers Dis. 2012;29(1): 177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menendez M Down syndrome, Alzheimer’s disease and seizures. Brain Dev. 2005. June;27(4):246–52. [DOI] [PubMed] [Google Scholar]
- 42.De Simone R, Puig XS, Gelisse P, et al. Senile myoclonic epilepsy: delineation of a common condition associated with Alzheimer’s disease in Down syndrome. Seizure. 2010. September;19(7):383–9. [DOI] [PubMed] [Google Scholar]
- 43.Mann DM, Yates PO, Marcyniuk B. Alzheimer’s presenile dementia, senile dementia of Alzheimer type and Down’s syndrome in middle age form an age related continuum of pathological changes. Neuropathol Appl Neurobiol. 1984. May-Jun; 10(3): 185–207. [DOI] [PubMed] [Google Scholar]
- 44.Lemere CA, Blusztajn JK, Yamaguchi H, et al. Sequence of deposition of heterogeneous amyloid beta-peptides and APOE in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol. Dis. 3, 16–32 (1996). [DOI] [PubMed] [Google Scholar]
- 45.Leverenz JB and Raskind MA Early amyloid deposition in the medial temporal lobe of young Down syndrome patients: a regional quantitative analysis. Exp. Neurol. 150, 296– 304(1998). [DOI] [PubMed] [Google Scholar]
- 46.Abrahamson EE, Head E, Lott IT, et al. Neuropathological correlates of amyloid PET imaging in Down syndrome. Dev Neurobiol. 2019. July;79(7):750–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cataldo AM, Peterhoff CM, Troncoso JC, et al. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and Down syndrome: Differential effects of APOE genotype and presenilin mutations. Am J Pathol 2000;157:277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thai DR, Rub U, Orantes M, et al. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002. June 25;58(12):1791–800. [DOI] [PubMed] [Google Scholar]
- 49.Davidson YS, Robinson A, Prasher VP, et al. The age of onset and evolution of Braak tangle stage and Thai amyloid pathology of Alzheimer’s disease in individuals with Down syndrome. Acta Neuropathol Commun. 2018. Jul 4;6(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alonso AD, Grundke-Iqbal I and Iqbal K (1996) Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nature Medicine, 2, 783–787. [DOI] [PubMed] [Google Scholar]
- 51.Alonso AC, Zaidi T, Novak M, et al. (2001) Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proceedings of the National Academy of Sciences of the United States of America, 98(12), 6923–69238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilcock DM, Griffin WS. Down’s syndrome, neuroinflammation, and Alzheimer neuropathogenesis. J Neuroinflammation. 2013;10:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilcock DM. Neuroinflammation in the aging down syndrome brain; lessons from Alzheimer’s disease. Curr Gerontol Geriatr Res. 2012;2012:170276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilcock DM, Hurban J, Helman AM, et al. Down syndrome individuals with Alzheimer’s disease have a distinct neuroinflammatory phenotype compared to sporadic Alzheimer’s disease. Neurobiol Aging. 2015;36(9):2468–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Landt J, D’Abrera JC, Holland AJ, et al. Using positron emission tomography and Carbon 11-labeled Pittsburgh Compound B to image Brain Fibrillar β-amyloid in adults with down syndrome: safety, acceptability, and feasibility. Arch Neurol. 2011. July;68(7):890–6. [DOI] [PubMed] [Google Scholar]
- 56.Handen BL, Cohen AD, Channamalappa U, et al. Imaging brain amyloid in nondemented young adults with Down syndrome using Pittsburgh compound B. Alzheimers Dement. 2012. November;8(6):496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rafii MS, Wishnek H, Brewer JB, et al. The down syndrome biomarker initiative (DSBI) pilot: proof of concept for deep phenotyping of Alzheimer’s disease biomarkers in down syndrome. Front Behav Neurosci. 2015. Sep 14;9:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jennings D, Seibyl J, Sabbagh M, et al. Age dependence of brain β-amyloid deposition in Down syndrome: An [18F] florbetaben PET study. Neurology. 2015. February 3;84(5):500–7. [DOI] [PubMed] [Google Scholar]
- 59.Annus T, Wilson LR, Hong YT, et al. The pattern of amyloid accumulation in the brains of adults with Down syndrome. Alzheimers Dement. 2016. May;12(5):538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cole JH, Annus T, Wilson LR, et al. Brain-predicted age in Down syndrome is associated with beta amyloid deposition and cognitive decline. Neurobiol Aging. 2017. August;56:41– 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lao PJ, Betthauser TJ, Hillmer AT, et al. The effects of normal aging on amyloid-β deposition in nondemented adults with Down syndrome as imaged by carbon 11-labeled Pittsburgh compound B. Alzheimers Dement. 2016. April;12(4):380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lao PJ, Handen BL, Betthauser TJ, et al. Longitudinal changes in amyloid positron emission tomography and volumetric magnetic resonance imaging in the nondemented Down syndrome population. Alzheimers Dement (Amst). 2017. May 23;9:l–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klunk WE, Price JC, Mathis CA, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. Version 2. J Neurosci. 2007. Jun 6;27(23):6174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Villemagne VL, Ataka S, Mizuno T, et al. High striatal amyloid beta-peptide deposition across different autosomal Alzheimer disease mutation types. Arch Neurol. 2009. December;66(12): 1537–44. [DOI] [PubMed] [Google Scholar]
- 65.Cohen AD, McDade E, Christian B, et al. Early striatal amyloid deposition distinguishes Down syndrome and autosomal dominant Alzheimer’s disease from late-onset amyloid deposition. Alzheimers Dement. 2018. June;14(6):743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scholl M, Lockhart SN, Schonhaut DR, et al. PET Imaging of Tau Deposition in the Aging Human Brain. Neuron. 2016;89(5):971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lowe VJ , Curran G, Fang P , et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun 2016:4, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rafii MS, Lukic AS, Andrews RD, et al. PET Imaging of Tau Pathology and Relationship to Amyloid, Longitudinal MRI, and Cognitive Change in Down Syndrome: Results from the Down Syndrome Biomarker Initiative (DSBI). J Alzheimers Dis. 2017;60(2):439–450. [DOI] [PubMed] [Google Scholar]
- 70.Forman MS , Zhukareva V , Bergeron C, et al. Signature tau neuropathology in gray and white matter of corticobasal degeneration. Am J Pathol 2002:160, 2045–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haier RJ, Head K, Head E, et al. Neuroimaging of individuals with Down’s syndrome at-risk for dementia: evidence for possible compensatory events. Neuroimage. 2008;39(3): 1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matthews DC, Lukic AS, Andrews RD, et al. Dissociation of Down syndrome and Alzheimer’s disease effects with imaging. Alzheimers Dement (N Y). 2016;2(2):69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meltzer CC, Zubieta JK, Brandt J, et al. Regional hypometabolism in Alzheimer’s disease as measured by positron emission tomography after correction for effects of partial volume averaging. Neurology. 1996. August;47(2):454–61. [DOI] [PubMed] [Google Scholar]
- 74.Hof PR, Bouras C, Perl DP, et al. Age-related distribution of neuropathologic changes in the cerebral cortex of patients with Down’s syndrome. Quantitative regional analysis and comparison with Alzheimer’s disease. Arch Neurol. 1995;52(4):379–391. [DOI] [PubMed] [Google Scholar]
- 75.Teipel SJ, Alexander GE, Schapiro MB, et al. Age-related cortical grey matter reductions in non-demented Down’s syndrome adults determined by MRI with voxel-based morphometry. Brain. 2004;127(Pt 4):811–824. [DOI] [PubMed] [Google Scholar]
- 76.Mullins D, Daly E, Simmons A, et al. Dementia in Down’s syndrome: an MRI comparison with Alzheimer’s disease in the general population. J Neurodev Disord. 2013;5(1):19. Published 2013 Aug 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krasuski JS, Alexander GE, Horwitz B, et al. Relation of medial temporal lobe volumes to age and memory function in nondemented adults with Down’s syndrome: implications for the prodromal phase of Alzheimer’s disease. Am J Psychiatry. 2002;159(1):74–81. [DOI] [PubMed] [Google Scholar]
- 78.Beacher F, Daly E, Simmons A, et al. Alzheimer’s disease and Down’s syndrome: an in vivo MRI study. Psychol Med. 2009;39(4):675–684. [DOI] [PubMed] [Google Scholar]
- 79.Schupf N, Patel B, Pang D, et al. Elevated plasma beta-amyloid peptide Abeta(42) levels, incident dementia, and mortality in Down syndrome. Arch Neurol. 2007;64(7): 1007– 1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schupf N, Zigman WB, Tang MX, et al. Change in plasma AB peptides and onset of dementia in adults with Down syndrome. Neurology. 2010;75(18): 1639–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dekker AD, Fortea J, Blesa R, et al. Cerebrospinal fluid biomarkers for Alzheimer’s disease in Down syndrome. Alzheimers Dement (Amst). 2017;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fortea J, Carmona-Iragui M, Benejam B, et al. Plasma and CSF biomarkers for the diagnosis of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. Lancet Neurol. 2018;17(10):860–9. [DOI] [PubMed] [Google Scholar]
- 83.Strydom A, Heslegrave A, Startin CM, et al. Neurofilament light as a blood biomarker for neurodegeneration in Down syndrome. Alzheimers Res Ther. 2018; 10(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rafii MS, Donohue MC, Matthews DC, et al. Plasma Neurofilament Light and Alzheimer’s Disease Biomarkers in Down Syndrome: Results from the Down Syndrome Biomarker Initiative (DSBI). J Alzheimers Dis. 2019;70(1): 131–8. [DOI] [PubMed] [Google Scholar]
- 85.Mapstone M, Gross TJ, Macciardi F, et al. Metabolic correlates of prevalent mild cognitive impairment and Alzheimer’s disease in adults with Down syndrome. Alzheimers Dement (Amst). 2020;12(l):el2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Handen BL. The Search for Biomarkers of Alzheimer’s Disease in Down Syndrome. Am J Intellect Dev Disabil. 2020;125(2):97–99. [DOI] [PubMed] [Google Scholar]
- 87.Esbensen AJ, Hooper SR, Fidler D, et al. ; Outcome Measures Working Group. Outcome Measures for Clinical Trials in Down Syndrome. Am J Intellect Dev Disabil. 2017. May; 122(3):247–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sperling RA, Jack CR Jr., Aisen PS. Testing the right target and right drug at the right stage. Sci. Transl. Med 2011:3:111–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rafii MS, Aisen PS. Alzheimer’s Disease Clinical Trials: Moving Toward Successful Prevention. CNS Drugs. 2019;33(2):99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Strydom A, Coppus A, Blesa R, et al. Alzheimer’s disease in Down syndrome: An overlooked population for prevention trials. Alzheimers Dement (N Y). 2018;4:703–713. Published 2018 Dec 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moran JA, Rafii MS, Keller SM, et al. The National Task Group on Intellectual Disabilities and Dementia Practices consensus recommendations for the evaluation and management of dementia in adults with intellectual disabilities. Mayo Clin Proc. 2013;88(8):831–840. [DOI] [PubMed] [Google Scholar]
- 92.Walsh DM, Doran E, Silverman W, et al. Rapid assessment of cognitive function in down syndrome across intellectual level and dementia status. J Intellect Disabil Res. 2015;59(11): 1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sheehan R, Sinai A, Bass N, et al. Dementia diagnostic criteria in Down syndrome. Int J Geriatr Psychiatry. 2015;30(8):857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fenili D, Brown M, Rappaport R, et al. Properties of scyllo-inositol as a therapeutic treatment of AD-like pathology. J Mol Med (Berl) 2007;85:603–11. [DOI] [PubMed] [Google Scholar]
- 95.McLaurin J, Golomb R, Jurewicz A, et al. Inositol stereoisomers stabilize an oligomeric aggregate of Alzheimer amyloid beta peptide and inhibit abeta-induced toxicity. J Biol Chem. 2000;275:18495–502. [DOI] [PubMed] [Google Scholar]
- 96.Townsend M, Cleary JP, Mehta T, et al. Orally available compound prevents deficits in memory caused by the Alzheimer amyloid-beta oligomers. Ann Neurol. 2006;60:668–76. [DOI] [PubMed] [Google Scholar]
- 97.Salloway S, Sperling R, Keren R, et al. ELND005-AD201 Investigators A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer disease. Neurology. 2011;77:1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rafii MS, Skotko BG, McDonough ME et al. A Randomized, Double-Blind, Placebo-Controlled, Phase II Study of Oral ELND005 (scyllo-inositol) in Young Adults with Down Syndrome without Dementia. J Alzheimers Dis. 2017;58(2):401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muhs A, Hickman DT, Pihlgren M, et al. Liposomal vaccines with conformation-specific amyloid peptide antigens define immune response and efficacy in APP transgenic mice. Proc Natl Acad Sci USA. 2007. June 5;104(23):9810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Orgogozo JM, Gilman S, Dartigues JF, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003. July 8;61(1):46–54. [DOI] [PubMed] [Google Scholar]


