Abstract
OBJECTIVES
This study determined the impact of excess epicardial adipose tissue (EAT) in patients with the obese phenotype of heart failure with preserved ejection fraction (HFpEF).
BACKGROUND
Patients with HFpEF and an elevated body mass index differ from n patients, but beyond generalized obesity, fat distribution may be more important. Increases in EAT are associated with excess visceral adiposity, inflammation, and cardiac fibrosis, and EAT has been speculated to play an important role in the pathophysiology of HFpEF, but no study has directly evaluated this question.
METHODS
Patients with HFpEF and obesity (n = 169) underwent invasive hemodynamic exercise testing with expired gas analysis and echocardiography. Increased EAT was defined by echocardiography (EAT thickness ≥9 mm).
RESULTS
Compared with obese patients without increased EAT (HFpEFEAT−, n = 92), obese patients with HFpEF with increased EAT (HFpEFEAT+; n = 77) displayed a higher left ventricular eccentricity index, indicating increased pericardial restraint, but similar resting biventricular structure and function. In contrast, hemodynamics were more abnormal in patients with HFpEFEAT+, with higher right atrial, pulmonary artery, and pulmonary capillary wedge pressures at rest and during exercise compared with those of patients with HFpEFEAT−. Peak oxygen consumption (VO2) was reduced in both groups but was 20% lower in patients with HFpEFEAT+ (p < 0.01).
CONCLUSIONS
Among patients with the obese phenotype of HFpEF, the presence of increased EAT is associated with more profound hemodynamic derangements at rest and exercise, including greater elevation in cardiac filling pressures, more severe pulmonary hypertension, and greater pericardial restraint, culminating in poorer exercise capacity. Further study is needed to understand the biology and treatment of excessive EAT in patients with HFpEF.
Keywords: epicardial fat, heart failure, hemodynamics, HFpEF, obesity, pericardium, pulmonary hypertension
Obesity is an important risk factor in the development of heart failure with preserved ejection fraction (HFpEF) (1,2). Individuals with obesity and HFpEF display a distinct pathophysiological phenotype that differs from individuals with HFpEF who are not obese (3). Excess fat content in specific locations, such as the visceral and epicardial adipose tissue (EAT) depots, may be particularly detrimental, because these tissues act as metabolically active endocrine organs that promote inflammation and may be associated with remodeling and dysfunction in both the heart and vasculature (4).
EAT lies directly over the surface of the myocardium, with no intervening fascial plane. Accordingly, EAT shares the same microcirculation as the epicardium, setting the stage for greater adipocyte myocyte interactions (4,5). EAT has been speculated to play an important role in cardiac energy regulation in health, but with excessive accumulation, there may be increased risk of atrial fibrillation, microvascular dysfunction and rarefaction, myocardial impairment, and cardiac fibrosis (5–9).
Previous studies have demonstrated that EAT is increased in patients with HFpEF compared with control subjects (7,8) and in obese patients with HFpEF relative to non- obese patients with HFpEF (3). Although it has been speculated that excess EAT plays an important role in HFpEF (6), it has not yet been determined whether or how excess EAT might affect cardiac structure, function, hemodynamics, and exercise capacity in HFpEF. We hypothesized that among patients with HFpEF and obesity, patients with excess EAT would demonstrate more severe hemodynamic derangements, increased pericardial restraint, and poorer aerobic capacity compared with those of obese patients with HFpEF without excess EAT.
METHODS
STUDY POPULATION.
Consecutive patients referred for invasive cardiopulmonary exercise testing at the Mayo Clinic Cardiac Catheterization laboratory between 2000 and 2014 were retrospectively analyzed. Patients with HFpEF and obesity were identified, defined by clinical symptoms of HF (dyspnea and fatigue), preserved EF ($50%), and elevated left-heart filling pressures (pulmonary capillary wedge pressure [PCWP] ≥15 mm Hg at rest and/or ≥25 mm Hg with exercise), together with an elevated body mass index (BMI) ($30 kg/m2). Patients with any history of reduced EF (<50%), isolated right-sided HF, significant valvular heart disease (greater than moderate left-sided regurgitation, greater than mild stenosis), unstable coronary disease or recent revascularization, constrictive pericarditis, high-output HF, or cardiomyopathy were excluded. The Mayo Clinic Institutional Review Board approved this study, and written informed consent was provided by all participants.
ASSESSMENT OF CARDIAC STRUCTURE AND FUNCTION.
Two-dimensional, Doppler, and tissue Doppler echocardiography were performed within 4 weeks of cardiac catheterization in a blinded fashion without knowledge of subjects’ characteristics. Excess EAT was defined by epicardial fat pad thickness ≥9 mm measured on the free wall of the right ventricle (RV) by echocardiography in the parasternal long-axis view (Figure 1) (10,11). Obese patients with HFpEF were then categorized into those with increased EAT (HFpEFEAT+) and those without increased EAT (HFpEFEAT−).
FIGURE 1. Echocardiographic Measurement of EAT.
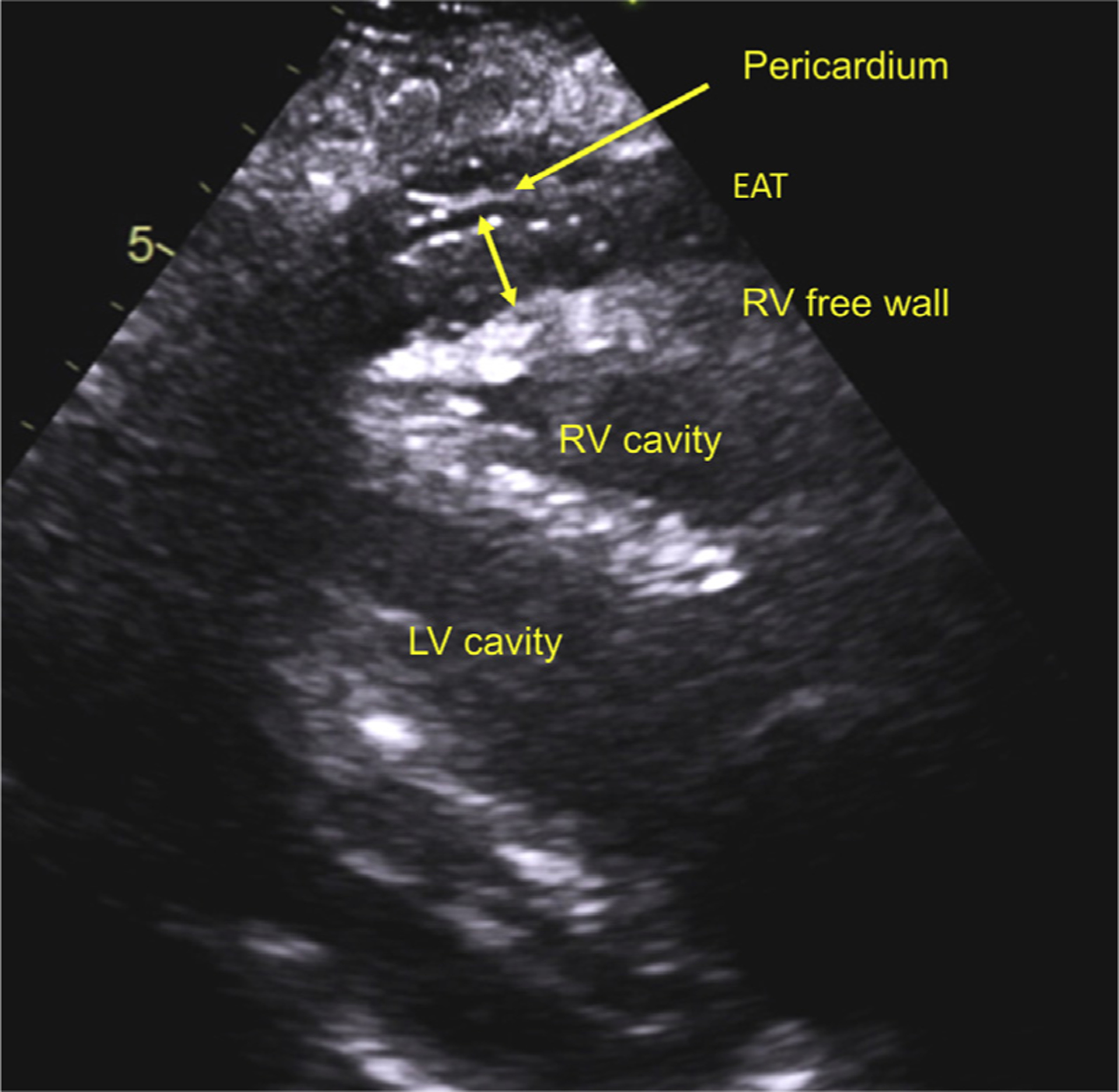
Example with landmarks taken from the parasternal long-axis view. EAT = epicardial adipose tissue; LV = left ventricle; RV = right ventricle.
Myocardial deformation analyses were performed off-line with commercially available software (Syngo, Siemens Medical Solutions, Munich, Germany). Left ventricular (LV) longitudinal strain was measured from 2 apical views (12). Strain values represented the mean of 3 beats and were expressed as positive absolute values, where higher LV longitudinal strain indicated better systolic function.
RV basal, mid-cavity, and longitudinal dimensions were measured at end-diastole with RV-focused views. RV systolic function was assessed by RV fractional area change. Total epicardial volume was estimated from 2 hemi-ellipsoids containing both atria and ventricles with the apical 4-chamber view (13).
ASSESSMENT OF VENTRICULAR INTERDEPENDENCE AND PERICARDIAL RESTRAINT.
Increases in EAT may enhance coupling between the LV and RV (ventricular interdependence) by reducing the amount of potential space within the pericardial sac (3,14). Ventricular interdependence was quantified in the parasternal short-axis view on 2-dimensional echocardiography by the LV eccentricity index (LVecc) and using planimetry to calculate idealized and actual LV radii (Rideal/Ractual), whereby higher values of both indexes indicated greater septal flattening, enhanced ventricular interdependence, and increased pericardial restraint (Supplemental Figure 1).
INVASIVE HEMODYNAMIC ASSESSMENT.
Patients were examined while remaining on their long-term medications, in the fasted state after minimal sedation in the supine position as previously described (15–17). Right heart catheterization was performed through a 9-F sheath via the right internal jugular vein using high-fidelity micromanometers. Right atrial (RA), pulmonary artery (PA), and PCWP were measured at end-expiration (mean of ≥3 beats). LV transmural pressure, a measure of net LV distending pressures, was estimated as the difference between PCWP and RA pressure, as in previous studies (18,19). Following baseline hemodynamic assessment, subjects underwent invasive exercise assessment until volitional exhaustion. The initial stage of exercise was performed for 5 min at a workload of 20 W, followed by 20-W increments in workload (3-min stages) to subject-reported exhaustion. Pressure tracings were digitized (240 Hz) and stored for offline analysis.
Oxygen consumption (VO2) was measured from expired gas analysis (MedGraphics, St. Paul, Minneapolis). Arterial and mixed venous blood was directly sampled to measure oxygen content (saturation × hemoglobin × 1.34). Arterial-venous oxygen content difference (A-VO2diff) was calculated as the difference between systemic and PA oxygen content. Cardiac output (Q) was quantified by the direct Fick method (Q = VO2/A-VO2diff) and was scaled to body surface area to determine the cardiac index (CI). Pulmonary and systemic vascular function was assessed by the pulmonary vascular resistance index (PVRI = [mean PA − PCWP]/CI). The PA compliance index (PACI = SVI/[PA pulse pressure], where SVI indicates stroke volume index), the systemic vascular resistance index (SVRI = [mean arterial blood pressure − RA] × 79.9/CI), the total arterial compliance index (TACI = SVI/systemic pulse pressure), and the effective arterial elastance index (EaI = [0.9 × systolic blood pressure]/SVI) were also assessed.
The relationship between LV end-diastolic pressure and end-diastolic volume (EDP = αEDVβ) was assessed using invasive PCWP and echocardiographic LV volumes according to the single-beat method of Klotz et al. (20). This calculation yields the LV stiffness constant (β), a load-independent measure of LV diastolic chamber stiffness (21).
STATISTICAL ANALYSIS.
Data are reported as mean ± SD, median (interquartile range), or number (percent) unless otherwise specified. Between-group differences were compared by 1-way analysis of variance, Kruskal-Wallis test, or chi-square test, as appropriate. The Tukey honestly significant difference test or Steel-Dwass test was used to adjust for multiple testing. Linear and nonlinear regressions were used to assess associations between 2 variables. Linear regression models with an interaction term were performed to test the difference in the relationship between dependent and independent variables between 2 groups. For non-normally distributed variables entered into regression models, the assumption of normally distributed residuals was verified by quantile plots, and no violations were observed.
RESULTS
Compared with obese patients with HFpEFEAT− (n = 92), obese patients with HFpEFEAT+ (n = 77) had higher body weight and mean BMI, and slightly poorer renal function, but there were no other differences in clinical characteristics, prevalent comorbidities, medication use, or other laboratories (Table 1). EAT was directly correlated with BMI (r = 0.48; p < 0.001) (Supplemental Figure 2).
TABLE 1.
Baseline Characteristics
| HFpEFEAT− (n = 92) | HFpEFEAT+ (n = 77) | p Value | |
|---|---|---|---|
| Age, yrs | 66 ± 10 | 67 ± 12 | 0.48 |
| Female | 52 (57) | 52 (68) | 0.14 |
| Anthropometries | |||
| Height, cm | 167 ±9 | 168 ±10 | 0.37 |
| Ideal body weight, kg | 65 ± 12 | 63 ± 12 | 0.27 |
| Actual body weight, kg | 98 ± 15 | 112 ± 23 | <0.0001 |
| Body mass index, kg/m2 | 34.5 ± 4.2 | 39.9 ± 6.6 | <0.0001 |
| Comorbidities | |||
| Diabetes mellitus | 19 (21) | 23 (30) | 0.17 |
| Hypertension | 73 (79) | 65 (84) | 0.39 |
| Current atrial fibrillation | 16 (17) | 9 (25) | 0.24 |
| Medications | |||
| ACEI or ARB | 40 (43) | 35 (45) | 0.80 |
| Beta-blocker | 47 (51) | 43 (56) | 0.54 |
| Calcium-channel blocker | 17 (18) | 10 (13) | 0.35 |
| Loop diuretic | 42 (46) | 38 (49) | 0.63 |
| Laboratories | |||
| Hemoglobin, g/dl | 12.3 ± 1.7 | 12.2 ± 1.6 | 0.92 |
| eGFR, ml/min/1.73 m−2 | 63 ± 15 | 56 ± 20 | 0.02 |
| NT-proBNP, pg/ml | 719 ± 948 | 712 ± 956 | 0.97 |
Values are mean ± SD or n (%).
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; EAT = epicardial adipose tissue; eGFR = estimated glomerular filtration rate; HFpEF = heart failure with preserved ejection fraction; HFpEFEAT+ = HFpEF with increased EAT; HFpEFEAT− = without increased EAT; NT-proBNP = N-terminal pro−B- type natriuretic peptide.
CARDIAC STRUCTURE AND FUNCTION.
Compared with patients with HFpEFEAT−, patients with HFpEFEAT+ displayed similar LV dimensions, volumes, mass, EF, global longitudinal strain, and diastolic chamber stiffness (β), although estimated LV filling pressures assessed by the E/e’ ratio tended to be greater in patients with HFpEFEAT+ (Table 2). Similarly, RV size and function, and total epicardial heart volume were not significantly different between the 2 groups.
TABLE 2.
Cardiac Structure and Function
| HFpEFEAT− (n = 92) | HFpEFEAT+ (n = 77) | p Value | |
|---|---|---|---|
| LV structure and function | |||
| LV diastolic dimension, mm | 49 ± 5 | 49 ± 5 | 0.94 |
| LV end-diastolic volume, ml | 115 ± 28 | 116 ± 29 | 0.94 |
| LV end-diastolic volume index, ml/m2 | 56 ± 13 | 54 ± 12 | 0.27 |
| LV mass, g | 190 ± 47 | 205 ± 57 | 0.06 |
| LV mass index, g/m2.7 | 91 ± 20 | 95 ± 23 | 0.31 |
| LVEF, % | 63 ± 6 | 63 ± 6 | 0.90 |
| Mitral E-wave, m/s | 0.87 ± 0.30 | 0.91 ± 0.03 | 0.40 |
| Mitral annular é, cm/s | 0.07 ± 0.02 | 0.07 ± 0.02 | 0.41 |
| E/e’, ratio | 12.6 ± 5.5 | 14.4 ± 6.3 | 0.05 |
| LV longitudinal strain, % | 15.5 ± 2.9 | 15.4 ± 3.6 | 0.89 |
| LV stiffness, β, per 1 ml | 5.68 ± 0.17 | 5.72 ± 0.22 | 0.14 |
| RV structure and function | |||
| RV basal dimension, mm | 33 ± 7 | 33 ± 6 | 0.97 |
| RV mid cavity dimension, mm | 6 ± 6 | 26 ± 7 | 0.33 |
| RV longitudinal dimension, mm | 64 ± 7 | 66 ± 8 | 0.11 |
| RV fractional area change, % | 50 ± 10 | 49 ± 9 | 0.62 |
| Ventricular interdependence | |||
| Total epicardial heart volume, ml | 975 ± 268 | 1,026 ± 309 | 0.27 |
| Epicardial fat thickness, mm | 6.6 ± 1.5 | 10.5 ± 1.7 | - |
| Eccentricity index at end-diastole | 1.02 ± 0.11 | 1.07 ± 0.15 | 0.03 |
| Eccentricity index at end-systole | 1.02 ± 0.11 | 1.10 ± 0.21 | 0.002 |
| Ideal/actual radius at end-diastole | 1.13 ± 0.21 | 1.30 ± 0.43 | 0.001 |
| Ideal/actual radius at end-systole | 1.14 ± 0.26 | 1.28 ± 0.53 | 0.03 |
Values are mean ± SD.
LV = left ventricular; LVEF = left ventricular ejection fraction; RV = right ventricular; other abbreviations as in Table 1
Compared with the group with HFpEFEAT−, the HFpEFEAT+ group demonstrated greater ventricular interdependence, as evidenced by higher LVecc and a greater Rideal/Ractual, in both systole and diastole (Table 2, Figure 2). The systolic LVecc remained higher in patients with HFpEFEAT+ after adjusting for pulmonary vascular resistance index and RV fractional area change (p = 0.02), whereas the diastolic LVecc was no longer significantly different after adjustment. The relationship between LVecc and PVR was similar in patients with HFpEFEAT− and patients with HFpEFEAT+, but there was a significant interaction in the relationship with RV function, whereby there was more ventricular interdependence as RV function worsened in the HFpEFEAT+ group (Figure 2).
FIGURE 2. Ventricular Interdependence in HFpEF With Increased EAT.
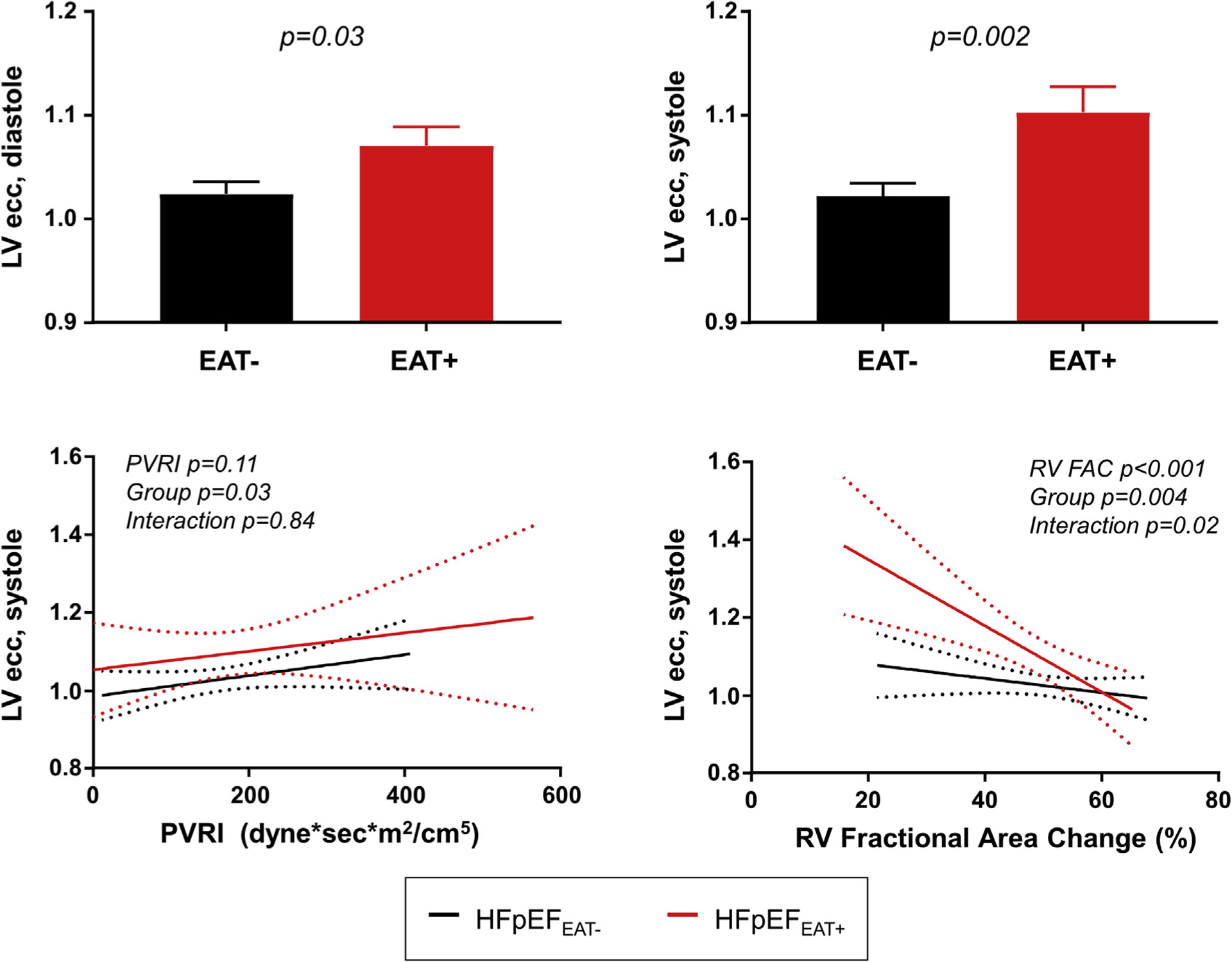
Compared with obese patients with heart failure with preserved ejection fraction (HFpEF) and no excess epicardial adipose tissue (EAT−, black), patients with increased EAT (red) displayed an increased left ventricular eccentricity index (LVecc) during diastole (top left) and systole (top right). Systolic LVecc was higher in patients with increased EAT for any pulmonary vascular resistance (PVR) (bottom left), and increased more with decreasing right ventricular fractional area change (RV FAC) in patients with high EAT (group × RV FAC interaction; p = 0.02) (lower right). Values are mean ± SE. EAT = epicardial adipose tissue.
RESTING HEMODYNAMICS.
At rest, arterial blood pressures and heart rate were similar between groups (Table 3). Compared with patients with HFpEFEAT−, patients with HFpEFEAT+ displayed higher right and left heart filling pressures, as well as higher PA pressures (Table 3, Central Illustration). Epicardial fat thickness was weakly but positively correlated with increasing RA pressure (r = 0.23; p = 0.003). There were no differences in LV transmural pressure or systemic and pulmonary vascular function. Resting CI, VO2, and A-VO2diff were similar between patients with HFpEFEAT− and patients with HFpEFEAT+.
TABLE 3.
Invasive Hemodynamics and Oxygen Delivery at Rest
| HFpEFEAT− (n = 92) | HFpEFEAT+ (n = 77) | p Value | |
|---|---|---|---|
| Vital signs | |||
| Heart rates, beats/min | 67 ± 10 | 67 ± 11 | 0.86 |
| Systolic BP, mm Hg | 147 ± 31 | 151 ± 31 | 0.51 |
| Mean BP, mm Hg | 99 ± 15 | 100 ± 18 | 0.67 |
| Central pressures | |||
| RAP, mm Hg | 9 ± 4 | 11 ± 5 | 0.002 |
| PA systolic pressure, mm Hg | 39 ±12 | 44 ±13 | 0.03 |
| PA mean pressure, mm Hg | 21 ± 4 | 29 ±8 | 0.0009 |
| PCWP, mm Hg | 15 ± 5 | 18 ± 7 | 0.0004 |
| RAP/PCWP ratio | 0.63 ± 0.18 | 0.64 ± 0.18 | 0.87 |
| LVTMP, mm Hg | 6 ± 3 | 7 ± 4 | 0.08 |
| Vascular function | |||
| Eal, mm Hg/m2/ml | 3.59 ±1.15 | 3.41 ± 1.27 | 0.44 |
| SVRI, dynes/m2/s/cm5 | 3,104 ± 814 | 3,070 ± 1,193 | 0.86 |
| TACI, ml/mm Hg/m2 | 0.66 ± 0.67 | 0.64 ± 0.37 | 0.84 |
| PVRI, dynes/m2/s/cm5 | 362 ± 174 | 373 ± 183 | 0.72 |
| PACI, ml/mm Hg/m2 | 1.83 ± 0.84 | 1.96 ± 1.12 | 0.42 |
| Oxygen delivery | |||
| Cardiac index, l/min/m2 | 2.53 ± 0.58 | 2.58 ± 0.72 | 0.62 |
| VO2 index, mL/min/kg | 3.69 ± 1.09 | 3.39 ± 0.77 | 0.19 |
| A-VO2diff, ml/dl | 4.48 ± 0.85 | 4.64 ± 0.96 | 0.26 |
Values are mean ± SD.
A-VO2diff = arterial-venous oxygen content difference; BP = blood pressure; EaI = effective arterial elastance index; LVTMP = left ventricular transmural pressure; PA = pulmonary artery; PACI = pulmonary artery compliance index; PCWP = pulmonary capillary wedge pressure; PVRI = pulmonary vascular resistance index; RA = right atrial; RAP = right atrial pressure; SVRI = systemic vascular resistance index; TACI = total arterial compliance index; other abbreviations as in Tables 1 and 2.
CENTRAL ILLUSTRATION.
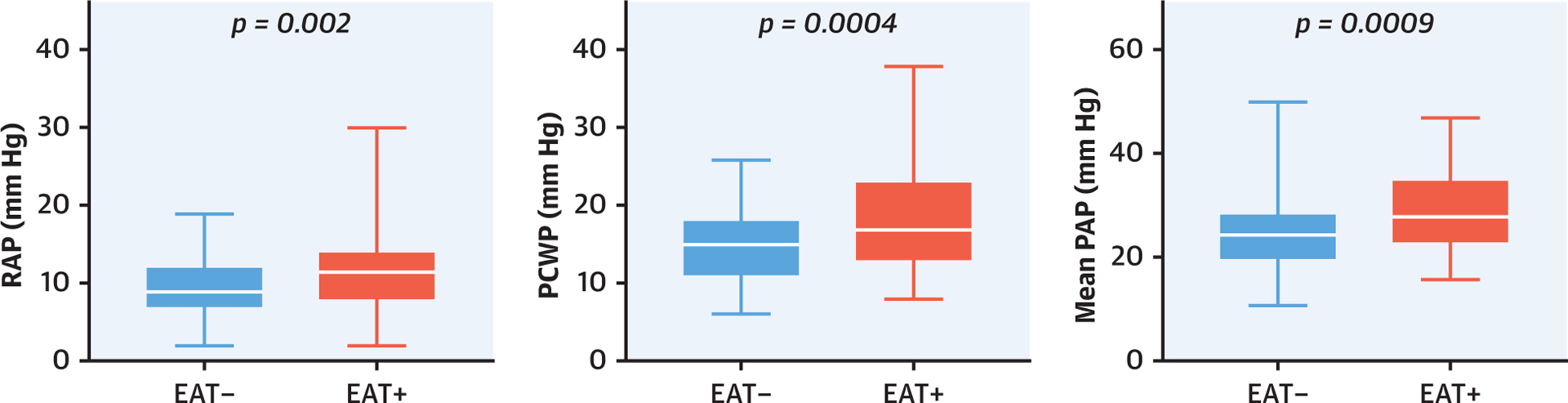
Impact of Increased Epicardial Adipose Tissue on Cardiac Hemodynamics in Heart Failure With Preserved Ejection Fraction. Patients with increased epicardial adipose tissue (EAT) (red) had higher right atrial pressures (RAPs), higher pulmonary capillary wedge pressure (PCWP), and higher mean pulmonary artery pressures (PAPs) compared with that of patients without increased EAT (blue).
EXERCISE HEMODYNAMICS AND AEROBIC CAPACITY.
With exercise, patients with HFpEFEAT+ again displayed higher RA pressures, PCWP, and PA pressures (Table 4). Compared with patients HFpEFEAT−, peak VO2 was 20% lower in patients with HFpEFEAT+. Differences of similar magnitude were observed in a subgroup of patients (n = 77) who underwent upright exercise treadmill testing before catheterization (Table 4). There was a significant inverse relationship between epicardial fat thickness and peak VO2 in regression analysis (Table 4, Figure 3). There was no interaction between age and EAT for peak VO2 (p = 0.80).
TABLE 4.
Invasive Hemodynamics And Oxygen Delivery With Exercise
| HFpEFEAT− (n = 92) | HFpEFEAT+ (n = 77) | p Value | |
|---|---|---|---|
| Vital signs | |||
| Heart rate, beats/min | 101 ± 19 | 97 ± 22 | 0.21 |
| Systolic BP, mm Hg | 187 ± 34 | 186 ± 38 | 0.86 |
| Mean BP, mm Hg | 118 ± 22 | 119 ± 23 | 0.82 |
| Central hemodynamics | |||
| RAP, mm Hg | 19 ± 6 | 22 ± 9 | 0.009 |
| PA systolic pressure, mm Hg | 58 ± 16 | 63 ± 13 | 0.14 |
| PA mean pressure, mm Hg | 46 ± 9 | 49 ± 11 | 0.03 |
| PCWP, mm Hg | 31 ± 6 | 34 ±7 | 0.001 |
| RAP/PCWP ratio | 0.60 ± 0.19 | 0.64 ± 0.21 | 0.29 |
| LVTMP, mm Hg | 13 ±7 | 12 ±8 | 0.85 |
| Vascular function | |||
| Eal, mm Hg/m2/ml | 4.52 ±1.50 | 4.25 ± 0.24 | 0.42 |
| SVRI, dynes/m2/s/cm5 | 2,135 ± 629 | 2,057 ± 833 | 0.62 |
| TACI, ml/mm Hg/m2 | 0.41 ± 0.24 | 0.48 ± 0.33 | 0.22 |
| PVRI, dynes/m2/s/cm5 | 324 ± 221 | 373 ± 346 | 0.31 |
| PACI, mL/mm Hg/m2 | 1.16 ± 0.55 | 1.07 ± 0.46 | 0.32 |
| Oxygen delivery | |||
| Cardiac index, l/min/m2 | 4.20 ± 1.25 | 4.09 ± 1.43 | 0.63 |
| A-VO2diff, ml/dl | 9.6 ± 2.0 | 9.7 ± 2.5 | 0.69 |
| Supine Peak V02, ml/min/kg* (available for n = 68/55) |
8.7 ± 2.9 | 7.4 ± 2.2 | 0.01 |
| Upright peak VO2, ml/min/kg† (available for n = 47/30) |
15.2 ± 5.3 | 12.0 ± 3.5 | 0.005 |
FIGURE 3. Impact Of Increased EAT On Aerobic Capacity.
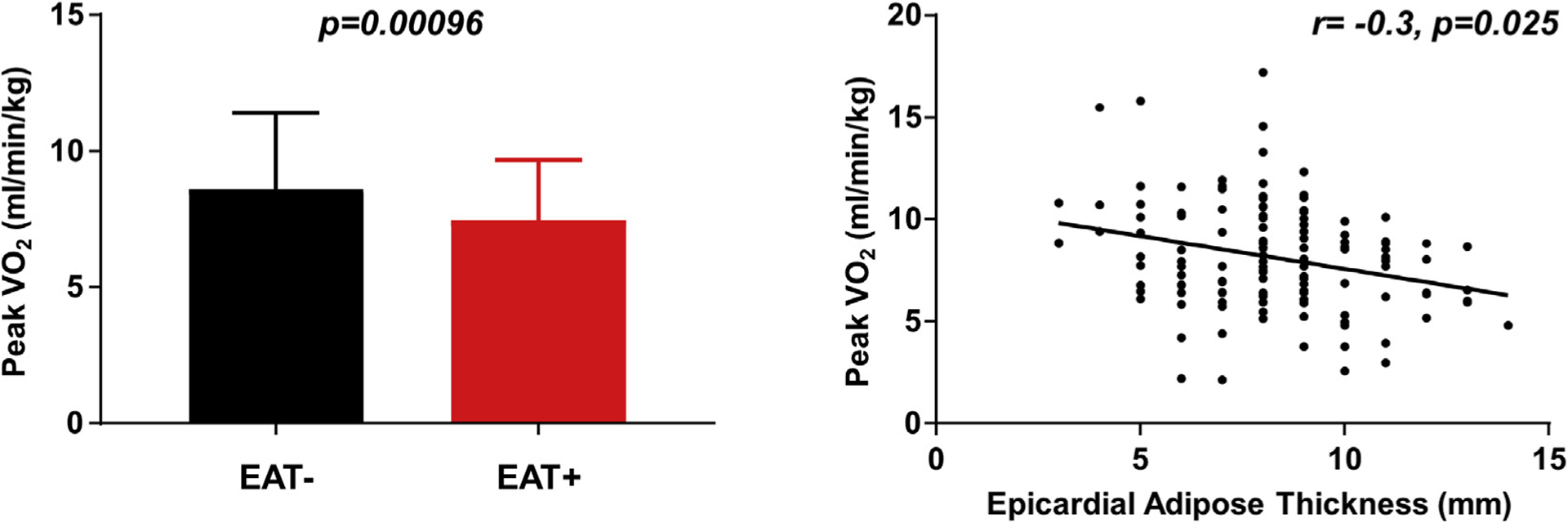
(Left panel) Peak oxygen consumption (VO2; available from invasive exercise testing in 68 patients with EAT− and 55 patients with EAT+) is significantly reduced in patients with HFpEF and increased EAT (red) compared with patients without increased EAT (black). The impairment in peak VO42 was correlated with greater EAT (right panel). EAT = epicardial adipose tissue; HFpEF = heart failure with preserved ejection fraction.
SENSITIVITY ANALYSIS.
Because of the difference in body weight between the HFpEFEAT+ and HFpEFEAT− groups, we compared hemodynamics restricted to patients in the overlapping BMI range of 32 to 38 kg/m2 (Supplementary Table 1). Mean BMI remained slightly higher in the HFpEFEAT+ group compared with that of the HFpEFEAT− group in this analysis, but the difference in point estimates for BMI was of much lower magnitude than the difference for EAT (3% vs. 31%), which allowed for a more specific exploration of the impact of EAT relative to BMI. Findings were similar to the entire population, with higher right and left heart filling pressures and PA pressures in the HFpEFEAT+ group compared with that of the HFpEFEAT− group at rest and during exercise (Supplemental Table 1).
DISCUSSION
We examined the effect of excess epicardial fat on cardiac structure and function, hemodynamics, and aerobic capacity in patients with the obese phenotype of HFpEF. Patients with HFpEFEAT+ demonstrated higher BMI, greater pericardial restraint, and enhanced ventricular interdependence compared with that of patients with HFpEFEAT−. Patients with HFpEF with excess epicardial fat also displayed worse peak aerobic capacity and more profound hemodynamic derangements, with higher biventricular filling pressures and more severe pulmonary hypertension at rest and during exercise, although measures of resting systolic and diastolic myocardial function were not significantly different between the 2 groups. These data identify a potentially important association between increased EAT, enhanced ventricular interdependence, abnormal central hemodynamics, and reduced exercise capacity in HFpEF. However, because of the higher generalized adiposity (BMI) among patients with increased EAT, the present data could not determine to what extent these abnormalities were specifically related to high EAT, increased total body fat content, or both. Further study is needed to better clarify the underlying biology and treatment for increased EAT in patients with HFpEF.
OBESITY, EPICARDIAL FAT, AND HFpEF.
Generalized obesity is associated with systemic inflammation, oxidative stress, neurohormonal activation, metabolic stress, volume expansion, and alterations in central hemodynamics, all of which may contribute to the development of HFpEF (21–28). Nearly 40% of all U.S. adults are obese, and although previous studies reported a high prevalence of obesity in HFpEF (32% to 46%), recent data suggested the prevalence may be twice as high (~60% to 80%) (29). Compared with nonobese subjects, subjects with obesity-related HFpEF demonstrated elevated plasma volume, more profound cardiac remodeling and dysfunction, marked hemodynamic abnormalities, worse exercise capacity, and increased EAT (3).
EAT is primarily composed of white adipose tissue and is known to be directly related to total body fat and visceral fat content (30). A central role for increased EAT in the pathophysiology HFpEF has been proposed (6). This hypothesis is supported by mechanistic studies in non-HFpEF populations (8–11) but has not yet been proven in HFpEF. Smaller studies have reported elevated EAT in patients with HF, including some with HFpEF, which has been associated with atrial dysfunction, atrial fibrillation, insulin resistance, systemic inflammation, and increases in myocyte injury (7,8). However, these studies have not included direct assessments of cardiac hemodynamics or aerobic capacity, and group differences were evaluated between cases and control subjects rather based upon the presence or absence of EAT.
The present data provide new insights into the potential role for excess EAT in the pathophysiology of HFpEF, showing that patients with obesity, HFpEF, and increased EAT displayed more severe hemodynamic derangements and poorer exercise capacity, which was assessed using robust invasive methods with simultaneous expired gas analysis. Notably, significant differences in diastolic and systolic ventricular function were not observed, at least when measured at rest (Table 2). However, it was also possible that myocardial reserve was more impaired in patients with excess EAT. A recent study reported that patients with increased visceral adipose tissue (which is associated with EAT) but no HF had greater myocardial steatosis and energetic abnormalities seen by magnetic resonance spectroscopy, which was associated with diastolic dysfunction and supports this possibility (31). Functional deficits related to excessive or dysfunctional EAT would expect to be amplified during exercise, in which energy availability becomes compromised secondary to increased myocardial oxygen demand, coronary microvascular dysfunction, and supply demand mismatch (32,33).
EPICARDIAL FAT AND DIASTOLIC VENTRICULAR INTERACTION.
Beyond the metabolic and inflammatory effects of EAT, its proximity to the myocardium might have direct effects on cardiac mechanics mediated by pericardial restraint (14). Previous studies demonstrated that RA pressure provides the best estimate of pericardial pressure (19). In the present study, RA pressure was weakly correlated with EAT and was elevated at rest and during exercise in patients with HFpEFEAT+ compared with that of patients with HFpEFEAT−. Considering this finding together with the observed increases in LV eccentricity and ideal/predicted chamber radius, these data strongly supported an important role for enhanced ventricular interaction in this cohort, as previously reported overall in patients with the obese phenotype of HFpEF (3). Approximately 75% of epicardial fat resides over the RV (30). We observed that the systolic LVecc increased to an even greater extent as RV function worsened in patients with increased EAT. This suggested that excess EAT might particularly exacerbate systolic ventricular interdependence in the setting of right heart failure, which is common and associated with adverse outcomes in HFpEF (34,35).
Although LV transmural pressures were similar between groups, patients with EAT+ demonstrated elevated PCWP at rest and during exercise, which suggested an uncoupling between LV filling pressure (PCWP) and LV pre-load (end-diastolic volume) (14). Ventricular interdependence and enhanced pericardial restraint play important roles in right-sided HF (18) and advanced HF with a reduced EF (36), and the present data indicated that patients with HFpEF and increased EAT also fall into this category. This is important because surgical therapies targeting pericardial restraint are currently being tested (Minimally Invasive Pericardiotomy as a New Treatment for Heart Failure; NCT03923673), and patients with HFpEF and EAT may be uniquely positioned to respond favorably to this type of interventional treatment (14,37).
AEROBIC CAPACITY AND EPICARDIAL FAT IN OBESE PATIENTS WITH HFpEF.
It was well established that compared with subjects without HFpEF, subjects with HFpEF displayed marked reductions in aerobic capacity; this was even more impaired in obese patients with HFpEF (3). The present study expanded upon these findings by demonstrating that among the broader population of obese patients with HFpEF, those with increased EAT displayed the poorest aerobic capacity. This finding contrasted with 1 study that demonstrated a paradoxical inverse relationship between EAT and aerobic capacity in HFpEF (38). The reasons for the discrepancy are unclear; however, in the latter study, EAT was lower in patients with HFpEF compared with control subjects, in contrast to previous studies that reported higher EAT in HFpEF (3,13,14). The inverse relationship observed might have been related more to the less typical group differences in that study.
Peak VO2 is normalized to body mass, which was greater in the EAT+ group and explained much of the difference between groups. However, it is known that larger individuals require greater oxygen metabolism to achieve the same amount of ergometric work; this must be considered when comparing gas exchange data. For example, a lean patient may achieve a total body VO2 of 600 ml/min cycling at 60 rpm at a 20-W workload, whereas a morbidly obese patient might require a VO2 of 1,000 ml/min to perform the same amount of work. The efficiency of converting metabolic (VO2) to ergometric work is known to be impaired in patients with the obese phenotype of HFpEF (3), which may partly explain their poorer quality of life and functional disability (39,40).
EPICARDIAL VERSUS TOTAL BODY FAT.
Mean BMI was higher in patients with HFpEFEAT+ compared with that of patients with HFpEFEAT−, which made it difficult to determine whether the abnormalities observed in the HFpEFEAT−group were specific to excess epicardial fat, greater general adiposity, or some combination. As total body fat increases, there is saturation of fat storage depots in the subcutaneous tissues and accumulation in alternative fat depots (e.g., the epicardial space and viscera), making it difficult to disentangle effects specific to EAT versus total fat content. EAT was strongly correlated with BMI, and because of this collinearity, the impact of each component in a multivariable regression model could not be accurately estimated. Because of the differences in mean BMI in the HFpEFEAT+ and HFpEFEAT− groups, it was also likely that other obesity-associated comorbid conditions would differ between groups (e.g., sleep apnea). This might have also influenced cardiac structure or function.
Although we could not conclude that functional and hemodynamic differences were uniquely ascribable to EAT versus excess adiposity, the present data clearly showed that the presence of increased EAT was associated with a more severe obese HFpEF phenotype. As such, increased EAT could function as a useful echocardiographic biomarker that could be used to identify patients best positioned to respond to novel therapies targeting weight loss in future studies.
STUDY LIMITATIONS.
These data were obtained from patients referred for invasive hemodynamic assessment at a single tertiary center, which introduced bias. The correlative nature of the data limited the ability to address causality in the relationships. Hemodynamic measures were obtained at end-expiration, which could overestimate filling pressures if there were positive end-expiratory intrathoracic pressure in some patients, but this would be unlikely to affect resting hemodynamics. It could be difficult to distinguish EAT from paracardial adipose tissue by echocardiography, which could lead to overestimation of EAT thickness, but the same method was applied to all patients in a similar fashion, which reduces bias. Alternative methods, such as cardiac computed tomography and cardiac magnetic resonance, provide greater precision and accuracy to quantify EAT and might distinguish it from paracardiac fat in future studies.
CONCLUSIONS
Subjects with HFpEF and obesity who have excess epicardial fat deposition demonstrate significantly greater impairments in rest and exercise hemodynamics, enhanced ventricular interdependence, and reduced exercise capacity compared with patients with obesity and HFpEF but without excess EAT. These data supported the emerging paradigm that excess EAT might contribute to the pathophysiology of patients with obesity-related HFpEF.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
In this cross-sectional analysis of patients with the obese phenotype of HFpEF, we found that the presence of increased EAT was associated with more adverse hemodynamic abnormalities, increased ventricular interdependence, and poorer exercise capacity compared with that of patients with HFpEF with obesity and no increased EAT.
TRANSLATIONAL OUTLOOK:
These data call for further study into the mechanisms by which EAT may influence cardiac function and worsen HFpEF severity, and into how it might be targeted therapeutically to improve clinical status.
Acknowledgments
Dr. Borlaug is supported by the National Institutes of Health (NIH) (R01 HL128526, R01 HL 126638, U01 HL125205, and U10 HL110262). Ms. Koepp is supported by the NIH (F31 HL143952). The authors have reported that they have no relationships relevant to the contents of this paper to disclose. The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Heart Failure author instructions page
ABBREVIATIONS AND ACRONYMS
- A- VO2diff
arterial-venous oxygen content difference
- BMI
body mass index
- CI
cardiac index
- EAT
epicardial adipose tissue
- HFpEF
heart failure with preserved ejection fraction
- HFpEFEAT+
HFpEF with increased EAT
- HFpEFEAT−
without increased EAT
- LV
left ventricular
- LVecc
left ventricular eccentricity index
- PA
pulmonary artery
- PCWP
pulmonary capillary wedge pressure
- RA
right atrial
- Rideal/Ractual
idealized to actual LV radius
- RV
right ventricular
- VO2
oxygen consumption
Footnotes
APPENDIX For supplemental figures and table, please see the online version of this paper.
REFERENCES
- 1.Ho JE, Lyass A, Lee DS, et al. Predictors of new- onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Failure 2013; 6:279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao VN, Zhao D, Allison MA, et al. Adiposity and incident heart failure and its subtypes: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol HF 2018;6:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Packer M Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol 2018;71:2360–72. [DOI] [PubMed] [Google Scholar]
- 5.Iacobellis G, Barbaro G. Epicardial adipose tissue feeding and overfeeding the heart. Nutrition 2019; 59:1–6. [DOI] [PubMed] [Google Scholar]
- 6.Greulich S, Maxhera B, Vandenplas G, et al. Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation 2012;126: 2324–34. [DOI] [PubMed] [Google Scholar]
- 7.Hung CL, Yun CH, Lai YH, et al. An observational study of the association among interatrial adiposity by computed tomography measure, insulin resistance, and left atrial electromechanical disturbances in heart failure. Medicine (Baltimore) 2016;95:e3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with midrange and preserved ejection fraction. Eur J Heart Fail 2018;20:1559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu CK, Tsai HY, Su MM, et al. Evolutional change in epicardial fat and its correlation with myocardial diffuse fibrosis in heart failure patients. J Clin Lipidol 2017;11:1421–31. [DOI] [PubMed] [Google Scholar]
- 10.Iacobellis G, Willens HJ, Barbaro G, Sharma AM. Threshold values of high-risk echocardiographic epicardial fat thickness. Obesity (Silver Spring) 2008;16:887–92. [DOI] [PubMed] [Google Scholar]
- 11.Iacobellis G Relation of epicardial fat thickness to right ventricular cavity size in obese subjects. Am J Cardiol 2009;104:1601–2. [DOI] [PubMed] [Google Scholar]
- 12.Shah AM, Claggett B, Sweitzer NK, et al. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation 2015;132:402–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melenovsky V, Borlaug BA, Rosen B, et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol 2007;49: 198–207. [DOI] [PubMed] [Google Scholar]
- 14.Borlaug BA, Reddy YNV. The role of the pericardium in heart failure: implications for pathophysiology and treatment. J Am Coll Cardiol 2019; 7:574–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J 2016;37: 3293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol 2015; 66:1672–82. [DOI] [PubMed] [Google Scholar]
- 17.Borlaug BA, Melenovsky V, Koepp KE. Inhaled sodium nitrite improves rest and exercise hemodynamics in heart failure with preserved ejection fraction. Circ Res 2016;119:880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen MJ, Nishimura RA, Borlaug BA. The hemodynamic basis of exercise intolerance in tricuspid regurgitation. Circ Heart Failure 2014;7: 911–7. [DOI] [PubMed] [Google Scholar]
- 19.Tyberg JV, Taichman GC, Smith ER, Douglas NW, Smiseth OA, Keon WJ. The relationship between pericardial pressure and right atrial pressure: an intraoperative study. Circulation 1986;73:428–32. [DOI] [PubMed] [Google Scholar]
- 20.Klotz S, Hay I, Zhang G, Maurer M, Wang J, Burkhoff D. Development of heart failure in chronic hypertensive Dahl rats: focus on heart failure with preserved ejection fraction. Hypertension 2006;47:901–11. [DOI] [PubMed] [Google Scholar]
- 21.Wong CY, O’Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation 2004;110:3081–7. [DOI] [PubMed] [Google Scholar]
- 22.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev 2008;88: 389–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bello NA, Cheng S, Claggett B, et al. Association of weight and body composition on cardiac structure and function in the ARIC Study (Atherosclerosis Risk in Communities). Circ Heart Fail 2016;9:e002978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borlaug BA, Redfield MM, Melenovsky V, et al. Longitudinal changes in left ventricular stiffness: a community-based study. Circ Heart Fail 2013;6: 944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitzman DW, Shah SJ. The HFpEF obesity phenotype: the elephant in the room. J Am Coll Cardiol 2016;68:200–3. [DOI] [PubMed] [Google Scholar]
- 26.Russo C, Jin Z, Homma S, et al. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol 2011;57:1368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selvaraj S, Martinez EE, Aguilar FG, et al. Association of central adiposity with adverse cardiac mechanics: findings from the hypertension genetic epidemiology network study. Circ Cardiovasc Imaging 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wohlfahrt P, Redfield MM, Lopez-Jimenez F, et al. Impact of general and central adiposity on ventricular-arterial aging in women and men. J Am Coll Cardiol 2014;2:489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borlaug BA, Anstrom KJ, Lewis GD, et al. Effect of inorganic nitrite vs placebo on exercise capacity among patients with heart failure with preserved ejection fraction: the INDIE-HFpEF randomized clinical trial. JAMA 2018;320:1764–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev 2007;8: 253–61. [DOI] [PubMed] [Google Scholar]
- 31.Rayner JJ, Banerjee R, Holloway CJ, et al. The relative contribution of metabolic and structural abnormalities to diastolic dysfunction in obesity. Int J Obesity 2018;42:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obokata M, Reddy YNV, Melenovsky V, et al. Myocardial injury and cardiac reserve in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2018;72:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah SJ, Lam CSP, Svedlund S, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J 2018; 39:3439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014;35:3452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obokata M, Reddy YNV, Melenovsky V, Pislaru S, Borlaug BA. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J 2019;40:689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janicki JS. Influence of the pericardium and ventricular interdependence on left ventricular diastolic and systolic function in patients with heart failure. Circulation 1990;81 suppl: III15 20. [PubMed] [Google Scholar]
- 37.Borlaug BA, Schaff HV, Pochettino A, et al. Pericardiotomy enhances left ventricular diastolic reserve with volume loading in humans. Circulation 2018;138:2295–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haykowsky MJ, Nicklas BJ, Brubaker PH, et al. Regional adipose distribution and its relationship to exercise intolerance in older obese patients who have heart failure with preserved ejection fraction. J Am Coll Cardiol HF 2018;6:640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddy YNV, Lewis GD, Shah SJ, et al. Characterization of the obese phenotype of heart failure with preserved ejection fraction: a RELAX trial ancillary study. Mayo Clin Proc 2019;94: 1199–209. [DOI] [PubMed] [Google Scholar]
- 40.Reddy YNV, Rikhi A, Obokata M, et al. Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur J Heart Fail 2020. March 9 [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


