Abstract
Objectives:
To provide updated and more detailed pooled IUD expulsion rates and expulsion risk estimates among women with postpartum IUD placement by timing of insertion, delivery type, and IUD type to inform current IUD insertion practices in the United States.
Data sources:
We searched PubMed, Cochrane Library, and ClinicalTrials.gov through June 2019.
Study eligibility criteria:
We included all studies, of any study design, that examined postpartum placement of Copper T380A (copper) or Levonorgestrel (LNG)-containing IUDs that reported counts of expulsion.
Study appraisal and synthesis methods:
We evaluated IUD expulsion among women receiving postpartum IUDs in the ‘immediate’ (within 10 minutes), ‘early inpatient’ (greater than 10 minutes to less than 72 hours), ‘early outpatient’ (72 hours to less than 4 weeks) and interval (4 weeks or greater) time periods after delivery. We assessed study quality using the U.S. Preventive Services Task Force evidence grading system. We calculated pooled absolute rates of partial and complete IUD expulsion separately and estimated adjusted relative risks by the timing of postpartum placement, delivery type and IUD type using log-binomial multivariable regression.
Results:
We identified 48 level I to II-3 studies of poor to good quality that reported a total of 7,661 IUD placements. Complete IUD expulsion rates varied by timing of placement: 10.2% (range 0.0–26.7) for immediate, 13.2% (3.5–46.7) for early inpatient, 0% for early outpatient, and 1.8% (0.0–4.8) for interval placements. Complete IUD expulsion rates also varied by delivery type: 14.8% (range 4.8–43.1) for vaginal and 3.8% (0.0–21.1) for cesarean deliveries. Among immediate postpartum vaginal placements, the expulsion rate for LNG-IUDs was 27.4% (18.8–45.2) and 12.4% (4.8–43.1) for copper IUDs.
Compared with interval placement, immediate and early postpartum placements (inpatient and outpatient combined) were associated with greater risk of complete expulsions (adjusted RR (aRR), 8.33; 95% CI, 4.32–16.08 and aRR, 5.27; 95% CI, 2.56–10.85, respectively). Among immediate postpartum placements, risk of expulsion was greater for placement after vaginal compared with cesarean deliveries (aRR, 4.57; 95% CI, 3.49–5.99). Among immediate placements at the time of vaginal delivery, LNG-IUDs were associated with a greater risk of expulsion compared with copper IUDs (aRR, 1.90; 95% CI, 1.36–2.65).
Conclusion:
While IUD expulsion rates vary by timing of placement, type, and mode of delivery, IUD insertion can take place at any time. Understanding the risk of IUD expulsion at each time period will enable women to make an informed choice about when to initiate an IUD in the postpartum period based on her own goals and preferences.
Keywords: contraception, copper, delivery, early, expulsion, immediate, interval, IUD, Levonorgestrel, postpartum
Condensation:
When IUDs are placed in the postpartum period, expulsion rates vary by timing of placement, delivery type, and IUD type.
Introduction
Intrauterine devices (IUDs) provide highly effective contraception and are commonly placed at an interval postpartum visit typically 4–6 weeks after delivery for women who desire intrauterine contraception. However, the timing of providing postpartum contraception around six weeks after delivery is based on historical precedent, not evidence.1 By six weeks postpartum, more than half of women have resumed intercourse.2–4 Non-breastfeeding women may ovulate as early as 25 days postpartum and at least 30% will have ovulated by 8 weeks.5 Therefore, delaying access to postpartum contraception until six weeks postpartum may increase the risk of rapid repeat pregnancy.
Immediate postpartum IUD placement, within 10 minutes of delivery, is safe and effective as well as convenient for providers and patients.6 Despite the benefits of immediate IUD insertion, there are significant barriers to widespread implementation, including barriers to receiving insurance reimbursement for devices placed in the hospital and lack of standardized provider training on the technique for device placement.7 In addition, there are gaps in knowledge about the risks of expulsion for immediate postpartum IUD placement. For example, the risk of expulsion is greater among women receiving immediate IUDs compared with interval placement8 but it is unclear if risk of expulsion varies by IUD type.9
Efforts are currently in place to increase access to immediate postpartum IUDs, including practice guidance from the American College of Obstetricians and Gynecologists (ACOG) and expanded state Medicaid payment strategies that improve reimbursement for inpatient devices9–11. However, expanding the timeframe when IUDs are placed in the postpartum period beyond the early and interval time periods may allow for increased access to highly effective contraception among postpartum women. The early postpartum period, from 10 minutes to 4 weeks after delivery, provides additional convenient times for women to receive contraception, including IUDs. An IUD can be placed any time before a woman leaves the hospital after delivery or at a postpartum visit within the first few weeks after delivery. The U.S. Medical Eligibility Criteria for Contraceptive Use supports the safety of IUD placement during this early time period.12 Providing IUDs during the early postpartum period, rather than waiting for an interval postpartum placement at greater than 4 weeks may offer additional benefits, including: 1) it is unlikely that women are pregnant at this time; 2) a visit can be co-located with other health visits, such as well-baby visits13; and, 3) timing may offer opportunities to screen women for postpartum depression or to evaluate cesarean delivery incisions. Finally, recent recommendations from the American College of Obstetricians and Gynecologists support contact between women and a maternal health provider within the first three weeks after delivery, so women may increasingly be seen for routine early postpartum visits in the United States.14
A previous meta-analysis of 48 studies suggested that the risk of expulsion may be greater among women receiving early postpartum IUDs between 10 minutes and 4 week postpartum compared with immediate post-placental placement (within 10 minutes) and both were significantly greater than interval placement (≥4 weeks after delivery); it also provided pooled rates of expulsion by placement timing, delivery method and IUD type.8 Given the new ACOG recommendations and interest in more detailed analyses15, we aimed to update the previous analysis by calculating pooled absolute rates of expulsion for immediate placements by IUD type and delivery type, and for early placements, divided into more clinically relevant time periods, and assessed by IUD type. In addition, we focused on IUD types currently used in the United States to better inform patient-centered counseling in the United States.
Objective
The purpose of this updated review and secondary analysis was to calculate more detailed pooled absolute expulsion rates among women with postpartum IUD placement and to estimate relative risk for expulsion in further detail. We sought to calculate updated pooled expulsion rates for immediate postpartum placements (< 10 minutes) and provide new pooled expulsion rates for immediate postpartum placements by delivery type and IUD type. We also sought to calculate pooled expulsion rates for early placements—further presented as early inpatient (greater than 10 minutes to less than 72 hours) and early outpatient (72 hours to less than 4 weeks) placements, and additionally categorized by IUD type. Finally, we aimed to focus our analysis on IUD types currently available in the United States.
Methods
Information sources
We updated the previous search8 in PubMed, Cochrane Library, and ClinicalTrials.gov, published from May 2018 through June 2019 that examined placement of IUDs in the postpartum period.
Search strategy
We searched using the search strategy previously published8:((((“Intrauterine Devices” [Mesh] OR “Intrauterine Devices, Copper” [Mesh] OR “Intrauterine Devices, Medicated” [Mesh] OR ((intrauterine OR intrauterine) AND (device OR system OR contracept*)) OR IUD OR IUC OR IUCD OR IUS OR mirena OR Skyla OR liletta OR paragard OR “Copper T380” OR CuT380 OR “Copper T380a” OR “Cu T380a”) AND (postpartum OR Puerperium*) NOT (“Animals” [Mesh] NOT “Humans” [Mesh])))). We searched Cochrane Library and ClinicalTrials.gov for any published reviews or additional studies including “Postpartum AND IUD.” No methodological filters were used. We hand-searched relevant articles and reviews for additional references. Interim results were not included, only trials with full data were included. We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting.16
Study selection
Our population of interest was women who received a postpartum IUD after a vaginal or cesarean delivery. IUD placement data were stratified by ‘immediate’ (within 10 minutes), ‘early inpatient’ (greater than 10 minutes to less than 72 hours), ‘early outpatient’ (72 hours or greater to less than 4 weeks) and interval (4 weeks or greater). Our primary outcome of interest was complete expulsion of the IUD. We included all primary research studies with any study design, in any language, that reported counts of IUD expulsion after postpartum IUD placement that clearly defined the timing of IUD placement by hours, days or weeks from delivery. Studies that straddled the early and interval timing categories of interest were excluded. We included studies with any length and rate of follow-up.
We only included studies that reported expulsion rates for IUD types currently available in the United States, including copper (CuT380A) and LNG-IUDs in order to focus analyses on current practice in the United States. We excluded studies that evaluated IUDs that were modified from their standard structure. We included studies evaluating IUD placement after vaginal and/or cesarean delivery.
Data extraction
Results from the initial search of the previous publication and the new search were reviewed by two co-authors (S.H.A., and Y.E.) including titles, abstracts and full text articles when necessary, to determine whether the studies met inclusion criteria. Two co-authors (S.H.A., and T.C.J.) independently reviewed newly identified studies and extracted: author, year of publication, country, study design, IUD type, timing of IUD placement, delivery type, length of study follow-up, number of women enrolled or randomized, number of IUDs initially placed, number of women with any follow-up, and counts of expulsion (overall, complete, and partial).
Assessment of risk of bias
Newly identified studies were independently assessed for quality separately by two co-authors (S.H.A., and T.C.J.) according to the U.S. Preventive Services Task Force system17 as previously described.8 To assess study quality, two coauthors independently reviewed each study to evaluate study design and risk of bias, such as potential for selection bias (eg, groups not comparable at baseline for randomized controlled trials), misclassification (eg, outcome of expulsion diagnosed inconsistently by nonblinded health care provider), and confounding (eg, parity and breastfeeding status not collected or adjusted for). Studies were classified as “good”, “fair”, or “poor”, based on the risk of bias assessment. Any discrepancies between authors for selection, abstraction, or risk of bias assessment were resolved through discussion.
Data synthesis
We calculated pooled expulsion rates by dividing the total pooled number of expulsions by the total pooled number of IUD placements within each strata weighted by study sample size. For our primary analysis, we assumed expulsions were “complete” if not otherwise defined. We additionally collected counts of partial expulsion when reported by study authors or if counts were provided for IUDs visualized in the cervix by speculum or ultrasound examination in order to calculate pooled partial expulsion rates.
We calculated pooled expulsion rates for IUDs placed in each of the following time periods: immediate, early inpatient, early outpatient, early inpatient and early outpatient combined, mixed (immediate, early inpatient and early outpatient combined), or interval placement, and by delivery type (cesarean, vaginal, or either cesarean or vaginal [mixed]). We reported the rate of IUD expulsion over each time period and the range of expulsion rates reported. We stratified by IUD type when possible (copper, LNG-IUD, or either copper or LNG-IUD [mixed]), and by length of study follow-up.
We used a log-binomial regression model to estimate adjusted risk ratios (aRRs) of IUD expulsion (for complete and partial expulsions separately) with associated 95% confidence intervals. For IUDs placed in the immediate time period, we reported aRRs by delivery type and IUD type, and adjusted for the following covariates: World Health Organization study region18, study quality, and length of study follow-up. We adjusted for study region due to potential differences in regional practice in which type of clinicians provide IUDs and the technique they use to place them, as well as differences in IUD prevalence in different regions. For IUDs placed in the early inpatient time period, we reported aRRs by IUD type, adjusting for the same three covariates as immediate placements as well as for delivery type. Analyses were completed using SAS 9.4 software (Cary, NC: SAS Institute Inc.).
Results
Study selection
The previous meta-analysis included data from 48 studies. For this analysis, we excluded 4 studies from the previously published meta-analysis that described IUDs not currently available or in use in the United States (CuT200, Cu7)19–22 and 1 that included early postpartum placements but did not report expulsions by our pre-identified timing categories: immediate, early inpatient, early outpatient or interval.23 Therefore, we included 43 studies from the original meta-analysis.
We identified an additional 98 studies in PubMed published between May 1st, 2018 and June 1st, 2019. There were no new studies identified with published data from ClinicalTrials.gov or Cochrane reviews. We removed 3 duplicate studies and screened the titles and abstracts of 95 records. We excluded 84 articles not relevant to our search. We read the full-text of 11 additional articles. We excluded 6 that did not meet inclusion criteria because they did not specify IUD type or timing of IUD placement, or did not provide individual counts for expulsion. We included an additional 5 new studies24–28 in this analysis for a total of 48 studies (Figure 1).
Figure 1. Flow Diagram of publication selection for inclusion into the review.
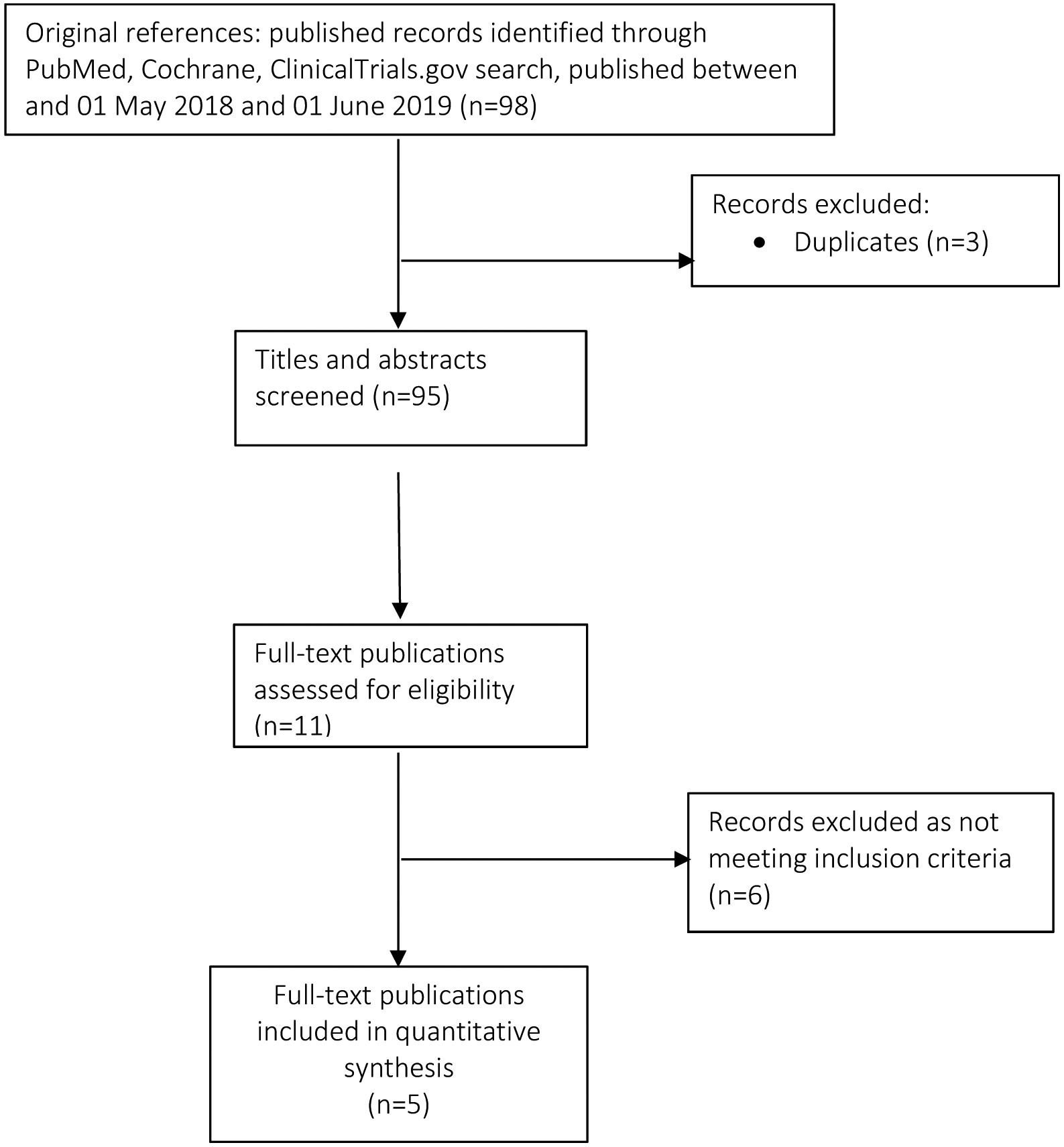
We relied upon the search from a previous systematic review [7] to identify all relevant studies published prior to 1 May 2018
Study characteristics
Studies included were published between 1999 and 2019 (Table 1). The level of evidence ranged from I to II-3. Five studies were rated as good quality24,26,29–31 and the remainder were fair or poor quality. The majority of studies examined IUDs placed in the immediate time period, while 6 examined early inpatient28,32–36 and 3 examined early outpatient placements37–39. Thirteen studies included IUDs placed in the interval time period as a comparison group30–35,37,38,40–44. Twenty-four studies included only copper IUDs24,26,28,29,32,34,41,45–61, 14 studies included only LNG-IUDs25,30,33,35,36,39,40,42–44,62–65, and 10 studies included data on both types of IUDs.27,31,37,38,66–71 Sixteen studies included only IUDs placed at cesarean delivery29,31,40–42,45,48,52,54–56,60,62,63,65,69, 14 studies included only IUDs placed at vaginal delivery24,26,30,32–36,43,57,59,64,68,70, and 18 studies included data on both types of delivery.25,27,28,37–39,44,46,47,49–51,53,58,61,66,67,71 Follow up ranged from 4 weeks to five years and study sample size ranged from 7 to 2,733 women.
Table 1.
Characteristics of Included Studies Reporting Counts of Expulsions among Postpartum Women
| Study Author, Year | Region* | Level of Evidence† | Study Quality‡ | IUD Type§ | Placement Timing∥ |
Delivery Type¶ | Length of Follow-up | Number Enrolled or Randomized | Number of Women with lUDs Placed | Number of Women with IUDs Placed with Follow-up (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Agarwal, 201745 | S.E. Asia | II-3 | Fair | CuT380A | Immediate | Cesarean | 3 months | 50 | 50 | 50 (100) |
| Baldwin, 201637 | N. America | I | Fair | Mixed | Mixed | Mixed | 6 months | 201 | 139 | unknown (66#) |
| Blumenthal, 201824 | S.E. Asia | II-3 | Good | CuT380A | Mixed | Vaginal | 6 weeks | 500 | 496 | 480 (97) |
| Braniff, 201540 | W. Pacific | I | Fair | LNG-IUD | Mixed | Cesarean | 6 months | 48 | 42 | unknown (84#) |
| Bryant, 201332 | Africa | I | Poor | CuT380A | Mixed | Vaginal | 12 weeks | 49 | 28 | 28 (100) |
| Celen, 201129 | E. Mediterranean | II-3 | Good | CuT380A | Immediate | Cesarean | 12 months | 245 | 245 | 245 (100) |
| Chen, 201030 | N. America | I | Good | LNG-IUD | Mixed | Vaginal | 6 months | 124 | 96 | 84 (88) |
| Chen, 201738 | N. America | II-2 | Poor | Mixed | Mixed | Mixed | 6 months | 74 | 74 | 59 (80) |
| Cohen, 201666 | N. America | II-3 | Poor | Mixed | Immediate | Mixed | 12 months | 82 | 82 | 67 (82) |
| Cole, 201925 | N. America | II-3 | Poor | LNG-IUD | Immediate | Mixed | 6 months | 116 | 116 | 87 (75) |
| Colwill, 201846 | N. America | II-2 | Fair | CuT380A | Immediate | Mixed | 6 weeks | 210 | 210 | 169 (80) |
| Dahlke, 201133 | N. America | I | Poor | LNG-IUD | Mixed | Vaginal | 6 months | 53 | 46 | 45 (98) |
| Dias, 201547 | S.E. Asia | II-2 | Poor | CuT380A | Immediate | Mixed | 6 weeks | 91 | 91 | 91 (100) |
| Eggebroten, 201767 | N. America | II-2 | Poor | Mixed | Immediate | Mixed | 6 months | 211 | 211 | 186 (88) |
| Elsedeek, 201262 | E. Mediterranean | II-3 | Fair | LNG-IUD | Immediate | Cesarean | 2 years | 65 | 65 | 62 (95) |
| Elsedeek, 201563 | E. Mediterranean | II-3 | Fair | LNG-IUD | Immediate | Cesarean | 5 years | 80 | 80 | 80 (100) |
| Eroglu, 200634 | E. Mediterranean | II-2 | Fair | CuT380A | Mixed | Vaginal | 12 months | 268 | 268 | 257 (96) |
| Goldthwaite, 201768 | N. America | II-2 | Fair | Mixed | Immediate | Vaginal | 12 weeks | 123 | 123 | 96 (78) |
| Gueye, 201348 | Africa | II-3 | Fair | CuT380A | Immediate | Cesarean | 6 months | 46 | 46 | 39 (85) |
| Gupta, 201461 | S.E. Asia | II-3 | Fair | CuT380A | Immediate | Mixed | 6 months | 100 | 100 | 92 (92) |
| Gurney, 201826 | N. America | II-3 | Good | CuT380A | Immediate | Vaginal | 6 months | 200 | 195 | 162 (83) |
| Hayes, 200764 | N. America | II-3 | Fair | LNG-IUD | Immediate | Vaginal | 10 weeks | 20 | 20 | 16 (80) |
| Heller, 201669 | Europe | II-3 | Fair | Mixed | Immediate | Cesarean | 12 months | 120 | 114 | 99 (87) |
| Hinz, 201927 | N. America | II-3 | Fair | Mixed | Immediate | Mixed | 6 months | 118 | 118 | 114 (97) |
| Hooda, 201649 | S.E. Asia | II-2 | Poor | CuT380A | Immediate | Mixed | 6 weeks | 593 | 593 | 171 (29) |
| Jatlaoui, 201470 | N. America | II-3 | Fair | Mixed | Immediate | Vaginal | 6 months | 99 | 99 | 88 (89) |
| Kumar, 201928 | S.E. Asia | II-3 | Poor | CuT380A | Mixed | Mixed | 12 months | 1200 | 1200 | 844 (70) |
| Kumar, 201450 | S.E. Asia | II-3 | Poor | CuT380A | Mixed | Mixed | 6 weeks | 2733 | 2733 | 1730 (63) |
| Lester, 201541 | Africa | I | Poor | CuT380A | Mixed | Cesarean | 6 months | 68 | 52 | unknown (90#) |
| Letti Muller, 200551 | S. America | II-2 | Fair | CuT380A | Immediate | Mixed | 1 month | 38 | 38 | 37 (97) |
| Levi, 201252 | N. America | II-3 | Fair | CuT380A | Immediate | Cesarean | 6 months | 90 | 90 | 42 (47) |
| Levi, 201531 | N. America | I | Good | Mixed | Mixed | Cesarean | 6 months | 112 | 87 | unknown (88#) |
| Mishra, 201453 | S.E. Asia | II-3 | Poor | CuT380A | Immediate | Mixed | 4–6 weeks | 564 | 564 | 434 (77) |
| Nelson, 200954 | N. America | II-3 | Fair | CuT380A | Immediate | Cesarean | 6 weeks | 7 | 7 | 7 (100) |
| Puzey, 200565 | Africa | II-3 | Poor | LNG-IUD | Immediate | Cesarean | 6 months | 33 | 33 | 20 (61) |
| Ragab, 201555 | E. Mediterranean | II-3 | Fair | CuT380A | Immediate | Cesarean | 12 months | 40 | 40 | 40 (100) |
| Singal, 201454 | S.E. Asia | II-3 | Fair | CuT380A | Immediate | Cesarean | 12 months | 300 | 300 | 300 (100) |
| Singh, 201657 | S.E. Asia | II-3 | Fair | CuT380A | Mixed | Vaginal | 8 weeks | 80 | 80 | 80 (100) |
| Soon, 201843 | N. America | I | Poor | LNG-IUD | Mixed | Vaginal | 6 months | 11 | 8 | 7 (88) |
| Stuart, 201236 | N. America | II-3 | Fair | LNG-IUD | Early | Vaginal | 6 months | 40 | 29 | 27 (93) |
| Stuart, 201535 | N. America | I | Poor | LNG-IUD | Mixed | Vaginal | 6 months | 35 | 31 | unknown (80#) |
| Sucak, 201558 | E. Mediterranean | II-2 | Fair | CuT380A | Immediate | Mixed | 12 months | 160 | 160 | 153 (96) |
| Turok, 201744 | N. America | I | Poor | LNG-IUD | Mixed | Mixed | 8 weeks | 285 | 228 | 214 (94) |
| Unal, 201860 | E. Mediterranean | II-3 | Fair | CuT380A | Immediate | Cesarean | 3 months | 70 | 70 | 68 (97) |
| Whitaker, 201442 | N. America | I | Poor | LNG-IUD | Mixed | Cesarean | 12 months | 42 | 37 | unknown (81#) |
| Woo, 201571 | N. America | II-3 | Poor | Mixed | Immediate | Mixed | 12 months | 76 | 76 | 43 (57) |
| Xu, 199959 | W. Pacific | II-3 | Fair | CuT380A | Immediate | Vaginal | 36 months | 384 | 384 | 381 (99) |
| Zerden, 201739 | N. America | II-3 | Fair | LNG-IUD | Delayed | Mixed | 6 months | 50 | 50 | 43 (86) |
Abbreviations: Cu= copper; IUD= intrauterine device; LNG= levonorgestrel
Based on World Health Organization (WHO) Regions with Region of the Americas separated into North and South American regions.
Level of evidence: I, a randomized, controlled trial; II-2, a cohort or case-controlled study that includes a comparison group; II-3, an uncontrolled descriptive study including case series.
Defined by U.S. Preventive Services Task Force (Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med 2001; 20:21–35)
Mixed=CuT380A and LNG IUD combined
Immediate = ≤10 minutes of placental delivery; Early = >10 minutes to <4 weeks postpartum; Mixed = Immediate and early placements; or, immediate or early placements and interval placements (≥ 4 weeks postpartum)
Mixed=vaginal or cesarean delivery
Number of women with IUDs placed having follow-up not reported, therefore percentage represents number of women with IUD placements among all women randomized.
Complete IUD Expulsion
The pooled rate of complete IUD expulsion varied by timing of placement.
Immediate:
For IUDs placed during the immediate period, within 10 minutes of the placenta, the pooled rate of complete IUD expulsion was 10.2% (range 0.0–26.7%, n=4460) among 39 studies (Table 2).
Table 2.
Pooled Complete Expulsion Rates by Study Follow-up Length
| All Studies | Study follow-up: longer than 6 months | Study follow-up: 3 – 6 months | Study follow-up: Less than 3 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placement Timing, Delivery Type, and IUD Type | Number of Studies† | Number of Women with IUDs placed with Follow-up | Complete Expulsion Rate (% Range among studies)* | Number of Studies† | Number of Women with IUDs placed with Follow-up | Complete Expulsion Rate (% Range among studies)* | Number of Studies† | Number of Women with IUDs placed with Follow-up | Complete Expulsion Rate (% Range among studies)* | Number of Studies† | Number of Women with IUDs placed with Follow-up | Complete Expulsion Rate (% Range among studies)* |
| Total | 48 | 7661 | 7.8 (0.0–37.0) | 13 | 2539 | 8.7 (0.0–25.4) | 22 | 1569 | 10.3 (0.0–37.0) | 13 | 3553 | 6.1 (0.0–19.8) |
| Interval Timing‡ | 13 | 502 | 1.8 (0.0–4.8) | 2 | 122 | 4.1 (0.0–4.8) | 9 | 262 | 0.8 (0.0–2.9) | 2 | 118 | 1.7 (0.0–2.0) |
| Mixed Timing‡$ | 3 | 2290 | 4.4 (3.6–7.5) | 0 | n/a | n/a | 0 | n/a | n/a | 3 | 2290 | 4.4 (3.6–7.5) |
| Early Timing‡ | 9 | 409 | 8.8 (0.0–46.7) | 2 | 204 | 6.9 (3.5–24.2) | 6 | 193 | 10.9 (0.0–46.7) | 1 | 12 | 8.3 |
| Inpatient | 6 | 273 | 13.2 (3.5–46.7) | 2 | 204 | 6.9 (3.5–24.2) | 3 | 57 | 36.8 (26.7–46.7) | 1 | 12 | 8.3 |
| ■ Copper-IUD | 3 | 216 | 6.9 (3.5–24.2) | 2 | 204 | 6.9 (3.5–24.2) | 0 | n/a | n/a | 1 | 12 | 8.3 |
| ■ LNG-IUD | 3 | 57 | 36.8 (26.7–46.7) | 0 | n/a | n/a | 3 | 57 | 36.8 (26,7–46.7) | 0 | n/a | n/a |
| Outpatient | 3 | 136 | 0.0 | 0 | n/a | n/a | 3 | 136 | 0.0 | 0 | n/a | n/a |
| Immediate Timing‡ | 39 | 4460 | 10.2 (0.0–26.7) | 13 | 2213 | 9.1 (0.0–25.4) | 17 | 1114 | 12.4 (0.0–26.7) | 9 | 1133 | 10.1 (0.0–21.4) |
| Vaginal deliveries | 18 | 1885 | 14.8 (4.8–43.1) | 4 | 1026 | 13.0 (4.8–23.5) | 8 | 479 | 20.3 (8.0–43.1) | 6 | 380 | 12.6 (7.8–22.2) |
| ■ Copper-IUD | 13 | 1586 | 12.4 (4.8–37.5) | 4 | 1026 | 13.0 (4.8–23.5) | 4 | 251 | 13.2 (8.0–37.5) | 5 | 309 | 9.7 (7.8–22.2) |
| ■ LNG-IUD | 8 | 299 | 27.4 (18.8–45.2) | 0 | n/a | n/a | 6 | 228 | 28.1 (19.4–45.2) | 2 | 71 | 25.4 (18.8–27.3) |
| Cesarean deliveries | 25 | 1733 | 3.8 (0.0–21.1) | 9 | 1077 | 3.9 (0.0–21.1) | 11 | 449 | 4.5 (0.0 −11.8) | 5 | 207 | 1.5 (0.0–3.2) |
| ■ Copper-IUD | 17 | 1320 | 3.8 (0.0 −15.0) | 5 | 817 | 4.0 (2.0–15.0) | 7 | 296 | 4.7 (0.0–11.8) | 5 | 207 | 1.5 (0.0–3.2) |
| ■ LNG-IUD | 7 | 261 | 2.3 (0.0–21.1) | 3 | 161 | 2.5 (0.0–21.1) | 4 | 100 | 2.0 (0.0–4.2) | 0 | n/a | n/a |
| ■ Copper or LNG | 2 | 152 | 5.9 (5.1–7.6) | 1 | 99 | 5.1 | 1 | 53 | 7.6 | 0 | n/a | n/a |
| Vaginal or Cesarean& | 5 | 842 | 13.1 (9.0–25.4) | 2 | 110 | 23.6 (20.9–25.4) | 1 | 186 | 11.3 | 2 | 546 | 11.5 (9.0–21.4) |
Abbreviations: IUD= intrauterine device; LNG= levonorgestrel; n/a=not applicable
Among women with IUDs placed with any follow-up
Some studies included and reported more than one category
Immediate = ≤10 minutes of placental delivery; Early = >10 minutes to <4 weeks postpartum; Mixed =Immediate and early combined; Interval= ≥4 weeks postpartum
Two studies did not classify IUD placements between early and immediate timing
Seven studies did not specify delivery types
Early:
For IUDs placed during the early postpartum period (combined inpatient and outpatient), from greater than 10 minutes to less than 4 weeks, the pooled complete expulsion rate was 8.8% (range 0.0–46.7%, n=409) among 9 studies. Six studies contributed to the early inpatient pooled complete expulsion rate of 13.2% (range 3.5–46.7%, n=273). Three studies including 216 copper IUD placements contributed to a pooled complete expulsion rate of 6.9% (range 3.524.2%). Three studies including 57 LNG-IUD placements contributed to a pooled complete expulsion rate of 36.8% (range 26.7–46.7%).
For IUDs placed during the early outpatient period, greater than 72 hours but less than 4 weeks postpartum, there were no complete IUD expulsions among 136 woman in 3 studies. All early outpatient IUD placements occurred between 13 days and 28 days postpartum.
Interval:
For IUDs placed during the interval time period, the risk of complete expulsion was 1.8% (range, 0.0–4.8%, n=502) among 13 studies.
The pooled rate of complete IUD expulsion varied by type of IUD when placed after vaginal delivery.
Among women with IUDs placed immediately after vaginal deliveries, the pooled complete expulsion rates varied between women using LNG-IUDs, 27.4% (range 18.8–45.2%, n=299) among 8 studies, and 13 studies including women using copper IUDs 12.4% (range 4.8–37.5%, n=1586). However, among women with IUDs placed at the time of cesarean delivery, the expulsion rates were generally lower than after vaginal deliveries and were similar between women using LNG-IUDs and copper IUDs, [2.3% (range 0.0–21.1%, n=261, 7 studies) and 3.8% (range 0.0–15.0%, n=1320, 17 studies), respectively].
The adjusted relative risk of complete IUD expulsion varied by the timing of postpartum placement, delivery type and IUD type.
Timing of Delivery:
Compared with interval placement, immediate and early postpartum (combined inpatient and outpatient) placements were associated with increased risk of complete expulsion (aRR, 8.33; 95% CI, 4.32–16.08 and aRR, 5.27; 95% CI, 2.56–10.85, respectively) (Table 3). The risk of expulsion among early inpatient placements compared with interval placements did not reach statistical significance (aRR, 9.51; 95% CI, 0.63–19.52).
Table 3.
Adjusted Risk Ratio (95% CI)* for IUD Expulsion among Postpartum Women by Placement Timing
| Placement Timing | Complete Expulsions† aRR (95% CI)* |
|---|---|
| Interval Timing§ | 1.00 (Ref) |
| Early or Immediate Timing# | 4.49 (2.22 – 9.09) |
| Early Timing§ | 5.27 (2.56 – 10.85) |
| Early Inpatient | 9.51 (0.63–19.52) |
| Early Outpatient | n/a |
| Immediate Timing§ | 8.33 (4.32 – 16.08) |
Abbreviations: aRR = adjusted risk ratios; CI= confidence interval; n/a=not applicable
Adjusted for IUD type, delivery type, placement timing, study region, study quality and length of follow-up
Among women with IUDs placed with any follow-up from 48 studies
Immediate = within 10 minutes of placental delivery; Early = more than 10 minutes to <4 weeks postpartum; Interval = ≥4 weeks postpartum
Studies did not classify IUD placements between early and immediate timing
Delivery Type:
Among immediate postpartum placements, risk of expulsion was greater for placement after vaginal compared with cesarean deliveries (aRR, 4.57; 95% CI, 3.49–5.99).
IUD Type:
Among women initiating IUDs in the immediate postpartum time period at the time of vaginal delivery, women using LNG-IUDs had a greater risk of expulsion compared with copper IUDs (aRR, 1.90; 95% CI, 1.36–2.65). No statistically significant difference in risk of IUD expulsion between IUD types placed during cesarean delivery was demonstrated (aRR, 0.52; 95% CI 0.22–1.22) (Table 4).
Table 4.
Adjusted Risk Ratio (95% CI)* for IUD Expulsion among Postpartum Women by Placement Timing, Delivery Type, and IUD Type
| Placement Timing, Delivery Type, and IUD Type | Complete Expulsions† aRR (95% CI)* |
|---|---|
| Early Timing§ | |
| IUD type (Inpatient only) | |
| Copper | 1.00 (Ref) |
| LNG | 1.91 (0.96 – 3.78) |
| Immediate Timing§ | |
| Delivery type | |
| Cesarean | 1.00 (Ref) |
| Vaginal | 4.57 (3.49 – 5.99) |
| Cesarean or Vaginal | 4.03 (2.76 – 5.86) |
| IUD type (vaginal deliveries) | |
| Copper | 1.00 (Ref) |
| LNG | 1.90 (1.36 – 2.65) |
| IUD type (cesarean deliveries) | |
| Copper | 1.00 (Ref) |
| LNG | 0.52 (0.22 – 1.22) |
Abbreviations: aRR = adjusted risk ratios; CI= confidence interval; IUD= intrauterine device; LNG= levonorgestrel
Adjusted for IUD type, delivery type, placement timing, study region, study quality and length of follow-up
Among women with IUDs placed with any follow-up from 48 studies
Immediate = within 10 minutes of placental delivery; Early = more than 10 minutes to <4 weeks postpartum; Interval = ≥4 weeks postpartum
Studies did not classify IUD placements between early and immediate timing
Partial Expulsion
When assessing partial IUD expulsions, for IUDs placed during the immediate, early (combined inpatient and outpatient), and interval time periods, the pooled partial expulsion rates were 6.3% (0.0–37.3%, n=2111, 21 studies), 13.8% (range 0.0–66.7%, n=196, 6 studies) and 1.9% (0.05.6%, n=319, 8 studies) respectively (data not shown). When early placement was further categorized into the early inpatient period and outpatient postpartum time periods, the pooled partial expulsion rates were 26.4% (range 0.0–66.7%, n=87) among 4 studies and 3.7% (range 3.0–4.7%, n=109) among 2 studies, respectively. Expulsion rates after early inpatient placement varied by IUD type with pooled partial expulsion rates of 48.9% (range 0.0–66.7%, n=45) among 2 studies of women receiving copper IUDs, and 2.4% (range 0.0–3.7%, n=109) among 2 studies of women receiving LNG-IUDs. Compared with interval placement, immediate and early postpartum IUD placements were associated with increased risks of partial expulsion (aRR, 4.56; 95% CI, 1.98–10.94 and aRR, 13.34; 95% CI, 5.82–30.57 respectively) (data not shown).
Comment
This systematic review provides updated estimates of expulsion rates among women with postpartum IUD placement by timing of insertion, further described by delivery type, and/or IUD type when IUDs are placed within the immediate (< 10 minutes) ‘early inpatient’ (greater than 10 minutes to less than 72 hours) and ‘early outpatient’ (72 hours to less than 4 weeks) postpartum time periods compared with interval placements (>4 weeks).
The previous meta-analysis grouped all IUDs occurring between immediate and interval insertions as early postpartum IUD insertions, from greater than 10 minutes to less than 4 weeks after delivery, based on timing categories in the U.S. Medical Eligibility Criteria for Contraceptive Use.8 In this analysis, we further estimated the risk of early expulsion specifically in the time before a woman typically leaves the hospital (early inpatient) or at a follow up visit within 4 weeks of delivery (early outpatient). In addition, we included only IUDs currently available in the United States. We provided new pooled rates of IUD expulsion when IUDs are placed in the immediate postpartum time period, stratified by both IUD and delivery type, and when placed in the early inpatient and early outpatient time periods, stratified by IUD type.
Similar to a previous analysis, we found that the risks of expulsion after immediate and early postpartum IUD insertions were greater than interval insertion. The benefits of immediate and early IUD insertion, however, may outweigh the increased risk of expulsion if uptake, continuation or satisfaction are improved with earlier insertion. In addition, we found no complete expulsions among women with IUDs placed in the early outpatient time period between 2–4 weeks postpartum and the pooled rate of partial expulsion was low (3.7%). The precision of our pooled rates of IUD expulsion after early postpartum placement was limited by a small number of studies with sample sizes ranging from 12 to 171. These data suggest that the risk of IUD expulsion may be lower in the early outpatient time period compared with the early inpatient time period, and IUD placement during the early outpatient postpartum period warrants further study.
This analysis has many strengths. In pooling the counts of IUD expulsion, we are able to include IUD placements from over 7,500 postpartum women in the analysis, with more than 400 women having early postpartum IUD placements using currently available IUDs. In addition, we were able to adjust for length of follow up and study quality. Another strength of this analysis is that we were able to stratify the analysis of early postpartum IUD insertion to estimate the risk of expulsion specifically in the time shortly after birth before a woman leaves the hospital (early inpatient) or at a follow up visit within 4 weeks of delivery (early outpatient). In addition, we were able to estimate the risk of IUD expulsion after immediate postpartum placement by IUD and delivery type.
This analysis has several limitations. We were limited by the small number of studies reporting IUD expulsion after placement in the early postpartum period. With no expulsions reported after early outpatient IUD placement, we were unable to compare the risk of IUD expulsion with interval placement or with early inpatient placement. In addition, we were unable to calculate the pooled rates of IUD expulsion by delivery type when IUDs were placed in the early postpartum time period because these studies generally did not report expulsions by delivery type. Although we defined the early outpatient time period as 72 hours to less than 4 weeks postpartum, there were no studies evaluating the risk of IUD expulsion when IUDs were placed 72 hours to 12 days postpartum, so little is known about expulsion risk when IUDs are placed within that period. Further investigation is needed to assess for differences in risk of IUD expulsion within the early outpatient period. In addition, the definition and diagnosis of expulsion were not standardized across studies and there was significant variation particularly in the definition and diagnosis of partial IUD expulsion. Because we assumed expulsions were “complete” if not otherwise defined, it is possible we overestimated the rate of complete IUD expulsion for some studies. As with the previous review, there were differential lengths of follow-up making comparisons across studies challenging. Finally, as in the previous review, we were unable to adjust for potential confounders that were not reliably reported by studies including training and experience of providers, use of ultrasound, and insertion technique.
Given the large number of participants from studies conducted all over the world, external validity of these results is likely good; however, as we only included studies on copper and LNG-IUDs currently available in the United States, these results are not generalizable to other IUDs types.
The American College of Obstetricians and Gynecologists recommends that women be counseled about the increased expulsion risk with postpartum IUD insertion,10 but the reported rates of IUD expulsion when IUDs are placed specifically within the early inpatient and outpatient time periods, and the rates of IUD insertion by IUD and delivery type, vary between studies and these studies are limited by small sample size. The data presented here can be used by providers in counseling women about expulsion risk for IUDs placed at different time periods postpartum.
Providing women with access to long acting reversible contraception, including IUDs, at many times during the postpartum period can help women achieve their reproductive goals and prevent unintended pregnancy. In addition to interval placement 4 or more weeks after delivery, IUDs can be placed immediately after delivery, in the inpatient early postpartum period before a woman leaves the birth facility, and in the early outpatient time period if a woman returns for an early postpartum visit within 4 weeks of delivery. Understanding the risk of IUD expulsion at each time period will enable women to make an informed choice about when to initiate an IUD in the postpartum period based on her own goals and preferences.
AJOG at a Glance:
A. Why was the review conducted?
This review was conducted to provide more detailed estimates of expulsion risk among women with intrauterine device (IUD) placement in the postpartum period than previously published, further describing pooled rates and risk estimates by timing of insertion, IUD type and delivery type to better inform current IUD practices in the United States.
B. What are the key findings?
This analysis is consistent with previous findings that the risk of IUD expulsion is greater when IUDs are placed in the immediate and early postpartum periods compared with placement at an interval postpartum visit (4 weeks or greater).
Novel analyses demonstrate the risk of IUD expulsion after early inpatient postpartum placements is similar to immediate postpartum placements and greater than early outpatient or interval placements.
Three studies of early outpatient postpartum placements between 13–24 days postpartum demonstrated 0 expulsions after 136 placements with 6 months of follow-up.
New analyses comparing IUD types highlight that the risk of expulsion is greater when initiating Levonorgestrel (LNG)-containing IUDs compared with copper IUDs in the early inpatient postpartum period, and in the immediate postpartum period with vaginal delivery. IUD type was not associated with an increased risk of expulsion at the time of cesarean delivery for any time period.
C. What does this review add to what is already known?
This review provides new pooled rates of IUD expulsion after postpartum placement by timing of placement, delivery type, and IUD type with a focus on IUDs that are currently available in the United States to better inform patient-centered counseling in the United States.
This review provides new pooled rates of IUD expulsion and expulsion risk estimates when IUDs are placed in the early inpatient postpartum time period between 10 minutes and 72 hours after delivery and the early outpatient time period (from 72 hours to less than 4 weeks).
Acknowledgments
Dr. Averbach is supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) physician scientist career development award (K12 HD001259).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institutes of Health.
The authors report no conflict of interest.
Citations
- 1.Speroff L, Mishell DR. The postpartum visit: it’s time for a change in order to optimally initiate contraception. Contraception. 2008;78(2):90–98. [DOI] [PubMed] [Google Scholar]
- 2.Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(4):263–267. [DOI] [PubMed] [Google Scholar]
- 3.Robson KM, Brant HA, Kumar R. Maternal sexuality during first pregnancy and after childbirth. Br J Obstet Gynaecol. 1981;88(9):882–889. [DOI] [PubMed] [Google Scholar]
- 4.Egbuonu I, Ezechukwu CC, Chukwuka JO, Ikechebelu JI. Breast-feeding, return of menses, sexual activity and contraceptive practices among mothers in the first six months of lactation in Onitsha, South Eastern Nigeria. J Obstet Gynaecol. 2005;25(5):500–503. [DOI] [PubMed] [Google Scholar]
- 5.Gray RH, Campbell OM, Zacur HA, Labbok MH, MacRae SL. Postpartum return of ovarian activity in nonbreastfeeding women monitored by urinary assays. J Clin Endocrinol Metab. 1987;64(4):645–650. [DOI] [PubMed] [Google Scholar]
- 6.Committee Opinion No. 670 Summary: Immediate Postpartum Long-Acting Reversible Contraception. Obstet Gynecol. 2016;128(2):422–423. [DOI] [PubMed] [Google Scholar]
- 7.Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jatlaoui TC, Whiteman MK, Jeng G, et al. Intrauterine Device Expulsion After Postpartum Placement: A Systematic Review and Meta-analysis. Obstet Gynecol. 2018;132(4):895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitaker AK, Chen BA. Society of Family Planning Guidelines: Postplacental insertion of intrauterine devices. Contraception. 2018;97(1):2–13. [DOI] [PubMed] [Google Scholar]
- 10.Long-acting reversible contraception: implants and intrauterine devices. Practice Bulletin No. 186. American College of Obstetricians and Gynecologists. Obstet Gynecol 2017;130:e251–69. [DOI] [PubMed] [Google Scholar]
- 11.Department of Health and Human Services. CMCS informational Bulletin. available at: https://www.medicaid.gov/federal-policy-guidance/downloads/cib040816.pdf accessed October 14, 2019.
- 12.Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(3):1–103. [DOI] [PubMed] [Google Scholar]
- 13.Caskey R, Stumbras K, Rankin K, Osta A, Haider S, Handler A. A novel approach to postpartum contraception: a pilot project of Pediatricians’ role during the well-baby visit. Contracept Reprod Med. 2016;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKinney J, Keyser L, Clinton S, Pagliano C. ACOG Committee Opinion No. 736: Optimizing Postpartum Care. Obstet Gynecol. 2018;132(3):784–785. [DOI] [PubMed] [Google Scholar]
- 15.Blumenthal PD, Lerma K. Intrauterine Device Expulsion After Postpartum Placement: A Systematic Review and Meta-Analysis. Obstet Gynecol. 2019;133(3):582. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3 Suppl):21–35. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. https://www.who.int/healthinfo/global_burden_disease/definition_regions/en/. Accessed July 10, 2019.
- 19.Laes E, Lehtovirta P, Weintraub D, Pyörälä T, Luukkainen T. Early puerperal insertions of copper-T-200. Contraception. 1975;11(3):289–295. [DOI] [PubMed] [Google Scholar]
- 20.Lavin P, Waszak C, Bravo C. Preliminary report on a postpartum CuT 200 study, Santiago, Chile. Int J Gynaecol Obstet. 1983;21(1):71–75. [DOI] [PubMed] [Google Scholar]
- 21.Newton J, Harper M, Chan KK. Immediate post-placental insertion of intrauterine contraceptive devices. Lancet. 1977;2(8032):272–274. [DOI] [PubMed] [Google Scholar]
- 22.Shukla M, Qureshi S, Chandrawati. Post-placental intrauterine device insertion--a five year experience at a tertiary care centre in north India. Indian J Med Res. 2012;136(3):432–435. [PMC free article] [PubMed] [Google Scholar]
- 23.Bonilla Rosales F, Aguilar Zamudio ME, Cázares Montero MeL, Hernández Ortiz ME, Luna Ruiz MA. [Factors for expulsion of intrauterine device Tcu380A applied immediately postpartum and after a delayed period]. Rev Med Inst Mex Seguro Soc. 2005;43(1):5–10. [PubMed] [Google Scholar]
- 24.Blumenthal PD, Lerma K, Bhamrah R, Singh S, Dedicated PIWG. Comparative safety and efficacy of a dedicated postpartum IUD inserter versus forceps for immediate postpartum IUD insertion: a randomized trial. Contraception. 2018;98(3):215–219. [DOI] [PubMed] [Google Scholar]
- 25.Cole M, Thomas S, Mercer BM, Arora KS. Impact of training level on postplacental levonorgestrel 52 mg intrauterine device expulsion. Contraception. 2019;99(2):94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurney EP, Sonalkar S, McAllister A, Sammel MD, Schreiber CA. Six-month expulsion of postplacental copper intrauterine devices placed after vaginal delivery. Am J Obstet Gynecol. 2018;219(2):183.e181–183.e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinz EK, Murthy A, Wang B, Ryan N, Ades V. A prospective cohort study comparing expulsion after postplacental insertion: the levonorgestrel versus the copper intrauterine device. Contraception. 2019;100(2):101–105. [DOI] [PubMed] [Google Scholar]
- 28.Kumar S, Srivastava A, Sharma S, et al. One-year continuation of postpartum intrauterine contraceptive device: findings from a retrospective cohort study in India. Contraception. 2019;99(4):212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Çelen Ş, Sucak A, Yıldız Y, Danışman N. Immediate postplacental insertion of an intrauterine contraceptive device during cesarean section. Contraception. 2011;84(3): 240–243. [DOI] [PubMed] [Google Scholar]
- 30.Chen BA, Reeves MF, Hayes JL, Hohmann HL, Perriera LK, Creinin MD. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116(5):1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levi EE, Stuart GS, Zerden ML, Garrett JM, Bryant AG. Intrauterine Device Placement During Cesarean Delivery and Continued Use 6 Months Postpartum: A Randomized Controlled Trial. Obstet Gynecol. 2015;126(1):5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bryant AG, Kamanga G, Stuart GS, Haddad LB, Meguid T, Mhango C. Immediate postpartum versus 6-week postpartum intrauterine device insertion: a feasibility study of a randomized controlled trial. Afr J Reprod Health. 2013;17(2):72–79. [PubMed] [Google Scholar]
- 33.Dahlke JD, Terpstra ER, Ramseyer AM, Busch JM, Rieg T, Magann EF. Postpartum insertion of levonorgestrel--intrauterine system at three time periods: a prospective randomized pilot study. Contraception. 2011; 84(3):244–248. [DOI] [PubMed] [Google Scholar]
- 34.Eroğlu K, Akkuzu G, Vural G, et al. Comparison of efficacy and complications of IUD insertion in immediate postplacental/early postpartum period with interval period: 1 year follow-up. Contraception. 2006;74(5):376–381. [DOI] [PubMed] [Google Scholar]
- 35.Stuart GS, Lesko CR, Stuebe AM, Bryant AG, Levi EE, Danvers AI. A randomized trial of levonorgestrel intrauterine system insertion 6 to 48 h compared to 6 weeks after vaginal delivery; lessons learned. Contraception. 2015;91(4):284–288. [DOI] [PubMed] [Google Scholar]
- 36.Stuart GS, Bryant AG, O’Neill E, Doherty IA. Feasibility of postpartum placement of the levonorgestrel intrauterine system more than 6 h after vaginal birth. Contraception. 2012;85(4):359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baldwin MK, Edelman AB, Lim JY, Nichols MD, Bednarek PH, Jensen JT. Intrauterine device placement at 3 versus 6 weeks postpartum: a randomized trial. Contraception. 2016;93(4):356–363. [DOI] [PubMed] [Google Scholar]
- 38.Chen MJ, Hou MY, Hsia JK, Cansino CD, Melo J, Creinin MD. Long-Acting Reversible Contraception Initiation With a 2- to 3-Week Compared With a 6-Week Postpartum Visit. Obstet Gynecol. 2017;130(4):788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zerden ML, Stuart GS, Charm S, Bryant A, Garrett J, Morse J. Two-week postpartum intrauterine contraception insertion: a study of feasibility, patient acceptability and short-term outcomes. Contraception. 2017;95(1):65–70. [DOI] [PubMed] [Google Scholar]
- 40.Braniff K, Gomez E, Muller R. A randomised clinical trial to assess satisfaction with the levonorgestrel-releasing intrauterine system inserted at caesarean section compared to postpartum placement. Aust N Z J Obstet Gynaecol. 2015;55(3):279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91(3):198–203. [DOI] [PubMed] [Google Scholar]
- 42.Whitaker AK, Endres LK, Mistretta SQ, Gilliam ML. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89(6):534–539. [DOI] [PubMed] [Google Scholar]
- 43.Soon R, McGuire K, Salcedo J, Kaneshiro B. Immediate Versus Delayed Insertion of the Levonorgestrel Intrauterine Device in Postpartum Adolescents: A Randomized Pilot Study. Hawaii J Med Public Health. 2018;77(3):60–65. [PMC free article] [PubMed] [Google Scholar]
- 44.Turok DK, Leeman L, Sanders JN, et al. Immediate postpartum levonorgestrel intrauterine device insertion and breast-feeding outcomes: a noninferiority randomized controlled trial. Am J Obstet Gynecol. 2017;217(6):665 e661–665 e668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agarwal K, Dewan R, Mittal P, Aggarwal A. Visibility of Strings After Postplacental Intracesarean Insertion of CuT380A and Cu375 Intrauterine Contraceptive Device: A Randomized Comparative Study. J Obstet Gynaecol India. 2017;67(5):324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colwill AC, Schreiber CA, Sammel MD, Sonalkar S. Six-week retention after postplacental copper intrauterine device placement. Contraception. 2018;97(3):215–218. [DOI] [PubMed] [Google Scholar]
- 47.Dias T, Abeykoon S, Kumarasiri S, Gunawardena C, Padeniya T, D’Antonio F. Use of ultrasound in predicting success of intrauterine contraceptive device insertion immediately after delivery. Ultrasound Obstet Gynecol. 2015;46(1):104–108. [DOI] [PubMed] [Google Scholar]
- 48.Gueye M, Gaye YF, Diouf AA, et al. [Trancesarean intra-uterine device. Pilot study performed at Dakar teaching hospital]. J Gynecol Obstet Biol Reprod (Paris). 2013;42(6):585–590. [DOI] [PubMed] [Google Scholar]
- 49.Hooda R, Mann S, Nanda S, Gupta A, More H, Bhutani J. Immediate Postpartum Intrauterine Contraceptive Device Insertions in Caesarean and Vaginal Deliveries: A Comparative Study of Follow-Up Outcomes. Int J Reprod Med. 2016;2016:7695847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar S, Sethi R, Balasubramaniam S, et al. Women’s experience with postpartum intrauterine contraceptive device use in India. Reprod Health. 2014;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Letti Müller AL, Lopes Ramos JG, Martins-Costa SH, et al. Transvaginal ultrasonographic assessment of the expulsion rate of intrauterine devices inserted in the immediate postpartum period: a pilot study. Contraception. 2005;72(3):192–195. [DOI] [PubMed] [Google Scholar]
- 52.Levi E, Cantillo E, Ades V, Banks E, Murthy A. Immediate postplacental IUD insertion at cesarean delivery: a prospective cohort study. Contraception. 2012;86(2):102–105. [DOI] [PubMed] [Google Scholar]
- 53.Mishra S Evaluation of Safety, Efficacy, and Expulsion of Post-Placental and Intra-Cesarean Insertion of Intrauterine Contraceptive Devices (PPIUCD). J Obstet Gynaecol India. 2014;64(5):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelson AL, Chen S, Eden R. Intraoperative placement of the Copper T-380 intrauterine devices in women undergoing elective cesarean delivery: a pilot study. Contraception. 2009;80(1):81–83. [DOI] [PubMed] [Google Scholar]
- 55.Ragab A, Hamed HO, Alsammani MA, et al. Expulsion of Nova-T380, Multiload 375, and Copper-T380A contraceptive devices inserted during cesarean delivery. Int J Gynaecol Obstet. 2015;130(2):174–178. [DOI] [PubMed] [Google Scholar]
- 56.Singal S, Bharti R, Dewan R, et al. Clinical Outcome of Postplacental Copper T 380A Insertion in Women Delivering by Caesarean Section. J Clin Diagn Res. 2014;8(9):OC01–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh S, Das V, Agarwal A, et al. A Dedicated Postpartum Intrauterine Device Inserter: Pilot Experience and Proof of Concept. Glob Health Sci Pract. 2016;4(1):132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sucak A, Ozcan S, Çelen Ş, Çağlar T, Göksu G, Danışman N. Immediate postplacental insertion of a copper intrauterine device: a pilot study to evaluate expulsion rate by mode of delivery. BMC Pregnancy Childbirth. 2015;15:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu J, Yang X, Gu X, et al. Comparison between two techniques used in immediate postplacental insertion of TCu 380A intrauterine device: 36-month follow-up. Reprod Contracept. 1999;10(3):156–162. [PubMed] [Google Scholar]
- 60.Unal C, Eser A, Tozkir E, Wildemeersch D. Comparison of expulsions following intracesarean placement of an innovative frameless copper-releasing IUD (Gyn-CS®) versus the TCu380A: A randomized trial. Contraception. 2018. [DOI] [PubMed] [Google Scholar]
- 61.Gupta S, Malik S, Sinha R, Shyamsunder S, Mittal MK. Association of the Position of the Copper T 380A as Determined by the Ultrasonography Following its Insertion in the Immediate Postpartum Period with the Subsequent Complications: An Observational Study. J Obstet Gynaecol India. 2014;64(5):349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elsedeek MS. Puerperal and menstrual bleeding patterns with different types of contraceptive device fitted during elective cesarean delivery. Int J Gynaecol Obstet. 2012;116(1):31–34. [DOI] [PubMed] [Google Scholar]
- 63.Elsedeek MS. Five-year follow-up of two types of contraceptive device fitted during elective cesarean delivery. Int J Gynaecol Obstet. 2015;130(2):179–182. [DOI] [PubMed] [Google Scholar]
- 64.Hayes JL, Cwiak C, Goedken P, Zieman M. A pilot clinical trial of ultrasound-guided postplacental insertion of a levonorgestrel intrauterine device. Contraception. 2007;76(4):292–296. [DOI] [PubMed] [Google Scholar]
- 65.Puzey M Mirena at caesarean section. Eur J Contracept Reprod Health Care. 2005;10(3):164–167. [DOI] [PubMed] [Google Scholar]
- 66.Cohen R, Sheeder J, Arango N, Teal SB, Tocce K. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93(2):178–183. [DOI] [PubMed] [Google Scholar]
- 67.Eggebroten JL, Sanders JN, Turok DK. Immediate postpartum intrauterine device and implant program outcomes: a prospective analysis. Am J Obstet Gynecol. 2017;217(1):51 e51–51 e57. [DOI] [PubMed] [Google Scholar]
- 68.Goldthwaite LM, Sheeder J, Hyer J, Tocce K, Teal SB. Postplacental intrauterine device expulsion by 12 weeks: a prospective cohort study. Am J Obstet Gynecol. 2017;217(6):674.e671–674.e678. [DOI] [PubMed] [Google Scholar]
- 69.Heller R, Cameron S, Briggs R, Forson N, Glasier A. Postpartum contraception: a missed opportunity to prevent unintended pregnancy and short inter-pregnancy intervals. J Fam Plann Reprod Health Care. 2016;42(2):93–98. [DOI] [PubMed] [Google Scholar]
- 70.Jatlaoui TC, Marcus M, Jamieson DJ, Goedken P, Cwiak C. Postplacental intrauterine device insertion at a teaching hospital. Contraception. 2014;89(6):528–533. [DOI] [PubMed] [Google Scholar]
- 71.Woo I, Seifert S, Hendricks D, Jamshidi RM, Burke AE, Fox MC. Six-month and 1-year continuation rates following postpartum insertion of implants and intrauterine devices. Contraception. 2015;92(6):532–535. [DOI] [PubMed] [Google Scholar]


