Abstract
Background:
Bilateral cardiac sympathetic denervation (BCSD) is an effective therapy for ventricular arrhythmias (VAs) in cardiomyopathies (CMP). Following BCSD, residual autonomic (ANS) function is unknown.
Objective:
To assess ANS responses in CMP patients before and after BCSD compared to demographically matched healthy controls.
Methods:
Patients with CMP undergoing BCSD and matched healthy controls were recruited. Non-invasive measures [finger cuff beat-to-beat blood pressure (BP), electrocardiography, palmar electrodermal activity (EDA), and finger pulse volume (FPV)] were obtained at rest and during autonomic stressors (posture change, handgrip and mental stress). Maximal as well as specific responses to stressor were compared.
Results:
Eighteen CMP pts (54±14 years, 16 males, LVEF 36±14%) with refractory VAs and 8 matched healthy controls were studied; 9 CMP patients underwent testing before and after (median 28 days) BCSD, with comparable ongoing medication. Before BCSD, CMP patients (n=13) had lower resting systolic BP (SBP) and FPV than healthy controls (p<0.01). Maximal FPV and SBP reflex responses, expressed as % change were similar, while diastolic BP (DBP), mean BP (MBP) and EDA responses were blunted. Following BCSD resting measurements were unchanged relative to pre-surgical baseline (n=9). EDA responses to stressors were abolished confirming BCSD, while maximal FPV and BP responses were preserved. DBP, MBP and FPV responses to orthostatic challenge pointed toward a better tolerance of active standing after BCSD as compared to before. Responses to other stressors remained unchanged.
Conclusions:
CMP patients with refractory VAs on optimal medical therapy have detectable but blunted adrenergic responses, that are not disrupted by BCSD.
Keywords: autonomic reflexes, adrenergic responses, cardiac sympathetic denervation, ventricular arrhythmias, cardiomyopathies, autonomic nervous system
Introduction:
The autonomic nervous system (ANS) plays a pivotal role in cardiovascular stress response to maintain homeostasis. In patients with heart failure (HF) and reduced left ventricular ejection fraction (LVEF), the combination of ventricular dysfunction and abnormal afferent signaling promotes increased sympathetic and reduced parasympathetic tone1. Markers of sympathoexcitation, such as increased peripheral norepinephrine levels2 and reduced heart rate variability3 predict adverse cardiovascular outcomes in HF, including life-threatening ventricular arrhythmias (VAs). Accordingly, bilateral cardiac sympathetic denervation (BCSD), the resection of the inferior aspect of the stellate ganglion and 2nd to 4th thoracic sympathetic ganglia, is an established antiarrhythmic intervention in animal models4 and in patients with cardiomyopathy and refractory VAs5,6,7. However, the impact of the procedure on autonomic responses to stressors has not been evaluated. This assessment is relevant since HF patients show signs of abnormal autonomic responses, including reduced baroreflex sensitivity8, reduced chronotropic competence9, and reduced orthostatic tolerance10. These measures are inversely related to NYHA functional class, and the latter has been directly associated with a decreased quality of life9,10.
This study has two purposes first, to assess sympathetic responses to endogenous autonomic stressors in cardiomyopathy patients on optimal medical therapy undergoing BCSD for refractory VAs. Second, to assess the impact of BCSD on those responses.
Methods:
Patient population:
Autonomic reflexes were studied in cardiomyopathy patients undergoing BCSD at a single center between 2012 and 2015, and in healthy controls matched for age, sex and BMI. Cardiomyopathy was defined as LVEF <55%, and included hypertrophic (HCM), arrhythmogenic right ventricular (ARVC), sarcoid/inflammatory, and ischemic cardiomyopathy (ICM). BCSD was performed for VAs refractory to conventional treatment (antiarrhythmic drugs and/or catheter ablation). Additional exclusion criteria are reported in the supplemental material. The protocol was approved by the institutional review board and written consent was obtained from each subject before participation.
Surgical procedure:
BCSD was performed via a minimally invasive (video- or robot-assisted) thoracoscopic surgery approach as previously described9,10. The lower third to half of both stellate ganglia, together with T2–T4 thoracic ganglia were removed and confirmed by immunohistochemistry.
Autonomic testing:
Autonomic stressors were administered in a standardized order and fashion. All subjects avoided strenuous physical activity and the use of alcohol for at least 24 hours prior, and nicotine, coffee, tea, cola drinks, or a heavy meal were also avoided for at least 8 hours before the study. Cardiomyopathy patients were maintained on optimal medical and antiarrhythmic therapy to enable assessment of their autonomic function on such medications and to avoid triggering VAs. The lower rate interval (LRI) of the implantable cardioverter defibrillator (ICD) was not modified, but the rate response function, when active, was turned off. Environmental thermoneutrality was maintained.
Cardiac and peripheral non-invasive neurophysiologic measures were continuously obtained at baseline (5 minutes) and during 3 autonomic stressors (listed below), including: beat-to-beat non-invasive blood pressure (BP, assessed with CNAP® HD, BIOPAC, System Inc), heart rate (HR, measured from lead II of a 3 lead ECG), palmar electrodermal skin activity (EDA, assessed with EDA100C Electrodermal Activity Amplifier, BIOPAC, System Inc) and finger pulse volumetry (FPV, assessed by TS200 and PPG100C, BIOPAC, System Inc). EDA measures skin conductance level expressed in microSiemens (μS). FPV was expressed in arbitrary units (AU) as the pulse wave amplitude of the finger blood volume assessed via optical (photoplethysmographic) methods. Both EDA and FPV were obtained from the non-dominant hand. Additional details regarding autonomic testing are reported in the supplemental material.
Assessment of autonomic responses:
Measurements were acquired using the MP150 platform (BIOPAC, System Inc) and analyzed using Acknowledge 5.0 software (BIOPAC System Inc). Maximal adrenergic responses were defined as the difference between the highest value (or lowest for FPV only) recorded during any stressor and the baseline value (averaged over 5 minutes). Specific responses to each stressor are reported in supplemental table 1. Responses to stressors were quantified both via absolute change and as percentage change vs. baseline [%change = (absolute delta/baseline value) *100]. For the assessment of maximal adrenergic responses, both the absolute and percent change were reported, while for the rest of the analyses only the % changes were reported.
Follow up:
Follow up data after BCSD were obtained through medical records. Arrhythmic recurrence was defined as any of the following: ICD shock, sustained VT (≥ 30 seconds) below ICD detection, or anti-tachycardia pacing.
Statistical Analysis:
Continuous variables were expressed as mean ± SD or median and interquartile range (IQR). Categorical data were expressed as number and percentages. Continuous, unpaired, data were compared with Student’s t-test or Mann-Whitney U-test, as appropriate. The paired t-test or Wilcoxon signed rank test was used for paired comparisons. The one-way analysis of variance (ANOVA) or the Kruskal-Wallis H test was used for comparisons involving 3 groups. The level of statistical significance was set at P < 0.05. Statistical analyses were performed using GraphPad Prism 8.2.0 for Windows, GraphPad Software, California.
Results:
Eighteen patients with cardiomyopathy and refractory VAs (54±14 years, 16 males, 16 nonischemic cardiomyopathy, mean LVEF 36±14%) and 8 matched healthy controls were studied (table 1). Thirteen cardiomyopathy patients underwent ANS tests before BCSD; 14 after BCSD; among them, nine patients were studied both before and after BCSD. Baseline characteristics of the groups at the time of the ANS assessment are reported in table 1. Paired comparison of the characteristics of the 9 patients is reported in the supplemental table 2. Medications before and after BCSD were comparable as were LVEF and NYHA functional class. The type of cardiomyopathy and timing of testing in each patient are reported in supplemental table 3. No adverse events, including symptomatic orthostatic hypotension on postural challenge, occurred during autonomic testing. Supplemental figure number 1 shows representative tracings recording obtained in the same subject during postural challenge before and after BCSD.
Table 1:
Demographic and clinical characteristics at the time of autonomic testing
| Healthy controls, n=8 | Before BCSD, n=13 | After BCSD, n=14 | |
|---|---|---|---|
| Age | 48 ± 14 | 52 ±14 | 51 ± 13 |
| Female gender | 2 (25) | 2 (15) | 1 (7) |
| Body max index | 27 ± 5 | 27 ± 5 | 28 ± 5 |
| Median days between ANS tests an BCSD | 2 (1–9) | 39 (25–115) | |
| Hypertension | 4 (31) | 4 (29) | |
| ICMP/NICMP/mixed | 1/11/1 (8/84/8) | 0/14/0 (0/100/0) | |
| Permanent AF | 1 (8) | 0 (0) | |
| Sinus rhythm (no atrial or ventricular pacing) | 8 (100) | 3 (23) | 3 (21) |
| LVEF (%) | 36 ± 14 | 40 ± 13 | |
| NYHA class I/II/III/IV | 3/8/2/0 (23/62/15/0) | 3/10/1/0 (21/71/7) | |
| ICD | 13 (100) | 14 (100) | |
| ICD in secondary prevention | 7 (54) | 9 (64) | |
| CRT-D | 6 (46) | 5 (36) | |
| Atrial lead | 10 (77) | 11 (79) | |
| LRI pacing, pulse per minute (dual chamber devices) | 71± 11 | 66± 13 | |
| Ongoing drugs | |||
| Beta blockers | 13 (100) | 13 (93) | |
| – Metoprolol | 5 (38) | 8 (57) | |
| – Carvedilol | 7 (54) | 4 (29) | |
| – Metoprolol and Carvedilol | 1 (8) | 1 (7) | |
| > 1 AAD | 3 (23) | 5 (36) | |
| Amiodarone | 7 (54) | 4 (29) | |
| Sotalol | 2 (15) | 6 (43) | |
| Mexiletine | 3 (23) | 3 (21) | |
| Dofetilide | 1 (8) | 0 (0) | |
| Propafenone | 1(11) | 0 (0) | |
| Ranolazine | 0 (0) | 1 (11) | |
| ACE-inhibitors/ARB | 10 (77) | 11 (79) | |
Values are reported as mean ± SD, n (%), or median (interquartile range). AAD= antiarrhythmic drug; AF=atrial fibrillation; BCSD= bilateral cardiac sympathetic denervation; LVEF= left ventricular ejection fraction; ICMP= ischemic cardiomyopathy; CRT-D= cardiac resynchronization therapy-defibrillation; ES= electrical storm; LRI= lower rate interval; NICMP= nonischemic cardiomyopathy.
Evoked responses in cardiomyopathy patients before BCSD versus healthy controls
To examine ANS reflex function in cardiomyopathy patients on optimal medical therapy before BCSD, we compared their hemodynamic, FPV, and EDA responses to those of healthy controls. Resting measures are reported in table 2. Resting systolic BP (SBP) and FPV were lower in cardiomyopathy patients (n=13) than in healthy controls (120±16 versus 142±9 mmHg and 1.53±1.2 versus 2.58±1.43 AU, respectively, p<0.05), while HR was slightly higher (69±10 versus 60±7 bpm, p=0.04) likely related largely to the programmed LRI of ICDs. Supporting this assertion, resting HR in cardiomyopathy patients in sinus rhythm (not paced) were similar to healthy controls (60 ±7 vs 60 ± 4 bpm, p =0.99).
Table 2:
Resting values and maximal adrenergic responses across groups
| Healthy controls, n=8 | Before BCSD, n=13 | After BCSD, n=14 | ||||
|---|---|---|---|---|---|---|
| Resting values | Maximal Change | Resting values | Maximal change | Resting values | Maximal change | |
| SBP (mmHg) | 142 ± 9 | +24 ± 12 | 120± 16 * | +17 ± 7 | 127 ± 20 | +18 ± 12 |
| DBP (mmHg) | 83 ±6 | +39 ± 15 | 81 ±7 | +14 ± 9* | 83 ± 14 | +16 ± 7* |
| MBP (mmHg) | 103 ± 7 | +30 ± 12 | 94 ± 9 | +14 ± 7* | 98 ± 15 | +15 ± 7* |
| HR, all bpm | 60 ±7 | +29 ± 13 | 69 ± 10* | +12±10* | 64 ± 13 | +7 ± 7* |
| HR, not paced (bpm) | 60 ± 4, n=3 | 18 ± 16 | 53 ± 6, n=3 | 11± 14 | ||
| FPV (AU) | 2.58±1.43 | −1.53 ± 1.20 | 0.36 ±0.25* | −0.25 ± 0.14* | 0.42 ± 0.31* | −0.28 ± 0.23* |
| EDA (μS) | 5.54 ± 5.09 | +7.89 ± 4.4 | 2.83 ± 1.19 | +3.03 ±3.41* | 1.88 ± 0.48 | +0.15 ± 0.26* |
Absolute values are reported as mean ± SD. BCSD= bilateral cardiac sympathetic denervation; DBP= diastolic blood pressure; EDA=electrodermal activity; HR= heart rate; FPV=finger pulse volume; MBP= mean blood pressure; SBP=systolic blood pressure; AU= arbitrary unit.
p<0.05 vs healthy controls.
Maximal adrenergic responses evoked across all stressors are summarized in table 2 (absolute changes), and in figure 1 (percent change). Hemodynamic (DBP, MBP, and HR), FPV and EDA responses to autonomic provocations were blunted in cardiomyopathy patients vs. healthy controls in both absolute (table 2) and relative (figure 1) terms.
Figure 1: Maximal Adrenergic Responses across study groups.
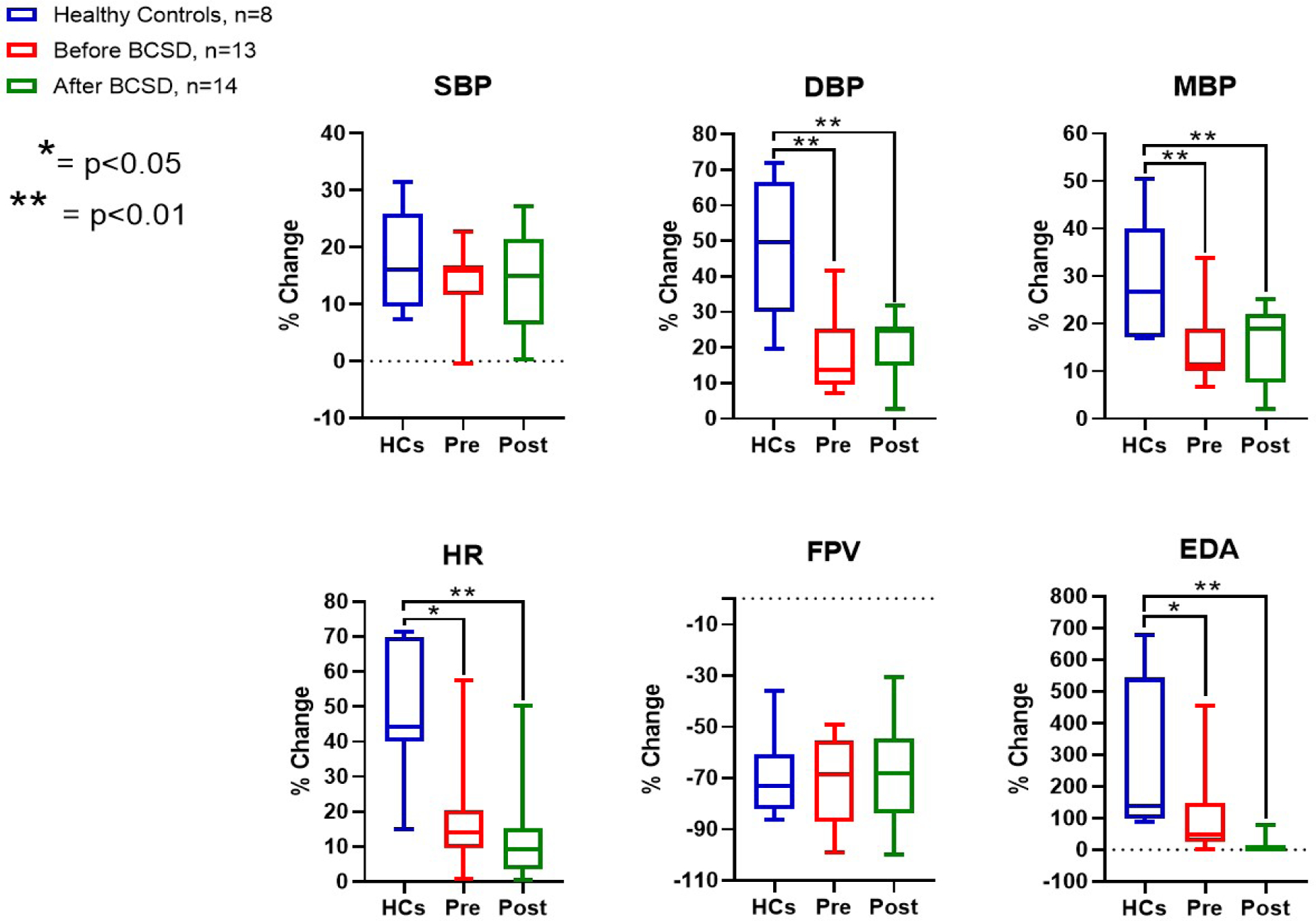
BCSD= bilateral cardiac sympathetic denervation; DBP= diastolic blood pressure; EDA=electrodermal activity; HR= heart rate; FPV=finger pulse volume; MBP= mean blood pressure; Pre= patients before BCSD; Post= patients after BCSD; SBP=systolic blood pressure. Each bar shows the range of values from the minimum to the maximum (the horizontal line within each bar represents the median value of the series)
Next, we examined responses to specific stressors in cardiomyopathy patients before BCSD vs healthy controls (supplemental table 4). Cardiomyopathy patients showed similar responses to postural challenge in blood pressure, FPV, and EDA. The percent increase in HR induced by standing, albeit significant in both groups, was lower in cardiomyopathy patients (+15±17% versus +21±10%, p <0.01) likely related to beta-adrenergic receptor blockade.
In healthy controls, handgrip induced a significant increase in HR and EDA relative to baseline, however, in cardiomyopathy patients, handgrip produced a significant rise in SBP, DBP and MBP while HR and EDA responses was mild but detectable, suggesting differentially integrated sympathetic outflow in cardiomyopathy patients on optimal medical therapy vs. healthy controls. Finally, HR and EDA responses to the math test were blunted in cardiomyopathy patients vs. healthy controls, while FPV responses were similar (−56% ± 28% in healthy controls versus −64% ± 17% in CMP, p=0.49).
In summary, these data show that cardiomyopathy patients undergoing BCSD, as compared to healthy controls, had blunted HR responses to all stressors and blunted EDA response to math test. In contrast, mean BP responses were preserved during postural challenge and handgrip. Finally, FPV responses to all stressors were similar. These finding suggest key differences in reflex sympathetic function in cardiomyopathy patients on optimal medical and antiarrhythmic therapy, reflecting an impact of both cardiomyopathy and medications on autonomic function.
Reflex responses before versus after BCSD
Cardiomyopathy patients underwent autonomic testing a median of 28 days (IQR 24–53 days) after BCSD. Compared to before, resting values remained unchanged after BCSD (supplemental table 5): FPV was 0.34 ± 0.16 AU before and 0.30 ± 0.23 AU after BCSD (p=0.35), while EDA was 2.73 ± 1.15 μS before and 2.08 ± 0.23 μS after BCSD (p=0.14). Resting hemodynamic values were similarly unchanged after BCSD.
To determine how BCSD impacts reflex sympathetic function in cardiomyopathy patients on optimal medical therapy, we examined maximal adrenergic responses across all stressors. As expected, following BCSD there were no EDA responses, as innervation to the sweat glands originates from the transected lower half of the stellate ganglion, confirming denervation. Other than blunted EDA response, no other differences in maximal absolute change were detected (supplemental table 5 and figure 2). In the 2 patients not paced, no differences in the maximal increase of HR could be detected; however, this was limited by the sample size.
Figure 2: Maximal Adrenergic Responses before versus after BCSD.

BCSD= bilateral cardiac sympathetic denervation; DBP= diastolic blood pressure; EDA=electrodermal activity; HR= heart rate; FPV=finger pulse volume; MBP= mean blood pressure; Pre= patients before BCSD; Post= patients after BCSD; SBP=systolic blood pressure. n=9.
Following BCSD, the percent drop in DBP and MBP induced by standing was significantly lower than before, accompanied by a blunted decrease in FPV (figure 3). The HR response to standing, already attenuated before BCSD, was not affected by the procedure. Except for EDA, the ANS reflex responses to the other 2 stressors, were not significantly different after BCSD as compared to before (supplemental table 6). Interestingly, the nearly null BP changes in response to math test before BCSD, became positive after BCSD, suggesting improvement in sympathetic reserve following BCSD.
Figure 3: Relative response to postural challenge before versus after BCSD.

BCSD= bilateral cardiac sympathetic denervation; DBP= diastolic blood pressure; EDA=electrodermal activity; HR= heart rate; FPV=finger pulse volume; MBP= mean blood pressure; Pre= patients before BCSD; Post= patients after BCSD; SBP=systolic blood pressure. n=9.
Evoked responses in CMP patients after BCSD versus healthy controls
Next, we performed a comparison of reflex responses to autonomic stressors in patients with cardiomyopathy after BCSD to healthy controls to assess the degree of normalization of autonomic deficits following surgery. At rest, the only significant difference between cardiomyopathy patients after BCSD (n=14) and healthy controls was a significantly lower FPV (0.42 ±0.3 versus 1.53 ± 1.20 AU, p<0.01), as reported in table 2.
Adrenergic Responses (percent change in MBP, DBP, and HR) in cardiomyopathy patients remained blunted relative to healthy controls, while SBP and FPV percentage responses were comparable (figure 1). As expected, EDA was suppressed following BCSD. Except for EDA, responses to each stressor (percent change) were not different between cardiomyopathy patients after BCSD and healthy controls (supplemental table 4).
Discussion:
The main findings of the present study are: 1) Patients with cardiomyopathy and refractory VAs on optimal medical treatment exhibit blunted but detectable responses to autonomic stressors before BCSD. 2) Reflex cardiovascular sympathetic responses to various autonomic stressors are preserved after BCSD. This study presents these data for the first time in this patient population and suggests that the antiarrhythmic effects of BCSD are not accompanied by compromised hemodynamic and reflex cardiac sympathetic responses.
Autonomic dysfunction in CMP patients on optimal medical therapy
Chronotropic response to physical effort5 and hemodynamic response to orthostatic challenge6 are blunted in patients with systolic HF, as were muscle sympathetic nerve activity (MSNA), BP and HR responses to mental stress test compared to healthy controls11. HF patients showed a pronounced increase in MSNA during static12 as well as dynamic exercise13, that was absent in healthy controls despite similar hemodynamic responses. Of note, these prior studies were all conducted in clinically stable HF patients; ongoing HF drugs were heterogeneous and were mostly interrupted at least 24 hours before autonomic testing.
In the present study, we assessed the short-term impact of BCSD on sympathetic responses to three stressors (active orthostatic standing, 30% isometric handgrip for 2 minutes and an arithmetic stress test) in patients with cardiomyopathy and refractory VAs. Hemodynamic responses were assessed through BP and HR measurements. Despite the high arrhythmic risk of the studied population, there were no episodes of sustained and/or treated VAs witnessed during testing, indicating the safety and tolerability of the tests.
At rest and on optimal medical therapy, cardiomyopathy patients awaiting BCSD showed consistently lower FPV values compared to controls, while EDA was not significantly different. FPV values at rest are likely related to the combined vasoconstrictor effects of pathological levels of plasma norepinephrine and ongoing beta-blocker (BB) therapy14, 15. Except for FPV, maximal cardiac and peripheral sympathetic responses were detectable but blunted in cardiomyopathy patients.
Specific differences in baseline responses to stressors observed in cardiomyopathy vs. healthy controls likely reflected the combined effects of medications and underlying autonomic imbalance related to HF status. During active standing, despite a similar maximal drop in BP and reduction in FPV (expressed as percent of change), the HR response was blunted in cardiomyopathy patients, consistent with previous findings6. More than half of the patients we studied were on alpha-adrenergic blocking therapy (carvedilol); nevertheless, BP adaptation during active standing was preserved, suggesting a high peripheral sympathetic efferent drive.
In our study we observed divergent group responses to isometric handgrip. BP significantly increased relative to baseline in cardiomyopathy patients only, while HR significantly increased in healthy controls. BP increases during handgrip in healthy controls are known to mostly reflect an increased cardiac output rather than a systemic vasoconstrictor response, with no significant associated changes in left ventricular end-diastolic pressure (LVEDP)16,17. The ability of isometric handgrip to evoke BP responses in healthy subjects is related to task duration, with a progressive increase in BP rise occurring up to the fifth minute of hand grip18. An early (after 2 minutes) significant increase in both systolic and diastolic BP in healthy controls in response to isometric handgrip was associated with future risk of hypertension19; its absence in our population suggests adequate selection of healthy controls.
BP increases evoked by handgrip in HF patients have been shown to be more pronounced than Healthy controls and tend to be associated with an increase in MSNA14, in vascular peripheral resistance and in LVEDP20. Of note, isometric handgrip longer than 3 minutes was associated with VAs (mostly PVCs but also VAs≥ 3 beats) in untreated patients with coronary artery disease and depressed LVEF21. Our data are therefore in agreement with previous findings, with the additional value that significant BP responses in our study were obtained despite high dosages of concurrent anti-adrenergic drugs.
Impact of BCSD on sympathetic responses to stressors
Paired comparisons of cardiomyopathy patients who received ANS tests both before and after BCSD showed no differences in resting parameters. The EDA response to stressors appeared to be completely abolished by BCSD, confirming the removal of sympathetic neuronal bodies projecting to the hand (T1 level). Besides, EDA, maximal adrenergic responses were not significantly affected by BCSD. These data support the idea that BCSD does not exert detrimental effects on cardiac and peripheral sympathetic responses to physiological stressors, even in patients who have blunted sympathetic reflexes at baseline. The mild HR increase observed before BCSD, likely mediated by circulating catecholamines, was also not compromised by BCSD.
Analysis of specific responses to each stressor before and after BCSD, was notable for an improved BP response to active standing, indicating improved orthostatic tolerance after BCSD. The improved BP response was associated with a reduction in the rapid FPV decrease induced by standing, likely reflecting the loss of the local contribution of neuronal released norepinephrine to FPV changes. Improved baroreflex gain and/or peripheral and cardiac response to catecholamines may explain these findings.
Limitations:
Due to the unique characteristics of the studied population, paired data could not be obtained for all patients, also medications and LRI of the ICD could not be modified before the ANS tests. The timing of ANS testing post-BCSD was not consistent across all patients, although majority had testing within the first 3 months after BCSD. Further, none were studied in the first 14 days after BCSD when neurodegenerative processes are expected to be still taking place22. Of note, autonomic responses may show intraindividual variability that may affect the reliability of a single assessment before and after a therapeutic intervention; however, the consistency among different tests and the biological plausibility of the observed results strongly argues against a pure effect of chance. Finally, due to feasibility and safety concerns, both active orthostatic challenge and isometric handgrip were performed for the minimum amount of time required for detectable autonomic responses.
Conclusions:
Cardiomyopathy patients with refractory VAs on optimal medical treatment have blunted adrenergic responses to autonomic stressors. Following BCSD, autonomic reflexes are not disrupted and the hemodynamic response to active standing is improved. This suggests that concern for the complete loss of cardiovascular autonomic reflexes after BCSD should not preclude physicians from recommending BCSD for the control of VAs in appropriate patients.
Supplementary Material
Acknowledgments:
This study was supported by NIH/NLHBI K08 HL125730 and DP2HL142045 to OAA, NIH OT2OD023848 to KS and OAA, and by NIH K23MH112949 and The William K. Warren Foundation to SSK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors report no conflicts of interest.
References
- 1.Shivkumar K, Ajijola OA, Anand I, et al. Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J Physiol 2016; 594:3911–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohn JN, Levine TB, Olivari MT, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 1984; 311:819–823. [DOI] [PubMed] [Google Scholar]
- 3.La Rovere MT, Pinna GD, Maestri R, al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 2003;107, 565–570. [DOI] [PubMed] [Google Scholar]
- 4.Irie T, Yamakawa K, Hamon D, Nakamura K, Shivkumar K, Vaseghi M. Cardiac sympathetic innervation via middle cervical and stellate ganglia and antiarrhythmic mechanism of bilateral stellectomy. Am J Physiol Heart Circ Physiol. 2017;312:H392–H405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaseghi M, Gima J, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and long-term follow-up. Heart Rhythm 2014; 11:360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaseghi M, Barwad P, Malavassi, et al. Cardiac sympathetic denervation for refractory ventricular arrhythmias. J Am Coll Cardiol 2017; 69:3070–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah R, Assis F, Alugubelli N, et al. Cardiac sympathetic denervation for refractory ventricular arrhythmias in patients with structural heart disease: A systematic review. Heart Rhythm. 2019; S1547–5271(19)30575–2. [DOI] [PubMed] [Google Scholar]
- 8.Mortara A, La Rovere MT, Pinna GD et al. Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation 1997; 96:3450–8. [DOI] [PubMed] [Google Scholar]
- 9.Zweerink A, Van der Lingen ACJ, Handoko ML, Van Rossum AC, Allaart CP. Chronotropic Incompetence in Chronic Heart Failure. Circ Heart Fail. 2018;11: e004969. [DOI] [PubMed] [Google Scholar]
- 10.Bronzwaer AGT, Bogert LWJ, Westerhof BE, Piek JJ, Daemen MJAP, van Lieshout JJ. Abnormal haemodynamic postural response in patients with chronic heart failure. ESC Heart Fail. 2017; 4: 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Middlekauff HR, Nguyen AH, Negrao CE, et al. Impact of acute mental stress on sympathetic nerve activity and regional blood flow in advanced heart failure: implications for ‘triggering’ adverse cardiac events. Circulation. 1997; 96:1835–42. [DOI] [PubMed] [Google Scholar]
- 12.Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol. 2001;280:H969–76. [DOI] [PubMed] [Google Scholar]
- 13.Notarius CF, Millar PJ, Murai H, et al. Divergent muscle sympathetic responses to dynamic leg exercise in heart failure and age-matched healthy subjects. J Physiol. 2015; 593:715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen RA, Coffman JD. Beta-adrenergic vasodilator mechanism in the finger. Circ Res. 1981; 49:1196–201. [DOI] [PubMed] [Google Scholar]
- 15.Grote L, Zou D, Kraiczi H, Hedner J. Finger plethysmography-a method for monitoring finger blood flow during sleep disordered breathing. Respir Physiol Neurobiol. 2003;136:141–52. [DOI] [PubMed] [Google Scholar]
- 16.Crawford MH, White DH, Amon KW. Echocardiographic evaluation of left ventricular size and performance during handgrip and supine and upright bicycle exercise. Circulation 1979; 59:1188–96. [DOI] [PubMed] [Google Scholar]
- 17.Grossman W, McLaurin LP, Saltz SB, Paraskos JA, Dalen JE, Dexter L. Changes in the inotropic state of the left ventricle during isometric exercise. Br Heart J 1973; 35:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khurana RK, Setty A. The value of the isometric hand-grip test studies in various autonomic disorders. Clin Auton Res.1996; 6: 211–8. [DOI] [PubMed] [Google Scholar]
- 19.Chaney RH, Eyman RK. Blood pressure at rest and during maximal dynamic and isometric exercise as predictors of systemic hypertension. Am J Cardiol. 1988;62:1058–61. [DOI] [PubMed] [Google Scholar]
- 20.Flessas AP, Ryan TJ. Cardiovascular Responses to Isometric Exercise in Patients With Mitral Stenosis Comparison With Normal Subjects and Patients With Depressed Ejection Fraction. Arch Intern Med.1982; 142:1629–33. [PubMed] [Google Scholar]
- 21.Atkins JM, Matthews OA, Blomqvist CG, Mullins CB. Incidence of arrhythmias induced by isometric and dynamic exercise. Br Heart J.1976; 38:465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andres KH, Düring M von, Jänig W, Schmidt RF. Degeneration Patterns of Postganglionic Fibers Following Sympathectomy. Anat Embryol (Berl) 1985; 172 (2), 133–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


