Abstract
BACKGROUND:
Improved patient outcomes and satisfaction associated with enhanced recovery after surgery protocols have increasingly replaced traditional peri-operative anesthesia care. Fast-track surgery pathways have been extensively validated in patients undergoing hysterectomies, yet impact on fertility-sparing laparoscopic gynecologic operations, particularly those addressing chronic pain conditions, has not been examined.
OBJECTIVE:
To determine the effects of enhanced recovery after surgery pathway implementation compared to conventional peri-operative care in women undergoing laparoscopic minimally invasive non-hysterectomy gynecologic procedures.
STUDY DESIGN:
We conducted a retrospective cohort study of women undergoing uterine-sparing laparoscopic gynecologic procedures for benign conditions (tubal/adnexal pathology, endometriosis or leiomyomas) during a 24-month period before and after enhanced recovery after surgery implementation at a tertiary care center. We compared immediate peri-operative outcomes and 30-day complications. The primary outcome was same day discharge rates. Factors influencing unplanned admissions, postoperative pain, sedation, nausea and vomiting represented secondary analyses.
RESULTS:
A total of 410 women (Enhanced recovery after surgery, n=196; Conventional perioperative care, n=214) met inclusion criteria. Following enhanced recovery after surgery implementation, same day discharge rates increased by 9.4% (P=.001). Reductions in postoperative pain and nausea/vomiting represented the primary driving factor behind lower unplanned admissions. Higher pre-operative anti-emetic medication administration in the enhanced recovery after surgery group resulted in a 57% reduction in post-anesthesia care unit anti-emetics (P<.001). Total peri-operative narcotic medication use was also significantly reduced by 64% (P<.001), and the enhanced recovery after surgery cohort still demonstrated significantly lower post-anesthesia unit care pain scores at hours 2 and 3 (P<.001). A nineteen-minute shorter post-anesthesia care unit stay was noted in the enhanced recovery after surgery cohort (P=.036). Increased same day discharge did not lead to higher postoperative complications or changes in 30-day emergency department visits or readmissions in enhanced recovery after surgery patients.
CONCLUSION:
Enhanced recovery after surgery implementation resulted in increased same day discharge rates and improved peri-operative outcomes without affecting 30-day morbidity in women undergoing laparoscopic minimally invasive non-hysterectomy gynecologic procedures.
Keywords: Enhanced recovery after surgery, ERAS, minimally invasive gynecologic surgery, MIGS, same day discharge, chronic pelvic pain, endometriosis
INTRODUCTION
Enhanced recovery after surgery (ERAS) is a multidisciplinary care team approach aimed to improve peri-operative patient outcomes, recovery and satisfaction. Evidence supporting decreased hospital length of stay, complications, health care cost and expedited recovery with faster return of bowel function, reduced pain and postoperative nausea and vomiting (PONV) has challenged conventional peri-operative care (CPC).1,2 ERAS protocols have now widely been adapted and modified throughout most surgical specialties including gynecologic care.1,3,4 Although first introduced in open gynecologic oncology procedures, urogynecologic and minimally invasive gynecologic surgeons have increasingly integrated ERAS care into standard peri-operative patient care for pelvic organ prolapse and hysterectomies.3,5-11 However, to date, no studies exist that specifically address outcomes in women undergoing minimally invasive gynecologic procedures treating chronic pelvic pain conditions such as endometriosis, fibroids, or tubal and adnexal pathology. Moreover, the true impact of ERAS protocols on outcome parameters such as length of stay or same day discharge (SDD) rates is difficult to determine given that extensive institutional practice shifts, patient education, and peri-operative care team attitudes towards outpatient major gynecologic surgery have primarily been credited for the success of ‘fast track surgery’ care. Hence, studying ERAS implementation in patients undergoing uterine-sparing gynecologic operations may provide insight into whether universal implementation of ERAS protocols for all gynecologic surgeries is effective. In contrast to the traditional twenty-four-hour observation period that until recently was considered standard of care following major gynecologic procedures, non-hysterectomy operations have long been accepted as outpatient surgeries and thus may overcome study limitations and inherent bias associated with ERAS implementation.12
The objective of this study was to determine whether ERAS implementation improved SDD rates among women undergoing minimally invasive non-hysterectomy gynecologic surgery for endometriosis, fibroids, or adnexal pathology as compared to CPC. Immediate peri-operative outcomes, factors influencing postoperative admission, pain and PONV management, as well as 30-day outcomes were also examined.
MATERIAL AND METHODS
We performed a retrospective, observational cohort study of women undergoing minimally invasive non-hysterectomy gynecologic procedure at Magee-Womens Hospital of UPMC before and after ERAS pathway implementation. Details of our institution specific minimally invasive gynecologic ERAS protocol have previously been published.8 Surgical cases were identified using surgical service calendars. Eligibility criteria included ages 18-100 and laparoscopic, non-robotic gynecologic surgeries for tubal/adnexal pathology, endometriosis and other pelvic pain conditions or leiomyoma removal by one of four minimally-invasive gynecologic fellowship-trained surgeons during a 24-month period before (July 2015-2016) and after (February 2017-2018) ERAS implementation. The surgeons remained constant throughout the study period. Caseloads did not change significantly before and after ERAS implementation, nor did the case types performed by each surgeon. Surgeries were performed at only one hospital. A six-month time interval between the CPC and ERAS group was omitted as ongoing modifications occurred to the ultimately final ERAS protocol used in this study. Other exclusion criteria included study subjects aged <18, known gynecologic malignancy, hysterectomy or urogynecologic procedures.
Data collection occurred retrospectively using electronic medical data from outpatient clinic notes, operative and anesthesia records, as well as emergency and inpatient documentation up to 30 days postoperatively. Patient demographics collected were comprised of age, gravidity, parity, body mass index, previous surgical history and medical comorbidities including coronary artery disease, congestive heart failure, obstructive sleep apnea, chronic obstructive pulmonary disease, diabetes, hypertension, asthma, venous thromboembolism, and liver disease. Pre-operative pain scores (scale 1-10) when available were recorded. Surgical and peri-operative care outcomes included primary indication for the procedure, estimated blood loss, as well as pre-, intra-, post-operative and post-anesthesia care unit (PACU) times. PONV and pain outcomes were analyzed utilizing medication records for anti-emetics as well as narcotic and non-narcotic pain medications. For standardization, all narcotic administrations were converted to oral morphine equivalent dosing. 30-day emergency room presentations and re-admissions rates were characterized by evaluation for pain, wound, venous thromboembolism, cardiac/chest pain, small bowel obstruction, ileus, constipation, PONV, gastrointestinal injury, urinary tract injury, voiding dysfunction or ovarian torsion. We hypothesized that ERAS implementation would significantly increase SDD rates, and hence this variable was selected as our primary outcome. A multivariate analysis adjusting for patient demographics, surgeon and surgery type was performed to ensure that these factors did not modify the primary outcome. Secondary outcomes analyzed included changes in peri-operative times, PONV and pain management as well as 30-day complication rates.
As SDD rates with CPC for non-hysterectomy minimally invasive gynecologic surgeries were not available from our institution prior to our analysis, sample size calculations were performed using data extrapolation from a similar patient population undergoing minimally invasive myomectomies with reports of outpatient procedure rates of 88%.13 We estimated that 102 women in each cohort were required to detect a 10% increase in SDD rates with 80% power at a 2-sided alpha of 0.05. This increase in SDD was predicted based on the observed increase of same day hysterectomy discharges following ERAS rollout at our institution. However, given potential institutional and procedural variances, data collection was extended to include all patients in a one-year period before and after ERAS implementation to ensure statistical robustness.
Both patient cohorts were characterized through descriptive statistics. Continuous variables were presented as means with standard deviations. Categorical variables were presented as frequencies in percentages. For comparisons between CPC and ERAS groups, t-tests were used for continuous variables, and chi-squared or Fisher's exact test were utilized for categorical variables. A mixed effects linear regression model adjusted for patient demographics, surgeon and surgery type was applied to analyze PACU pain (scale 1-10) and sedation (scale 0-3) scores at various time intervals of recovery and calculate the difference in score changes between CPC and ERAS at hours 1-3. A sensitivity analysis was performed using only people with complete data in the first three hours to confirm that missing values did not impact the results and their statistical accuracy. STATA version 15 (College Station, TX) was used for all analyses. P-values of less than 0.05 were considered statistically significant. The University of Pittsburgh Institutional Review Board approved this study.
RESULTS
During the 24-month study period, 410 women met inclusion criteria (CPC, n=214; ERAS, n=196). Demographic characteristics were similar in both groups (Table 1A). Chronic pelvic pain was the primary indication for surgery in 64.9% of all patients with no difference in the remaining operative interventions (Table 2A). Pre-operative pain scores were available in 55.9% of patients (CPC: 5.16±0.2 vs. ERAS: 4.60 ±0.24, P=.071). Patient cohorts underwent comparable rates of myomectomies, adnexal surgery including ovarian cystectomies or salpingo-oophorectomies, and excision of endometriosis with or without concurrent adnexal or bowel surgery (Table 2B).
Table 1.
Patient demographics of women undergoing CPC vs. ERAS in minimally invasive non-hysterectomy procedures
| Demographic Characteristic |
CPC (n = 214) |
ERAS (n = 196) |
P-value |
|---|---|---|---|
| Age | 33.6 10.2 | 35.1 11.3 | .154 |
| Gravidity | 1.4 1.8 | 1.2 1.6 | .433 |
| Parity | 0.9 1.3 | 0.8 1.1 | .134 |
| BMI | 28.0 7.5 | 28.2 7.8 | .743 |
| Previous pelvic surgery | 132 (61.7) | 111 (56.6) | .299 |
| Previous abdominal surgery | 61 (28.5) | 71 (36.2) | .095 |
| Medical comorbidities | 65 (30.4) | 64 (32.7) | .620 |
CPC, conventional peri-operative care; ERAS, enhanced recovery after surgery; BMI, body mass index
Data are n (%) or mean ± standard deviation
Table 2A.
Primary surgical indications for minimally invasive non-hysterectomy procedures in CPC vs. ERAS
| Indication | CPC (n = 214) |
ERAS (n = 196) |
P-value |
|---|---|---|---|
| Pelvic Pain – Endometriosis | 104 (48.6) | 97 (49.5) | .857 |
| Pelvic Pain – Non-Endometriosis | 47 (22.0) | 18 (9.2) | <.001 |
| Fibroids | 6 (2.8) | 17 (8.7) | .010 |
| Infertility | 2 (0.9) | 2 (1.0) | >.999 |
| Sterilization | 6 (2.8) | 6 (3.0) | .877 |
| Adnexal Cyst/Mass | 43 (20.1) | 47 (24.0) | .342 |
| Foreign Body | 1 (0.5) | 0 (0.0) | .338 |
| Prophylactic/ Breast Cancer | 5 (2.3) | 9 (4.6) | .209 |
CPC, conventional peri-operative care; ERAS, enhanced recovery after surgery; Data are n (%)
Table 2B.
Surgical demographics of women undergoing CPC vs. ERAS in minimally invasive non-hysterectomy procedures
| Surgery Type* | CPC (n = 214) |
ERAS (n = 196) |
|---|---|---|
| Adnexal/Tubal | 75 (35.0) | 62 (31.6) |
| Endometriosis + Adnexal PSN | 26 (12.2) | 34 (17.4) |
| Endometriosis PSN | 78 (36.5) | 64 (32.7) |
| Endometriosis + Bowel Adnexal PSN | 8 (3.7) | 3 (1.5) |
| Myomectomy | 9 (4.2) | 20 (10.2) |
| Other | 18 (8.4) | 13 (6.6) |
CPC, conventional peri-operative care; ERAS, enhanced recovery after surgery; PSN, presacral neurectomy
Data are n (%)
P-value .073; noting no difference in distribution between CPC and ERAS across any surgery types
ERAS implementation significantly increased SDD by 9.4% (Table 3A). ERAS remained superior with 96.9% of patients discharged on postoperative day 0 (POD0) (P=.005) after excluding planned postoperative admissions due to medical comorbidities or complex surgical interventions such as bowel surgery. While planned admission rates did not vary, unplanned admissions occurred more frequently in the CPC group due to unanticipated PACU complications (CPC: 43.3% vs. ERAS: 33.3%, P=.021). Postoperative pain accounted for 84.6% of unplanned PACU admissions in the CPC group while only one patient in the ERAS group required admission for pain management (data not shown). PONV was twice as common among unplanned PACU admissions in the CPC cohort, albeit with low rates given only four affected patients. Multivariate analysis adjusting for patient demographics, surgeon or surgery type did not lead to qualitative difference in the primary outcome.
Table 3A.
Peri-operative outcomes in women undergoing CPC vs. ERAS in minimally invasive non-hysterectomy procedures
| Outcomes | CPC (n=214) |
ERAS (n=196) |
P-value |
|---|---|---|---|
| Same day discharge rates | 184 (86.0) | 187 (95.4) | .001 |
| Admission rates | 30 (14.0) | 9 (4.6) | .001 |
| +Planned | |||
| Comorbidities | 3 (10.0) | 1 (11.1) | .625 |
| Surgery | 9 (30.0) | 2 (22.2) | .065 |
| ¥Unplanned | |||
| Intraoperative complication | 1 (3.3) | 2 (22.2) | .608 |
| Intraoperative surgery | 4 (13.3) | 1 (11.1) | .065 |
| PACU | 13 (43.3) | 3 (33.3) | .021 |
| *Adjusted same day discharge | n=184/202 (91.1) |
n=187/193 (96.9) |
.005 |
CPC, conventional peri-operative care; ERAS, enhanced recovery after surgery; PACU, post-anesthesia care unit; ED, emergency department
Data are n (%)
Planned admissions including anticipated bowel surgery and medical comorbidities requiring extended peri-operative care; data is n (%) of admitted patients
Unplanned admissions including unanticipated bowel surgery, intraoperative complications or PACU-related occurrences; data is n (%) of admitted patients
Adjusted for planned admissions
30-day ED presentation rates (CPC: 18.7% vs. ERAS: 16.8%; P=.624) and hospital readmission rates (CPC: 2.3% vs. ERAS 3.1%; P=.584) were similar (Table 3B). Although no statistical difference was noted between the two groups, patients in the CPC cohort had a slightly higher trend toward postoperative pain presentations (CPC: 62.5% vs. ERAS 51.5%; P=.316). In contrast, ERAS patients presented more frequently for incisional evaluation (CPC: 17.5% vs. ERAS: 30.3%; P=.490). Pure PONV was rarely the sole presenting complaint in either group (CPC: 12.5% vs. ERAS 6.1%, P=.304). No cases of postoperative voiding dysfunction or retention occurred.
Table 3B.
Peri-operative outcomes in women undergoing CPC vs. ERAS in minimally invasive non-hysterectomy procedures
| Outcomes | CPC | ERAS | P-value |
|---|---|---|---|
| 30-day ED presentation rate | n=40/214 (18.7) |
n=33/196 (16.8) |
.624 |
| Pain | 25 (62.5) | 17 (51.5) | .316 |
| Wound | 7 (17.5) | 10 (30.3) | .490 |
| Chest/Cardiac Pain/VTE | 2 (5.0) | 5 (15.2) | .267 |
| GI | 6 (15.0) | 5 (15.2) | .874 |
| GU | 0 (0.0) | 0 (0.0) | - |
| Other | 8 (20.0) | 5 (15.2) | .986 |
| 30-day readmission rate | n=5/214 (2.3) |
n=6/196 (3.1) |
.584 |
| Pain | 4 (80.0) | 4 (66.7) | >.999 |
| GI | 1 (25.0) | 2 (33.3) | .353 |
| Other | 2 (40.0) | 2 (33.3) | >.999 |
CPC, conventional peri-operative care; ERAS, enhanced recovery after surgery; PACU, post-anesthesia care unit; ED, emergency department; VTE, venous thromboembolism work-up; GI, gastrointestinal evaluations for ileus, bowel injury, small bowel obstruction, nausea/vomiting, constipation; GU, genitourinary evaluations for bladder and ureteral injury
Data are n (%) and allow for multiple presentation complaints
Peri-operative times were evaluated to assess whether ERAS implementation affected operative and PACU duration (Table 4). There were no significant changes in pre-operative anesthesia to surgery start time, surgery duration, or post-surgery anesthesia to PACU transfer. However, ERAS implementation shortened PACU stays by 19 minutes (P=.036) with an average PACU time of 3.6 hours.
Table 4.
Peri-operative times in women undergoing CPC vs. ERAS in minimally invasive non-hysterectomy procedures
| Peri-operative Time Duration (minutes) |
CPC (n = 214) |
ERAS (n =196) |
P-value |
|---|---|---|---|
| Pre-operative | 34.7 7.8 | 35.7 7.1 | .181 |
| Surgery | 95.2 76.8 | 90.5 57.5 | .476 |
| Post-operative | 16.7 7.5 | 17.7 8.8 | .213 |
| PACU | 235.9 89.1 | 217.0 83.3 | .036 |
CPC, conventional peri-operative care; ERAS, enhanced recovery after surgery; PACU, Post-anesthesia care unit
Data are mean ± standard deviation
Although, no differences were noted in the average total doses of anti-emetic medications received between the two cohorts (CPC: 3.37±1.3 vs. ERAS 3.48+1.0; P=.346), timing of PONV management significantly differed (Table 5). ERAS patients received an average of 0.82 additional doses of anti-emetic medications preoperatively (P<.001) with perphenazine as the distinguishing factor. No change in the use of scopolamine patches or aprepitant was observed. A slightly higher rate of intraoperative administration of anti-emetics occurred in the CPC cohort, but this was marginal in comparison to PACU data where one third of women in the CPC group required PONV treatment. In contrast, only one in five ERAS patients received PONV treatment in the PACU (CPC: 0.57±0.9 doses vs. ERAS: 0.25±0.6; P<0.001).
Table 5.
PONV management in women undergoing CPC vs. ERAS in minimally invasive non-hysterectomy procedures
| Antiemetic Use | CPC (n=214) |
ERAS (n=196) |
P-value |
|---|---|---|---|
| Pre-operative Medications | n=66/214 (30.8) | n=193/196 (98.5) | <.001 |
| Perphenazine | 12 (5.6) | 192 (98.0) | <.001 |
| Scopolamine patch | 38 (17.8) | 28 (14.3) | .339 |
| Aprepitant | 43 (20.1) | 28 (14.3) | .121 |
| Pre-operative Doses | 0.43 0.71 | 1.25 0.6 | <.001 |
| Intra-operative Medications | n=213/214 (99.5) | n=190/196 (96.9) | .043 |
| Dexamethasone | 191 (89.3) | 173 (88.3) | .752 |
| Ondansetron | 213 (99.5) | 190 (96.9) | .058 |
| Intra-operative Doses | 2.36 0.6 | 1.96 0.4 | <.001 |
| PACU Medications | n=77/214 (36.0) | n=41/196 (20.9) | .001 |
| Dexamethasone | 28 (13.1) | 1 (0.5) | <.001 |
| Ondansetron | 71 (33.2) | 39 (19.9) | .002 |
| Prochlorperazine | 15 (7.0) | 4 (2.0) | .017 |
| PACU Doses | 0.57 0.9 | 0.25 0.6 | <.001 |
| Total Doses | 3.37 1.3 | 3.48 1.0 | .346 |
CPC, conventional peri-operative care; ERAS, enhanced recovery after surgery; PONV, post-operative nausea and vomiting
Data are n (%) or mean ± standard deviation
Groups varied significant in the amount of narcotic and multi-modality non-narcotic pain medication use. ERAS resulted in a 64% decrease in total amounts of peri-operative oral morphine equivalents (OME) (CPC: 152.2±57.1 mg vs. ERAS: 55.3±21.1; P<.001) (Table 6). The use of 30 mg of preoperative OME resulted in a 98% and 60% deduction in intra-operative and PACU narcotic use, respectively. A 91% increase in alternative non-narcotic medications, including NSAID (Celecoxib, ketorolac, and Ibuprofen) and acetaminophen, was observed with ERAS. In the CPC group, these medications, if ordered, were primarily administered during surgery, while ERAS shifted their use to the pre- and post-operative phases.
Table 6.
Pain management in women undergoing CPC vs. ERAS in minimally invasive non-hysterectomy procedures
| Pain Medication Use | CPC (n=214) |
ERAS (n=196) |
P-value |
|---|---|---|---|
| Narcotics (OME) | |||
| Pre-operative | 0.0 0.0 | 28.3 7.6 | * |
| Intra-operative | 95.3 36.8 | 2.4 8.5 | <.001 |
| PACU | 56.9 36.4 | 23.0 16.7 | <.001 |
| Total | 152.2 57.1 | 55.3 21.1 | <.001 |
| Non-narcotic Pain Medications | |||
| Pre-operative | n=46/214 (21.5) | n=193/196 (98.5) | <.001 |
| NSAID | 36 (16.8) | 154 (78.6) | <.001 |
| Acetaminophen | 14 (6.5) | 192 (98.0) | <.001 |
| Intra-operative | n=175/214 (81.8) | n=6/196 (3.1) | <.001 |
| NSAID | 79 (36.9) | 4 (2.0) | <.001 |
| Acetaminophen | 156 (72.9) | 2 (1.0) | <.001 |
| PACU | n=69/214 (32.2) | n=177/196 (90.3) | <.001 |
| NSAID | 45 (21.0) | 162 (82.7) | <.001 |
| Acetaminophen | 47 (22.0) | 146 (74.5) | <.001 |
| Non-narcotic Doses | |||
| Total | 1.76 0.9 | 3.37 0.9 | <.001 |
CPC, conventional peri-operative care; ERAS, enhanced recovery after surgery; PACU, post-anesthesia care unit; OME, oral morphine equivalents; NSAID, non-steroidal anti-inflammatory drugs
Data are n (%) or mean ± standard deviation
No statistical analysis possible given no standard preoperative narcotic administration in the CPC group
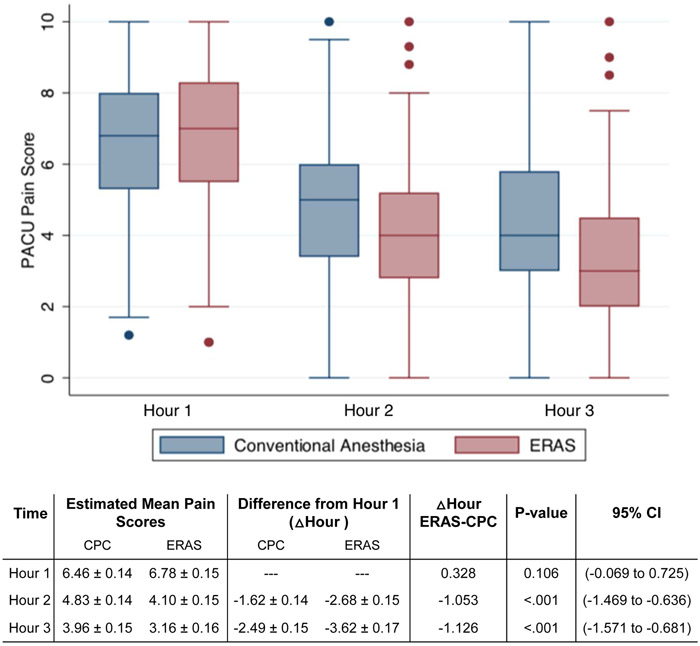
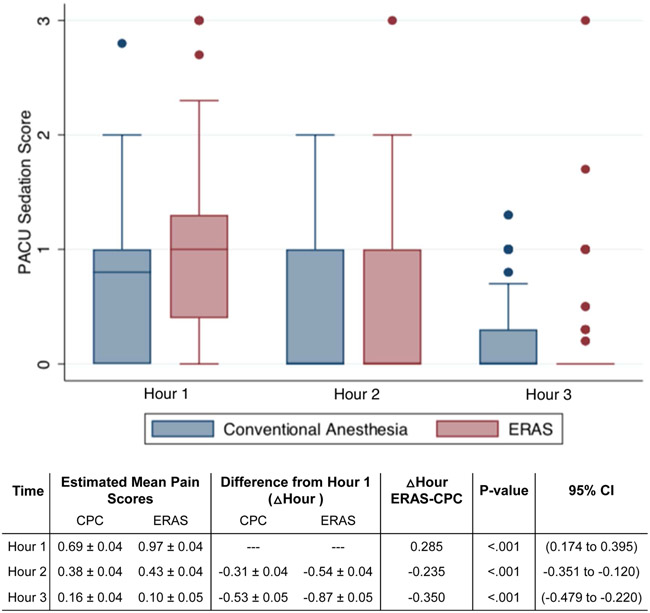
Visual analog pain score revealed that patients presented to the PACU with similar pain levels (Figure 1A). However, scores decreased more quickly at a rate of at least one additional pain score point at each subsequent hour in the ERAS cohort (P<.001). Sedation scores decreased in both groups over time, but ERAS participants experienced more sedation until hour 3 (P<0.001) when sedation levels reached low enough levels in both groups to meet discharge criteria (Figure 1B).
Figure 1A.
PACU pain scoring in women undergoing CPC vs. ERAS in minimally invasive non-hysterectomy procedures
CPC, conventional peri-operative care; ERAS, enhanced recovery after surgery; PACU, post-anesthesia care unit
Data are margins ± standard error
Δ Hour ERAS-CPC represent mixed effects linear regression coefficients estimating the difference in change of PACU pain scores at different time points compared to hour 1 between ERAS and CPC
Figure 1B.
PACU sedation scoring in women undergoing CPC vs. ERAS in minimally invasive non-hysterectomy procedures
CPC, conventional peri-operative care; ERAS, enhanced recovery after surgery; PACU, post-anesthesia care unit
Data are in margin ± standard error
Δ Hour CPC-ERAS represent mixed effects linear regression coefficients estimating the difference in change of sedation scores at different time points compared to hour 1 between CPC and ERAS
COMMENTS
Principal Findings
ERAS implementation for laparoscopic non-hysterectomy gynecologic surgeries leads to a significant increase in SDD rates and improved peri-operative patient outcomes without affecting 30-day morbidity. In our current study, 96.9% of ERAS protocol patients were discharged home following their procedure after adjusting for planned admissions while nearly one in 10 CPC pathway patients required admission. The major driving force behind unplanned admissions in CPC was inadequate pain control despite three times higher narcotic pain medication use. PONV was the second most common reason for admission, but this result was not statistically different between the groups. We also did not see a surge in emergency room presentations, post-operative complications or re-admissions in our ERAS non-hysterectomy minimally invasive gynecologic surgery population, suggesting that early discharge does not lead to increased 30-day complications.
Clinical and Research Implications
The significant reduction in post-operative overnight admissions without compromising patient safety and quality of care could substantially lower health care costs and improve patient satisfaction. Considering peri-operative costs alone, with an average difference of 19 minutes of PACU time favoring ERAS, and an estimated cost of $36.50 per minute spent on operative care, $693.50 health care dollars per patient alone could be saved (or $284,335 total for all 410 patients in a 24-months period).14 These cost savings will be even more substantial when considering additional costs of inpatient admissions avoided with ERAS implementation. A number needed to treat analysis revealed that only 17 patients are required to be treated per ERAS protocol to avoid one hospital admission experienced under CPC.
Moreover, our study is unique from others reported in the literature as it overcomes some of the biases and difficulties associated with interpreting ERAS protocol outcomes. The success of ‘fast track surgery’ pathways has in part been attributed to multifaceted changes in peri-operative care. Extensive institutional practice shifts, pre-operative patient education and care team attitudes towards supporting outpatient major gynecologic surgeries have been credited for increased SDD rates and the paradigm shift that 24-hour overnight observation is not necessary.1,5,8 By focusing on the outpatient non-hysterectomy gynecologic surgery patient population that has traditionally been discharged on POD0 in our practice, we had the rare opportunity to draw less biased conclusions on the efficacy of ERAS implementation. We demonstrate that all else equal, ERAS implementation significantly decreases admission rates, PACU pain scores, PONV and opioid consumption without impacting 30-day morbidity.
Our study is also the first to investigate peri-operative results in a non-hysterectomy minimally invasive gynecologic surgery population not involving urogynecology or oncology patients. In fact, nearly 65% of our patients who sought treatment presented for endometriosis or chronic pelvic pain evaluation and management. This patient population can often come with unique challenges in the post-operative period as opioid dependence is a common occurrence.15-17 ERAS implementation has successfully been shown to reduce opioid use in the recovery period without negatively affecting pain scores.11 We similarly observe a 64% reduction in oral morphine equivalent use in our ERAS cohort. Moreover, this reduction did not lead to higher post-operative pain scores, but rather improvement at PACU hours 2 and 3. Furthermore, only one unplanned admission for postoperative pain occurred in the ERAS group. This contrasts with the CPC cohort where the majority of unplanned post-operative admissions occurred for pain management. Interestingly, while excisions of endometriosis were ultimately performed in 52.4% of CPC patients, a disproportionate number (81.8%) of the unplanned admissions for pain control occurred in this patient subset, particularly if undergoing concurrent adnexal surgery. This patient population, which generally suffers from a higher level of disease severity and potentially increased propensity for chronic pelvic pain, may uniquely be served by ERAS.18 Use of additional non-narcotic pain medications may be critical in these distinct patient cohorts to overcome postoperative pain to achieve SDD. ERAS pathway implementation led to a significantly higher use of pre-operative and PACU non-narcotic pain medication administration while these agents were used less and primarily intraoperatively in CPC.
It has been stipulated that multimodal preoperative pain medication utilization preemptively blocks pain pathways leading to improved pain modulation, which in turn can minimize postoperative pain and opioid requirements.19 As public health concerns over the current opioid epidemic continue to rise, it is critical for surgeons to understand that narcotic use even in the postoperative period is not without risk of potential addiction and should be minimize whenever possible. There is increasing evidence that prescription opioids in the postoperative period maybe serve as the gateway to opioid dependence if not properly monitored.20-22 Hence, ERAS implementation will be a useful tool to reduce surgical opioid use across all specialties while also enhancing patient outcomes and satisfaction.
Lastly, our results investigated the positive effects of ERAS institution on PONV, which up to 70% of gynecologic surgery patients can experience.23 Although the total doses or perioperative antiemetic medications utilized did not differ between the cohorts, higher doses of pre-operative antiemetics resulted in a 56% reduction in PONV medication usage in the PACU. In our study, postoperative pain and PONV were the main driving factors dictating inpatient admission following non-hysterectomy laparoscopic gynecologic procedures. Consequently, outpatient surgery success and patient satisfactions can be maximized with fast track surgery pathways and should strongly be considered for all laparoscopic gynecologic operations and not reserved for hysterectomies alone.5,8
Strengths and Limitations
Weaknesses associated with this study are those inherent to a retrospective chart review, including inaccurate or missing data, as well as errors in data collection. Pre-operative pain scores were missing in equal proportions from both patient cohorts with only 55.9% of recorded scores available. This could be either due to patients not experiencing pain when presenting for surgery or errors in documenting of pain scores. Multivariate analyses were utilized to account for other potentially cofounding factors in order to overcome some of these limitations that otherwise can only be address by blinded randomization. Furthermore, our data is limited to one institution and four specialized fellowship-trained minimally invasive gynecologic surgeons, which reduces external validity. On the other hand, this homogeneity also controls for differences in surgeon skills that may impact operative times and practice patterns. It is important to note that although our readmission rates were slightly lower than those reported in the literature8,9, emergency room presentation were somewhat higher, albeit comparable between the groups. The majority of ED visits occurred either for wound or postoperative pain complaints. Due to the limited number of same day office appointments in our surgical practice, a large portion of these evaluations could have been performed in the office to decrease ED presentations. Morbidity analysis could also have been impacted by loss of patient follow up, which was not directly measured in our analysis. However, our postoperative no show appointment rates are overall low, and should not differ between the two groups as no postoperative practice changes occurred with ERAS implementation.
Strengths of the study include its large sample size and primary focus on a chronic pelvic pain population that can be challenging to manage postoperatively. Future studies investigating ERAS protocols in chronic pain patients suffering from narcotic addition may shed further light on preliminary associations observed in this cohort.
Conclusions
ERAS implementation has a significant positive impact on patient outcomes across multiple peri-operative parameters without affecting 30-day morbidity in laparoscopic non-hysterectomy operations. Improved postoperative pain is associated with increased SDD rates. Narcotic medication use is limited and ERAS adaptation across all specialties for any laparoscopic procedure may significantly reduce opioid dependence. ERAS utilization can have tremendous impact on health care saving and thus should be considered for all gynecologic laparoscopic procedures, not just hysterectomies.
Condensation: Enhanced recovery after surgery implementation in non-hysterectomy laparoscopic gynecologic surgeries increases same day discharge rates and improves peri-operative outcomes without affecting 30-day morbidity.
AJOG at a Glance:
- Why was the study conducted?
- To investigate the advantages of enhanced recovery after surgery in women undergoing fertility-sparing minimally invasive gynecologic procedures
- To explore perioperative care outcomes using enhanced recovery after surgery protocols with an emphasize on women with chronic pelvic pain conditions
- What are the key findings?
- Enhanced recovery after surgery implementation increases same day discharge rates without affecting 30-day morbidity in non-hysterectomy procedures
- Our enhanced recovery after surgery protocol resulted in a 64% and 57% reduction in opioid medication requirements and anti-emetics, respectively
- What does the study add to what was already known?
- Enhanced recovery after surgery protocols should be implemented for all gynecologic laparoscopic procedures and not reserved for laparoscopic/robotic hysterectomies alone
- Enhanced recovery after surgery administration prevents unplanned post-anesthesia care unit admissions by primarily targeting pain management in women undergoing extensive endometriosis surgery
- Health care costs could significantly be curbed with universal enhanced recovery after surgery initiatives
Acknowledgments
Funding: The National Institutes of Health through Grant Number UL1-TR-000005 supported the statistical analysis of this project.
Footnotes
Disclosures: The authors declare that they have no conflicts of interest and nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery a review. JAMA Surg. 2017;152(3):292–298. [DOI] [PubMed] [Google Scholar]
- 2.Chapman JS, Roddy E, Ueda S, Brooks R, Chen LL, Chen LM. Enhanced recovery pathways for improving outcomes after minimally invasive gynecologic oncology surgery. Obstet Gynecol. 2016;128(1):138–144. [DOI] [PubMed] [Google Scholar]
- 3.Trowbridge ER, Dreisbach CN, Sarosiek BM, et al. Review of enhanced recovery programs in benign gynecologic surgery. Int Urogynecol J. 2018;29(1):3–11. [DOI] [PubMed] [Google Scholar]
- 4.Carey ET, Moulder JK. Perioperative management and implementation of enhanced recovery programs in gynecologic surgery for benign indications. Obstet Gynecol. 2018;132(1):137–146. [DOI] [PubMed] [Google Scholar]
- 5.Modesitt SC, Sarosiek BM, Trowbridge ER, et al. Enhanced Recovery Implementation in Major Gynecologic Surgeries: Effect of Care Standardization. In: Obstetrics and Gynecology. Vol 128 ; 2016:457–466. [DOI] [PubMed] [Google Scholar]
- 6.Nelson G, Altman AD, Nick A, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations — Part I. Gynecol Oncol. 2016;140(2):313–322. [DOI] [PubMed] [Google Scholar]
- 7.Nelson G, Altman AD, Nick A, et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations--Part II. Gynecol Oncol. 2016;140(2):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter-Brooks CM, Du AL, Ruppert KM, Romanova AL, Zyczynski HM. Implementation of a urogynecology-specific enhanced recovery after surgery (ERAS) pathway. Am J Obstet Gynecol. 2018;219(5):495.e1–495.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keil DS, Schiff LD, Carey ET, et al. Predictors of Admission After the Implementation of an Enhanced Recovery After Surgery Pathway for Minimally Invasive Gynecologic Surgery. Anesth Analg. April 2018:1. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Asher V, Nair A, et al. Comparing the experience of enhanced recovery programme for gynaecological patients undergoing laparoscopic versus open gynaecological surgery: a prospective study. Perioper Med. 2018;7(15):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer LA, Lasala J, Iniesta MD, et al. Effect of an Enhanced Recovery After Surgery Program on Opioid Use and Patient-Reported Outcomes. Obstet Gynecol. 2018; 132(2):281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korsholm M, Mogensen O, Jeppesen MM, Lysdal VK, Traen K, Jensen PT. Systematic review of same-day discharge after minimally invasive hysterectomy. Int J Gynecol Obstet. 2017;136(2):128–137. [DOI] [PubMed] [Google Scholar]
- 13.Alton K, Sullivan S, Udaltsova N, Yamamoto M, Zaritsky E. Same-Day Discharge after Minimally Invasive Myomectomy. Obstet Gynecol. 2016;127(3):539–544. [DOI] [PubMed] [Google Scholar]
- 14.Childers CP, Maggard-Gibbons M. Understanding Costs of Care in the Operating Room. JAMA Surg. 2018;153(4):e176233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cichowski SB, Rogers RG, Komesu Y, et al. A 10-yr Analysis of Chronic Pelvic Pain and Chronic Opioid Therapy in the Women Veteran Population. Mil Med. 2018; 183(11– 12):e635–640. [DOI] [PubMed] [Google Scholar]
- 16.Steele A Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491–501. [DOI] [PubMed] [Google Scholar]
- 17.Madsen AM, Stark LM, Has P, Emerson JB, Schulkin J, Matteson KA. Opioid Knowledge and Prescribing Practices Among Obstetrician–Gynecologists. Obstet Gynecol. 2018; 131 (1): 150–157. [DOI] [PubMed] [Google Scholar]
- 18.Schliep KC, Mumford SL, Peterson CM, et al. Pain typology and incident endometriosis. Hum Reprod. 2015;30(10):2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long JB, Bevil K, Giles D. Preemptive analgesia in MIGS. J Minim Invasive Gynecol. 2019;26(2):198–218. [DOI] [PubMed] [Google Scholar]
- 20.Thiels CA, Anderson SS, Ubl DS, et al. Wide Variation and Overprescription of Opioids after Elective Surgery. Ann Surg. 2017;266(4):564–573. [DOI] [PubMed] [Google Scholar]
- 21.Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in us adults. JAMA Surg. 2017;152(6):e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Movilla PR, Kokroko JA, Kotlyar AG, Rowen TS. Postoperative Opioid Use Using Enhanced Recovery After Surgery Guidelines for Benign Gynecologic Procedures. J Minim Invasive Gynecol. 2019. doi: 10.1016/j.jmig.2019.04.017 [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee S, Rudra A, Sengupta S. Current Concepts in the Management of Postoperative Nausea and Vomiting. Anesthesiol Res Pract. 2011;2011:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]