Abstract
OBJECTIVE:
We performed a systematic review and meta-analysis to determine whether D-mannose reduces urinary tract infection (UTI) recurrence (i.e. cumulative incidence) in adult women with recurrent UTI compared to other prevention agents. Secondary outcomes included side effects and compliance with D-mannose use.
DATA SOURCES:
Ovid Medline 1946-, Embase 1947-, Scopus 1823-, Cochrane Library, Web of Science 1900-, and Clinicaltrials.gov were searched through 4/15/2020.
STUDY ELIGIBILITY CRITERIA:
Systematic review inclusion: randomized controlled trials (RCTs), prospective cohorts, and retrospective cohorts written in English of women ≥18 years old with recurrent UTI in which D-mannose was utilized as an outpatient prevention regimen. Systematic review exclusion: lab or animal-based research, study protocols only, and conference abstracts. Meta-analysis inclusion: stated D-mannose dose, follow-up time ≥6 months, a comparison arm to D-mannose, and data available from women ≥18 years of age.
STUDY APPRAISAL AND SYNTHESIS METHODS:
Two independent reviewers made abstract, full text, and data extraction decisions. Study methodologic quality was assessed using the Cochrane Risk of Bias tool. Relative risks (RRs), confidence intervals (CIs), and heterogeneity (I2) were computed.
RESULTS:
Searches identified 776 unique citations. Eight publications met eligibility: 2 using D-mannose only; 6 using D-mannose combined with another treatment. Seven studies were prospective: 2 RCTs, 1 randomized cross-over trial, and 4 prospective cohort studies. One retrospective cohort study was included. Three studies met meta-analysis eligibility (1 RCT, randomized cross-over trial, prospective cohort). Pooled RR of UTI recurrence comparing D-mannose to placebo was 0.23 (95%CI: 0.14–0.37; I2=0%; D-mannose n=125, placebo n=123). Pooled RR of UTI recurrence comparing D-mannose to preventative antibiotics was 0.39 (95%CI: 0.12–1.25; I2=88%; D-mannose n=163, antibiotics n=163). Adverse side effects were reported in 2 studies assessing D-mannose only (one study (n=10) reported none; the other reported a low incidence (8/103 participants) of diarrhea). Two studies reported compliance, which was high.
CONCLUSIONS:
D-mannose appears protective for recurrent UTI (versus placebo) with possibly similar effectiveness as antibiotics. Overall, D-mannose appears well tolerated with minimal side effects - only a small percentage experiencing diarrhea. Meta-analysis interpretation must consider the small number of studies with varied study design and quality and the overall small sample size.
Keywords: D-mannose, Non-antibiotic, Nutraceutical, Prevention, Recurrent Urinary Tract Infection, Urinary Tract Infection, UTI
Introduction
Eleven percent of women in the United States (US) report at least one physician-diagnosed urinary tract infection (UTI) per year.1 Up to half of women will experience an additional UTI within the first year after initial infection.1,2 In the US, annual societal costs of urinary tract infections (UTIs) in all patients are estimated to be at least $2.4 billion,3,4 with some estimates upwards of $3.5 billion.1 In addition to the financial implications, UTIs can negatively impact patients’ work productivity, personal and family responsibilities, quality of life, and sexual well-being.5–7
Recurrent UTI (rUTI) is defined as 2 UTI episodes in six months, or 3 UTI episodes in twelve months. Historically, antibiotics have been used as the primary method to prevent rUTI. These medications are associated with a wide range of side effects, including diarrhea, nausea, headache, vaginal burning and candidiasis.8,9 Although less common, more serious side effects can occur. For example, long-term nitrofurantoin use has been associated with pulmonary and hepatic toxicity and trimethoprim-sulfamethoxazole has been associated with numerous serious cutaneous reactions, including Stevens-Johnson syndrome, blood dyscrasias, and drug interactions.10–12 Long-term antibiotic use can also lead to alteration of normal flora and antibiotic resistance, making it difficult to treat future UTI. For all these reasons, non-antibiotic rUTI prevention regimens, such as D-mannose, have increasingly been the subject of research.
The most common rUTI uropathogen is Escherichia coli (E. coli). E. coli has specific virulence factors that promote infection through adhesion to urothelial cells; the most commonly expressed virulence factor is type 1 fimbriae.12,13 Also known as type 1 pili, these virulence factors promote bacterial adhesion to urothelial cells and an early inflammatory response by recruiting neutrophils to the urinary tract.13–16 D-mannose is a promising non-antibiotic prevention strategy because it binds to the tip of type 1 pili and saturates the adhesin FimH, thereby preventing bacterial adhesion to the urothelium.12,17,18 FimH interaction with the urothelium is also believed to initiate a signaling cascade promoting uropathogenic E. coli urothelial invasion.19 Saturation of FimH by D-mannose should theoretically prevent this invasion by preventing induction of the signaling cascade. New research suggests that D-mannose may also act as an immune modulator.15 The majority of research surrounding type 1 fimbriae and UTIs focuses on E. coli. However, type 1 pili have been documented on other members of the Enterobacteriaceae family, including Klebsiella pneumoniae, Shigella flexneri, Salmonella typhimurium, Serratia marcescens, and Enterobacter cloacae 20,21 Many of these are common rUTI uropathogens.
Our objective was to systematically review and combine data from published original literature evaluating the effectiveness of D-mannose compared to other agents for rUTI prevention in adult women. Secondary objectives were to evaluate side effects and compliance with D-mannose use. A systematic review and meta-analysis (SRMA) is needed to provide a compiled source of evidence to guide clinical practice. We hypothesized D-mannose would have similar efficacy as antibiotics in preventing rUTI and that it would be well tolerated.
Methods
This SRMA was submitted to the Washington University in St Louis School of Medicine institutional review board and determined to not be human subjects research. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed. An a priori protocol was written and followed.
Information sources and search strategy
A comprehensive literature search was performed with a medical librarian using search strategies, standardized terms, and keywords for the concepts of recurrent urinary tract infection and mannose. These strategies were executed in Ovid Medline 1946-, Embase 1947-, Scopus 1823-, Cochrane Library, Web of Science 1900-, and Clinicaltrials.gov. All searches were completed on February 5, 2018, March 5, 2019, and again on April 15, 2020. Database-supplied English language limits were applied. Endnote was used for deduplication. Manual checks and comparisons were also performed to determine whether abstracts were unique or duplicates. If there was any uncertainty at the abstract level, the two authors reviewed the full-text article and came to consensus. The full search strategies can be found in the appendix and they followed a similar strategy as the one used for Ovid-Medline April 2020 search: (exp urinary tract infections/) or (urinary adj1 tract adj1 infection*) or bacteriuria or pyuria or schistosomiasis haematobia or cystalgia or cystitis or pyelocystitis or exp cystitis/or (recurrent adj1 urinary adj1 tract adj1 infection*) AND (mannose/or mannose or mannosides or exp mannosides/or methylmannosides or d-mannose).
Study selection and eligibility criteria
Abstracts and full text publications were screened with a predetermined list of eligibility criteria. Inclusion criteria were as follows: a.) original clinical research (Randomized controlled trials [RCTs], including cross-over trials, prospective cohorts, and retrospective cohorts - not including case reports, case series, or conference abstracts) for women receiving care in an outpatient setting for rUTI (defined as at least 2 UTI in 6 months or at least 3 UTI in 12 months); b.) participant age of 18 years or older; and c.) a study arm including D-mannose as a UTI prevention intervention. Exclusion criteria included: a.) laboratory or animal-based research; b.) publications written in languages other than English; c.) duplicate publications; and d.) published study protocols. If a study met all criteria, it was included. For our meta-analysis (MA), we only included studies with clearly stated D-mannose dosing, follow-up time ≥6 months, and a comparator arm. Studies that lacked follow-up, a comparator arm, or combined D-mannose with additional supplements were included in the systematic review (SR) but not the MA.
Abstract and full text review, data extraction and quality assessment
Abstracts from the systematic literature search were independently screened by two authors (SL and MB) using the stated eligibility criteria. Full text of selected abstracts were independently reviewed by the same two authors. Data extraction was also independently performed by two authors. In the event of disagreement, the two authors reviewed the study together and a third reviewer was available if a consensus could not be made. Each of the clinicaltrials.gov search results was reviewed to ensure that any known unpublished data were included in our MA. At the beginning of data extraction, an application for Prospero registration was submitted with our a priori protocol. Due to the extended timeline for processing and reviewing the application, this study was not selected to be registered as the registry felt the study was too close to completion by the time the application was reviewed.
We performed a SRMA to determine whether D-mannose reduces UTI recurrence (i.e. cumulative incidence) in adult women with rUTI compared to other prevention agents. Secondary outcomes included side effects and compliance with D-mannose use. The primary outcome was defined as the proportion of participants experiencing at least one UTI in a 6-month or greater time frame of treatment (cumulative incidence). We referred to the first UTI during study follow up as the incident UTI. Extracted information included title, year of publication, author names, study information and duration, including timing of measurements, demographics, and baseline characteristics, information about the intervention and/or control arms, treatment outcomes, side effects reported in participants using D-mannose, and compliance with D-mannose use.
We assessed the methodologic quality of each study using predefined criteria from a three-tier system in which studies were graded as good, fair, or poor based on scientific merit, the likelihood of biases, and the completeness of reporting. This grading was completed according to the Cochrane Risk of Bias tool (as either high, low or unclear) and relevant questions from the Newcastle-Ottawa Scale.22,23 Selective study reporting within studies was assessed as part of the risk of bias assessment.
Data synthesis and analysis
Aggregate data from published reports were used to determine the effect of D-mannose compared to other treatments (such as a placebo or antibiotic). At minimum, two studies were required for each MA performed. Our a priori protocol had initially planned for relative risks (RRs) to be computed using a fixed effects model where published estimates are combined using a weighted average (weighted according to the number of participants in each study). However, due to the small number of studies identified for this MA, the decision was made to compute RRs using a random effects model with robust variance estimation since I2 can be falsely low with a small number of studies.24 Confidence intervals (CI) for the log RRs were determined and then exponentiated to obtain the confidence interval for the RR. These were computed and graphed in R, using the metafor and forestplot libraries. Two separate MAs were done: a MA of D-mannose versus placebo, and MA of D-mannose versus antibiotics. Data are presented similarly to other SRMA with comparable primary outcomes.25
Results
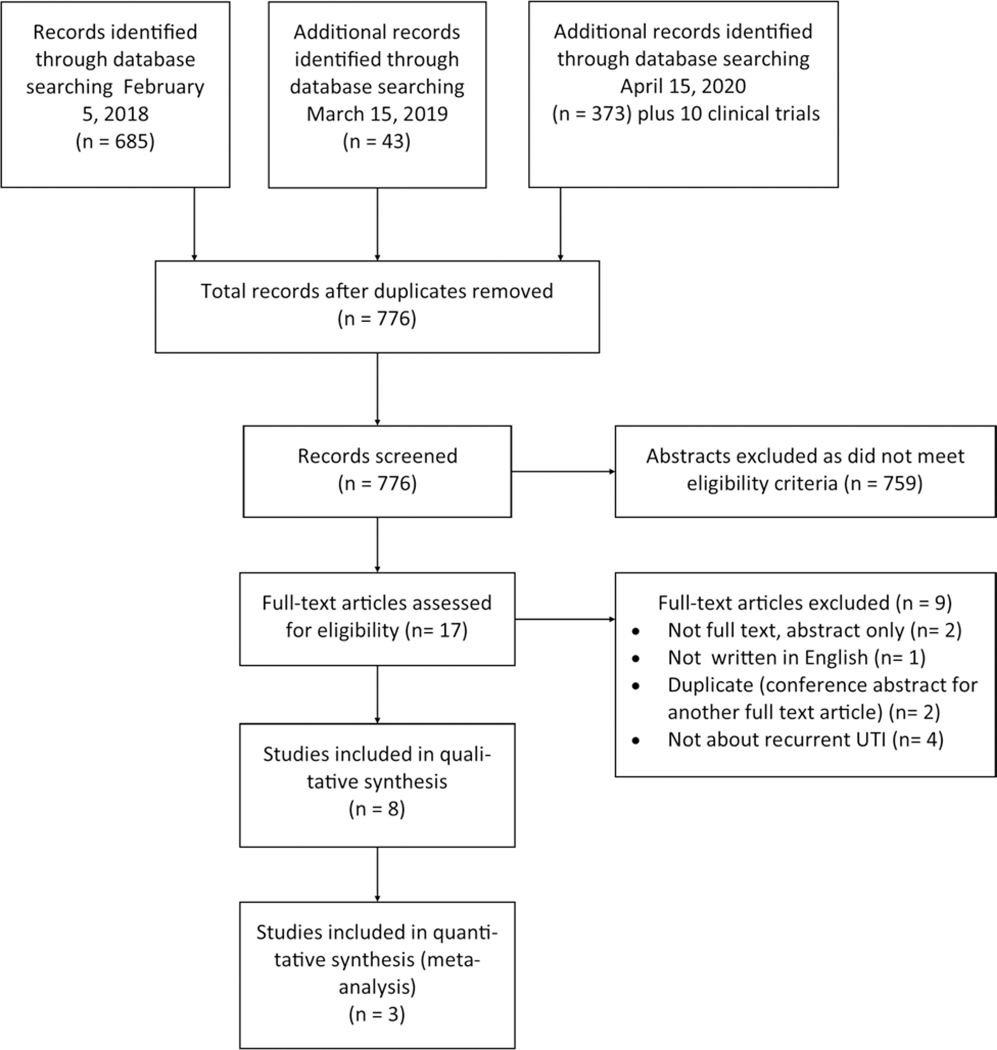
The literature search yielded 776 unique abstracts, of which 17 were reviewed in full text (Figure 1, PRISMA diagram). Of the 17 full text publications reviewed, three were conference abstracts only (no accompanying full text), one was non-English (only abstract was written in English), and one was a duplicate study; leaving 12 full text articles for further review and data extraction. Four of the 12 articles evaluated patient populations other than adult women with rUTI, leaving a total of 8 articles that met the SR eligibility criteria (Table 1). Seven studies were prospective: 2 RCTs, 1 randomized cross-over trial, and 4 prospective cohort studies. The other study was a retrospective cohort study. Cochrane assessment of bias of each study (Table 2) was used to determine each study’s grade of evidence. Evaluation of selective reporting within studies is shown in Table 2. The definition of rUTI and incident UTI as defined in each study is outlined in Table 3 as well as the study follow up time. Of note, the authors identified a few additional studies during abstract and full text review that demonstrated possible additional uses of D-mannose for preventing UTI after urodynamic office procedures and for treating UTI and urinary stones.26–28 These were not included in the SRMA as they did not use D-mannose for prevention of recurrent uncomplicated infections. An additional article was found to be written in Italian, despite an English abstract. Ten studies were identified in our clinicaltrials.gov search, including two rUTI studies evaluating D-mannose that are still currently recruiting and two studying non-antibiotic rUTI prevention regimens that have not yet begun recruiting. No other studies were relevant. All completed relevant studies were included in our SRMA.
Figure 1:
PRISMA flow diagram
Table 1:
Characteristics of Included Studies
| Authors, Year | Study design | Study Population | Inclusion Criteria | Exclusion Criteria | D-mannose group (dose, other components, duration) | Compared group(s) | Grade of Evidencea |
|---|---|---|---|---|---|---|---|
| Meta-analysis Studies | |||||||
| Domenici et al, 201631 | Prospective cohort | Women, age 18–65 yo (mean 46.7 ± 5.7 y) n=45 | Acute cystitis and/or history of recurrent UTIs b | urinary tract anomalies, pregnancy/breastfeedi ng, symptoms of pyelonephritis, upper tract infection, hormone therapy, diabetes, use of CISC, previous antibiotic prophylaxis | All participants received oral Mannocist® (D-mannose 1.5 g, sodium bicarbonate, sorbitol and silicon dioxide) BID for 3 days, then daily for 10 days at same dose. No antibiotics were given. | Poor | |
| Prophylaxis: oral Mannocist® daily (one week every other month) × 6 months (n=22) | Prophylaxis: None (n=21) | ||||||
| Kranjcec et al, 20148 | Randomized controlled trial | Women age ≥18 yo (median 48–52, range 29–58) n=308 | Acute cystitis and history of recurrent UTIs b | Pregnant/breastfeeding symptoms of pyelonephritis, urinary tract anomalies, diabetes, current hormone therapy/contraception, previous antibiotic prophylaxis | All participants first rec ciprofloxacin 500 mg B eived oral D × 7 days | Fair | |
| Prophylaxis: 2 g of oral D-mannose powder (diluted in 200 ml of water) daily × 6 months (n=103) | Active prophylaxis: 50 mg of Nitrofurantoin daily × 6 months (n=103) or Control prophylaxis: none(n=102) | ||||||
| Porru et al, 201437 | Randomized cross-over trial | Women age ≥18 yo (median age 42 y, range 22–54) n=46 | Current acute symptomatic UTI and history of recurrent UTIs b | Symptoms of pyelonephritis, renal disease, anatomical abnormalities, prior gynecological surgery, immunosuppressive medications or diseases, pregnant/breastfeeding | Oral D-mannose 1 g TID Prophylaxis: TID × 2 weeks then BID × total 6 months (n=30) | Oral TMP/SMX 160/800 mg BID × 5 days Prophylaxis: TMP/SMX 160 mg/800 daily (one week each month) × total 6 months (n=30) | Fair |
| 6 month: Cross-over to other group for additional 6 months | |||||||
| Other studies | |||||||
| Genovese et al, 20 1 738 | Randomized three-arm parallel group | Women (no ages reported) n=72 | Current acute UTI and history of recurrent cystitisb | Pregnancy/lactation, abnormalities of the upper urinary tract, permanent urinary catheter, stage 5 chronic kidney disease | A: oral D-mannose 420 mg + berberine, arbutin, birch (n=24) B: oral D-mannose 420 mg + berberine, arbutin, birch, forskolin (n=24) C: oral D-mannose 500 mg + proanthocyanidins (n=24) All (A, B, and C) for 12 week duration | None (all arms with D-mannose) | Poor |
| Phe et al, 20 1 732 | Open label feasibility, prospective study | Men or women (median age 50.0, IQR 46.2–59.0) n=22 | clinically stable MS ≥ 3 mo, history of recurrent UTIs b | Pregnancy, urinary tract anomalies, diabetes mellitus, current UTI or vaginal infection, allergies to D-mannose | Oral D-mannose 1.5 g BID × 16 weeks (n=22) | None | Poor |
| Del Popolo et al, 201816 | Prospective cohort | Men or women seen in neuro-urology clinic(mean age 45.2 y, range 22–78) Neurogenic bladder (n=33 female) Non-neurogenic bladder (n=39 female) | Symptomatic UTI, history History of recurrent UTIs,b previous unsuccessful treatment with D-mannose and/or cranberry | Non-E. coli UTI at the screening, pregnancy/breastfeedi ng, hematuria, fever, urogenital abnormalities, allergies to salicylates and/or D-mannose, antibiotics in the last two weeks | All participants receivec mannose + 200 mg of Ci (salicin) TID for 5 Cays | 1000 oral mg D-y willow extract | Poor |
| All received 700 mg oral D-mannose + 50 mg (1×109 CFU) of oralLactobacillus acidophilus (La-14) BID for 15 Cays monthly for 2 months | |||||||
| Efros et al, 20 1 030 | Prospective, dose-escalation trial | Women (mean age 46.5, SD 12.8 y) n=28 | History of History of recurrent UTIs b | current UTI or vaginitis, allergy to cranberry, predisposition to kidneys stones, diabetes on insulin, immunosuppressive disease/corticosteroid use, catheterization, pregnant/breastfeeding, abnormalities of the urinary tract, recent prophylactic antibiotics, warfarin | Study agent of oral liquid dietary supplement UTI-STAT (3875 mg Proantinox [cranberry concentrate 4:1, ascorbic aciC, D-mannose, fructo-oligosacchariCes, and bromelain]/30 mL) Participants were given 15 mL of UTI-STAT with Proantinox then increasing by 15 mL to maximal dose target of 90 mL/d (only reached 75 ml/d due to adverse events (n=28) | Poor | |
| Marchiori et al, 20 1 733 | Retrospective cohort study | Women (no ages reported) n=60 | History of recurrent UTIs, physiological menopause and childbearing age, breast cancer | Not reported | 1: oral D-mannose 500 mg, n-acetylcysteine 100 mg, and morinda citrifolia fruit extract 200 mg (NDM) BID × 2 months then daily × 4 months with antibiotic regimen as needed(n=40) | 2: no prophylaxis (either Fosfomycin, nitrofurantoin, or ciprofloxacin for acute cystitis) (n=20) | Poor |
Methodologic quality of each study was assessed using predefined criteria from a three-tier system in which studies were graded as good, fair, or poor based on the Cochrane Risk of Bias tool and relevant questions from the Newcastle-Ottawa Scale
Recurrent UTI defined as ≥2 episodes of acute cystitis/last 6 months and/or 3 episodes/last 12 months
Abbreviations: BID, twice daily; CISC, clean intermittent self-catheterization; IQR, interquartile range; LUTS, lower urinary tract symptoms; rUTI: recurrent urinary tract infection; TID, three times a day; TMP/SMX, trimethoprim/sulfamethoxazole; UTI, urinary tract infection
Table 2:
Summary of risk bias assessment and grade of evidence
| Authors, Year | Random Sequence Generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Grade of Evidence |
|---|---|---|---|---|---|---|---|
| Meta-analysis Studies | |||||||
| Domenici et al, 201631 | ↑ | ↑ | ↑ | Unclear | ↓ | Unclear | Poor |
| Kranjcec et al, 20148 | ↑ | ↑ | ↑ | Unclear | ↓ | Unclear | Fair |
| Porru et al, 201437 | ↓ | Unclear | ↑ | Unclear | ↓ | ↓ | Fair |
| Other studies | |||||||
| Genovese et al, 201738 | ↓ | ↑ | ↑ | ↑ | ↓ | Unclear | Poor |
| Phe et al, 20 1 732 | ↑ | ↑ | ↑ | ↑ | ↓ | ↓ | Poor |
| DelPopolo et al, 201816 | ↑ | ↑ | ↑ | ↑ | ↑ | Unclear | Poor |
| Efros et al, 20 1 030 | ↑ | ↑ | ↑ | ↑ | ↑ | Unclear | Poor |
| Marchiori et al, 201733 | ↑ | ↑ | ↑ | ↑ | ↑ | Unclear | Poor |
Risk of bias graded as either high (↑), low (↓) or unclear
Table 3:
Definitions used for recurrent UTI, UTI recurrence, and study follow up time
| Meta-analysis | Other Studies | |||||||
|---|---|---|---|---|---|---|---|---|
| Study | Domenici et al, 201631 | Kranjcec et al, 20148 | Porru et al, 201437 | Genovese et al, 201738 | Phe et al, 201732 | Del Popolo et al, 201816 | Efros et al, 201030 | Marchiori et al, 201730 |
| Definition of recurrent UTI used by each study | ||||||||
| ≥2 UTI/6 mo and/or ≥3 UTI/12 mo (Sx + Ucx) | • | • | • | • | • | • | ||
| ≥2 UTI/12 mo (+ Ucx) | • | |||||||
| Not reported | • | |||||||
| Definition of incident UTI during study follow up | ||||||||
| +Sx (no UCx, or not reported) | • | |||||||
| + Sx and Ucx | • | • | • | • | • | • | ||
| Not reported/Not evaluated | • | |||||||
| Definition of study follow up time | ||||||||
| 16 weeks | • | • | ||||||
| 6 months | • | • | • | |||||
| 24 weeks | • | |||||||
| 12 months | • | |||||||
| Not reported/Not applicable | • | |||||||
For all studies with UCx, definition of positive urine culture ranged from ≥103 to ≥105
Abbreviations: UTI, urinary tract infection; UCx, urine culture; Sx, lower urinary tract symptoms; mo, months
For included studies, D-mannose dose, frequency and length of treatment varied, ranging from as low as 420 mg to 2 g, once daily to three times per day, and for one week per month to daily. (Tables 1 and 4). When D-mannose was evaluated with another treatment, the concomitant therapy was typically another supplement or probiotic. None of the studies evaluated D-mannose in the context of vaginal estrogen therapy use (a standard non-antibiotic rUTI prevention method for post-menopausal women with rUTI). These articles were assessed to identify eligibility for the MA that required data comparing D-mannose to a comparator (either placebo or antibiotic prevention of rUTI). Ultimately, 3 studies were included in the MA and had sample sizes ranging from 43 to 308 participants. In the D-mannose arms of the 3 MA studies, the proportion of women with at least one UTI during follow up (cumulative incidence) ranged from 4.5% to 20%. In two studies, the mean time to symptomatic UTI was between 43 days (± 5.4 SD) and 200 days (±50.7) and the other study reported a median time to recurrence of 30 days (range 20–41). All studies were single centers and were undertaken in Croatia or Italy. One study (a prospective cohort) assessed the effect of D-mannose for rUTI prophylaxis versus no prophylaxis, the second study (a randomized cross-over trial) compared D-mannose to antibiotic prophylaxis (trimethoprim/sulfamethoxazole), and the third study (a RCT) had three arms and compared D-mannose to no treatment and to antibiotic prophylaxis (nitrofurantoin). We used data from these three studies to perform two MAs, one comparing D-mannose to placebo and one comparing D-mannose to antibiotics.
Table 4:
Reported adverse events by study
| Authors, Year | D-mannose group (dose, other components, duration)a | Compared group(s)a | AE reported in D-mannose group | AE reported in compared group | Reported difference between groups |
|---|---|---|---|---|---|
| D-mannose alone | |||||
| Kranjcec et al, 20148 | All participants first received ciprofloxacin 500 mg BID × 7 days | 8 of 103 participants taking D-mannose experienced diarrhea (7.8%); No nausea, headache, skin rash, or vaginal burning reported | 29 of 103 participants taking
nitrofurantoin reported AE (27.2%); • Diarrhea (n=10) • Nausea (n=6) • Headache (n=3) • Skin rash (n=1) • Vaginal burning (n=9) |
Risk of AE in D-mannose group compare to nitrofurantoin group: RR 0.276, 95% CI 0.132–0.574, P<0.0001 | |
| Prophylaxis: 2 g of D-mannose powder (diluted in 200 ml of water) daily × 6 months (n=103) | Active prophylaxis: 50 mg of Nitrofurantoin daily × 6 months (n=103) Control prophylaxis: none (n=102) | ||||
| Phe et al, 201732 | D-mannose 1.5 g BID × 16 weeks (n=22) | None | No adverse events were reported | n/a - no comparator group | n/a - no comparator group |
| Porru et al, 201437 | D-mannose 1 g TID Prophylaxis: TID × 2 weeks then BID × total 6 months (n=30) |
TMP/SMX 160/800 mg BID × 5 days
Prophylaxis: TMP/SMX 160 mg/800 daily (one week each month) × total 6 months (n=30) |
Not reported
specifically for this study. Generalized statement in discussion section: “No significant side effects limiting long-term consumption of mannose have been reported.” |
||
| 6 month: Cross-over to over group for additional 6 months | |||||
| D-mannose combined with other ingredients | |||||
| Domenici et al, 20 1 631 | All participants received Mannocist® (D-mannose 1.5 g, sodium bicarbonate, sorbitol and silicon dioxide) BID for 3 days, daily 10 days | Generalized statement for study: “Treatment did not presentany side effect also in a long-term schedule” | |||
| Prophylaxis: Mannocist® daily (one week every other month) × 6 months (n=22) | Prophylaxis: None (n=21) | ||||
| Genovese et al, 20 1 738 | A: D-mannose 420 mg + berberine, arbutin,
birch (n=24) B: D-mannose 420 mg + berberine, arbutin, birch, forskolin (n=24) C: D-mannose 500 mg + proanthocyanidins (n=24) All for 12 weeks |
None (all arms with d-mannose) | Not reported | ||
| Del Popolo et al, 201816 | All participants received 1000 mg D-mannose + 200 mg of dry willow extract (salicin) TID for 5 days | “No significant side effects” were reported | |||
| Neurogenic bladder (n=4, 33
female) Non-neurogenic bladder (n=39) All received 700 mg D-mannose + 50 mg (1×109 CFU) of Lactobacillus acidophilus (La-14) BID for 15 days monthly for 2 months | |||||
| Efros et al, 201030 | Study agent of liquid dietary
supplement UTI-STAT (3875 mg Proantinox [cranberry concentrate 4:1,
ascorbic acid, D-mannose, fructo-oligosaccharides, and bromelain]/30
mL) Participants were given 15 mL of UTI-STAT with Proantinox then increasing by 15 mL to maximal dose target of 90 mL/d (only reached 75 ml/d due to adverse events (n=28) |
At a dose of 75 mL/d 3 of 3
patients developed diarrhea, headache, and heartburn. All other adverse
events during the 15–60 mL/d regimens were mild to moderate in
severity, and mostly GI related and resolved with either taking the
study drug with food or spontaneous • Nausea (n=1; resolved when taken with food) • Heartburn (n=1; resolved when taken with food) • Diarrhea (n=1; resolved in ≥4 weeks) • Dyspepsia (n=4; resolved when taken with food) • Headache (n=1; resolved in ≥4 weeks) • Back pain (n=1; resolved in ≥4 weeks) |
|||
| Marchiori et al, 201733 | 1: D-mannose 500 mg, n-acetylcysteine 100 mg, and morinda citrifolia fruit extract 200 mg (NDM) BID × 2 months then daily × 4 months with antibiotic regimen as needed(n=40) | 2: no prophylaxis (either Fosfomycin, nitrofurantoin, or ciprofloxacin for acute cystitis) (n=20) | Not reported | ||
Column contains same data as shown in Table 1
Abbreviations: AE, adverse event; BID, twice daily; d, day; g, grams; IQR, interquartile range; LUTS, lower urinary tract symptoms; mg, milligram; mL, milliliter; RR, relative risk; TID, three times a day; TMP/SMX, trimethoprim/sulfamethoxazole; UTI, urinary tract infection
The three MA studies were published between 2013 and 2016. With respect to patient demographics, two studies reported median age with range between 42 and 48 and the other study reported a mean age of 46.7. All three MA studies only included women that had an acute UTI and a history of rUTI (all defined as ≥2 UTIs in the last 6 months and/or ≥3 in the last 12 months). Reporting of key indicators of study quality was limited, with all studies providing few details about the process of blinding if it was randomized. All studies involved an initial treatment course followed by prophylaxis with D-mannose in a range of doses (Tables 1 and 4). One study utilized Mannocist® once daily only one week per month for 6 months, another utilized 2 g daily for 6 months, and finally the third utilized 3 g total (1g TID) × 2 weeks then 2 g daily for a total of 6 months. Some of the studies assessed patient populations at higher risk of rUTI, such as patients with neurogenic bladders or breast cancer patients that are likely in a hypoestrogenic state due to breast cancer therapy. The remaining studies that met SR criteria were excluded from the MA for the following reasons: they did not report number of patients with UTI (n=3) or dose of D-mannose administered (n=1), or they compared different D-mannose formulations (n=1).
Effectiveness Outcomes
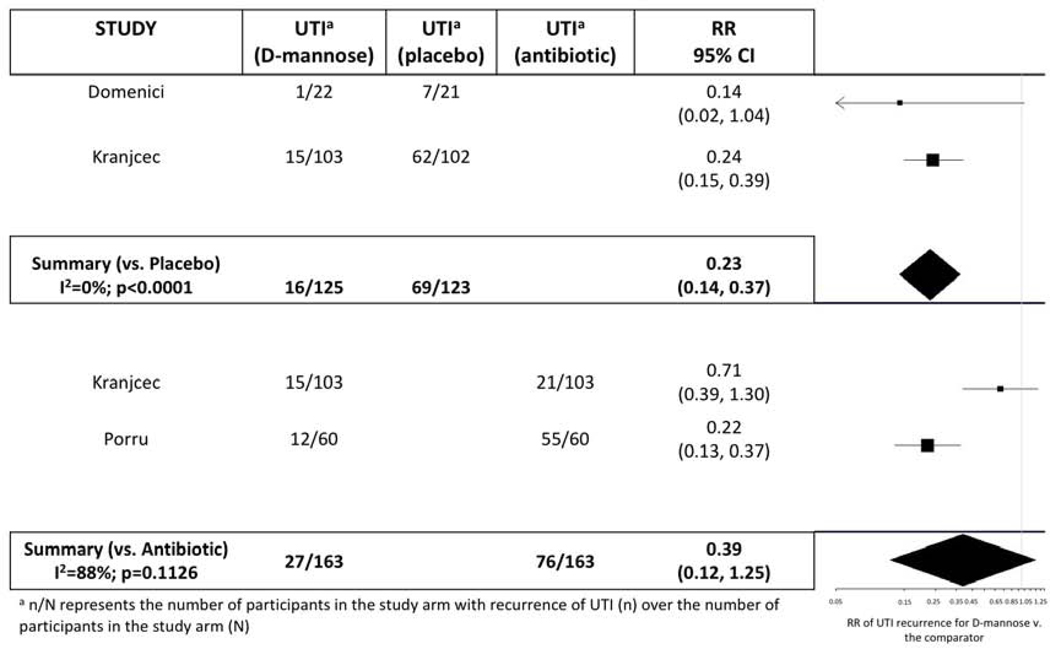
We performed two separate MAs comparing D-mannose to placebo/control and D-mannose to antibiotic prophylaxis. The relative risk (RR) of rUTI for those on D-mannose compared to placebo varied from 0.14 (95% CI: 0.02–1.04) to 0.24 (95% CI: 0.15–0.39). When pooled, there were 125 participants taking D-mannose and 123 taking placebo. The overall RR was 0.23 (95% CI: 0.14–0.37) showing a protective effect of D-mannose compared to placebo (Figure 2). The MA versus placebo had an I of 0%.
Figure 2:
Meta-analysis with forest plot of D-mannose versus other agents for recurrent urinary tract infection prevention
The RR of rUTI for those on D-mannose compared to preventative antibiotics varied from 0.22 (95% CI: 0.13–0.37) to 0.71 (95% CI: 0.39–1.30). When pooled, there were 163 participants taking D-mannose and 163 taking antibiotics. The overall RR was 0.39 (95% CI: 0.12–1.25) showing possibly similar effectiveness of D-mannose compared to preventative antibiotics (Figure 2). The MA versus antibiotics had an I2 of 88%. Although funnel plots generally appeared symmetric (Figure 3), evaluation of symmetry is most meaningful for MAs with at least 10 studies.29
Figure 3:
Funnel plots
Side Effects
All 8 studies in the SR were assessed for reported side effects of D-mannose. Adverse events (AEs) were reported in 2 studies assessing D-mannose alone (i.e. not in combination with other ingredients) (Table 4). One study (n=22) reported no AEs to D-mannose and the other study (n=103) reported a low incidence of diarrhea (7.8%). Importantly, one study reported a lower risk of side effects during D-mannose prophylaxis as compared to nitrofurantoin (RR 0. 276, 95% CI: 0.132–0.574, p<0.0001).8 We were unable to report a pooled analysis of AEs for D-mannose due to heterogeneity of reporting. When D-mannose was combined with other ingredients, side effects were either not reported or reported as insignificant, except in a dose-escalation study with mild to moderate gastrointestinal related AEs when attempting to reach a maximal target dose.13–16,30,31
Compliance with D-mannose use
Of the 8 studies in the SR, only two reported compliance. One study assessing rUTI in patients with multiple sclerosis (MS; n=22) reported participant compliance with twice daily D-mannose over 16 weeks.32 In MS patients not using catheters (n=10) mean compliance was 99.7% (range 97.8–100, median 100% [IQR 99.7–100]). Their comparator group (MS patients using catheters, reported as both n=11 and n=12 in different parts of the study) had a mean compliance of 99.4% (range 93.7–100, median 100% [IQR 100–100]). Overall, the cited reasons for non-compliance were failure to remember (n=2), generally feeling unwell (n=1), feeling sleepy (n=1), feeling sick (n=1), and seizure recurrence in an epileptic patient known to have frequent seizures (n=1). Another study assessing dose escalation of a 4:1 cranberry concentrate with ascorbic acid, D-mannose, fructo-oligosaccharides, and bromelain did not report exact compliance but stated that none of their 28 participants were withdrawn for compliance less than 80%.30
Other studies evaluating high risk patient populations
Certain patient characteristics are risk factors for developing rUTI, such as neurogenic bladders or hypoestrogenic state due to breast cancer therapy. Two of the 8 SR studies focused on these patient populations at higher risk for rUTI. One study was a single-center, open-label, feasibility study that enrolled patients with multiple sclerosis (MS) using (n=12) and not using (n=10) urinary catheters with rUTI (≥3 in 1 year or ≥2 in 6 months). Participants were given D-mannose powder 1.5 grams twice daily for 16-weeks. The number of monthly proven UTIs significantly decreased in both groups (P < 0.01), by 75% in the group not using catheters and by 63% in the group using catheters. Another study was an observational retrospective clinical study that was conducted on 60 patients with recurrent cystitis and breast cancer, but no detailed inclusion or exclusion criteria were presented. One group (n=40) included patients treated with D-mannose 500 mg, N-acetylcysteine 100 mg, and Morinda citrifolia fruit extract 200 mg (NDM) for a total of 6 months along with antibiotic therapy (either Fosfomycin, nitrofurantoin, or ciprofloxacin for acute cystitis episodes).33 The alternate group (n=20) only took antibiotics as needed. Of those in the D-mannose group, only 5 (12.5%) had positive urine culture at 2-month follow-up, whereas of those not using D-mannose, 18 (90%) had positive urine culture at 2-month follow-up.
Comment
The goal of this study was to compare D-mannose to other agents and placebo for rUTI prevention in adult women and to combine evidence for its effectiveness, side effects, and compliance. We performed a SR of 8 original research publications that met eligibility criteria. Ultimately, 3 of 8 studies in the SR had available data for further evaluation in a MA. Overall, our MA suggests that D-mannose is protective for recurrent UTI (versus placebo) with possibly similar effectiveness as antibiotics, but this should be interpreted in the setting of an overall small number of studies with varying study design and quality. D-mannose also appears well tolerated with minimal adverse side effects - only a small percentage experiencing diarrhea. There is a lack of studies evaluating D-mannose in the context of post-menopausal women with rUTI. The initial standard of care for these patients is to use vaginal estrogen therapy and none of the studies in the SR evaluated D-mannose in the presence of vaginal estrogen therapy use.
To the authors’ knowledge, this is the first peer-reviewed MA performed on D-mannose for rUTI prevention, which adds to the growing body of literature available on non-antibiotics and rUTI prevention. Our results add to our understanding of D-mannose and its use as a non-antibiotic prevention method for rUTI. Although the pooled patient cohorts are small, the results are promising for possible application of D-mannose to prevent rUTI, particularly when alternative options such as antibiotic suppression have significant limitations.
This SR also demonstrates the variation in D-mannose dosing used in studies. Among the studies evaluated, dosing ranged from as low as 420 mg daily to 2 g three times per day, with frequency of dosing ranging from daily to one week per month. In some countries, such as the United States, supplements are not regulated in the same way as prescription medications. This may add to difficulty of dose standardization for future studies. In addition to dosing, formulation of D-mannose for oral intake can be either a powder dissolved in liquid and then drank or a capsule. Future studies should investigate the bioavailability differences of powder versus capsule to see whether formulation alters efficacy.
Overall, D-mannose appears well tolerated with minimal adverse side effects - only a small percentage experiencing diarrhea or gastrointestinal upset. As with many other treatments, adherence to treatment appears to be related to patient tolerance of side effects, which can be assessed through reported adverse events. A 1997 study investigating potential therapies for Carbohydrate-Deficient Glycoprotein Syndrome (CDGS) type 1 demonstrated that half of the participants in their cohort of individuals without CDGS experienced gastrointestinal disturbances such as watery diarrhea and bloating 1–2 hours after ingestion of mannose doses greater than 0.2 g mannose/kg body weight.34 Two participants in the same study also noted dizziness at doses greater than 0.2 g mannose/kg body weight. When the dose was decreased to 0.15 g/kg body weight the percent of participants that experienced gastrointestinal disturbances decreased to 10%. Data from the National Health and Nutrition Examination Survey in 2015–2016 demonstrated the mean weight of women age 20 and older to be 77.3 kg (crude, age adjusted mean weight 77.4 kg).35 Using this mean weight, a weight based dose of 0.15 g/kg would mean a daily dose of just more than 11.5 g D-mannose. This is considerably higher than the total daily dose used by any of the studies included in this SR.
This SRMA is limited by the small number of studies with small sample size evaluating D-mannose to prevent rUTI in women. The heterogeneity of D-mannose intervention and dosing and even smaller number of studies that reported data that could be pooled further limited the MA. Addition of data from future studies evaluating D-mannose efficacy in rUTI prevention would strengthen findings of a MA. Limiting studies in the SRMA to those written in English may have added bias or caused data to be missed; however, we made the decision to limit to English language manuscripts as English is the primary language used for many medical publications.36 In addition, as many rUTI prevention regimens use multiple strategies, such as vaginal estrogen and oral antibiotic prophylaxis, further studies are needed to evaluate the effectiveness of D-mannose in combination with other methods of rUTI prophylaxis.
Conclusion
Before beginning our SRMA, we hypothesized that D-mannose would have similar efficacy to antibiotics for rUTI prevention, would be well tolerated, and would have good compliance. Consistent with this hypothesis, our MA results suggest that D-mannose is protective for rUTI (versus placebo) in adult women with possibly similar effectiveness as antibiotic prophylaxis. However, this must be interpreted in light of a relatively small number of studies with small sample sizes and varied study designs (1 RCT, 1 randomized cross-over trial, and 1 prospective cohort) and quality. Overall, D-mannose appears well tolerated with minimal adverse side effects - only a small percentage experiencing diarrhea. Compliance with D-mannose use was also high. Addition of data from future studies evaluating D-mannose efficacy in rUTI prevention would strengthen our findings.
AJOG at a Glance:
A. Why was this study conducted?
To systematically review and combine data from published original literature evaluating the effectiveness, side effects, and compliance of D-mannose for recurrent urinary tract infection prevention in adult women.
B. What are the key findings?
Small number of studies: 8 in systematic review with 3 in meta-analysis
In the meta-analysis, D-mannose appears protective for recurrent urinary tract infection versus placebo with possibly similar effectiveness as preventative antibiotics.
D-mannose appears well tolerated with minimal adverse side effects.
C. What does this study add to what is already known?
This is the first systematic review and meta-analysis of the effectiveness of D-mannose for recurrent urinary tract infection prevention.
Acknowledgements:
The authors thank Ratna Pakpahan for her assistance with organization of abstract search results.
Funding Source: Dr. Sutcliffe was supported by the Foundation for Barnes-Jewish Hospital, CTSA Grant UL1 TR002345, and the Alvin J. Siteman Cancer Center (P30 CA091842). Dr. Bertolet was supported through the National Institutes of Health Grant Number UL1TR001857. These funding sources were not involved in the research, collection, analysis, data interpretation, manuscript writing, or decision to submit the article for publication. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1 TR002345. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Paper Presentation information: An abstract of this paper was accepted for poster presentation at the Society of Gynecologic Surgeons 46th Annual Scientific Meeting (Jacksonville, FL, March 29-April 1, 2020).
Appendix:
Full search strategies used on February 5, 2018 and March 5, 2019 searches:
Ovid Medline 1946 to the present
(exp urinary tract infections/) OR (cystalgia) OR (exp cystitis/) OR (recurrent adj 1 urinary adj 1 tract adj 1 infection*) AND (exp mannose/) OR (exp mannosides/) OR (d-mannose)
Embase 1947 to the present
‘urinary tract infections’/exp OR ‘urinary tract infections’ OR ‘cystalgia’/exp OR cystalgia OR ‘recurrent next urinary next tract next infection*’ OR ‘cystitis’/exp OR cystitis and ‘mannose’/exp OR mannose OR ‘mannosides’/exp OR mannosides OR ‘d-mannose’/exp OR ‘d-mannose’
Scopus 1823 - to the present
(TITLE-ABS-KEY (“recurrent urinary tract infection*”) AND (mannose) OR (mannoside*)
Web of Science 1990-to the present
(“recurrent urinary tract infection*”) AND (mannose or mannoside*)
Cochrane Library
Urinary tract infections and mannose
Clinicaltrial.gov
Urinary tract infections and mannose
Updated full search strategies (based on peer review) used on April 15, 2020:
Ovid Medline (1946 to the present)
(exp urinary tract infections/) or (urinary adj1 tract adj1 infection*) or bacteriuria or pyuria or schistosomiasis haematobia or cystalgia or cystitis or pyelocystitis or exp cystitis/or (recurrent adj1 urinary adj1 tract adj1 infection*) AND (mannose/or mannose or mannosides or exp mannosides/or methylmannosides or d-mannose)
Embase (1947 to the present)
(‘urinary tract infections’/exp/mj OR ‘urinary tract infections’ OR ‘urinary tract infection*’ OR ‘cystalgia’ OR ‘cystalgia’/exp OR cystalgia OR ‘recurrent next urinary next tract next infection*’ OR ‘cystitis’ OR ‘cystitis’/exp OR cystitis OR ‘bacteriuria’/exp OR bacteriuria OR ‘pyuria’/exp OR pyuria OR ‘schistosomiasis next haematobia’ OR ‘pyelocystitis’/exp OR pyelocystitis) AND (‘mannose’ OR ‘mannose’/exp OR mannose OR ‘mannosides’ OR ‘mannosides’/exp OR mannosides OR ‘d-mannose’/exp OR ‘d-mannose’ OR ‘methylmannosides’/exp OR methylmannosides) AND [english]/limit
Scopus (1823 to the present)
TITLE-ABS-KEY (recurrent W/1 urinary W/1 tract W/1 infection*) OR TITLE-ABS-KEY (“urinary tract infection*”) OR TITLE-ABS-KEY (cystalgia) OR TITLE-ABS-KEY (cystitis) OR TITLE-ABS-KEY (bacterium) OR TITLE-ABS-KEY (pyuria) OR TITLE-ABS-KEY (schistosomiasis W/1 haematobia) OR TITLE-ABS-KEY (pyelocystitis) AND TITLE-ABS-KEY (mannose) OR TITLE-ABS-KEY (mannoside*) OR TITLE-ABS-KEY (d-mannose) OR TITLE-ABS-KEY (methylmannosides) AND (LIMIT-TO (LANGUAGE, “English”) Web of Science 386
Web of Science (1990-to the present)
TS=((“recurrent urinary tract infection”*) OR (recurrent NEAR/1 urinary NEAR/1 tract NEAR/1 infection*) OR (“urinary tract infection*”) OR (urinary NEAR/1 tract NEAR/1 infection*) OR cystitis OR bacteriuria OR pyuria OR (“schistosomiasis haematobia”) OR cystalgia OR pyelocystitis AND (mannose OR mannoside* OR methylmannosides))
Cochrane 17 trials
Urinary tract infection* AND mannose No limits
Clinical trials 10 trials
Search: urinary tract infection and mannose OR mannoside
No limits
Footnotes
Condensation:
D-mannose appears protective for recurrent UTI versus placebo, is possibly as effective as preventative antibiotics, and is well tolerated.
Short version of title: D-mannose systematic review and meta-analysis
Disclosure Statement: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American College of O, Gynecologists. ACOG Practice Bulletin No. 91: Treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111(3):785–794. [DOI] [PubMed] [Google Scholar]
- 2.Beerepoot M, Geerlings S. Non-Antibiotic Prophylaxis for Urinary Tract Infections. Pathogens. 2016;5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eells SJ, Bharadwa K, McKinnell JA, Miller LG. Recurrent urinary tract infections among women: comparative effectiveness of 5 prevention and management strategies using a Markov chain Monte Carlo model. Clin Infect Dis. 2014;58(2):147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7(12):653–660. [DOI] [PubMed] [Google Scholar]
- 5.Ciani O, Grassi D, Tarricone R. An economic perspective on urinary tract infection: the “costs of resignation”. Clin Drug Investig. 2013;33(4):255–261. [DOI] [PubMed] [Google Scholar]
- 6.Keating KN, Perfetto EM, Subedi P. Economic burden of uncomplicated urinary tract infections: direct, indirect and intangible costs. Expert Rev Pharmacoecon Outcomes Res. 2005;5(4):457–466. [DOI] [PubMed] [Google Scholar]
- 7.Renard J, Ballarini S, Mascarenhas T, et al. Recurrent Lower Urinary Tract Infections Have a Detrimental Effect on Patient Quality of Life: a Prospective, Observational Study. Infect Dis Ther. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kranjcec BP D: Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: A randomized clinical trial. World Journal of Urology. 2014;32(1):79–84. [DOI] [PubMed] [Google Scholar]
- 9.Gupta K, Trautner BW. Diagnosis and management of recurrent urinary tract infections in non-pregnant women. BMJ. 2013;346:f3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho JM, Juurlink DN. Considerations when prescribing trimethoprim-sulfamethoxazole. CMAJ 2011;183(16):1851–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarty M, Rosso JQ. Chronic administration of oral trimethoprim-sulfamethoxazole for acne vulgaris. J Clin Aesthet Dermatol. 2011;4(8):58–66. [PMC free article] [PubMed] [Google Scholar]
- 12.Aydin AA K: Zaman I: Khan MS: Dasgupta P. Recurrent urinary tract infections in women. International Urogynecology Journal. 2015;26(6):795–804. [DOI] [PubMed] [Google Scholar]
- 13.Blomgran R, Zheng L, Stendahl O. Uropathogenic Escherichia coli triggers oxygen-dependent apoptosis in human neutrophils through the cooperative effect of type 1 fimbriae and lipopolysaccharide. Infect Immun. 2004;72(8):4570–4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cusumano CK, Pinkner JS, Han Z, et al. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci Transl Med. 2011;3(109):109ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang D, Chia C, Jiao X, et al. D-mannose induces regulatory T cells and suppresses immunopathology. Nat Med. 2017;23(9):1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Popolo GN F. Recurrent bacterial symptomatic cystitis: A pilot study on a new natural option for treatment. Archivio Italiano di Urologia e Andrologia. 2018;9(2): 101–103. [DOI] [PubMed] [Google Scholar]
- 17.Bouckaert J, Berglund J, Schembri M, et al. Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Mol Microbiol. 2005;55(2):441–455. [DOI] [PubMed] [Google Scholar]
- 18.King SS, Young DA, Nequin LG, Carnevale EM. Use of specific sugars to inhibit bacterial adherence to equine endometrium in vitro. Am J Vet Res. 2000;61(4):446–449. [DOI] [PubMed] [Google Scholar]
- 19.Spaulding CN, Hultgren SJ. Adhesive Pili in UTI Pathogenesis and Drug Development. Pathogens. 2016;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones CHP JS: Roth R: Heuser J: Nicholes AV: Abraham SN: Hultgren SJ. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(6):2081–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan YT, Xu B, Rice K, Smith S, Jackson R, Elbein AD. Specificity of the high-mannose recognition site between Enterobacter cloacae pili adhesin and HT-29 cell membranes. Infect Immun. 1997;65(10):4199–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. [DOI] [PubMed] [Google Scholar]
- 24.Kontopantelis E, Springate DA, Reeves D. A re-analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta-analyses. PLoS One. 2013;8(7):e69930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price JR, Guran LA, Gregory WT, McDonagh MS. Nitrofurantoin vs other prophylactic agents in reducing recurrent urinary tract infections in adult women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215(5):548–560. [DOI] [PubMed] [Google Scholar]
- 26.Proietti S, Giannantoni A, Luciani LG, Sortino G, Graziotti P, Giusti G. Cystoman(R) and calculi: a good alternative to standard therapies in preventing stone recurrence. Urolithiasis. 2014;42(4):285–290. [DOI] [PubMed] [Google Scholar]
- 27.Palleschi G, Carbone A, Zanello PP, et al. Prospective study to compare antibiosis versus the association of N-acetylcysteine, D-mannose and Morinda citrifolia fruit extract in preventing urinary tract infections in patients submitted to urodynamic investigation. Arch Ital Urol Androl. 2017;89(1):45–50. [DOI] [PubMed] [Google Scholar]
- 28.Milandri R, Maltagliati M, Bocchialini T, et al. Effectiveness of D-mannose, Hibiscus sabdariffa and Lactobacillus plantarum therapy in prevention of infectious events following urodynamic study. Urologia. 2019;86(3):122–125. [DOI] [PubMed] [Google Scholar]
- 29.Cochrane Handbook for Systematic Reviews of Interventions: 10.4.3.1 Recommendations on testing for funnel plot asymmetry. https://handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asy mmetry.htm
- 30.Efros MB W: Cossu L: Nakeleski E: Katz AE. Novel concentrated cranberry liquid blend, UTI-STAT with Proantinox, might help prevent recurrent urinary tract infections in women. Urology. 2010;76(4):841–845. [DOI] [PubMed] [Google Scholar]
- 31.Domenici LM M: Bracchi C: Giorgini M: Colagiovanni V: Muzii L: Benedetti Panici P. D-mannose: a promising support for acute urinary tract infections in women. A pilot study. European Review for Medical & Pharmacological Sciences. 2016;20(13):2920–2925. [PubMed] [Google Scholar]
- 32.Phe VP M: Haslam C: Gonzales G: Curtis C: Porter B: Chataway J: Panicker JN. Open label feasibility study evaluating D-mannose combined with home-based monitoring of suspected urinary tract infections in patients with multiple sclerosis. Neurourology and Urodynamics. 2017;36(7):1770–1775. [DOI] [PubMed] [Google Scholar]
- 33.Marchiori DZ PP. Efficacy of N-acetylcysteine, D-mannose and morinda citrifolia to treat recurrent cystitis in breast cancer survivals. In Vivo. 2017;31(5):931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alton G, Kjaergaard S, Etchison JR, Skovby F, Freeze HH. Oral ingestion of mannose elevates blood mannose levels: a first step toward a potential therapy for carbohydrate-deficient glycoprotein syndrome type I. Biochem MolMed. 1997;60(2): 127–133. [DOI] [PubMed] [Google Scholar]
- 35.Fryar CD, Kruszon-Moran D, Gu Q, Ogden CL. Mean Body Weight, Height, Waist Circumference, and Body Mass Index Among Adults: United States, 1999–2000 Through 2015–2016. Natl Health Stat Report. 2018(122):1–16. [PubMed] [Google Scholar]
- 36.Baethge C. The languages of medicine. Dtsch ArzteblInt. 2008;105(3):37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porru DP A: Tinelli C: Barletta D: Choussos D: Di Franco C: Bobbi V: Bassi S: Miller O: Gardella B: Nappi RE: Spinillo A: Rovereto B. Oral D-mannose in recurrent urinary tract infections in women: A pilot study. Journal of Clinical Urology. 2014;7(3):208–213. [Google Scholar]
- 38.Genovese CD S: Mangano K: Tempera G: Nicolosi D: Corsello S: Vergalito F: Tartaglia E: Scapagnini G: Di Marco R. Effects of a new combination of plant extracts plus d-mannose for the management of uncomplicated recurrent urinary tract infections. Journal of Chemotherapy. 2017:1–8. [DOI] [PubMed] [Google Scholar]